Abstract
Extinction training is a form of inhibitory learning that allows an organism to associate a previously aversive cue with a new, safe outcome. Extinction does not erase a fear association, but instead creates a competing association that may or may not be retrieved when a cue is subsequently encountered. Characterizing the conditions under which extinction learning is expressed is important to enhancing the treatment of anxiety disorders that rely on extinction-based exposure therapy as a primary treatment technique. The ventromedial prefrontal cortex, which plays an important role in the expression of extinction memory, has been shown to be functionally impaired after stress exposure. Further, recent research in rodents found that exposure to stress led to deficits in extinction retrieval, although this has yet to be tested in humans. To explore how stress might influence extinction retrieval in humans, participants underwent a differential aversive learning paradigm, in which one image was probabilistically paired with an aversive shock while the other image denoted safety. Extinction training directly followed, at which point reinforcement was omitted. A day later, participants returned to the lab and either completed an acute stress manipulation (i.e., cold pressor), or a control task, before undergoing an extinction retrieval test. Skin conductance responses and salivary cortisol concentrations were measured throughout each session as indices of fear arousal and neuroendocrine stress responses, respectively. The efficacy of our stress induction was established by observing significant increases in cortisol for the stress condition only. We examined extinction retrieval by comparing conditioned responses during the last trial of extinction (day 1) with that of the first trial of re-extinction (day 2). Groups did not differ on initial fear acquisition or extinction, however, one day later participants in the stress group (n = 27) demonstrated significantly less extinction retrieval (i.e., greater fear recovery) than those in the control group (n = 25). Our results suggest that acute stress impairs extinction memory retrieval and offers insight into why treatment strategies used in the clinic may be challenging to recruit in daily life where stress is pervasive.
Keywords: extinction retrieval, fear conditioning, stress, cortisol
1. Introduction
The capacity to regulate fear responses to cues as they change from predicting danger to safety is critical to shaping healthy, adaptive behavior. Extinction learning refers to the process by which conditioned fear responses to a cue (conditioned stimulus, or CS) that has acquired affective properties after being paired with an aversive outcome (unconditioned stimulus, or US) gradually diminish as the cue is repeatedly presented without reinforcement (Pavlov, 1927; Bouton, 2004). Since extinction training leads to the suppression of fear responses by creating a new, safe CS-association, it does not eliminate the original CS-US association, leaving extinction learning vulnerable to retrieval deficits that enable the recovery of conditioned fear responses (Quirk & Mueller, 2008). Characterizing the conditions under which extinction learning is susceptible to retrieval impairments is critical because this learning comprises the foundation of exposure therapy, a therapeutic technique used by clinicians to treat anxiety disorders such as phobias and posttraumatic stress disorder (PTSD) (Rothbaum & Schwartz, 2002; Vervliet, Craske & Hermans, 2013). Importantly, deficits in retrieving extinction learning are thought to underlie the re-emergence of anxiety symptoms in these populations after treatment (Shin & Liberzon, 2010; Vervliet et al, 2013).
Research across species has shown that the failure to retrieve extinction training can occur over time (spontaneous recovery), after a change in context (renewal), or after exposure to the US (reinstatement) (Bouton, 2004). Another important, but relatively unexplored, factor that may promote impaired extinction retrieval is exposure to stress. Stressful life events and experiences often precede the onset of anxiety disorders and can trigger the return of clinical symptoms after successful treatment (Cisler et al, 2009; Shin & Liberzon, 2010; Vervliet et al, 2013). Importantly, the brain regions that support extinction learning and retrieval overlap extensively with those that modulate neuroendocrine responses to stress, rendering these processes especially sensitive to the influence of stress. Nonetheless, the relationship between acute stress and the expression of extinction memory has not been directly examined in humans.
Stress and stress hormones (i.e., glucocorticoids) have long been implicated in modulating learning and memory processes, but these effects differ considerably depending on the stage at which stress is assessed (i.e., acquisition, consolidation, retrieval) (McGaugh, 2004; Roozendaal, McEwen, & Chattarji, 2009). For example, across a wide range of declarative and episodic learning and memory tasks, stress and stress hormones (i.e., glucocorticoids) have been shown to enhance the consolidation of memory but lead to memory deficits when administered directly before retrieval (for review, see: Roozendaal, 2002; Wolf, 2009). These divergent effects are thought to be due to interactions between the brain regions that support different learning and memory phases and the influence of glucocorticoids during stress exposure (McGaugh, 2004; Wolf, 2009; Roozendaal, McEwen, & Chattarji, 2009; Roozendaal & McGaugh, 2011). However, little work has assessed stress-related retrieval effects in the context of associative learning and memory processes (Rodrigues, LeDoux & Sapolsky, 2009). Neurobiological research in non-human animals has provided a detailed understanding of how stress interacts with the neural circuitry that supports each phase of cued-based fear conditioning and extinction, offering insight into how stress can differentially affect the way in which aversive associations are learned, extinguished and later retrieved.
The acquisition, consolidation and retrieval of cued fear conditioning rely on the amygdala, which serves as the site of CS-US convergence (LeDoux 2000; Maren 2001). During stress exposure, noradrenaline release in the brain enhances amygdala function and interacts with circulating glucocorticoids to modulate fear learning and consolidation (Roozendaal, McEwen, & Chattarji, 2009). Consistent with this, work in rodents has shown stress exposure or administration of glucocorticoids facilitates both the acquisition (Wilson et al, 1975; Shors et al, 1992; Shors, 2001) and consolidation (Hui et al. 2004; Rau & Fanselow, 2009) of cued fear learning.
Extinction learning and consolidation involve both the amygdala and ventromedial prefrontal cortex (vmPFC) (Myers & Davis, 2007; Quirk & Mueller, 2008; Sierra-Mercado et al, 2011). Brief exposure to stress can produce morphological changes to neurons in the infralimbic (IL) cortex (homologous to the vmPFC in humans) (Izquierdo et al, 2006) and impaired plasticity between the vmPFC and amygdala (Maroun & Richter-Levin, 2003). Empirical work examining how stress affects extinction learning has been inconsistent, however, with some finding no effect of stress on learning (Miracle et al, 2006; Garcia et al, 2008; Knox et al, 2012) and others reporting impairments (Izquierdo et al 2006; Akirav & Maroun, 2007; Maroun et al 2013). The consolidation of extinction learning, however, is enhanced with glucocorticoid administration, suggesting that despite the variable effects of stress on extinction learning, stress hormones facilitate the long-term storage of such training (Cai et al 2006; Yang et al, 2006). The vmPFC also plays a key role in the retention and later retrieval of extinction learning (see Milad & Quirk, 2012, for review). Chronic stress (Miracle et al, 2006; Garcia et al, 2008; Wilber et al, 2011) and single, prolonged stress exposure (Knox et al, 2012) has been shown to impair extinction retention after intact training. Additionally, recent work has shown that a single episode of acute stress induced directly before an extinction retention test leads to extinction retrieval deficits and the re-emergence of extinguished fear (Deschaux et al, 2013).
