Abstract
Objective
Growing evidence suggests that neurodegenerative diseases are associated with metabolic disorders, but the mechanisms are still unclear. Better comprehension of this issue might provide a new strategy for treatment of neurodegenerative diseases. We investigated possible roles of adiponectin (APN), the antidiabetes protein, in the pathogenesis of α-synucleinopathies.
Methods
Using biochemical and histological methods, we investigated autopsy brain of α-synucleinopathies including Parkinson's disease (PD) and dementia with Lewy bodies (DLB), and analyzed the effects of APN in cellular and in mouse models of α-synucleinopathies.
Results
We observed that APN is localized in Lewy bodies derived from α-synucleinopathies, such as Parkinson's disease and dementia with Lewy bodies. In neuronal cells expressing α-synuclein (αS), aggregation of αS was suppressed by treatment with recombinant APN in an AdipoRI-AMP kinase pathway-dependent manner. Concomitantly, phosphorylation and release of αS were significantly decreased by APN, suggesting that APN may be antineurodegenerative. In transgenic mice expressing αS, both histopathology and movement disorder were significantly improved by intranasal treatment with globular APN when the treatment was initiated in the early stage of the disease. In a mouse model, reduced levels of guanosine and inosine monophosphates, both of which are potential stimulators of aggregation of αS, might partly contribute to suppression of aggregation of αS by APN.
Interpretation
Taken together, APN may suppress neurodegeneration through modification of the metabolic pathway, and could possess a therapeutic potential against α-synucleinopathies.
Introduction
In our aged/superaged societies, there is an urgent need for the discovery of therapy against neurodegenerative diseases, while there are so far no effective ways available. Evidence has been accumulating to suggest that neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD), have aspects of lifestyle disorders. For instance, it had been known that some lifestyle interventions, such as exercise and low calorie diet, were beneficial for neurodegenerative diseases.1 Furthermore, epidemiological studies have shown that increased risk of neurodegenerative diseases is associated with metabolic disorders that may develop in adulthood.2 In particular, type II diabetes mellitus is a risk factor for AD2–4 and for other neurodegenerative diseases, such as PD5,6 and Huntington's disease.7 In this context, it is of note that adiponectin (APN), an antidiabetes factor, may be systematically increased in AD8 and PD9 patients, as well as in female centenarians.10 APN is an adipocytic protein that is structurally homologous to collagen VIII and X and complement C1q, and has many biological actions, including glucose utilization, insulin sensitization, and antiinflammatory properties.11 The APN signaling pathway has been described as an “exercise mimetic” that may be is protective against metabolic disorders, including type II diabetes, atherosclerosis, and obesity.12 However, little is known about the effect of APN against neurodegeneration in vivo, although in vitro APN protects neuroblastoma cells against neurotoxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine13 and ß-amyloid.14 The protective properties of APN are may be ideal for neurodegenerative diseases that currently have no curable treatments, and thus the main objective of this study was to determine whether APN is protective against α-synucleinopathies such as PD.
Materials and Methods
Use of autopsy brains was approved by the Human Ethics Committee of Fukushimura Hospital and Tokyo Metropolitan Institute of Medical Science, and the family of each subject provided informed consent for the postmortem analysis. All animal procedures were approved and conducted in accordance with Animal Ethics Review Committee regulations of the Tokyo Metropolitan Institute of Medical Science.
Reagents
Recombinant human APN (full-length APN or gAPN) (ProSpec, East Brunswick, NJ) was used for cells and transgenic mice experiments. SB203580 (Promega, Madison, WI) and compound C (Calbiochem, San Diego, CA) were applied to cell cultures at the indicated concentrations. Unless otherwise noted, all other chemicals were obtained from Sigma (St. Louis, MO).
Antibodies
The following primary antibodies were used in the study: polyclonal anti-C-terminal APN antibody (Novus Biologicals, Littleton, CO), anti-N-terminal APN antibody (Abcam, Cambridge, UK), anti-AdipoR1 (C-14) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-alix (Cell Signaling, Beverly, MA), anti-αS (Cell Signaling) and monoclonal anti-αS (syn-1; BD Biosciences, Franklin Lakes, NJ), anti-pαS (Wako Pure Chemical Industries, Osaka, Japan), anti-flottilin-1 (BD Biosciences), and anti-ß-actin (Sigma) antibodies. The secondary antibodies were Alexa Fluor 488-conjugated antirabbit antibody and Alexa Fluor 594-conjugated antimouse antibody (Invitrogen, Carlsbad, CA).
