Abstract
Objective
Systemic lupus erythematosus (SLE) is a multifaceted disease characterized by immune dysregulation and unpredictable disease activity. This study evaluated changes in plasma concentrations of soluble mediators preceding clinically-defined disease flares.
Methods
Soluble mediators (n=52) were examined, including cytokines, chemokines, and soluble receptors, using validated multiplex bead-based or enzyme-linked immunosorbent assays in plasma from European American SLE patients who developed disease flare 6 or 12 weeks after baseline assessment were compared to 28 matched SLE patients without impending flare and 28 matched healthy controls (n=84). For a subset, mediators within samples preceding SLE disease flare and during a clinically stable period from the same individual were compared.
Results
Compared to clinically stable patients, patients with impending flare had significant (p≤0.01) alterations in 27 soluble mediators at baseline with significantly higher levels of pro-inflammatory mediators, including Th1, Th2, and Th17-type cytokines, several weeks before clinical flare. Baseline levels of regulatory cytokines, including IL-10 and TGF-β, were higher in non-flare SLE patients, while baseline levels of soluble TNFRI, TNFRII, Fas, FasL, and CD40L were significantly greater in pre-flare patients (p≤0.002). A normalized and weighted combined soluble mediator score was significantly higher in pre-flare SLE patients versus those with stable disease (p≤0.0002).
Conclusion
Pro-inflammatory adaptive cytokines and shed TNF receptors, are elevated prior to disease flare, while regulatory mediators are elevated during periods of stable disease. Alterations in the balance between inflammatory and regulatory mediators may help identify patients at risk of disease flare and help decipher SLE pathogenic mechanisms.
Keywords: SLE, disease flare, cytokines
Systemic lupus erythematosus (SLE) is a multifaceted autoimmune disease characterized by variable immune dysregulation, disabling symptoms and progressive organ damage (1). Given the heterogeneous nature of SLE, recognition and early treatment to prevent tissue and organ damage is clinically challenging. Validated disease activity clinical instruments assess and weigh changes in signs and symptoms within each organ system. The Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI (2)) is a reliable measure of clinical disease activity (1). However, the traditional biomarkers incorporated in the SELENA-SLEDAI are not necessarily the earliest, or sufficient biologic signals of worsening disease. In addition, proposed serologic markers of disease activity, alone or in combination, including anti-dsDNA, complement, complement split products, and inflammatory markers (ESR and CRP), are limited in their clinical utility due to inconsistent correlation with disease activity (3, 4). Despite clinical instruments of disease activity and improved treatment regimens to temper chronic inflammation, SLE patients may experience an average of 1.8 disease flares annually (5). Treatment typically relies on rapidly acting, toxic agents such as steroids. Earlier identification and treatment of flares might prevent significant organ damage and improve the quality of life for patients with SLE (6). Further, uncovering early markers of clinical flares may provide mechanistic insight to improve the development and selection of targeted preventative treatments.
Certain cytokines and chemokines are known to be involved in SLE pathogenesis and disease flare. IL-6, TNF-α, and IL-10, as well as Th1 and Th2 type cytokines, are implicated in SLE disease activity (7–9); elevated IL-12 has been detected prior to disease flare (10). Th17 pathway mediators are elevated in samples from SLE patients with increased disease activity (11) and sequelae, including cutaneous (12), serositis (12), and renal (13) manifestations. In addition to an increase in inflammatory mediators with elevated disease activity, regulatory pathways are diminished, including decreased TGF-β (14) and the potential for altered T-regulatory cell populations (15–19) and/or activity (20), suggesting an imbalance between inflammatory and regulatory mediators in promoting flares (21). This study builds on previous work by concurrently evaluating soluble inflammatory and regulatory mediators in the context of altered disease activity with ensuing SLE disease flare.
In addition to soluble mediators of inflammation, SLE flares might also involve altered regulation of membrane-bound or soluble receptors expressed by activated immune cells (7). Members of the TNF(R)eceptor superfamily act as co-stimulatory molecules on B and T-lymphocytes and form a prototypic pro-inflammatory system (reviewed in (22)). The ligand/receptor pairings are either membrane bound or can be cleaved by proteases as soluble proteins that cluster as trimers to either block ligand/receptor interactions or to initiate receptor-mediated signal transduction. Multiple members of the TNFR superfamily are implicated in SLE. The classical ligand TNF-α interacts with two TNFRs, TNFRI (p55) and TNFRII (p75), both of which have been associated with altered SLE disease activity (7). In addition, expression and cleavage of Fas, FasL (23), and CD40L [CD154] (24) are increased in SLE patients. BLyS and APRIL, key regulators of B cell survival and differentiation, are important SLE therapeutic targets (25). In a study of 245 SLE patients followed for two years, with power to account for some confounding factors such as medications, increased BLyS levels associated with increased disease activity (4, 26). Furthermore, a neutralizing anti-BLyS monoclonal antibody can reduce risk of disease flare over time (27), suggesting that BLyS may help regulate disease activity in some patients (28). However, their roles in ensuing disease flares are presently unknown.
This study explores the inflammatory and regulatory pathways potentially dysregulated early in lupus flare before clinical symptoms are reported. Plasma samples and clinical data were evaluated from SLE patients and matched, healthy controls participating in the SLE Influenza Vaccination Cohort (29). Using an xMAP multiplex approach, European-American (EA) SLE patients with impending disease flare 6 or 12 weeks after vaccination were found to have increased pre-flare inflammatory adaptive cytokines, chemokines, and shed TNFR superfamily members, with decreased regulatory mediators of inflammation, compared to matched patients with stable disease. These results enabled the development of a combined soluble mediator score that reflects pre-flare immune status in SLE patients who go on to flare.