An emerging body of research examining the effects of stress and glucocorticoids on human fear conditioning and extinction has been largely consistent with these findings. Stress exposure (Jackson et al., 2006), high levels of endogenous cortisol (Zorawski et al., 2005; Zorawski et al., 2006; Grillon et al., 2006) or exogenous administration of cortisol (Kuehl et al., 2010) have all been shown to facilitate fear learning and consolidation, although gender specific effects have been reported (Jackson et al., 2006; Zorawski et al., 2005; Kuehl et al., 2010). Few studies in humans have examined the effect of stress on extinction learning and consolidation, however work in phobic patients has shown that glucocorticoid administration prior to exposure therapy leads to reduced cued-related fear responses that persists after treatment at follow up, suggesting that glucocorticoids enhance extinction learning and consolidation (Soravia et al., 2006; de Quervain et al., 2011). The effects of stress on fear memory retrieval and extinction processes were recently examined in healthy humans (Bentz et al., 2013). In this study, participants underwent a fear-conditioning task and returned a day later for a retrieval test and extinction training. Stress induced directly before extinction training led to lower US-expectancy ratings at the start of extinction in males who showed robust cortisol responses to stress, suggesting that stress impaired fear memory retrieval compared to controls. In contrast, female stress participants showed little cortisol response and intact fear memory retrieval. Perhaps due to the limited number of extinction trials, extinction learning was not demonstrated in either group, so prolonged effects of stress on fear memory retrieval were instead examined the following day. Men in the stress condition again showed reduced US-expectancy ratings the following day as compared to controls, whereas women showed intact expectancy ratings across conditions. However, since participants did not extinguish fear expectancy responses, stress-related effects on extinction learning and extinction retrieval could not be assessed.
The objective of the current study was to assess the influence of acute stress on the retrieval of extinction memory. Although research in humans has shown that patients diagnosed with PTSD are subject to deficits in extinction retrieval (Milad et al 2009), it remains unclear how explicitly manipulating stress levels directly before extinction retrieval might alter the expression of this safety learning in healthy humans. We addressed this question using a two-day classical fear-conditioning paradigm. Participants first acquired and extinguished conditioned fear as assessed with skin conductance response (SCR), an index of sympathetic nervous system arousal. A day later, participants underwent a stress (or control) manipulation shortly before an extinction retrieval test. Salivary cortisol concentrations were assayed across sessions to probe neuroendocrine responses to stress. Since acute stress exposure has been shown to enhance amygdala activity (Rodrigues, LeDoux & Sapolsky, 2009), impair the functional integrity of the prefrontal cortex (Arsten, 2009), and disrupt connectivity between the amygdala and vmPFC (Clewett, Schoeke, & Mather, 2013), we hypothesized that inducing stress before an extinction retrieval test would lead to poorer extinction retrieval in stressed participants than controls, leading to the re-emergence of fear responses that had been extinguished the previous day.
2. Materials and Methods
2.1 Participants
One hundred and twenty-seven healthy participants were recruited using flyers posted around the NYU campus or postings on NYU’s psychology department study website. Due to the involvement of electric shock as well as the stress induction technique, participants were ineligible for the study is they were pregnant, had heart or blood pressure problems in the past or were currently being treated for such conditions. Participants were also ineligible if they were being treated with any form of psychotropic medication. Consistent with previous research on aversive learning in both animals (Yang et al, 2006; Parnas, Weber & Richardson, 2005) and humans (LaBar & Phelps, 2005; Kalisch et al, 2006; Dunsmoor et al, 2009), participants were excluded before day 2 if they failed to show measurable levels of skin conductance response (non-responders; n = 23), or if they failed to acquire (mean CS+ > CS− by at least 0.1µS) or extinguish (mean CS+ ≥ CS− by less than 0.1µS) differential fear responses (non-acquirers: n = 24; non-extinguishers: n = 13). These criteria were necessary because we were unable to assess extinction learning if conditioned fear was never acquired, nor could we assess extinction retrieval if extinction learning had not occurred. On day 2, participants were eliminated if they were unable to complete the stress manipulation (n = 4; see section 2.4 Stress manipulation). Six participants were excluded due to experimenter or technical error, and four did not return on day 2 for the follow up session. One final participant was eliminated prior to analysis because their baseline cortisol level on day 2 was greater than 3 standard deviations from the mean. Our final sample included a total of 52 healthy participants (20 males) with a mean age of 24.44 (SD = 7.45; range=18–50). All participants gave informed consent by signing a form approved by New York University’s Committee on Activities Involving Human Subjects and were compensated for their time.
2.2 Fear acquisition and extinction
To create a novel fear association, we used a simple discrimination fear-conditioning task with delay conditioning and partial reinforcement (see Figure 1 for experimental protocol and timing). Conditioned stimuli (CSs) were two square images depicting abstract, fractal geometry (Figure 1). A mild electric wrist-shock served as the unconditioned stimulus (US). Our index of fear arousal was skin conductance response (SCR), an assay of sympathetic nervous system arousal that reflects changes over discrete intervals consistent with the autonomic arousal characteristic of fear (Critchley, 2002).
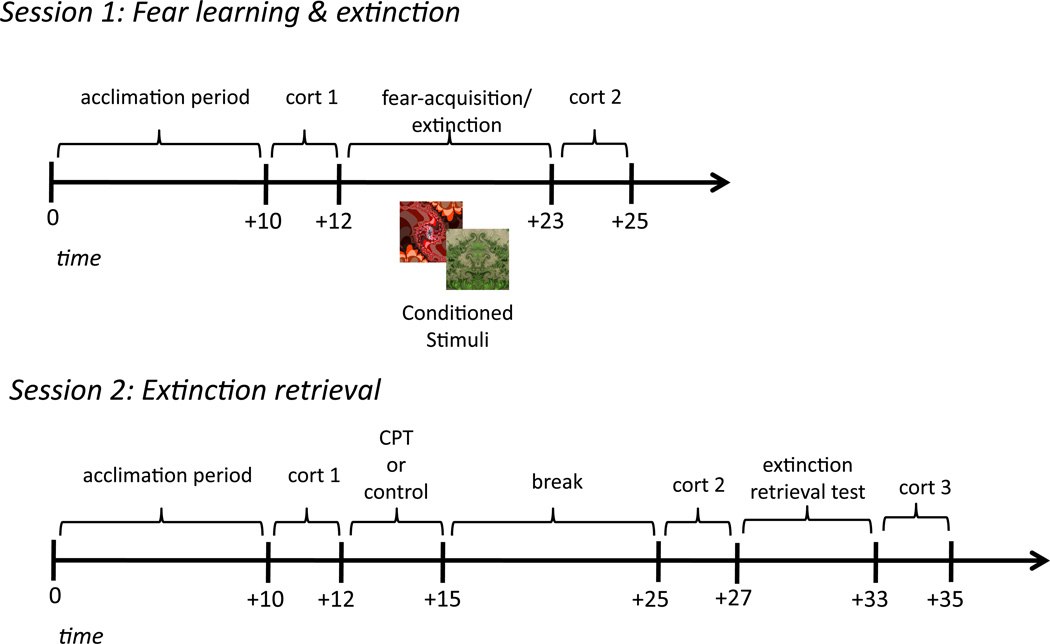
Figure 1. Experimental procedure.