Autopsy brains
Sporadic PD (Yahr stage II), sporadic DLB, and age-matched controls were used in the study. Diagnoses of PD and DLB were based on previously described criteria15 and were ultimately confirmed by autopsy. The postmortem interval was less than 2 h in each case. The substantia nigra of PD brains and the cingulate cortex of DLB brains were fixed in 4% paraformaldehyde. Brain samples were then embedded in paraffin and sectioned into 4-μm slides. Sections were then deparaffinized and analyzed by H&E staining or immunostaining. Alternatively, brain samples were frozen at −80°C until later use for immunoblotting.
Cell cultures
B103 rat neuroblastoma cells expressing αS were routinely cultured as previously described.16 Typically, cells were incubated under serum-free conditions for 2 h and were treated with rec. APN (5 μg/mL: this concentration was decided, based on the previous publications17,18 or phosphate buffer saline (PBS) for an additional 18 h). Cells were then harvested and fractionated into tris-buffered saline (TBS), 1% sodium dodecyl sulfate (SDS), and 70% formic acid (FA) fractions.16 In some experiments, cells were dissolved in lysis buffer: 1% Nonidet P-40, 50 mmol/L HEPES, 150 mmol/L NaCl, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L ethylene glycol tetraacetic acid (EGTA), and 100 mmol/L sodium fluoride-containing protease inhibitor mixture (Nacalai Tesque, Tokyo, Japan). For the analysis of conditioned medium (CM), the CM samples were collected from the cell monolayer and centrifuged at 10,000g for 30 min to remove cell debris. Alternatively, exosome fractions were semipurified from the CM using a Total Exosome isolation kit (Invitrogen). For siRNA experiments, siRNA targeting rat AdipoR1 or a nontarget control (Santa Cruz Biotechnology) was transfected using Lipofectamine 2000 (Invitrogen).
Immunological procedures
Immunohistochemistry, immunofluorescence, and immunoblotting were performed as described elsewhere19 with minor modifications.
Animal experiments
Transgenic mice expressing wild-type human αS under the control of the Thy-1 promoter were used.20There were no differences in serum APN levels between mice expressing αS at 2–3 months and controls (data not shown), and tests for glucose in urine were negative in both groups (data not shown). These mice were previously shown to have accumulation and aggregation of αS in the frontal cortex and limbic system, with accompanying motor deficits.20 Genomic DNA was extracted and analyzed as previously described.19 The control mice were littermates of the same age and mixed gender. Typically, the αS tg mice (male at 3 months of age) were intranasally treated with gAPN every 3 days for 2 months. Alternatively, mice at 5 months of age were intranasally treated with gAPN every 3 days for 1 month. When performing nasal administration, a transmucosal absorption enhancer (10 μL of PBS containing 5 mg/mL poly-l-arginine hydrochloride, molecular mass >70,000)21 was administered nasally. Thirty minutes later, the mice were nasally treated with gAPN (10 μL of PBS containing 0.1 mg/mL of gAPN) or PBS alone. After behavioral tests at the indicated time points, the mice were analyzed histologically and biochemically. During the experimental period (∼8 months old, male), the levels of activation of astrocytes and microglia were too low to be detected in the brains of the αS tg mice15 (not shown).
Motor behavioral analyses
The motor behavioral analyses were performed under blind conditions. A rotarod test was performed for 3-, 4-, and 5-month-old mice, as previously described.19 Assessment of motor functions was supplemented by a beam walking test, as previously described22 with modifications. Briefly, a 90-cm beam with a flat surface of 12- or 5-mm width was set 50 cm above a table. A black box was placed at the end of the beam as a finishing point. The starting point was identified by the light of a lamp. Groups of mice had a training day of five trials on the 12-mm beam and five trials on the 5-mm beam. On the test day, the time to cross the 5-mm beam was measured and the number of paw slips that occurred during this process was recorded.
Metabolome analysis
Sample preparations were performed according to the manufacturer's instructions (Human Metabolome Technologies, Inc., Tsuruoka, Japan). Approximately 50 mg of mice brain (frozen at −80°C) was plunged into 1500 μL of 50% acetonitrile/Milli-Q water containing internal standards at 0°C to inactivate enzymes. The tissue was homogenized thrice at 1500 rpm for 120 s using a tissue homogenizer (Microsmash MS100R; Tomy Digital Biology Co., Tokyo, Japan) and then the homogenate was centrifuged at 2300g and 4°C for 5 min. Subsequently, 800 μL of the upper aqueous layer was filtered centrifugally through a Millipore 5-kDa cutoff filter at 9100g and 4°C for 120 min to remove proteins. The filtrate was concentrated centrifugally and resuspended in 50 μL of Milli-Q water for capillary electrophoresis/mass spectrometry (CE/MS) analysis. Metabolome measurements were carried out at Human Metabolome Technology Inc.