METHODS
Study population
Experiments were performed in accordance with the Helsinki Declaration and approved by the Institutional Review Boards of the Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center. Study participants were enrolled in the SLE Influenza Vaccination Cohort (29) after written informed consent. Female EA SLE patients (meeting ≥ 4 ACR classification criteria (29)) with disease flare 6–12 weeks post-vaccination (age 46.9 ± 14.0 years, n=28, Supplementary Table 1) were matched by age (± 5 years), race, gender, and time of disease assessment to patients with stable disease (age 47.2 ± 12.3 years, n=28, Supplementary Table 1), as well as unrelated healthy controls (age 46.8 ± 13.5 years, n=28). Samples from 13 SLE patients pre-flare were compared to samples drawn from the same individuals in a different year with no associated SELENA-SLEDAI flare, Supplementary Table 1.
Clinical data and sample collection
Demographic and clinical information were collected as previously described (29), including humoral response to influenza vaccination, medication usage, clinical laboratory values, disease activity, and SELENA-SLEDAI defined flare (Supplementary Table 1); severe flares were uncommon and not assessed independently (29). Patients were evaluated at baseline/pre-vaccination and 6 and 12 weeks post-vaccination for disease activity by SELENA-SLEDAI (29). Blood was collected from each participant before vaccination, and at 2, 6, and 12 weeks after vaccination. Plasma was isolated and stored at −20°C until further use.
Soluble analyte determination
Plasma levels of BLyS (R&D Systems, Minneapolis, MN) and APRIL (eBioscience/Affymetrix, San Diego, CA) were determined by enzyme-linked immunosorbent assay (ELISA), per the manufacturer protocol. An additional fifty analytes, including innate and adaptive cytokines, chemokines, and soluble TNFR superfamily members (Supplementary Table 2), were assessed by xMAP multiplex assays (Panomics/Affymetrix, Santa Clara, CA) (30).
Data were analyzed on the Bio-Rad BioPlex 200® array system (Bio-Rad Technologies, Hercules, CA), with a lower boundary of 100 beads per sample/analyte. Median fluorescence intensity for each analyte was interpolated from 5-parameter logistic nonlinear regression standard curves. Analytes below the detection limit were assigned a value of 0.001 pg/mL. Well-specific validity was assessed by AssayCheX™ QC microspheres (Radix BioSolutions, Georgetown, TX, USA) to evaluate non-specific binding. A known control serum was included on each plate (Cellgro human AB serum, Cat#2931949, L/N#M1016). Mean inter-assay coefficient of variance (CV) of multiplexed bead-based assays for cytokine detection has previously been shown to be 10–14% (31, 32), and a similar average CV (10.5%) across the analytes in this assay was obtained using healthy control serum. Intra-assay precision of duplicate wells averaged <10% CV in each 25-plex assay.
Statistical Analysis
Plasma mediator concentration
Concentrations of plasma mediators were compared between pre-flare SLE patients and matched non-flare patients or self non-flare samples by Wilcoxon matched-pairs test and adjusted for multiple comparisons using the False Discovery Rate (FDR) via the Benjamini-Hochberg procedure (using R version 2.15.3). Differences between pre-flare patients, matched non-flare patients or self non-flare samples, and matched healthy controls were determined by Friedman test with correction by Dunn’s multiple comparison. Except where noted, analyses were performed using GraphPad Prism 6.02 (GraphPad Software, San Diego, CA).
Soluble mediator score
To compare the overall level of inflammation in pre-flare vs. non-flare SLE patients (at baseline/pre-vaccination) in relationship to disease activity at flare (post-vaccination), a soluble mediator score was derived by the cumulative contribution of all pre-flare 52 plasma mediators assessed in relationship to SELENA-SLEDAI disease activity at flare, following an approach previously used for rheumatoid arthritis (33). Briefly, the concentration of all 52 plasma analytes were log-transformed and standardized; (observed value)-(mean value of all SLE patients assessed [Flare and NF or SNF])/(standard deviation of all SLE patients assessed [Flare and NF or SNF]). Spearman coefficients of each analyte were generated from a linear regression model testing associations between the flare SELENA-SLEDAI disease activity scores and each pre-flare soluble mediator. The transformed and standardized soluble mediator levels were weighted by the respective Spearman coefficients and summed for a total, global soluble mediator score (33). By generating the weights, the inflammatory mediators that were most differentially altered at baseline between pre-flare and non-flare SLE patients in their associations with SELENA-SLEDAI scores at time of disease flare contributed most to the score and therefore the overall level of inflammation correlating with disease flare, Supplementary Tables 3–4.
RESULTS
Inflammatory mediators and regulatory cytokines are altered prior to SLE disease flare
SLE patients within our cohort were followed longitudinally and evaluated for evidence for SELENA-SLEDAI disease flare. We hypothesized that clinical changes in disease activity are the result of a perturbation in the already dysregulated immune system of SLE patients. To test whether markers of immune dysregulation might precede clinical disease flares, 52 soluble analytes were compared in 28 EA SLE patients in whom flare was detected after influenza vaccination, matched SLE patients who did not experience flare for at least 12 weeks post-vaccination, and matched healthy individuals. All SLE patients, with or without subsequent flare, had similar SELENA-SLEDAI scores (3.8 ± 3.7 flare vs. 2.6 ± 3.2 non-flare [NF], p = 0.2451 by Wilcoxon matched-pairs test) and ESR levels (25.5 ± 21.3 flare vs. 16.8 ± 9.6 NF, p = 0.0870 by Wilcoxon match-pairs test) at baseline (Supplementary Table 1).