Timeline of experiment protocol on day 1 (learning) and day 2 (retrieval).
During acquisition, one image (hereafter referred to as the CS+) co-terminated with the US on a subset of trials, while the other image (CS-) was designated as a safety signal and never paired with shock. Participants were instructed to pay attention to the computer screen and monitor the relationship between what they were viewing on the screen and when/if they received a shock. The acquisition session comprised a total of 26 trials: 10 CS− trials, 10 unreinforced CS+ trials, and 6 reinforced CS+ trials. Partial reinforcement enabled unreinforced CS+ trials be analyzed for conditioned responses, without contamination by the distinct physiological response induced by the US. The acquisition session was followed immediately and without warning (i.e., no break or signal) by extinction training, during which 10 unreinforced CS+ trials and 10 CS− trials were presented.
On each trial, the CS was presented for 4 seconds, followed by a variable 8–12s inter-trial interval, during which a white fixation cross was presented at the center of a black screen. Two distinct, pseudo-randomized trial orders were used such that no trial of the same kind occurred more than two times consecutively. CS assignment and trial orders were counterbalanced across participants.
2.3 Physiological stimulation and recording
Mild electric shocks (200ms) were delivered through a bar electrode (Biopac Systems) attached with a Velcro strap to participants’ wrist. Bar electrode wells were manually filled with NaCl electrolyte gel to enhance conductance and attached to a SD9 Square Pulse Stimulator (Grass Technologies). To identify each individual’s shock level, mild shocks were manually triggered and increased in increments of 5V until the participant reported the shock to be uncomfortable, but not painful, or 60V were reached. To record SCR, shielded Ag-AgCl electrodes filled with standard NaCl electrolyte gel were applied to participant’s left index and middle fingers. SCR was sampled at a rate of 200Hz and recorded using an MP100 Data Acquisition module (BIOPAC Systems) connected to an Apple computer.
2.4 Stress Manipulation
Participants were randomly assigned to the stress (n = 27; 15 females) or control (n = 25; 19 females) condition on day 1, prior to the initial learning session. On day 2, participants in the stress condition completed the cold pressor task (CPT), which entailed submerging their right hand to elbow in ice-cold water (0–4°C) for three minutes. The CPT is widely used to model the effects of mild to moderate stress and reliably induces sympathetic nervous system and HPA-axis arousal as measured by increased physiological, endocrine (i.e., cortisol), and subjective levels of stress (Lovallo, 1975; Velasco, Gomez, Blanco, and Rodriguez, 1997; Lupien, Maheu, Fiocco, & Schramek, 2007; McRae et al., 2006). If a participant was unable to complete the CPT, the experiment was terminated. Participants in the control condition submerged their right hand to elbow in room-temperature water (30–35°C) for three minutes. To assess subjective levels of stress, all participants rated how unpleasant they felt during the CPT or control task on a scale from 1 (not unpleasant) to 10 (highly unpleasant).
2.5 Extinction retrieval test
Ten minutes after the CPT or control task, when cortisol levels began to rise, participants were set up for an extinction retrieval test. SCR and shock electrodes were prepared and attached, and participants’ shock amplitude was set to the same level as that of the previous day. On day 2, no additional workup procedure was conducted to avoid reinstating fear responses and no further instructions were given. A second extinction session commenced, in which participants were presented with 10 unreinforced trials of each CS while SCRs were recorded. The first trial of the re-extinction session was designated as a CS− to absorb the initial orienting response that commonly occurs at the start of the session, and was therefore disregarded before all day 2 analyses. The second trial was counterbalanced between CS+ and CS− across participants.
2.6 Neuroendocrine assessment
Saliva samples were collected throughout the experiment in order to assay neuroendocrine levels indicative of stress response. To control for diurnal rhythms in cortisol levels, all participants were invited to participate between 12:00 and 5:00 pm, at the same time on each day. Saliva was collected using an absorbent oral swab that participants placed under their tongues for two minutes. Before arriving to the laboratory, participants were instructed to abstain from eating or drinking for one hour. When participants arrived to the laboratory each day, they were placed in a sound-attenuated, temperature-controlled experiment room for 10 minutes to acclimate to the laboratory setting. During this time, they were given a 6-ounce glass of water to drink in order to clear out residual saliva before baseline samples were taken.
On day 1, saliva samples were collected at baseline and again after the fear-conditioning/extinction task to ensure that stress hormones, widely known to influence the consolidation of such learning, did not differ between groups. On day 2, saliva samples were collected at baseline, again 10 minutes after the stress/control manipulation when cortisol elevations were expected to rise, and finally, 20 minutes after the stress/control manipulation to confirm that cortisol concentrations remained stable until the end of the session.
All samples were preserved immediately after collection in a sterile tube that was stored in a freezer set to −20°C. Samples were shipped frozen to Salimetrics Testing Services (State College, PA), a CLIA-certified analytical laboratory where samples were analyzed using high-sensitivity enzyme immunoassay kits. Samples were brought to room temperature and centrifuged at approximately 3,000RPM for 15 minutes before testing. To ensure our neuroendocrine measures were accurate, samples underwent duplicate assay and the average of these two values was used in our analyses.
2.7 Physiological analysis
Conditioned fear responses were assessed offline using Acqknowledge software (BIOPAC System). Each individual’s SCR data was preprocessed using AcqKnowledge software (BIOPAC systems) prior to analysis by low-pass filtering (cutoff frequency 25Hz) and mean-value smoothing using a 3-sample window. Fear responses were measured by taking the largest base-to-peak waveform amplitude response (in microsiemens, µS) within the 0.5 to 4.5s interval after CS onset. Responses lower than a pre-determined criterion of 0.02 µS were recorded as zero. Raw SCR amplitudes were square root transformed to reduce skew and were subsequently divided by individuals’ mean US response in order to account for individual differences in shock reactivity (Lykken, & Venables, 1971). To assess how day 1 learning developed over time, both acquisition and extinction phases were divided into an early (first half of trials) and late (latter half of trials) phase. We calculated conditioned fear responses by subtracting mean SCR to the baseline stimulus from that of the threat-related stimulus (CS+ minus CS−).
2.8 Statistical Analysis
Analysis of variance (ANOVA) with repeated measures was used to analyze all SCR and cortisol data. Separate analyses were conducted to assess differential SCR across each conditioning phase (acquisition, extinction, retrieval) and mean cortisol concentrations across each day. Further, since gender has been shown to modulate emotional learning and stress response, all analyses included gender as a between-subjects factor. Post hoc comparisons were conducted using Student t -tests when appropriate. All tests were two-tailed and considered statistically significant when p < .05. All analyses were conducted using SPSS (version 20.0, 2011; IBM Corp., Armonk, NY).