In vitro aggregation assay
Recombinant human αS was produced in E.coli and an aggregation assay in the cell-free system was performed as previously described.23 Aggregation of αS (0.2 μmol/L in 20 μL sodium buffer, pH 7.0) was induced at high temperature (65°C, 18 h) in the presence of various reagents.
Statistical methods
Differences between groups were evaluated by Student's t-test or one-way ANOVA followed by a Tukey post hoc test. For metabolome analysis, results are expressed as mean ± SD. All other results are expressed as mean ± SEM.
Results
Immunostaining of APN in human brains in cases of α-synucleinopathies
To determine whether APN is involved in the pathogenesis of α-synucleinopathies, we analyzed postmortem brains of cases of PD and dementia with Lewy bodies (DLB) histologically (Fig.1). Immunohistochemistry occasionally showed anti-APN-positive immunoreactivities in Lewy bodies (Fig.1C and F, G–I), which were weaker than those of S129-phosphorylated α-synuclein (pαS), the hallmark of α-synucleinopathies24 (Fig.1B and E). Indeed, double-immunofluorescence studies revealed that the immunoreactivity of APN colocalized with that of pαS in the majority (∼80%) of brainstem Lewy bodies in PD (Fig.1J–l) and to a lesser extent (∼10%) in neocortical Lewy bodies in DLB (Fig.1M–O). It is possible that APN in the Lewy bodies might be derived from adipose tissues. An alternative, but not mutually exclusive, possibility is that small amounts of APN locally expressed in the brain might be increased under neurodegenerative conditions. In either case, it is unlikely that APN is directly involved in aggregation of α-synuclein (αS) because anti-APN immunoreactivity was negative in both Lewy neurites and pale bodies, the precursors of mature Lewy bodies25 (Fig. S1A). However, an indirect role cannot be ruled out from these data.
Figure 1.
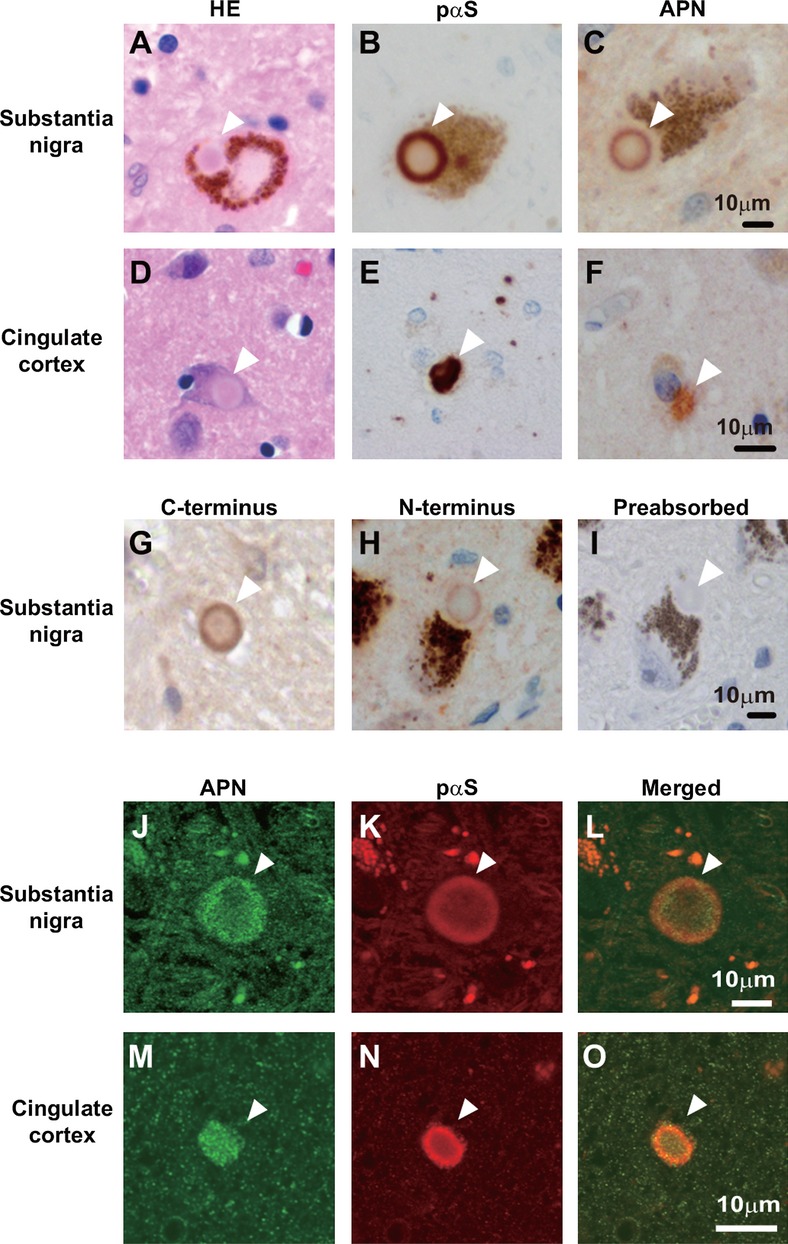
Immunostaining of APN in human brains in cases of α-synucleinopathies. Substantia nigra of PD brains (A–C, G–I, J–L) and cingulate cortex of DLB brains (D–F, M–O) were analyzed by hematoxylin and eosin (H&E) staining (A and D) and immunohistochemistry using anti-C-terminal APN (C, F, G, J) or anti-N-terminal APN (H), or anti-pαS (B, E, K). (I) The immunoreactivity of anti-C-terminal APN disappeared by preabsorption with rec. APN. Double immunofluorescence studies (J–L, M–O) showed that APN was immunopositive in the pαS-positive Lewy bodies (16 of 21 in 4 PD brains and 8 of 84 in 6 DLB brains, respectively, denoted by arrowheads). APN, adiponectin; PD, Parkinson's disease; DLB, dementia with Lewy bodies.
Immunoblot analysis of APN in human brains in cases of α-synucleinopathies
