Abstract
A new procedure for room-temperature storage of DNA was evaluated whereby DNA samples from human tissue, bacteria, and plants were stored under an anoxic and anhydrous atmosphere in small glass vials fitted in stainless-steel, laser-sealed capsules (DNAshells®). Samples were stored in DNAshells® at room temperature for various periods of time to assess any degradation and compare it to frozen control samples and those stored in GenTegra™ tubes. The study included analysis of the effect of accelerated aging by using a high temperature (76°C) at 50% relative humidity. No detectable DNA degradation was seen in samples stored in DNAshells® at room temperature for 18 months. Polymerase chain reaction experiments, pulsed field gel electrophoresis, and amplified fragment length polymorphism analyses also demonstrated that the protective properties of DNAshells® are not affected by storage under extreme conditions (76°C, 50% humidity) for 30 hours, guaranteeing 100 years without DNA sample degradation. However, after 30 hours of storage at 76°C, it was necessary to include adjustments to the process in order to avoid DNA loss. Successful protection of DNA was obtained for 1 week and even 1 month of storage at high temperature by adding trehalose, which provides a protective matrix. This study demonstrates the many advantages of using DNAshells® for room-temperature storage, particularly in terms of long-term stability, safety, transport, and applications for molecular biology research.
Introduction
Current methodologies for maintaining frozen DNA samples require materials, space, and energy, rendering the technology expensive without guaranteeing long-term viability. Moreover, transporting DNA samples requires caution (e.g., dry ice), increasing cost, and in the case of delays, could result in sample degradation. The development of new technological approaches to ensure nondestructive, long-term stability of DNA and appropriate storage in biobanks is critical to meet present and future requirements for modern science. In recent years, several room-temperature, anhydrobiosis-based DNA storage technologies such as QIAsafe (QIAGEN), GenTegra™ (IntegeneX®), and DNAstable™ (BioMatrica®) have demonstrated their effectiveness in protecting DNA samples.1–4
Mechanisms of DNA damage during storage have recently been reviewed.5,6 These studies demonstrated the stability of DNA at room temperature if appropriately purified and protected against factors that may alter it, essentially water, oxygen, temperature, and light. Based on this concept, Imagene (Evry, France) has obtained a worldwide patent for an innovative method of DNA storage. The technique involves encapsulation of the genomic material such that it can be stored dry at room temperature, in small, watertight, oxidation-proof metal capsules. We evaluated this technology by testing the integrity (quality and quantity) of DNA samples from human tissues, plants, and bacteria after encapsulation and storage during various time periods. The efficiency of this storage method was also estimated by accelerated aging. In order to simulate long-term storage, DNA samples were subjected to high temperatures according to the Arrhenius model which links time, temperature, and the degradation constant, allowing estimation of the degradation constant of DNA at 25°C, by extrapolation.6 The Arrhenius model is generally followed when dealing with a simple chemical reaction. The main degradative event for DNA is depurination. The temperature dependence of this reaction has been studied in various conditions for DNA6–13 and adenosine;14 in all cases, the data fit the Arrhenius model. The authors used it to extrapolate degradation rates to room temperature or 37°C. In some examples, the model has been shown adequate to 54°C,13 40°C,8 or 25°C.10 The measurements relied on here were done between 140°C and 70°C,6 so there is good reason to be confident in the validity of the model and the degradation rates obtained by extrapolation.
Materials and Methods
DNA studies
Twenty-three DNA samples from plants, bacteria, and human tissues were prepared by several different French laboratories: INRA, the French National Institute for Agricultural Research (Montpellier); the Collection de l'Institut Pasteur (Paris); the Banque d'ADN et de cellules du Généthon (Evry); and the Centre de Biotechnologie Cellulaire (Groupe Hospitalier Est) (Bron). DNA samples used in the study are listed in Table 1; some degraded DNA samples were included in the study.
Table 1.
DNA Samples and Their Source
| DNA samples | DNA prepared by : | Origin, Tissues |
|---|---|---|
| H01 to H12 and H16 to H18 | Généthon | Human, blood |
| H13 to H15 and H19 to H20 | Généthon | Human, cell culture |
| H21 to H23*§ | Groupe hospitalier Est | Human, myoblast |
| B01 to B23* | Collection de l'Institut Pasteur | Bacterial strains (cocci and bacilli, Gram positive or Gram negative) |
| P01 to P23* | INRA Montpellier | Plant Grapes, young leaves |
The letters H, B, and P refer to human, bacterial, and plant DNA samples, respectively.
Trehalose (T) was added to the following plant samples during encapsulation: P01T, P03T, P04T, P06T, P07T, P012T, and P015T.
H21 to H23, B21 to B23 and P21 to P23 were dried both with and without trehalose.
§Human DNA samples H13 to H20 were degraded.
DNA extractions
Genomic DNA from bacteria was prepared by using the DNeasyR tissue kit (Qiagen, Alameda, CA) or the Promega kit, according to the manufacturer's instructions. DNA was dissolved in AE elution buffer or DNA rehydration solution according to the kit instructions, and stored at −20°C.