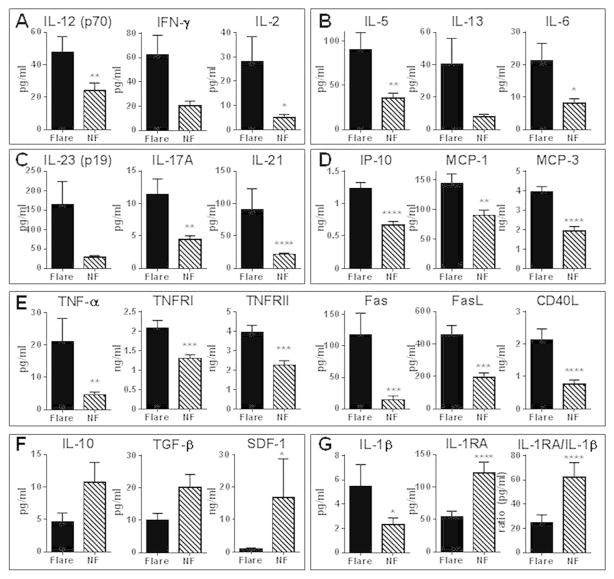
At baseline and follow-up, non-flare SLE patients had levels of T cell mediators IFN-γ (Th1), IL-13 (Th2), as well as IL-17A and IL-21 (Th17), that were similar to those in healthy controls, despite significantly higher levels of cytokines from antigen presenting cells (APC), including IL-12, IL-5, and IL-6 (Supplementary Figure 1A–C). However, in those who later experienced a flare, baseline levels of several proinflammatory mediators were increased (Figure 1), including Th1, Th2, and Th17 type cytokines (Figure 1A–C and Supplementary Table 3). Patients with impending flare also had higher baseline levels of IP-10, MCP-1, and MCP-3 (Figure 1D), as well as IL-8 and soluble ICAM-1 (Supplementary Figure 1H). While levels of soluble TNF receptors TNFRI and TNFRII and CD40L were increased in all SLE patients compared to healthy controls (Supplementary Figure 1E), baseline levels of several soluble TNF superfamily members, including TNFRI, TNFRII, TNF-α, Fas, FasL, and CD40L, were significantly higher in patients with subsequent flare compared to non-flare patients (Figure 1E and Supplementary Table 3).
Figure 1.
Increased adaptive immunity pathways and soluble TNF superfamily members, and decreased levels of regulatory mediators, in SLE patients with impending flare. Plasma was procured at baseline from SLE patients who exhibited disease flare 6 to 12 weeks later (black bar) and demographically matched SLE patients who did not exhibit flare (NF, stripped bar). Levels of Th1 (A), Th2 (B), and Th17 (C) type cytokines, as well as chemokines (D), soluble TNF superfamily members (E), regulatory mediators (F), and IL-1RA:IL-1β ratio (G) in 56 EA SLE patients (mean ± SEM) were measured. Significance was determined by Wilcoxon matched-pairs test. * p< 0.05, **p < 0.01, *** p < 0.001, **** p, 0.0001
In contrast to proinflammatory mediators, regulatory cytokines were higher in stable SLE patients compared to patients with subsequent flare or to healthy controls. At baseline (Figure 1F) and follow-up (Supplementary Figure 1F), patients with no flare within 12 weeks had relatively higher levels of regulatory cytokines IL-10 and TGF-β and chemokine SDF-1 compared to both SLE patients with subsequent flare (Figure 1F) and healthy controls (Supplementary Figure 1F). Furthermore, the balance between inflammatory (IL-1α and IL-1β) and regulatory (IL-1 receptor antagonist; IL-1RA) IL-1 family cytokines was significantly altered. Plasma levels of IL-1α and IL-1β were significantly higher in pre-flare compared to non-flare SLE patients (Figure 1G and Supplementary Figure 1H), while non-flare patients had a 2–3 fold mean increase in plasma IL-1RA compared to SLE patients with flare (Figure 1G and Supplementary Table 3) and healthy individuals (Supplementary Figure 1G). IL-1RA levels were similar in pre-flare patients and matched healthy controls (Supplementary Figure 1G). IL-1 receptor antagonist (IL-1RA) downregulates IL-1 mediated immune activation, binding to IL-1 receptor type I (IL-1R1) and preventing binding of IL-1 and subsequent signaling through the receptor (reviewed in (34)). Given that an increased circulating IL-1RA:IL-1β ratio would favor an anti-inflammatory state (34), the mean 2.5- and 3.2-fold increase in IL-1RA:IL-1β ratio in non-flare patients compared to pre-flare SLE patients (Figure 1G) and healthy individuals (Supplementary Figure 1G), respectively, implicates an enhanced, regulatory anti-inflammatory state in stable periods of SLE.