3. Results
3.1 Day 1 Analyses
3.1.1 Neuroendocrine responses1
Cortisol concentrations across the day 1 session did not differ as a function of condition or gender. A repeated-measures ANOVA using a within-subject factor of time of cortisol measurement (baseline, post-conditioning) and between-subject factors of gender (male, female) and condition (control, stress) revealed no main effect of time (F(1,44) = 0.32, p = .57), condition (F(1,44) = 0.57, p = .45), or gender (F(1,44) = 0.58, p = .45), nor any interactions (all ps > .23). Importantly, this confirms that groups did not differ in cortisol concentrations throughout the day 1 learning session, suggesting that any differences in extinction retrieval on day 2 cannot accounted for by differences in glucocorticoid levels that might affect the consolidation of learning on day 1. Further, cortisol did not increase across day 1, suggesting that while the fear conditioning and extinction task elicited emotional arousal, it did not lead to enhanced HPA-axis activity.
3.1.2 Fear acquisition
To confirm that initial fear conditioning did not differ between condition or gender, we assessed skin conductance responses during the early phase (first half of trials) and late phase (latter half of trials) of the acquisition session. Across groups, differential SCRs were significantly greater during late acquisition than those during early acquisition (F(1,48) = 10.99, p = .002) with no effect of gender (F(1,48) = 0.16, p = .69) or group (F(1,48) = 2.38, p = .13), nor any interactions (all ps > .63). Planned comparisons confirmed that both groups showed significantly greater mean SCR to the CS+ relative to the CS− across late acquisition trials (control: t(24) = 5.59, p < .0001; stress: t(26) = 7.32, p < .0001). Further, across acquisition, this differential responding did not differ between groups (t(50) = 0.79, p = .43) or gender (t(50) = 0.28, p = .77), suggesting that all participants initially acquired comparable levels of differential fear responses.
3.1.3 Fear extinction
All participants extinguished fear responses by the latter half of the session (F(1,48) = 23.30, p < .0001). Importantly, the extent of this learning did not differ between condition (F(1,48) = 0.48, p = .49), or gender (F(1,48) = 0.54, p = .47), nor did it result in any interactions (all ps > .35). Planned comparisons verified that conditioned fear responses significantly decreased in both groups during late as compared to early extinction (control: t(24) = 3.15, p = .004; stress: t(26) = 3.82, p = .0007) and that differential responding across the extinction phase did not differ between condition (t(50) = 1.30, p = .20) or gender (t(50) = 0.81 p = .42).
3.2 Day 2 Analyses
3.2.1 Neuroendocrine response2
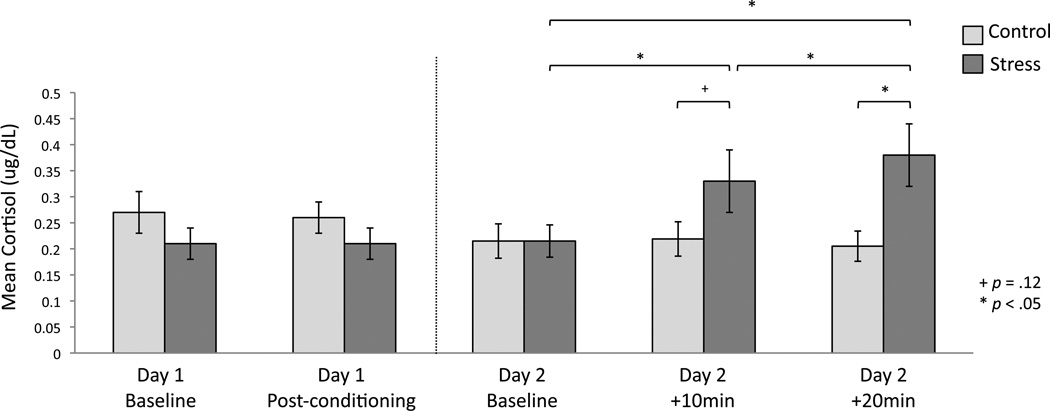
Mean cortisol concentrations for the three samples collected on Day 2 (i.e., baseline, +10min, +20min) are plotted in Figure 2. Critically, we found a main effect of time of cortisol measurement (F(2,88) = 7.97, p = .001) as well as a time X condition interaction (F(2,88) = 5.65, p = .005). There was no main effect of group (F(1,44) = 0.74, p = .39) or gender (F(1,44) = 1.21, p = .28), nor any interactions with gender (time X gender: F(2,88) = 0.90, p = .41; time X gender X group: F(2,88) = 0.42, p = .66). Mean cortisol did not differ between conditions at baseline (t (47) = −0.002 p = .99), however, 10 minutes after the CPT, participants in the stress condition demonstrated a trend toward higher cortisol concentrations (t (47) = −1.56, p = .12), which reached significance 20 minutes after the CPT (t (48) = −2.33, p = .02). Paired samples t -tests confirmed that participants in the stress group showed significant elevations in cortisol relative to baseline levels both 10 minutes (t(25) = −4.26, p = .0002) and 20 minutes (t(25) = −3.90, p = .001) after the stress induction. No elevations from baseline were demonstrated in the control group (+10min; t(21) = −0.22, p = .82; +20min: t(22) = 0.53, p = .60).
Figure 2. Cortisol measurements.
Mean cortisol concentrations on day 1 and day 2. On day 1, cortisol was measured at baseline and after fear conditioning /extinction. On day 2, cortisol was measured at baseline and again 10 and 20 minutes after the stress/control task. Across the experimental session, groups differed only after the stress/control manipulation. *p < .05; errors bars denote standard error of the mean.
3.2.2 Subjective stress responses
Consistent with previous research (Lovallo, 1975; Velasco, Gomez, Blanco, and Rodriguez, 1997; McRae et al., 2006), participants in the stress condition rated the CPT as more unpleasant (M = 7.47, SD = 2.03) than did participants in the control condition (M = 1.80, SD = 1.26) indicating that the task elicited a subjective stress response.