Plant genomic DNA was extracted from 1 g of leaves of different genotypes of grape vine (vitis vinifera) according to the DNeasy Plant Maxi Kit (Qiagen) with the following modification: 1% polyvinylpyrrolidone (PVP 40,000) and 1% 2-β-mercaptoethanol were added to buffer AP1 (buffer in the kit). DNA was dissolved in TE buffer (10 mM TrisHCl pH 8, 1 mM EDTA) and stored at −20°C.
Human DNA was extracted following a standard protocol using an AutoGen machine to reduce manual handling of phenol/chloroform. Red blood cells were lysed in buffer (20 mM TRIS pH 7.6, 10 mM MgCl2, 20 mM NaCl). White blood cells were lysed by stirring overnight at 37°C in 3 M NaCl, 20 mM TRIS pH 7.6, 20 mM EDTA pH 8, 1% SDS, and 100 μg proteinase K. Phenol/chloroform extraction was performed according to the AutoGen procedure: extraction first with AutoGen AG00312 (phenol/chloroform) and then precipitation of DNA with AutoGen AG00410. The resulting pellet was washed once with isopropanol and twice with 70% ethanol. The DNA was dissolved in TE 10−1 and incubated overnight at 37°C with agitation to homogenize. DNA was stored at −20°C for long-term preservation, or at 4°C for immediate use.
Each type of DNA, extracted according to the different protocols, may include specific pollutant compounds (i.e., polyphenols and polysaccharides for plant, glycoproteins for bacteria, and proteins for human DNA).
DNA encapsulation
Aliquots of DNA solution were sent to Imagene and treated using the DNAshells® encapsulation procedure. In brief, 1 μg of DNA was deposited on the bottom of a 0.2 mL cylindrical glass insert placed in an oxidation-proof metallic capsule (DNAshells®). All samples were vacuum-dried for 30 min to 1 h in a Thermo-Savant Explorer concentrator until desiccation was complete. Samples were then introduced into a confinement chamber and packaged under a controlled, inert atmosphere. Each capsule was hermetically sealed using a laser to weld the junction between the shell and the adapted metallic cap under an anhydrous, anoxic inert atmosphere. This procedure ensures a completely air-tight capsule. A data-matrix code was engraved onto each capsule with a laser to ensure permanent, unalterable labeling. For some samples, the desiccation process before encapsulation was modified. In these cases, DNA was dried in the presence of trehalose (40 molecules per nucleotide)6 (see Table 1). The rest of the DNAshells® procedure was followed.
GenTegra™ DNA tubes
GenTegra™ DNA tubes contain an inorganic mineral matrix with oxidation protection and antimicrobial activity for storage of purified DNA at room temperature. DNA in GenTegra™ tubes is designed to tolerate temperatures of −80°C to 76°C during transport. This method, previously tested in 20092 and in 2011,3 was used for comparison in some experiments. For this purpose, 1 μg of DNA was added to each GenTegra™ DNA tube and dried down in a FastDryer or a SpeedVac according to the GenTegra™ DNA protocol. This step was carried out by GenVault for human, bacterial, and plant samples 01 to 20 and by INRA for human, bacterial, and plant samples 21 to 23. Genvault, which was the first company to distribute GenTegra™ DNA tubes, was acquired by IntegenX® (Pleasanton, CA, USA), in January 2011.
Experimental design
In order to assess DNA degradation, DNAshells® were stored at room temperature for two time periods (1 month and 18 months) and were subjected to high temperature (76°C at 50% relative humidity (RH)) for 30 hours, 1 week, or 1 month. In this case, samples were stored in a constant climate chamber KBF 720 (Binder). The results were compared not only with those of control samples stored frozen or at 4°C, but also with those of DNA samples stored in the same conditions in GenTegra™ tubes. After storage, dry DNA was rehydrated by adding water without further purification and then returned to the appropriate partner laboratory for analysis using standard tests.
Standard tests
Fluorometry, agarose gel electrophoresis, pulsed field gel electrophoresis (PFGE), polymerase chain reaction (PCR), and amplified fragment length polymorphism (AFLP) were performed to evaluate DNA stability in terms of quality, quantity and integrity.
Fluorometric measurements and agarose gel electrophoresis
Before and after each experiment, all DNA samples were subjected to quality and quantity tests by performing agarose gel electrophoresis and spectrofluorometric measurements. Double strand (ds) DNA fluorometric measurements were taken with the Quant-iT™ PicoGreen® (Invitrogen) or the Hoesch-3325815 using the dsDNA protocol with a Tecan Genios spectrofluorometer. Absorbances from 220 to 350 nm were measured with a Nanodrop 8000 spectrophotometer.
Based on an assumed initial quantity prior to any treatment, an equivalent amount of DNA from each capsule, storage tube, and control was run on agarose gels (0.8% agarose gel, in TAE 1X buffer stained with ethidium bromide). A 1 kb DNA Ladder (Invitrogen) was used for size reference and Lambda DNA was used as integrity control. Each lane was scored according to the presence or absence of a band, size of the band, and whether the band was smeared. A single unsmeared band is referred to as undegraded DNA.