Plasma mediator patterns differ in the same patient during stable vs. pre-flare periods
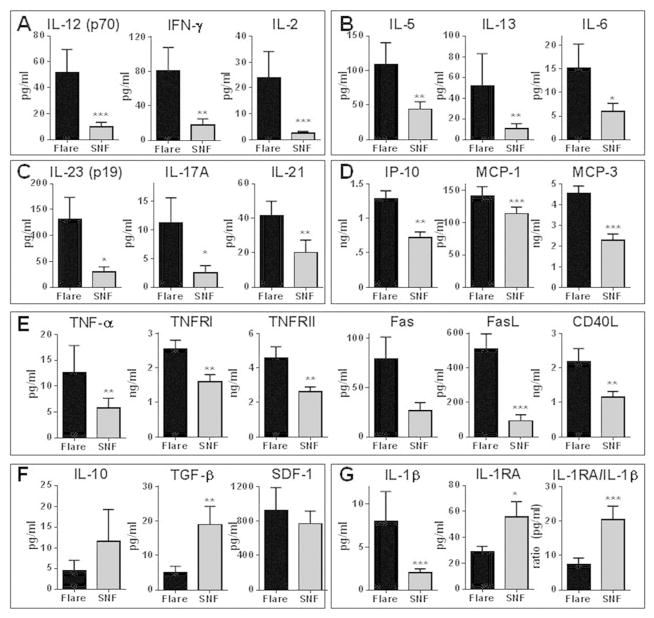
Of the 28 patients with impending flare, 13 participated in the study in multiple years and had at least one flare and one non-flare year. No significant difference in baseline SELENA-SLEDAI scores (3.0 ± 4.3 flare vs. 2.9 ± 2.0 self non-flare [SNF], p = 0.7065) or ESR levels (31.3 ± 23.0 flare vs. 27.0 ± 21.4 SNF, p = 0.5967) preceded a flare compared to an observed non-flare period in the same patients (Supplementary Table 1). In contrast, consistent with the results above, levels of several inflammatory mediators varied between pre-flare and non-flare periods (Figure 2 and Supplementary Table 4). Impending flares were associated with increased Th1, Th2, and Th17 (Figure 2A–C) type cytokines, compared to both self non-flare and matched healthy control samples (Supplementary Figure 2A–C). In addition, levels of plasma IP-10, MCP-1 and MCP-3 (Figure 2D), along with IL-8 and ICAM-1 (Supplementary Figure 2H), were significantly elevated in pre-flare periods compared to periods of stable disease. Levels of T-lymphocyte secreted IL-2, IFN-γ, IL-5, IL-13, and the Th17 type cytokines were similar in healthy controls and SLE patients during non-flare periods (Supplementary Figure 2A–C), while APC-secreted IL-12 and IL-6 were higher in SLE patients in both pre-flare and non-flare periods compared to matched healthy controls (Supplementary Figure 2A–C).
Figure 2.
SLE patients have altered baseline mediators in adaptive immunity pathways and soluble TNF superfamily members during pre-flare periods compared to the same patients during non-flare periods. Plasma was procured at baseline from 13 SLE patients who exhibited disease flare 6 to 12 weeks later (black bar) and from the same patients in a separate year of the study when they did not exhibit disease flare (SNF, gray bar). Plasma Th1 (A), Th2 (B), and Th17 (C) type cytokines, as well as chemokines (D), soluble TNF superfamily members (E), regulatory mediators (F), and IL-1RA:IL-1β ratio (G) were measured (mean ± SEM). Significance was determined by Wilcoxon matched-pairs test. * p< 0.05, **p < 0.01, *** p < 0.001, **** p, 0.0001
During non-flare periods, levels of soluble TNF-α and Fas in SLE patients were not significantly different than in matched healthy controls, while TNFRI, TNFRII, FasL, and CD40L were persistently elevated in SLE patients regardless of impending flare (Supplementary Figure 2E). Compared to periods of stable disease, pre-flare periods were marked by increases in soluble TNF-α and sFas (nonsignificant) and further increases in TNFRI, TNFRII, FasL, and CD40L (Figure 2E and Supplementary Table 4). Levels of SDF-1 were similar during pre-flare and non-flare periods. TGF-β significantly decreased during pre-flare periods, but the apparent decrease in IL-10 levels was not significant (Figure 2F). A significant pre-flare increase in IL-1β and decrease in IL-1RA resulted in a 2.7-fold decrease in the IL-1RA:IL-1β ratio compared to stable periods in the same patient (Figure 2G). Thus, when followed longitudinally, an altered balance of proinflammatory and regulatory cytokines precedes SLE flares.
Not all inflammatory mediators increase prior to flare
BLyS and APRIL, TNFR superfamily ligands that support B cell survival, differentiation and autoantibody production (35), were increased in SLE patients compared to healthy controls at baseline (Figure 3) and follow-up (data not shown). However, levels of these mediators were not different between pre-flare and non-flare patients in this study. Levels of IL-15 and IL-2Rα (CD25), along with MIG, MIP-1α, and MIP1-β, were also similar between both groups of SLE patients and higher in SLE patients than healthy controls (Figure 3). In addition there was no difference between SLE patients and healthy controls for 16 assessed analytes (Supplementary Table 2).
Figure 3.