3.2.3 Extinction retrieval
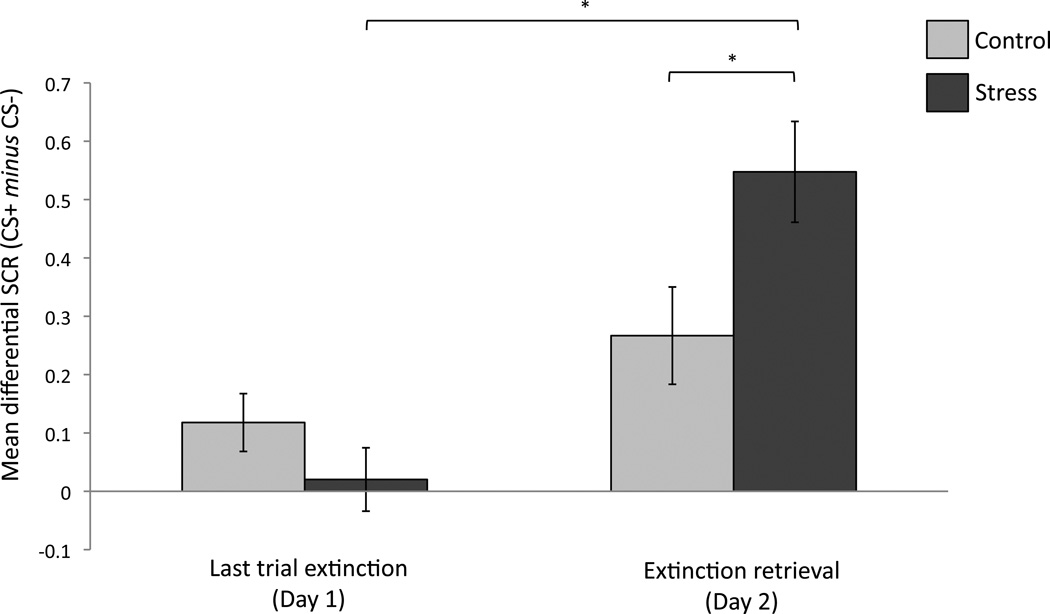
We examined the extent to which extinction memory was retrieved a day later by comparing participants’ conditioned responses on the last trial on day 1, when fear responses were extinguished, with those on the first trial of day 2 when participants were initially presented with each CS (Figure 3). Since trial order was counterbalanced across participants after the initial orienting trial, such that half the participants were then presented with a CS+ and half with a CS−, trial order was included as a covariate to account for any differences that this presentation order may have had on initial fear responses. We note that conditions did not differ in regards to their initial orienting response to this trial (t(50)=1.18; p=.24).
Figure 3. Retrieval of extinction memory.
Extinction retrieval was indexed by comparing conditioned responses on the last extinction trial on day 1 to the first extinction retrieval trial on day 2. *p < .01; errors bars denote standard error.
An ANOVA with repeated measures of trial (last trial of extinction, first trial retrieval) and between subject factors of gender and condition revealed no effect of trial (F(1,47) = 0.15, p = .70), gender (F(1,47) = 0.003, p = .96), or trial order (F(1,47) = 0.009, p = .92), and no interactions with gender or order (all ps > .24). However, we did find a marginal effect of condition (F(1,47) = 331, p = .07) and, critically, a condition X trial interaction (F(1,47) = 7.21, p = .01), suggesting that extinction retention differed as a function of condition. Follow up t-tests demonstrated that while fear responses did not differ between conditions on the last trial of extinction (t(50) = 1.27, p = .21), they differed between conditions at the start of day 2 (t(50) = −2.34, p = .02), such that stressed participants showed significantly lower extinction retrieval (i.e., higher fear recovery) relative to controls. Further, within the stress condition, differential fear responses on the first extinction retrieval trial were significantly higher relative to the last extinction trial on day 1 (t(26) = −5.21, p = .00002), while responses in the control condition did not differ across trials (t(24) = −1.52, p = .14), indicating that participants in the control condition showed better retrieval of extinction memory across sessions, while stressed participants showed marked impairments. An exploratory analysis revealed that this first retrieval trial did not differ from that of late acquisition in the control condition, indicating that although control participants did not show evidence for significant fear recovery relative to the final extinction trial, they did show some preservation of initial fear responses prior to extinction training. In contrast, participants in the stress condition showed a trend toward significantly higher fear responses at extinction retrieval compared to late acquisition (t(26) = 1.84, p = .07), indicating that fear arousal at retrieval exceeded that demonstrated during late acquisition.
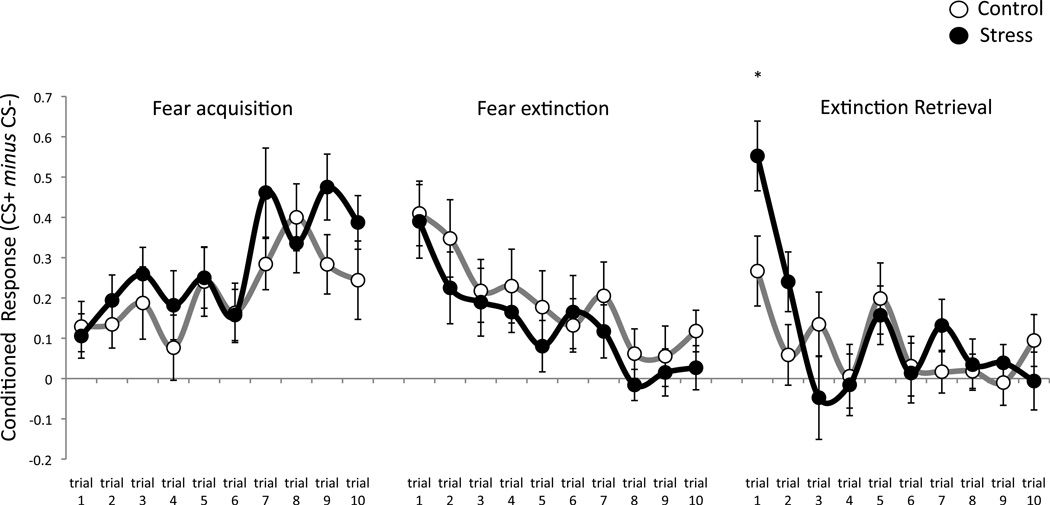
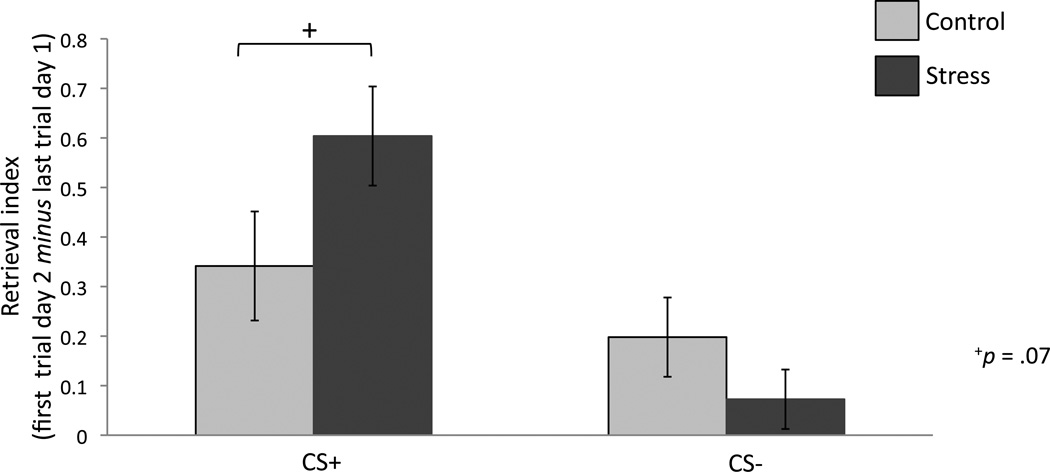
Trial-by-trial differential SCRs for each condition are depicted in Figure 4. The only trial on which conditions differed is that of the first retrieval trial at the start of day 2. To assess whether group differences in the recovery of conditioned responses emerged from CS+ or CS− responses, we compared the extent of fear recovery between conditions for each individual CS. Fear recovery between the last trial of extinction and the first retrieval trial trended toward significantly higher in the stress condition as compared to controls for the CS+ only (t(50) = −1.84 p = .07), while CS− responses did not differ (t(50) = 0.84 p = .40), suggesting that stress selectively increased responses to the cue previously paired with threat and not that which signaled safety (Figure 5).