Pulsed field gel electrophoresis (PFGE)
PFGE (Biorad System) was performed on human DNA samples in order to test DNA integrity. From each capsule, 0.4 μg of quantified DNA was deposited. The size controls used were Lambda ladder PFG marker and Mid-range PFG marker 50 μg/mL (Biolabs).
PCR experiments
Classical PCR was used to evaluate the functional quality of DNA. PCR analyses were conducted on plant samples by amplifying a part of the O-methyltransferase 3 (1600 pb) locus with the grapevine specific primers OMT 3.1.2F 5′-AAGTTACAGCTAAACATACTCAATCTT-3′ and OMT 3.1.3R 5′-GCCCATATAAGTTCCGACC-3′, and on bacterial samples by amplifying rrs (1500 pb) locus with the universal primers16 A 5′-AGAGTTTGATCATGGCTCAG-3′ (position 8 to 27 Escherichia coli numbering) and H 5′-AAGGAGG TGATCCAACCGCA-3′ (position 1541 to 1522).
PCR experiments were performed in a total volume of 25 μL containing about 25 ng DNA, 1X PCR buffer including 2 mM MgCl2, 10 pmol of each primer, 0.2 mM of each dNTP, and 0.75 U Taq Polymerase (Qiagen), on an Eppendorf MasterCycler using the following conditions: 2 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C, followed by a final extension of 5 min at 72°C. Amplified fragments were detected on 1.5% agarose gel.
Amplified fragment length polymorphism analysis
In order to test enzymatic digestions, amplified fragment length polymorphism (AFLP) analysis was performed on plant DNA samples with the AFLP® Core Reagent Kit (Invitrogen), following the manual with some modifications. Two-hundred-fifty ng of genomic DNA was double-digested with EcoRI and MseI and the resulting fragments ligated to adaptors specific for the EcoRI and MseI restriction sites. A pre-selective amplification was carried out with EcoRI+A and MseI+C primers on an Eppendorf MasterCycler PCR-machine under the following conditions: 2 min at 94°C followed by 30 cycles of 30 sec at 94°C, 60 sec at 56°C, and 60 sec at 72°C. The PCR products were then diluted 10-fold with water and used as the template for selective amplifications using EcoRI+ACG and MseI+CAT primers. EcoRI+ACG primer was 5′ end labeled with the fluorescent dye 6-FAM. Amplified products were detected on an ABI 3130xl Genetic Analyser (Applied Biosystems). For each DNA, samples were prepared by mixing 1 μL of diluted (10-fold with water) PCR products with 0.15 μL GenSize 500HD Rox and 18.85 μL water. Profiles were read using the GENEMAPPER V3.7 software (Applied Biosystems).
Results
Storage in DNAshells® for 1 month or 18 months at room temperature
Human, bacterial, and plant DNA samples (H01 to H20, B01 to B20, and P01 to P20) were stored in DNAshells® for 1 month or 18 months at room temperature without special control of temperature or humidity. In order to investigate whether the storage time affected sample recovery, rehydrated DNA samples were analyzed using gel electrophoresis or PFGE for human DNA samples and compared to frozen or 4°C control samples. Some comparisons were also conducted with the same DNA samples stored in GenTegra™ tubes.
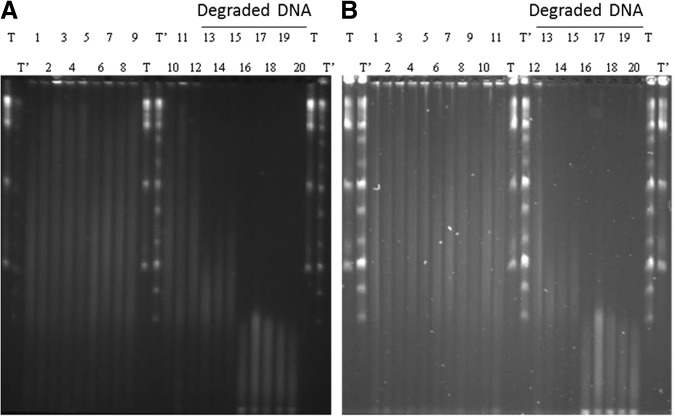
After 1 month of storage at room temperature, no degradation was seen in dried DNA (whatever the origin) protected in DNAshells® when compared to control samples and to DNA samples stored in GenTegra™ tubes. Representative examples of the results obtained are shown in Figure 1,wells 2, 3, and 6. In the same manner, the PFGE analysis of human DNA samples presented in Figure 2 showed that after 18 months of storage in DNAshells®, no visible difference of the samples (degraded or not) occurred compared to control.
FIG. 1.