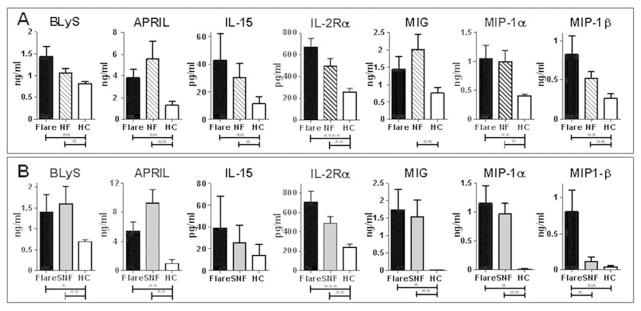
Soluble mediators of inflammation in SLE patients which are elevated compared to healthy controls, but which do not discriminate between impending disease flare and non-flare. Plasma levels of BLyS, APRIL, IL-15, IL-2Rα, MIG, MIP-1α, and MIP-1β were measured and compared between (A) pre-flare SLE patients (black bar), matched non-flare SLE patients (NF, striped bar), and matched healthy controls (HC, white bar) or (B) SLE patients during a pre-flare period (black bar), the same SLE patients during a non-flare period (SNF, gray bar), and matched healthy controls (HC, white bar). Data are shown as mean ± SEM; significance between SLE patients (Flare and NF/SNF) and HC was determined by Wilcoxon matched-pairs test. * p< 0.05, **p < 0.01, *** p < 0.001, **** p, 0.0001
A weighted global soluble mediator score correlates with impending flare
To determine the correlation and relative contribution of pre-flare inflammatory and regulatory soluble analytes to SLE disease flare risk, we developed a combined soluble mediator score based on a previously described approach used to identify individuals at increased risk of developing rheumatoid arthritis (33). A distinct advantage of this approach is that it does not require cut-offs for each cytokine/chemokine to establish positivity, and gives impact to those untransformed pre-flare analytes with stronger correlations (Spearman correlation coefficients) to disease activity at time of flare (Supplementary Tables 3–4, center panel). Twenty-eight of 52 pre-flare analytes assessed, as well as the IL-1RA:IL-β ratio, were significantly altered in SLE patients with impending flare compared to matched, non-flare patients, or the same patients during a non-flare period (with 25 of 52 significant after controlling for false discovery rate; Supplementary Tables 3–4, far left panel).
The soluble mediator score was significantly higher in SLE patients with impending flare versus matched stable patients (median soluble mediator score 4.14 [pre-flare] vs. −1.70 [non-flare], p< 0.0001; Table 1A and Figure 4A) or from the same patients during non-flare periods (median soluble analyte score 7.41 [pre-flare] vs. −3.09 [self non-flare], p= 0.0002; Table 1B and Figure 4B–C). Compared to stable patients or to non-flare periods in the same patients, pre-flare patients were 13.8 or 11.1 times more likely, respectively, to have a positive soluble mediator score (Table 1). Twenty-one of 28 SLE patients with impending flare have positive soluble mediator scores, while 23/28 non-flare SLE patients have negative soluble mediator scores (Figure 4A). Ten of 13 SLE patients with samples available at pre-flare and non-flare periods have positive soluble mediator scores at time of impending flare (Figure 4B), all of which decrease during a comparable periods of non-flare (Figure 4C).
Table 1.
Association between Soluble Mediator Score and SLE Disease Activity
| Soluble Mediator Score
|
|||||||
|---|---|---|---|---|---|---|---|
| Median | SD | p valuea | ORb | 95% CI | P valuec | ||
|
| |||||||
| A. | Flare subjects (n = 28) | 4.14 | 4.40 | < 0.0001 | 13.8 | 3.79 to 50.2 | < 0.0001 |
| NF subjects (n = 28) | −1.70 | 4.64 | |||||
|
| |||||||
| B. | Flare subjects (n = 13) | 7.41 | 8.12 | 0.0002 | 11.1 | 1.79 to 68.9 | 0.0169 |
| SNF subjects (n = 13) | −3.09 | 8.47 | |||||
A. SLE patients with flare vs. non-flare [NF] post-vaccination); B. SLE patients with flare vs. a self non-flare (SNF) period.
Wilcoxon Matched-Pairs test (2-tailed);
Odds Ratio (# of Flare vs. NF [or SNF] subjects with positive or negative soluble analyte score);
Fisher’s Exact test (2-tailed)
Figure 4.
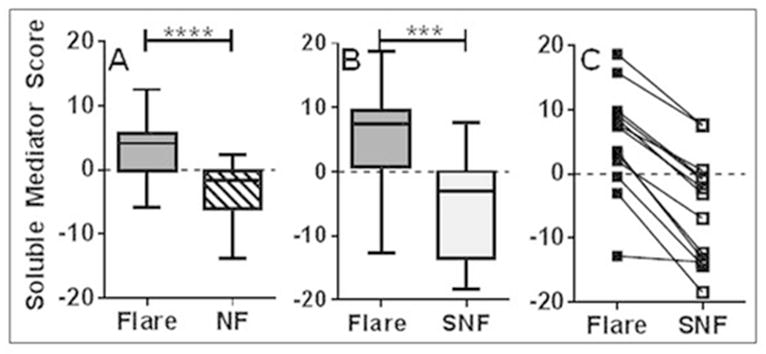
Higher Soluble Mediator Scores in SLE patients with impending flare. Soluble Mediator Scores from baseline (pre-vaccination) plasma levels were determined for each SLE patient who exhibited disease flare within the following 12 weeks relative to (A) a demographically matched SLE patient who did not exhibit disease flare (NF, p < 0.0001 by Wilcoxon matched-pairs test) or (B) the same SLE patient in a separate year of the study with no observed disease flare (SNF, p = 0.002 by Wilcoxon matched-pairs test). Data presented as Box and Whisker (median ± max and min) graphs. C. Soluble Mediator Scores for each SLE patient were compared between year of impending disease flare (Flare) and year of non-flare (SNF) in B.