Figure 4. Trial-by-trial data.
Differential SCRs for each trial across the acquisition, extinction and extinction retrieval session. Fear responses did not differ between conditions on any trial except the first trial of re-extinction, after the stress/control manipulation. *p < .05; errors bars denote standard error of the mean.
Figure 5. Retrieval Index.
Extinction retrieval for each CS was indexed by comparing mean SCR on the last extinction trial on day 1 to the first retrieval trial on day 2. The difference between these two trials (first trial re-extinction – last trial extinction) constitutes our retrieval index and trended toward differing significantly between conditions for the CS+ only. +p = .07; errors bars denote standard error
3.2.4 Correlational analyses
To assess whether day 2 cortisol concentrations were related to extinction retrieval or conditioned responses during extinction retrieval, we conducted a correlation analysis between cortisol levels at both time-points after the CP/control task and our extinction retrieval index on day 2. Cortisol levels were not associated with the first extinction retrieval trial on day 2 in either condition. However, cortisol levels measured at both time points after the CP/control manipulation were associated with conditioned responses (control: 10min: r=.64, p=.002; +20min: r=.51, p=.01; stress: +10min: r=.46, p=.02; +20min: r=.50, p=.01) and mean CS+ responses (control: +10min: r=.58, p=.005; +20min: r=.33, p=.12; stress: +10min: r =.54, p=.005; +20min: r=.63, p=.001) across the extinction retrieval session.
4. Discussion
The current study investigated the influence of an acute stress induction on the retrieval of extinction training in a cued aversive conditioning paradigm. We found that while initial fear acquisition and extinction training did not differ between conditions, extinction retrieval was impaired a day later in those participants who underwent an acute stress manipulation.
Participants in the control condition demonstrated stronger extinction retrieval as evidenced by only a moderate increase in differential fear arousal that did not differ significantly from that of the extinction session. Stressed participants, however, showed marked impairments in extinction expression as manifested by greater fear recovery relative to the end of the extinction phase the previous day. Notably, fear arousal at the start of this retrieval session did not differ from the late stage of acquisition in either group, suggesting all participants showed some degree of fear arousal that was comparable to initial acquisition, however, this was significantly higher in stressed participants relative to controls. Cortisol concentrations extracted from participants’ saliva samples confirmed that the stress induction successfully evoked enhanced HPA-axis activity throughout the extinction retrieval session in the stress condition only. These findings are consistent with recent work in rodents (Deschaux et al, 2013) showing that acute stress leads to extinction memory deficits when induced directly before retrieval. Our results are unique in that they investigate the effect of stress on extinction retrieval in healthy humans within the context of cued classical fear conditioning, providing a viable model for the return of fear in clinical populations suffering from affective psychopathology.
These data are consistent with a substantial body of research across species showing that stress or glucocorticoid exposure leads to memory retrieval impairments (Kirschbaum et al., 1996; Diamond et al., 1999; de Quervain et al., 1998, 2000; Kuhlman et al., 2005a, 2005b; Schilling et al., 2013; see: Roozendaal, 2002; Het et al., 2005; Wolf, 2009, for review). These retrieval impairments, however, have typically been demonstrated in the context of declarative or episodic memory retrieval, which critically rely on the hippocampus. Consistent with this, the prominent neural substrate thought to underlying these retrieval impairments is supraoptimal levels of glucocorticoid receptor activation in the hippocampus, which can impair hippocampal function (see Roozendaal, 2002 or Wolf, 2009, for review). Like Bentz and colleagues (2013), our results extend these retrieval deficits to that of cued-based fear and extinction learning, a form of associative learning that has been shown to rely critically on the amygdala and its functional interaction with the vmPFC (Quirk & Mueller, 2008), rather than the hippocampus, which instead plays a larger role in the contextual modulation of fear and extinction learning and retrieval. Our results are novel in that we demonstrate this effect in the context of extinction memory retrieval using an implicit measure of fear expression that enables the assessment of phasic cue-evoked physiological responses driven by autonomic nervous system arousal rather than measures that assess participant explicit predictions of an aversive outcome.
Substantial work has now demonstrated a pivotal role for the vmPFC in the retention and retrieval of extinction learning (see Quirk & Mueller, 2008; Milad & Quirk, 2012, for review). Neuroimaging work in humans has shown that vmPFC activity increases during extinction training and correlates with the magnitude of later extinction retention (Milad et al., 2007). The vmPFC is also active during extinction retrieval (Phelps et al., 2004; Kalisch et al., 2006) and cortical volume in this region has been shown to correlate with the magnitude of extinction retrieval (Milad et al., 2005; Hartley et al., 2011), confirming an important role for this region in the successful retrieval of extinction training. Although the precise neural mechanism underlying a stress-related retrieval deficit have not been well characterized in humans, work in non-human animals has shown that the vmPFC contains a high concentration of glucocorticoid receptors (Meaney & Aitken, 1985) and is acutely sensitive to the deleterious effects of stress (Arnsten, 2009). Acute exposure to stress is sufficient to produce neuronal alterations (i.e., dentritic retraction) in IL neurons (Izquierdo, Wellman & Holmes, 2006), and a single dose of glucocorticoids is enough to cause prolonged expansion of basolateral amygdala neurons that correlate with increased anxietylike behavior (Mitra & Sapolsky, 2008). Further, IL lesion in rats produce extinction retrieval deficits that are comparable to those seen after stress induction (Farrell et al, 2010). Since the vmPFC exerts an inhibitory influence on the amygdala via direct projections that activate circuits that gate fear expression, stress may influence similar circuitry in humans by enhancing amygdala function and attenuating the inhibitory influence of the vmPFC, resulting in greater fear expression and less retrieval of extinction memory.