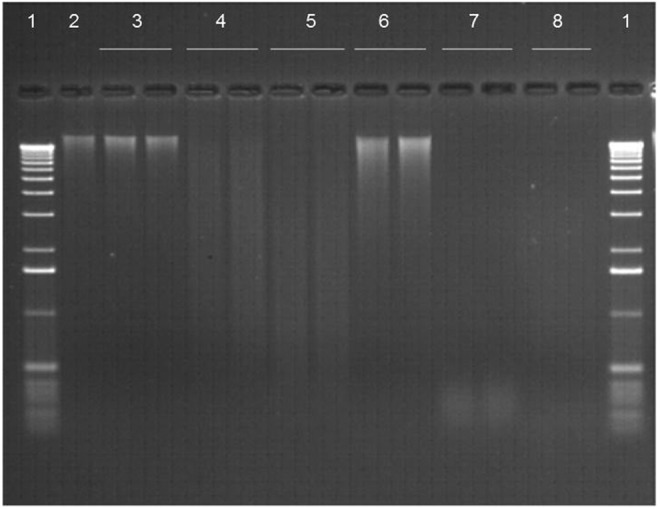
Gel analysis of DNA recovered from plant sample P02 stored in DNAshells® (IM) or GenTegra™ DNA tubes (GT) at room temperature for 1 month (RT1); or 1 week (761W) or 1 month (761M) at 76°C, 50% RH. Wells: 1. DNA ladder; 2. Control DNA stored frozen at −20°C; 3. IMRT1; 4. IM761W; 5. IM761M; 6.GTRT1; 7. GT761W; 8.GT761M. Samples in wells 3–8 were done in duplicate.
FIG. 2.
Pulsed field gel analysis of 20 human DNA samples stored in DNAshells® before (A) and after (B) 18 months at room temperature. T, Lambda ladder PFG marker; T', Mid-range PFG marker. Wells 1–20 represent samples H01 to H20, respectively.
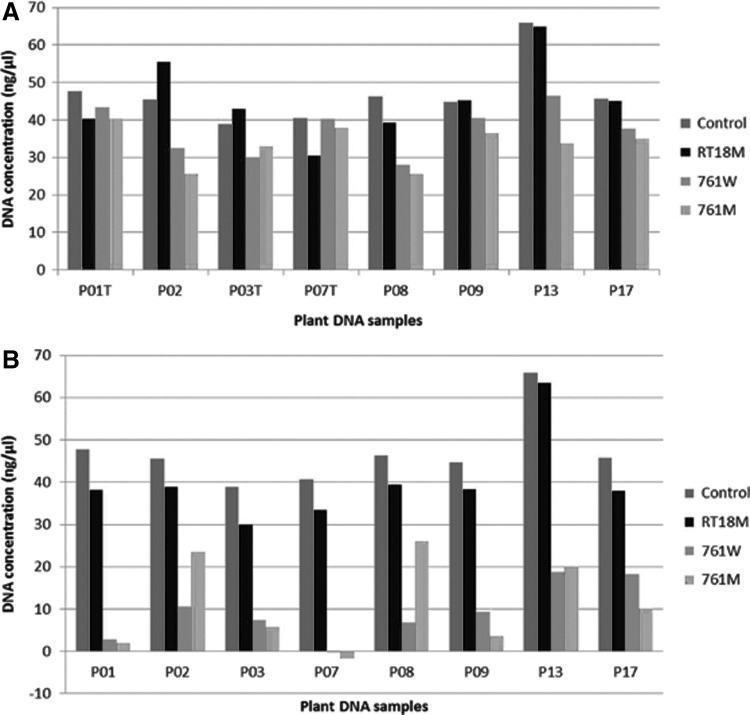
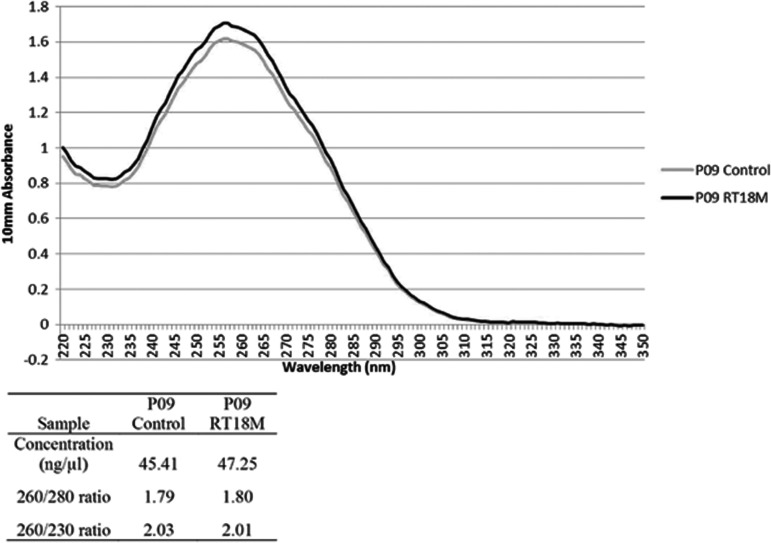
Recovered samples quantified following dry storage in DNAshells® versus control DNA samples confirmed the gel analysis results (Fig. 3A, red columns). Room temperature storage of DNashells® altered neither the amount of dsDNA nor the A260/A280 ratio (DNA purity of P09 is shown in Figure 4 as an example of the results).