DISCUSSION
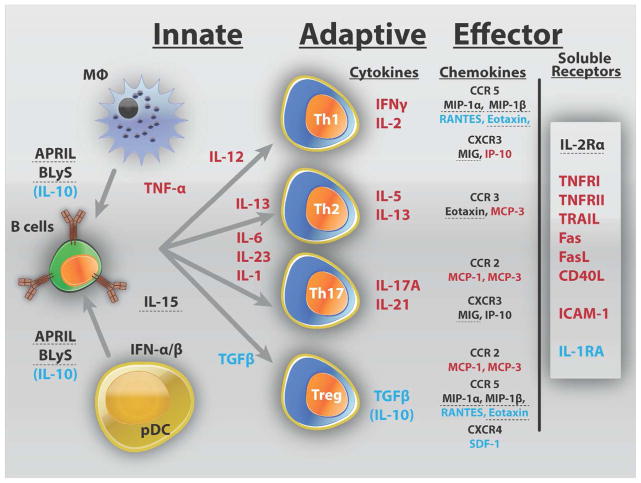
Delays in treating SLE flares may potentiate chronic inflammation, leading to recurrent illness and end-organ damage. Immune dysregulation in SLE likely precedes clinical disease, and in some cases, low grade active inflammation could persist over time, contributing to progressive organ damage in the absence of overt clinical flare. Our data point to the nature of the immune response that either leads to impending disease flare (inflammation) or allows for periods of non-flare (regulation). In this study, elevated levels of shed TNF receptors and/or pro-inflammatory Th adaptive pathway cytokines were found in nearly all SLE patients prior to impending flare (Figure 5). While a predominant inflammatory pathway is evident for a subset of patients, a number of patients had elevated inflammatory mediators from multiple pathways, which may help explain variability among previous reports of inflammatory mediators in SLE patients with active disease (9, 10, 12). In this study, regulatory factors were less likely to be elevated prior to a flare, suggesting an altered balance of inflammatory and regulatory mediators.
Figure 5.
Summary of altered soluble mediators in SLE patients prior to disease flare. Inflammatory mediators which were significantly higher in SLE patients with impending disease flare (compared to NF/SNF and HC) are listed in red, while those significantly higher in the NF/SNF groups (compared to pre-flare and HC) are listed in blue. Those mediators which were found to be higher in SLE patients compared to HC, but not different between groups of SLE patients, are dashed. SLE patients with impending disease flare have increased innate and adaptive mediators of inflammation, including those from Th1, Th2, and Th17 pathways. In addition inflammatory chemokines and soluble TNFR superfamily members are elevated. SLE patients who are in a period of non-flare (NF/SNF groups) have higher regulatory mediators, including IL-10, TGF-β, and IL-1RA
Significantly higher levels of IL-1β and IL-1α, as well as lower levels of IL-1RA, preceding a disease flare suggest that diminished downregulation of innate cytokines may contribute to increased disease activity during SLE flares. The IL-1RA/IL-1β ratio may reflect changes in either inflammatory IL-1β or regulatory IL-RA levels. Pre-flare SLE patients have significantly higher IL-1β levels than either non-flare SLE patients or the same SLE patients during a non-flare period (SNF), as well as HC (non-flare SLE patients have similar levels of IL-1β as HC). The opposite is true with respect IL-1RA, which are significantly higher in SLE patients during non-flare periods (pre-flare SLE patients have IL-1RA levels similar to HC). That HC individuals do not have altered IL-1β or IL-1RA levels highlights the state of immune dysregulation in SLE patients that is further unbalanced in the pre-flare state. Additionally, T-regulatory cells require TGF-β and IL-10 for their development and propagation (reviewed in (36)). In this study, the lower levels of TGF-β and IL-10, with increased inflammatory cytokines, may reflect a failure of active regulation in the period before disease flare. IL-10 and TGF-β levels are higher in stable SLE patients, suggesting the possibility of context-dependent regulatory roles for these cytokines. Future studies will assess whether SLE patients with impending flare have varied numbers or function of T-regulatory cells (15–19), or possibly T-effector cells that are resistant to T-regulatory cell influence (20).
TNFR superfamily members are a context-dependent group of ligand-receptor pairs (22) and we detect significantly elevated levels of soluble members, including TNF-α and its receptors TNFRI and TNFRII, Fas and FasL, and CD40L/CD154 in pre-flare SLE patients. Ectodomain shedding of TNFR family members occurs through the activation of ADAM (a disintegrin and metalloprotease) family members, most notably ADAM-17 (TNF-α converting enzyme [TACE]), which is upregulated in response to cellular activation (37). TNFRI and TNFRII shedding suggests a reactive process to cellular activation in SLE patients with impending flare. Soluble TNF-α interacts primarily with TNFRI on a variety of cell types (22). TNFRII, activated optimally by membranous TNF-α (22), lowers the threshold of activation on T-effector cells, while contributing to the suppressive function of T-regulatory cells (38), in part from TNFRII shedding (39).