This proposal is consistent with recent work in humans that has examined the effects of stress on amygdala-centered functional connectivity using resting-state fMRI. These studies reported decreased connectivity between the amygdala and prefrontal regions such as the vmPFC and OFC after stress induced by the cold-pressor task (Clewett et al., 2013) and aversive films (Eryilmaz, et al., 2011). Another study (van Marle et al., 2010) reported enhanced functional coupling after stress between the amygdala and locus coeruleus, as well as the dorsal anterior cingulate cortex (dACC), brain regions implicated in promoting vigilance and the expression of conditioned fear, respectively (Milad et al., 2007). Collectively, these studies suggest that stress shifts amygdala-based connectivity away from regions critical to the retrieval and expression of extinction memory, and enhances connectivity with those that promote fear expression. Interestingly, glucocorticoids have been shown to restore adaptive amygdala connectivity both through endogenous cortisol release after stress (Veer et al., 2011) or exogenous administration of cortisol (Henckens et al., 2010, 2012).
Functional neuroimaging studies assessing stress in healthy humans have shown variable effects on the brain depending on a number of factors, including the type of stressor used. Nonetheless, some consistencies have emerged. For example, a number of these studies have reported increases in dACC activity and decreases in hippocampal and medial/orbitofrontal regions during or after stress exposure (see Dedovic et al., 2009, for review). Although few studies have examined the effects of stress and glucocorticoids on the neural circuitry underlying fear learning and extinction, those that have generally report that stress or cortisol administration leads to enhanced responses to conditioned stimuli in brain regions involved in fear learning and extinction (e.g., amygdala, dACC, hippocampus, vmPFC) in female participants and reduced activity in these regions in men (Stark et al., 2006; Tabbert et al., 2010; Merz et al., 2012; 2013). This may be related to the propensity of male participants to experience stronger cortisol responses to acute stress than females (Kirschbaum et al., 1999; Merz et al., 2013; Bentz et al, 2013), leading to greater attenuation of conditioned response related activity in these brain regions. Neuroimaging studies that have been conducted in patients diagnosed with PTSD have reported that these populations demonstrate impaired extinction recall, despite intact fear learning and extinction (Milad et al., 2008, 2009). These extinction retrieval impairments were related to hypoactivation in the vmPFC and hyperactivation in the dACC (Milad et al., 2009). These findings are consistent with the above work assessing stress on brain function and support the provisional model of prefrontal-amygdala interactions as underlying extinction learning and retention.
In the current investigation we assessed extinction retrieval rather than extinction learning, however there is some suggestion that the same stress hormones we found to correlate with conditioned responses during extinction retrieval can exert different effects on the learning and consolidation phases of extinction. Specifically, studies that have administered glucocorticoids to patients with anxiety disorders have shown that these hormones can enhance extinction learning and consolidation, suggesting that the administration of glucocorticoids may theoretically provide a way to buffer individuals from stress-induced retrieval deficits in extinction memory by strengthening initial extinction learning and storage. In one study, glucocorticoids administered exogenously to patients with PTSD led to reduced traumatic memory retrieval and trauma-related symptoms (Aerni et al., 2004; Suris et al., 2010). In another, phobic patients who were administered glucocorticoids before exposure to fear-inducing stimuli or experiences reported significant reductions in fear responses throughout the exposure session that were maintained at follow-up 48-hr later (Soravia et al., 2006). Finally, de Quervain and colleagues (2011) showed that an oral doses of cortisol administered prior to a series of exposure therapy sessions resulted in the persistent attenuation of phobic fear responses in patients with height phobia. Interestingly, the therapeutic effects of cortisol in these studies were selective to anxiety patients (i.e., no benefit was seen in healthy controls), suggesting that glucorticoids may be most effective in patients suffering from stress-related psychopathology. This is consistent the substantial work showing that PTSD patients have lower basal cortisol levels than healthy controls, which is thought to arise from hypersensitivity to glucocorticoid release through dysfunctional negative feedback inhibition (see Yehuda, 2009, for review). This suggests that exogenously elevating cortisol levels through glucocorticoid administration in such populations may benefit extinction processes by promoting optimal glucocorticoid levels that facilitate more adaptive emotional responses and lead to stronger consolidation of safety learning.
To date, the effect of glucocorticoids on extinction retrieval has not been investigated. Here, we found cortisol release to be associated both with conditioned responding and mean CS+ responses during extinction retrieval, suggesting a relationship between cortisol and extinction memory impairments. Since we assessed extinction retrieval in healthy populations, this result may indicate that our sample attained supraoptimal glucocorticoid levels after stress that impaired extinction memory retrieval. However, it should be noted that an acute stress induction, such as the one used here, engenders different physiological and neuroendocrine responses than that of exogenous cortisol administration alone. This is because stress engages the rapid activation of the autonomic nervous system that evokes catecholamine release (e.g., noradrenaline) as well as the subsequent engagement of the hypothalamic-pituitary-adrenal (HPA) axis that triggers circulating glucocorticoids, which interact with and potentiates these rapid catecholamine responses (Ulrich-Lai & Herman, 2009). It is therefore possible that administering glucocorticoids without this noradrenergic influence may produce different results.
Unlike many other studies that have reported gender effects on emotional learning and stress response, we did not find such effects in this investigation. This may be due to the stressor we used (i.e., physiological rather than psychosocial), may simply reflect a power issue in our sample or may be related to the strict criteria we applied to participants’ acquisition and extinction performance on day 1. It is possible that the exclusion of these participants before day 2 eliminated some portion of the variance that might have been present between genders. However, these criteria were essential in that we were not able to assess extinction training without evidence of differential fear arousal during initial learning, nor could we assess the retrieval of extinction memory if evidence for successful extinction learning was not demonstrated.
One notable limitation of our study is that we did not collect information concerning female participants’ menstrual cycle phase or hormonal contraceptive use. Research has demonstrated that females with higher levels of estrogen (i.e., those typically in the luteal or early follicular phase of the menstrual cycle) show stronger extinction retrieval that is comparable to that of males, as compared to women with lower levels of estrogen (i.e., typical of the late follicular phase) (for review, see: Lebron-Milad & Milad, 2013). Hormonal contraceptive use has also been shown to alter stress responses and its potential impact on emotional memory process. Specifically, females using such contraceptives show blunted cortisol responses after stress exposure relative to men (Merz et al., 2013; Bentz et al., 2013) and naturally cycling women (Kirschbaum et al., 1999), and diminished effects of stress on emotional memory processes (Nielsen et al, 2013). Therefore, further investigation into whether these factors also modulate stress-induced extinction retrieval deficits will be critical to fully characterize how stress affects memories of safety learning. Importantly, such work may lead to more effective and tailored interventions by highlighting ways that endogenous or exogenous sex hormones may make women more resilient or vulnerable to the effects of stress on extinction memories.