To determine whether APN is aggregated in Lewy bodies, we analyzed the APN concentrations in DLB and non-DLB brains. The immunoreactive of APN was significantly increased in the SDS fraction from DLB brains than those of non-DLB brains. However, little APN was detected in FA fractions using immunoblot analysis (Fig. S1B and C). Thus, one possibility is that APN might be sequestered by αS into Lewy bodies, which corresponds to the increased APN level in the SDS fraction of DLB brains.
APN ameliorates neurodegeneration in a cell model for α-synucleinopathies
To investigate the effect of APN, we incubate the cellular model of α-synucleinopathy with recombinant APN. Despite the uncertainties in the autopsy brain samples, APN clearly suppressed neurodegeneration in B103 neuroblastoma cells stably expressing αS (Fig.2). Aggregation of αS was significantly reduced by treatment with recombinant APN, as assessed by immunoblot analysis of αS accumulated in FA-extractable fractions (Fig.2A). Mechanistically, the AdipoRI26-AMP kinase (AMPK) pathway may be important because suppression of αS aggregation by APN was abolished by siRNA knockdown of AdipoRI expression (Fig.2B), as well as by pharmacological inhibition of AMPK but not of P38 (Fig.2C). AdipoRI is widely expressed in nonneuronal and neuronal tissues/cells,11,17,27 as well as the current cell model of α-synucleinopathies (Fig.2B). APN has been shown to stimulate phosphorylation of several signaling molecules, such as AMPK, P38, and GSK-3 (Fig. S2A), while GSK-3β was situated downstream of P38 (Fig. S2B). Consistent with a notion that APN might be antineurodegenerative in cell cultures, the level of pαS24 was significantly decreased by APN treatment in an AdipoRI-dependent manner (Fig.2D–F). Moreover, the level of αS released into the CM was significantly reduced by APN, with a clear decrease in exosome-associated release of αS28 (Fig.2G). As exosome might play a critical role for the cell-to-cell transmission of the αS oligomers,29 our finding provides a view that APN might be suppressive on the propagation of the αS pathology. Finally, APN modestly stimulated the activity of proteasomes, but not that of lysosomes (Fig. S2C and D). A further study showed that APN was neuroprotective. Cell viability was significantly recovered in the presence of APN when cells were treated with various types of neurotoxins that may mimic neurotoxicities in PD, including an endoplasmic reticulum stressor, a mitochondrial toxin, and a proteasome inhibitor (Fig. S2E). In the present experimental setting, APN had little effects on mitochondria based on the mitochondrial complex I activity (Fig. S2F) and mRNA levels for PPAR-γ co-activator-1 (PGC-1) α and cytochrome C (Fig. S2G). However, given the central role of mitochondria in the pathogenesis of PD,30 further improvements of the experimental system might allow detection of a positive effect of APN on mitochondria.