While individual markers may not universally correlate with development of flares, the overall balance between inflammatory and regulatory mediators could be a predictor of impending flares. Varying pre-flare levels of specific soluble plasma mediators were significantly altered in SLE patients who flared and/or correlated with disease activity at time of disease flare (Supplementary Tables 3–4). The normalized, weighted, soluble inflammatory mediator score developed in this study enhanced our ability to discern factors that significantly contributed to downstream clinical sequelae in SLE patients with impending flare (Table 1 and Figure 4). Although our findings are promising, further study of outliers and how to recognize them may be warranted. This is evidenced by the finding that 7 of 28 pre-flare SLE patients had negative soluble mediator scores and 5 of 28 non-flare SLE patients had a positive soluble mediator scores. However, all of the 13 SLE patients with disease flare followed longitudinally had decreased soluble mediator scores during periods of non-flare. Future evaluation of inflammatory and regulatory mediators in the soluble mediator score utilizing larger, diverse cohorts will allow for the consideration of race and age in the combined mediator score.
In addition, future refinement of the combined mediator score utilized in SLE patients longitudinally must also address the effects of real-life parameters that may perturb immune regulation, such as medication regimens, infections, or vaccinations. No significant difference was found in the SLE-specific medications utilized by pre-flare vs non-flare SLE patients, nor in the same SLE patients between pre-flare and non-flare periods of assessment, Supplementary Table 1. The current study utilizes samples from SLE patients and matched healthy controls collected prior (pre-flare) and subsequent to vaccination (flare time point). Although it is possible that some of the SLE patient disease flares could be associated with vaccination, we previously demonstrated the rate of flare in this vaccination cohort is similar to that of non-vaccination cohort studies (29) and others have demonstrated no increase in flare rate with vaccination (40, 41). Further, we see limited difference in soluble mediators between pre-flare (pre-vaccination) and flare (follow-up) time points in SLE patients who flare at either 6 or 12 weeks post-vaccination, suggesting that vaccination does not contribute to immune perturbations that lead to disease flare This is further supported by similar pre- and post-vaccination (6 or 12 weeks) soluble mediator levels in SLE patients during non-flare periods, as well as in matched healthy controls.
If larger prospective studies validate this approach, an optimized mediator score could become a valuable prognostic tool in experimental SLE trials and in lupus clinical care. Such an approach has been validated in RA (42), and holds promise for improved clinical management of disease activity (43). For SLE patients with stable disease and relatively low risk of impending flare, it may be relatively safe to reduce treatments with significant side effects. Depending on the comprehensive clinical picture of an individual patient, early detection of risk for SLE flare could prompt closer monitoring, preventative treatments, or inclusion in clinical trials for targeted biologics relevant to pathways altered within the mediator score. In the future, chronic suppression of critical flare pathways and/or augmentation of regulatory pathways might promote longer periods of remission, decreased accumulation of organ damage over time, and better quality of life for SLE patients.
Supplementary Material
Acknowledgments
Grant support: This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute of Allergy and Infectious Diseases with co-funding by the Office of Research on Women’s Health, and the National Institute of General Medicinal Sciences of the National Institutes of Health under award numbers P30AR053483, U19AI082714, U01AI101934, P30GM103510, S10RR026735, and HHSN266200500026C.
We would like to thank all of the study participants for their time and commitment to the study. We would like to thank the referring physicians and Amy Dedeke, MD for their assistance. We would also like to thank Virginia Roberts, Jourdan Anderson, and Wade DeJager for clinical or technical assistance. Finally, we would like to thank J. Donald Capra, MD for critically reading the manuscript, Julie M. Robertson, PhD for scientific editing, and Dustin Fife, PhD for review of statistical analyses.
Footnotes
Disclosure: The authors report no conflicts of interest
AUTHOR CONTRIBUTIONS
JMG, LFT, JTM, and JAJ assembled the SLE Influenza Vaccination Cohort and assembled part of the dataset. ESV, JTM, and JAJ collected clinical patient data. MEM designed and carried out the soluble mediator experiments. MEM and JTM carried out the statistical analysis. All participating authors were involved in the writing, revision and approval of the manuscript. All authors read and approved the manuscript.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Lam GK, Petri M. Assessment of systemic lupus erythematosus. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S120–32. [PubMed] [Google Scholar]
- 2.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 3.Liu CC, Ahearn JM. The search for lupus biomarkers. Best Pract Res Clin Rheumatol. 2009;23(4):507–23. doi: 10.1016/j.berh.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petri MA, van Vollenhoven RF, Buyon J, Levy RA, Navarra SV, Cervera R, et al. Baseline Predictors of Systemic Lupus Erythematosus Flares: Data From the Combined Placebo Groups in the Phase III Belimumab Trials. Arthritis Rheum. 2013;65(8):2143–53. doi: 10.1002/art.37995. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Singh S, Tesfasyone H, Malik A. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol. 2009;36(11):2476–80. doi: 10.3899/jrheum.090019. [DOI] [PubMed] [Google Scholar]
- 6.Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol. 2009;5(7):400–4. doi: 10.1038/nrrheum.2009.106. [DOI] [PubMed] [Google Scholar]
- 7.Davas EM, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis PC. Serum IL-6, TNFalpha, p55 srTNFalpha, p75srTNFalpha, srIL-2alpha levels and disease activity in systemic lupus erythematosus. Clin Rheumatol. 1999;18(1):17–22. doi: 10.1007/s100670050045. [DOI] [PubMed] [Google Scholar]