FIG. 3.
Examples of plant DNA recovered after storage in DNAshells® (A) or in GenTegra™ tubes (B). Control DNA was stored at −20°C in plastic tubes. T, trehalose treated samples; RT18M, 18 months at room temperature; 761W, 1 week at 76°C, 50% RH; 761M, 1 month at 76°C, 50% RH.
FIG. 4.
Characteristics of the quality of the plant DNA sample P09, stored frozen at −20°C (control) and in DNAshells® for 18 months at room temperature (RT18M).
Accelerated aging
Storage in DNashells® for 30 h at 76°C
In order to simulate long-term storage, DNAshells® were subjected to high temperature over a period of time which, by extrapolation, could correspond to 100 years at 25°C according to the Arrhenius model. These experiments were conducted on three human (H21–H23), bacterial (B21–B23), and plant (P21–P23) DNA samples sealed in capsules and heated at 76°C under controlled humidity conditions (50% RH), for 30 h. Based on gel analysis, the bacterial and plant DNA samples stored in these conditions demonstrated identical characteristic banding patterns expected of undegraded genomic DNA, and PFGE analysis confirmed the absence of significant degradation of almost all the human DNA samples treated this way (data not shown).
Fluorometric analysis indicated that overall there was little or no difference in the amount of dsDNA recovered following storage as compared with control samples. The results obtained with P22 are presented as an example in Table 2. As any cut in the target sequence will prevent its amplification, bacterial and plant DNA samples were also subjected to PCR analysis to determine whether the DNA was degraded. PCR results for all samples tested were the same as for the control sample stored at −20°C; an example of the results is shown in Figure 5.
Table 2.
DNA Concentration and Characteristics of the Quality of Plant DNA Sample P22
| P22 30H76 | P22T 30H76 | P22 RT | P22T RT | P22 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tubes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Concentration (ng/μL) | 47.00 | 48.68 | 41.61 | 49.10 | 55.09 | 43.99 | 54.80 | 54.51 | 45.41 | 39.49 | 47.56 |
| DO 260/280 | 1.73 | 1.73 | 1.70 | 1.74 | 1.80 | 1.78 | 1.76 | 1.79 | 1.82 | 1.71 | 1.74 |
| DO 260/230 | 2.03 | 2.01 | 1.99 | 2.03 | 1.85 | 1.92 | 1.98 | 1.93 | 2.09 | 1.81 | 1.92 |
T, With trehalose treatment; 30H76, After storage in DNAshells® for 30 hours at 76°C, 50% relative humidity; RT, Room temperature.
All encapsulated DNA samples were resuspended in a volume of water equal to the volume of initial deposit. Tube 11 contained control DNA sample P22, stored frozen at −20°C.
FIG. 5.
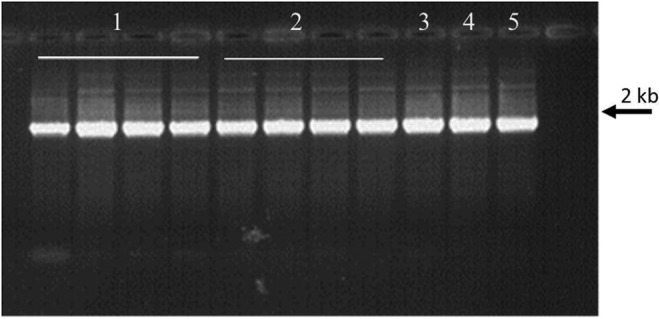
Amplification of the 16S rRNA gene (rrs): bacterial DNA sample B21 with (T) and without trehalose treatment, stored in DNAshells® for 30 hours at room temperature (RT) and at 76°C, 50% RH (30H76). A 10-μL aliquot of each PCR reaction was run on a 0.8% agarose gel stained with ethidium bromide. Primers A and H were used for amplification. Wells: 1. B21 30H76; 2. B21T 30H76; 3. B21 RT; 4. B21T RT; 5. Control DNA sample B21 stored frozen at −20°C.
For comparison, DNA samples were stored in GenTegra™ tubes at 76°C for 30 h. While no degradation was observed for DNA stored under dry conditions (<5% humidity) when run on agarose gels, DNA samples stored in GenTegra™ tubes subjected to 50% RH did show degradation (Fig. 6).
FIG. 6.