Figure 2.
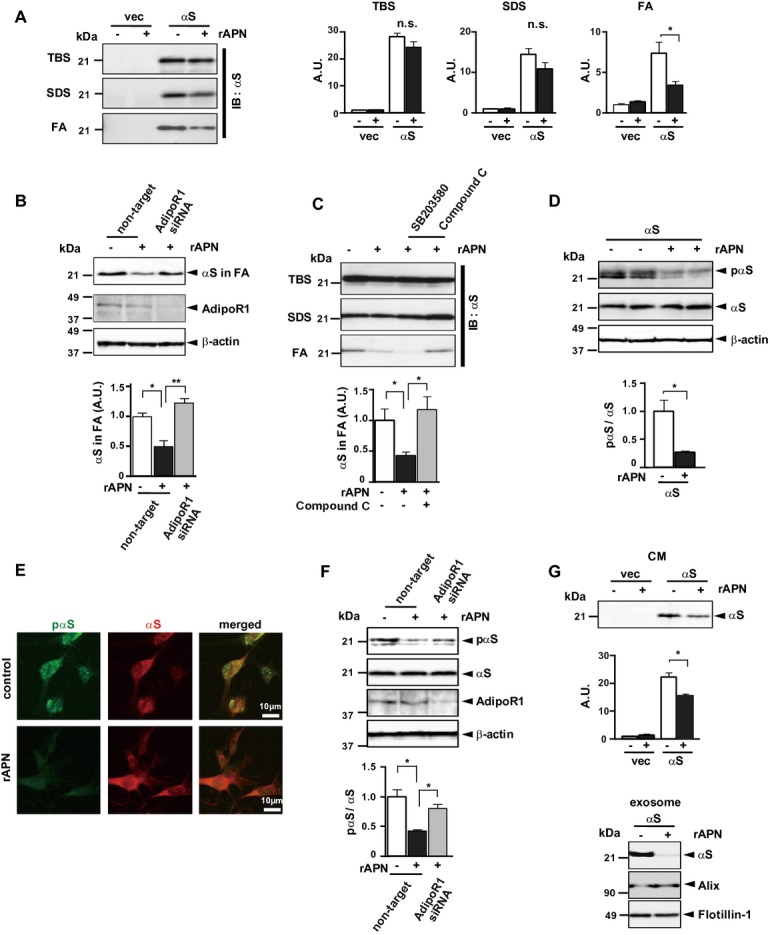
APN ameliorates neurodegeneration in a cell model of α-synucleinopathies. B103 neuroblastoma cells expressing human αS or empty vector were treated with rec. APN (5μg/ml) or PBS. Cells were fractionated into TBS-, SDS-, and FA-extractable fractions)16 (A–C) and analyzed by immunoblotting using anti-αS. Uncropped blots of (a) are presented in Figure S4. In (C), cells were pretransfected with siRNA of AdipoRI or nontarget (control), while in (C) cells were preincubated with a p38 inhibitor SB203580 (1 μmol/L) or a AMPK inhibitor compound C (1 μmol/L). In (D–F), phosphorylation of αS was evaluated by immunoblotting (D and F) or immunofluoresence (E) using anti-pαS or anti-αS. In (E), representative image of double immunofluorescence showed that colocalization of pαS with αS was reduced by APN. In (F), cells were pretransfected with siRNA for AdipoRI or nontarget. In (G), the suppressive effect of APN on release of αS in the conditioned medium (CM) was evaluated, while exosomes were semipurified from the CM and analyzed for αS and two exosome markers: alix and flotillin-1. The intensities of the immunoreactivities of αS were quantified (A: all fractions, B and C: FA fraction, mean ± SEM, n = 3–5, n.s; not significant, *P < 0.05, **P < 0.01). In (F) and (G), the intensities of pαS versus those of αS, and the intensities of αS in the CM were quantified, respectively (mean ± SEM, n = 5, *P < 0.05). APN, adiponectin; FA, formic acid; AMPK, AMP kinase.