- 8.Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27(5):461–6. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 9.Gomez D, Correa PA, Gomez LM, Cadena J, Molina JF, Anaya JM. Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factor alpha protective? Semin Arthritis Rheum. 2004;33(6):404–13. doi: 10.1016/j.semarthrit.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Tokano Y, Morimoto S, Kaneko H, Amano H, Nozawa K, Takasaki Y, et al. Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE)--relation to Th1- and Th2-derived cytokines. Clin Exp Immunol. 1999;116(1):169–73. doi: 10.1046/j.1365-2249.1999.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(2):R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok MY, Wu HJ, Lo Y, Lau CS. The Relation of Interleukin 17 (IL-17) and IL-23 to Th1/Th2 Cytokines and Disease Activity in Systemic Lupus Erythematosus. J Rheumatol. 2010 doi: 10.3899/jrheum.100293. [DOI] [PubMed] [Google Scholar]
- 13.Chen DY, Chen YM, Wen MC, Hsieh TY, Hung WT, Lan JL. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus. 2012;21(13):1385–96. doi: 10.1177/0961203312457718. [DOI] [PubMed] [Google Scholar]
- 14.Becker-Merok A, Eilertsen GO, Nossent JC. Levels of Transforming Growth Factor-{beta} Are Low in Systemic Lupus Erythematosus Patients with Active Disease. J Rheumatol. 2010 doi: 10.3899/jrheum.100180. [DOI] [PubMed] [Google Scholar]
- 15.Alexander T, Sattler A, Templin L, Kohler S, Gross C, Meisel A, et al. Foxp3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann Rheum Dis. 2013;72(9):1549–58. doi: 10.1136/annrheumdis-2012-202216. [DOI] [PubMed] [Google Scholar]
- 16.Golding A, Hasni S, Illei G, Shevach EM. The Percentage of FoxP3+Helios+ Treg Cells Correlates Positively With Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheum. 2013;65(11):2898–906. doi: 10.1002/art.38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonelli M, von Dalwigk K, Savitskaya A, Smolen JS, Scheinecker C. Foxp3 expression in CD4+ T cells of patients with systemic lupus erythematosus: a comparative phenotypic analysis. Ann Rheum Dis. 2008;67(5):664–71. doi: 10.1136/ard.2007.074690. [DOI] [PubMed] [Google Scholar]
- 18.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175(12):8392–400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 19.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27(2):110–8. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Vargas-Rojas MI, Crispin JC, Richaud-Patin Y, Alcocer-Varela J. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17(4):289–94. doi: 10.1177/0961203307088307. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29(11):1251–8. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 22.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12(2):147–68. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinazzi E, Puccetti A, Gerli R, Rigo A, Migliorini P, Simeoni S, et al. Serum DNase I, soluble Fas/FasL levels and cell surface Fas expression in patients with SLE: a possible explanation for the lack of efficacy of hrDNase I treatment. Int Immunol. 2009;21(3):237–43. doi: 10.1093/intimm/dxn142. [DOI] [PubMed] [Google Scholar]
- 24.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillon SR, Harder B, Lewis KB, Moore MD, Liu H, Bukowski TR, et al. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthritis Res Ther. 2010;12(2):R48. doi: 10.1186/ar2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58(8):2453–9. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa G, Cervera R. Belimumab, a BLyS-specific inhibitor for the treatment of systemic lupus erythematosus. Drugs Today (Barc) 2010;46(12):891–9. doi: 10.1358/dot.2010.46.12.1544336. [DOI] [PubMed] [Google Scholar]
- 28.Qin Q, Chang Y, Wang D, Wu Y, Zhang LL, Wei W. TACI-Ig induces immune balance of Th cells in MLN via BLyS/APRIL-receptors signaling in rats with adjuvant-induced arthritis. Int Immunopharmacol. 2011;11(12):2167–75. doi: 10.1016/j.intimp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Crowe SR, Merrill JT, Vista ES, Dedeke AB, Thompson DM, Stewart S, et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum. 2011;63(8):2396–406. doi: 10.1002/art.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Transl Med. 2013;11(1):93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66(2):175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods. 2009;350(1–2):125–32. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Hughes-Austin JM, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolove J, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA) Ann Rheum Dis. 2013;72(6):901–7. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 35.Chu VT, Enghard P, Schurer S, Steinhauser G, Rudolph B, Riemekasten G, et al. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum. 2009;60(7):2083–93. doi: 10.1002/art.24628. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto A, Fujio K, Okamura T, Yamamoto K. Regulatory T-cell-associated cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:463412. doi: 10.1155/2011/463412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doedens JR, Mahimkar RM, Black RA. TACE/ADAM-17 enzymatic activity is increased in response to cellular stimulation. Biochem Biophys Res Commun. 2003;308(2):331–8. doi: 10.1016/s0006-291x(03)01381-0. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Oppenheim JJ. The phenotypic and functional consequences of tumour necrosis factor receptor type 2 expression on CD4(+) FoxP3(+) regulatory T cells. Immunology. 2011;133(4):426–33. doi: 10.1111/j.1365-2567.2011.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, et al. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol. 2008;180(5):2747–51. doi: 10.4049/jimmunol.180.5.2747. [DOI] [PubMed] [Google Scholar]
- 40.Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72(5):659–64. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Shakra M, Zalmanson S, Neumann L, Flusser D, Sukenik S, Buskila D. Influenza virus vaccination of patients with systemic lupus erythematosus: effects on disease activity. J Rheumatol. 2000;27(7):1681–5. [PubMed] [Google Scholar]
- 42.Curtis JR, van der Helm-van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64(12):1794–803. doi: 10.1002/acr.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peabody JW, Strand V, Shimkhada R, Lee R, Chernoff D. Impact of rheumatoid arthritis disease activity test on clinical practice. PLoS One. 2013;8(5):e63215. doi: 10.1371/journal.pone.0063215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.