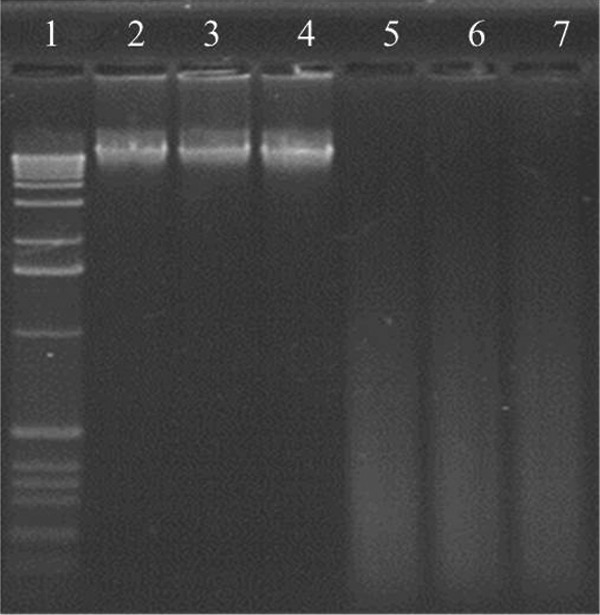
Gel analysis of DNA recovered from plant sample P23 stored in GenTegra™ DNA tubes for 30 h at 76°C under dry conditions (<5% humidity) and under controlled humidity (50% RH). Wells: 1. DNA ladder; 2. Control DNA sample stored frozen at −20°C; 3,4. Dry conditions; 5,6. Controlled humidity; 7. DNA in water solution stored for 30 h at 76°C under controlled humidity.
Storage in DNAshells® for 1 week or 1 month at 76°C
In order to test the response of DNA to very aggressive conditions, DNAshells® were stored at 76°C under controlled humidity (50% RH) for 1 week or 1 month, corresponding by extrapolation to 500 and 2000 years respectively, at 25°C. For comparison, in parallel, these extreme conditions of storage were also applied to GenTegra™ DNA tubes containing the same DNA. After rehydration, DNA samples were run on gel electrophoresis. A representative gel analysis is shown in Figure 1, wells 4, 5, 7, and 8. The following conclusions can be drawn: 1) encapsulated DNA was degraded after 1 week of storage; 2) dsDNA stored in GenTegra™ DNA tubes was already completely degraded after 1 week; and 3) these findings were confirmed by dsDNA quantification (Fig. 3B).
Effect of trehalose
Trehalose was added to a part of the encapsulated DNA samples (Table 1) in order to assess its effects on dsDNA stability during storage at high temperature under controlled humidity. Storage was carried out for 30 h, 1 week, or 1 month. Figure 7 allows comparison of the results obtained for DNA samples with or without trehalose. It was very interesting to note that encapsulated DNA samples stored for more than 30 h at 76°C, 50% RH in the presence of trehalose exhibited no detectable degradation, whereas in the absence of trehalose, DNA was degraded and appeared as a smear on an agarose gel (Fig. 7A and B, wells 4 and 5). DNA was degraded but an important part remains as dsDNA. This last result was confirmed by the fact that the average yield of dsDNA recovered following DNAshells® storage at high temperature for 1 week or 1 month without addition of trehalose was obviously decreased compared to that with addition of trehalose but remains between 30% and 90% of the control (Fig. 3A).
FIG. 7.
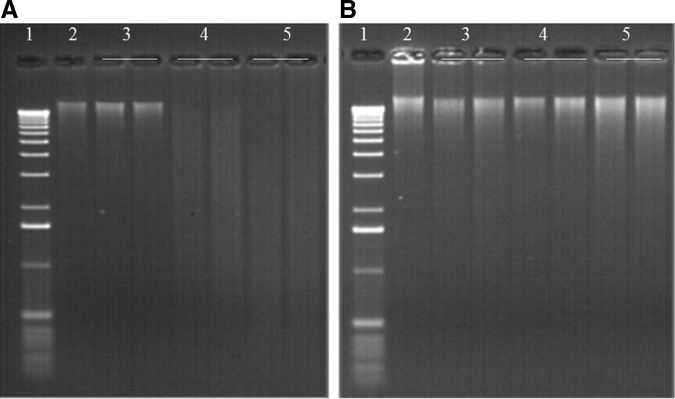
Gel electrophoresis of the same plant DNA sample stored in DNAshells® without trehalose P08 (A) and with trehalose P07T (B). Wells: 1. DNA ladder; 2. Control DNA sample stored at −20°C; 3. Storage at room temperature for 1 month; 4. Storage at 76°C, 50% RH for 1 week; 5. Storage at 76°C, 50% RH for 1 month.