Extinction serves as a viable model of how associative learning can be flexibly modified and updated to promote adaptive functioning in every day life. Understanding how extinction training is expressed over time is of particular importance because it serves as the foundation of behavioral-based clinical interventions that aim to reduce anxiety and fear related to affective psychopathology. The ability of stress to induce deficits in extinction retrieval in otherwise healthy participants demonstrates how vulnerable this safety learning is to stressful circumstances that often arise in everyday life. Since stress is both a catalyst and cardinal symptom of anxiety disorders, understanding and further characterizing how stress interacts with the neural circuitry underlying extinction learning, retention and retrieval will be critical to refining treatment options for patients suffering with anxiety-related disorders. Persistent fear responses and impaired regulation from inhibitory regions are the hallmark of such disorders, especially cue-specific phobias and PTSD. Since stress is ubiquitous outside of the clinic, examining ways to enhance extinction retrieval, either by reducing stress exposure, or strengthening the consolidation of extinction memory to facilitate subsequent retrieval, will be critical to enhancing the efficacy of such extinction based therapies.
Highlights.
-
○
Extinction retrieval deficits underlie the re-emergence of anxiety after treatment
-
○
Stress exposure can impair the brain regions critical to extinction retrieval
-
○
Participants completed an aversive-learning task followed by extinction training
-
○
Participants underwent a stress or control task before a retrieval test a day later >
-
○
Stress exposure led to increased cortisol and impaired extinction memory retrieval
-
○
Physiological arousal was associated with salivary cortisol in both conditions
-
○
Highlights challenges using treatment outside the clinic where stress is pervasive
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
At least one of the Day 1 samples from three (2 female) participants in the control condition and one male participant from the stress condition contained insufficient saliva and could not be analyzed, therefore these samples were excluded from this analysis.
At least one the Day 2 samples from two female participants in the control condition contained insufficient saliva and could not be analyzed, therefore these samples were excluded from this analysis.
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007 doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, Willhelm FH, Hartmann FR, Kunz S, von Rohr IRR, de Quervain DJ-F. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013;38:1186–1197. doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2004;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. The Journal of Neuroscience. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment. 2010;32:68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Schoeke A, Mather M. Amygdala functional connectivity is reduced after the cold pressor task. Cognitive Affective Behavior Neuroscience. 2013 doi: 10.3758/s13415-013-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, et al. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, D’Aguiar C, Pruessner JC. What stress does to your brain: A review of neuroimaging studies. Canadian Journal of Psychiatry. 2009;54:6–15. doi: 10.1177/070674370905400104. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Zheng X, Lavigne J, Nachon O, Cleren C, Moreau JL, et al. Post-extinction fluoxetine treatment prevents stress-induced reemergence of extinguished fear. Psychopharmacology. 2013;225:209–216. doi: 10.1007/s00213-012-2806-x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CP, Herman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Diaz-Mataix L, Debiec J, LeDoux JE, Doye`re J. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. The Journal of Neuroscience. 2011;31:9538–9543. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryilmaz H, Van de Ville D, Schwartz S, Vuilleumier P. Impact of transient emotions on functional connectivity during subsequent resting state: A wavelet correlation approach. NeuroImage. 2011;54:2481–2491. doi: 10.1016/j.neuroimage.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiology of Learning & Memory. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning and Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Baas JMP, Lawley M, Ellis V, Charney DS. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology. 2006;186:434–441. doi: 10.1007/s00213-005-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay L, Zelikowsky M, Blair HT, Fanselow M. Reinstatement of extinguished fear by an unextinguished conditional stimulus. Frontiers in Behavioral Neuroscience. 2012;6:1–7. doi: 10.3389/fnbeh.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joëls M, Fernández G. Time-dependent effects of corticosteroids on human amygdala processing. Journal of Neuroscience. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joëls M, Fernández G. Corticosteroid induced decoupling of the amygdala in men. Cerebral Cortex. 2012;22:2336–2345. doi: 10.1093/cercor/bhr313. [DOI] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning and Memory. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. Journal of Neuroscience. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress Differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, et al. Context dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal Network. Journal of Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress-and treatment induce elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learning & Memory. 2012;19:43–49. doi: 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl LK, Lass-Hennemann J, Richter S, Blumenthal TD, Oitzl M, Schachinger H. Accelerated trace eyeblink conditioning after cortisol IV-infusion. Neurobiology of Learning and Memory. 2010;94:547–553. doi: 10.1016/j.nlm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oralcortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiology of Learning and Memory. 2005a;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005b;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of Mood & Anxiety Disorders. 2013;2:3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lovallo W. Cold pressor task and autonomic function: A review and integration. Psychophysiology. 1975;12:268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Fiocco A, Schramek T. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain & Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. The Journal of Neuroscience. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. European Journal of Neuroscience. 2013 doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Reviews of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor & Trier Social stressors. Human Psychopharmacology. 2006;21:377–85. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. Dexamethasone binding in rat frontal cortex. Brain Research. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive Affective Neuroscience. 2012;7:819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Stark R, Vaitl D, Tabbert K, Wolf OT. Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biological Psychology. 2013;92:82–89. doi: 10.1016/j.biopsycho.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of Psychiatric Research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in post-traumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M, Quirk G. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra RM, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NY, Elzinga BM, Wolf OT, de Ruiter MB, Damoiseaux JS, Kuijer JP, Veltman DJ, Scheltens P, Rombouts SA. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behavior. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiology of Learning & Memory. 2005;8:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. Oxford University Press: Oxford. 1927 [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influences of stress hormones on fear circuitry. Annual Review of Neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews: Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Memory Modulation. Behavioral Neuroscience. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. American Journal of Psychotherapy. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- Schilling TM, Kolsch M, Larra MF, Zech CM, Blumenthal TD, Frings C, Schachinger H. For whom the bell (curve) tolls: Cortisol rapidly affects memory retrieval by an inverted U-shaped dos-response relationship. Psychneuroendocrinology. 2013;38:1565–1572. doi: 10.1016/j.psyneuen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. Neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacology. 2013;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Shors T. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol N, Lovibond PF. Cross-US reinstatement of human conditioned fear: Return of old fears or emergence of new ones? Behaviour Research and Therapy. 2012;50:313–322. doi: 10.1016/j.brat.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (self-evaluation questionnaire) Consulting Psychologists Press: Palo Alto. 1970 [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of glucocorticoid on combat-related PTSD symptoms. Annals of Clinical Psychiatry. 2010;22:274–279. [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiology of Learning and Memory. 2010;94:392–401. doi: 10.1016/j.nlm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YA, Herman JP. Neural regulation of endocrine and autonomic responses to stress. Nature Reviews. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SARB. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2011b;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Velasco M, Gomez J, Blanco M, Rodriguez I. The cold pressor test: Pharmacological and therapeutic aspects. American Journal of Therapeutics. 1997;4:34–38. doi: 10.1097/00045391-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: State of the art. Annual Review of Clinical Psychology. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LM, Wilson R, DiCara LV. Pavlovian conditioned cardiodecelerations following preshock in immobilized rats. Physiology & Behavior. 1975;15:653–658. doi: 10.1016/0031-9384(75)90115-8. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory: Twelve years of progress? Brain Research. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in pos-traumatic stress disorder. Annals of the New York Academy of Science. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cognitive Affective Behavioral Neuroscience. 2005;5:191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learning & Memory. 2006;13:441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]