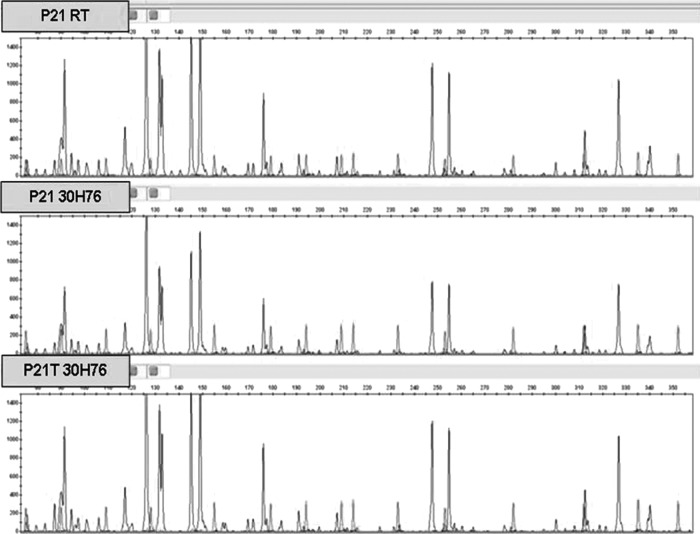
Based on these results, the AFLP analysis was intended to check whether the presence of trehalose in DNA samples would generate problems with the digestion (partial or complete inhibition) by the restriction enzymes. It was only performed with plant DNA sample P21 stored in DNAshells® with and without trehalose and incubated for 30 h at 76°C, 50% RH. Figure 8 shows that DNA samples did not exhibit any interference or inhibition during AFLP analysis.
FIG. 8.
AFLP analysis of plant DNA sample P21 with (T) and without trehalose treatment, stored in DNAshells® for 30 h at room temperature (RT) and at 76°C, 50% RH (30H76).
Discussion
The results presented here demonstrate that the protective properties of DNAshells® are not affected by storage under extreme conditions (76°C, 50% RH) for 30 h, guaranteeing 100 years without degradation so the DNA can be used in downstream applications such as PCR, gel electrophoresis, and AFLP analysis.
The presumption is often made that if nucleic acids are dried, they are stable for long periods of time. Therefore, the stability of DNA stored in DNAshells® and in GenTegra™ DNA tubes was examined after exposure to high temperature during long periods of time (1 week or 1 month) under normal humidity. As GenTegra™ DNA tubes are not watertight, unlike DNAshells®, the degree of humidity used, which mimics normal conditions, could explain the important loss of dsDNA stored for 1 week in GenTegra™ tubes. Indeed, this result is consistent with other results17 demonstrating that dsDNA exposed to moist air is rapidly degraded, even in the presence of trehalose as a protective matrix. In the same way, it had been previously shown that DNA can undergo chemical changes such as depurination, hydrolysis, and oxidation even at low moisture content.13,18 The type of container (glass for DNAshells® and plastic for GenTegra™ DNA tubes) may also play a role.19 With regard to the sealing of the DNAshells®, it is clear that the only parameter that could be considered in the degradation/loss of DNA stored in DNAshells® is the high temperature. Considering the usefulness of trehalose, recent evidence has suggested that trehalose could preserve intact cells in vitro in the dry state.20 In 2005, a comparison of different storage conditions with the addition of potential preserving agents was conducted.21 The results indicated that dry storage with an additive such as trehalose may improve DNA recovery. It has also been reported that trehalose has a strong stabilizing effect on DNA secondary structure during and after dehydratation.22 According to these authors, the stabilization effect of trehalose may be explained by its ability to strongly interact with DNA through hydrogen bonding to phosphates and other polar groups. These interactions stabilize the double helix by screening the large phosphate–phosphate repulsion and stabilizing base stacking. In our tests, it is clear that the intense heat is responsible for degradation of DNA and loss of dsDNA in DNAshells®, and that trehalose provides successful protection of DNA after 1 week and even after 1 month at high temperature, as shown in Figure 7.
We have also shown that partially degraded DNA from human samples stored using DNAshells® technology does not undergo further degradation as shown by PFGE analysis. As human samples are precious, it is important to be able to retain as much DNA as possible even if it has been somewhat degraded during the extraction.
We also demonstrate that DNAshells® technology addresses the need for long-term DNA storage at room temperature while maintaining DNA integrity. The addition of a trehalose protective matrix guarantees DNA stability over a long period of time whatever the environmental conditions, and thus DNAshells® allows secure exchanges at room temperature even if temperature increases during transport.
Moreover, if the cost of long-term DNA preservation is compared between freezing at −20°C or −80°C in specific plastic tubes versus DNA encapsulation, the latter may be economically advantageous in the long term. In the case of DNAshells®, there is a one-time charge of between 4 and 5€ per DNA sample incurred at the time of encapsulation, depending on the service chosen (Imagene). By comparison, a GenTegra™ DNA tube costs about 1€; however, there are no additional costs associated with subsequent long-term storage (e.g., purchase and maintenance of freezers).
Conclusion
Based on our results, we recommend the use of room-temperature storage of precious DNA in DNAshells®. This provides an alternative to conventional cold storage and makes it possible to create large-capacity (several million samples) DNA biobanks with numerous advantages for long-term stability, safety, transport, and applications for molecular biology research. Optimal storage of DNA makes possible retrospective (retesting) or prospective (downstream analysis with additional or new genetic markers) testing. Moreover, it is an opportunity for laboratories to store their samples for long periods of time in a highly sustainable environment, with reduced cost, and optimized use of space, lower energy costs, and fewer greenhouse emissions when compared to freezer storage.
Finally, the tests performed show unambiguously that the dual DNAshells®/trehalose treatment gives high protection to DNA samples, even in a very aggressive environment. The protection of the dsDNA molecule for long-term storage is thus much better than that provided by the conventional procedures used for storage at low temperature or at room temperature on inert resins, or whatever the conditions of the future.
Acknowledgments
This research has been funded by IBiSA (Infrastructures Biologie Santé et Agronomie) (R01-HG01720) and the European Community's Seventh Framework Programme (FP7, 2007-2013), Research Infrastructures action, under the grant agreement No. FP7-228310 (EMbaRC project). The products from Genvault were provided as free supplies by the manufacturer; however, the study was designed and performed independently by the authors. We thank M. T. Zabot and the Groupe Hospitalier Est de Bron for preparation of DNA from myoblasts.
Author Disclosure Statement
None of the authors have a financial interest in Imagene or Genvault.
References
- 1.Howlett SE, Castillo HS, Gioeni LJ, et al. Evaluation of DNAstableTM for DNA storage at ambient temperature. Forensic Sci Int Genet 2014;8:170–117 [DOI] [PubMed] [Google Scholar]
- 2.Wan E, Akana M, Pons J, et al. Green technologies for room temperature nucleic acid storage. Curr Issues Mol Biol 2009;12:135–142 [PMC free article] [PubMed] [Google Scholar]
- 3.Frippiat C, Zorbo S, Leonard D, et al. Evaluation of novel forensic DNA storage methodologies. Forensic Sci Int Genet 2011;5:386–392 [DOI] [PubMed] [Google Scholar]
- 4.Lee SB, Clabaugh KB, Silva B, et al. Assessing a novel room temperature DNA storage medium for forensic biological samples. Forensic Sci Int Genet 2012;6:31–40 [DOI] [PubMed] [Google Scholar]
- 5.Lee SB, Crouse CA, Kline MC. Optimizing storage and handling of DNA extracts. Forensic Sci Rev 2010;22:131–144 [PubMed] [Google Scholar]
- 6.Bonnet J, Colotte M, Coudy D, et al. Chain and conformation stability of solid state DNA: Implications for room temperature storage. Nucleic Acid Res 2010;38:1531–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilenchik MM. Studies of DNA damage and repair of thermal- and radiation-induced lesions in human cells. Int J Radiat Biol 1989;56:685–689 [DOI] [PubMed] [Google Scholar]
- 8.Vilenchik MM, and Knudson AG., Jr.Inverse radiation dose-rate effects on somatic and germ-line mutations and DNA damage rates. Proc Natl Acad Sci USA 2000;97:5381–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RK, Xu Z, Bohannon KE, et al. Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J Pharm Sci 2000;89:76–87 [DOI] [PubMed] [Google Scholar]
- 10.Lindahl T, Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry 1972;11:3618–3623 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Ohsumi S, Makino K. Mechanistic studies on depurination and apurinic site chain breakage in oligodeoxyribonucleotides. Nucleic Acids Res 1994;22:4997–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res 2002;30:1354–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrone A, Ballantyne J. Hydrolysis of DNA and its molecular components in the dry state. Forensic Sci Int Genet 2010;4:168–177 [DOI] [PubMed] [Google Scholar]
- 14.Stockbridge RB, Schroeder LV, Wolfenden R. The rate of spontaneous cleavage of the glycosidic bond of adenosine. Bioorg Chem 2010;38:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daxhelet GA, Coene MM, Hoet PP, et al. Spectrofluorometry of dyes with DNAs of different base composition and conformation. Anal Biochem 1989;179:401–403 [DOI] [PubMed] [Google Scholar]
- 16.Böttger EC. Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA. FEMS Microbiol Lett 1989;65:171–176 [DOI] [PubMed] [Google Scholar]
- 17.Colotte M, Coudy D, Tuffet S, et al. Adverse effect of air exposure on the stability of DNA stored at room temperature. Biopreserv Biobanking 2011;9:47–50 [DOI] [PubMed] [Google Scholar]
- 18.Lindahl T. Instability and decay of the primary structure of DNA. Nature 1993;362:709–715 [DOI] [PubMed] [Google Scholar]
- 19.Kline MC, Duewer DL, Redman JW, et al. Results from the NIST 2004 DNA Quantitation Study. J Forensic Sci 2005;50:570–578 [PubMed] [Google Scholar]
- 20.Wolkers WF, Tablin F, Crowe JH. From anhydrobiosis to freeze-drying of eukaryotic cells. Comp Biochem Physiol A Mol Integr Physiol 2002;131:535–543 [DOI] [PubMed] [Google Scholar]
- 21.Smith S, Morin PA. Optimal storage conditions for highly dilute DNA samples: A role for trehalose as a preserving agent. J Forensic Sci 2005;50:1101–1108 [PubMed] [Google Scholar]
- 22.Zhu B, Furuki T, Okuda T, et al. Natural DNA mixed with trehalose persists in B-form double-stranding even in the dry state. J Phys Chem B 2007;111:5542–5544 [DOI] [PubMed] [Google Scholar]