APN ameliorates neurodegeneration in a mouse model for α-synucleinopathies
On the basis of the findings in the autopsy brains and cell-based study, we wished to investigate the effect of APN on α-synucleinopathies in vivo. For this purpose, recombinant globular domain of APN (gAPN), a physiological product of C-terminal APN,11 was administered intranasally to tg mice expressing αS (Fig.3). We used gAPN not simply because gAPN is shorter than full length of APN but because suppressive effect of gAPN on the aggregation of αS in cell cultures was comparable to that of full length of APN (Fig. S3A). Furthermore, intranasally injected FLAG-tagged gAPN was shown to reach various areas of the brain in mice (Fig. S3B and C). Indeed, such a noninvasive delivery of therapeutics directly to the brain has been considered for many reagents, including insulin treatment for dementia.31 With this background, the APN nasal treatment was initiated in the αS tg mice at 3 months of age without severe movement disorder (Fig.3, male, n = 8–9). Compared to the mice that did not receive gAPN, the mice at 5 months of age that received gAPN had significantly improved weight loss (Fig.3A) and retarded the progression of movement disability, as assessed by a rotarod test (Fig.3B), followed by an examination by beam walking assay (Fig.3C). In addition to the behavior analyses, αS pathology was remarkably ameliorated in the mice treated with gAPN compared to the mice that received vehicle injection as evaluated by immunohistochemistry; accumulation of pαS was reduced in both cortex and olfactory bulb, while αS-positive globule formation in thalamus was remarkably suppressed (Fig.3D). Furthermore, these results were confirmed by immunoblot analysis showing that accumulation of αS in the FA-extractable fractions of the cortex of the αS tg mice brain was remarkably suppressed by treatment with gAPN (Fig.3E). During the experimental periods, the activation of glial cells, including astrocytes and microglia, was not extensive as to be quantified (not shown). In contrast to the current protocol of experiment, movement disorder was little improved when the intranasal treatment of APN was initiated in the αS tg mice at 5 months of age which had severer movement disorders compared to the mice at 3 months of age (not shown). Taken together, these results suggest that intranasal gAPN protects against neurodegeneration in the early stage in the αS tg mouse.
Figure 3.
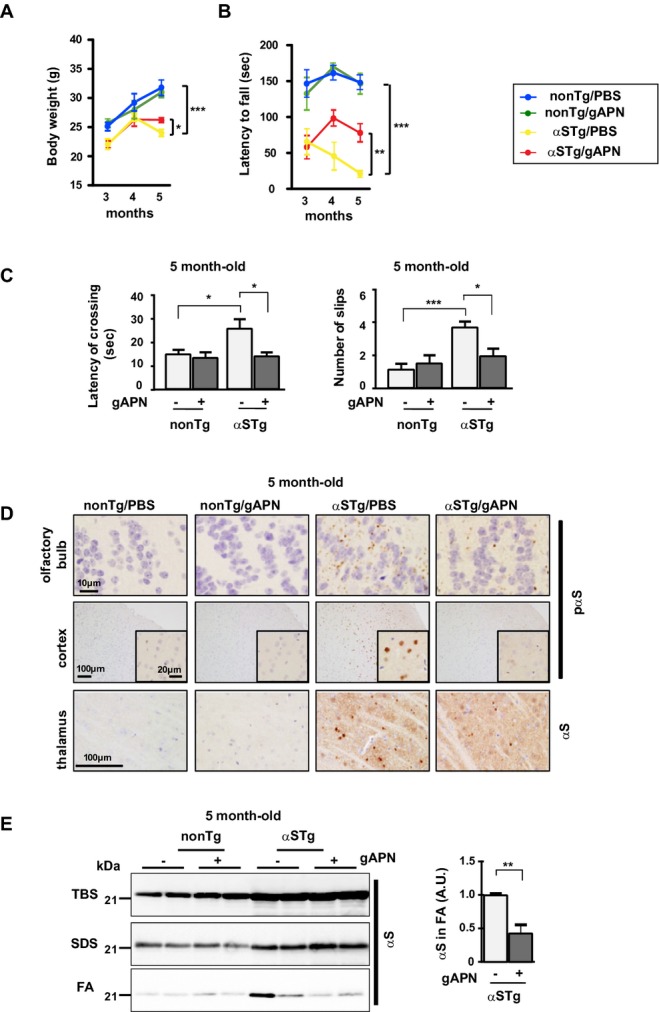
APN ameliorates neurodegeneration in a mouse model of α-synucleinopathies. gAPN (0.1 mg/mL in 10 μL PBS) or PBS alone (10 μL) was injected into the nasal cavities of αS tg mice (male, 3-month-old) or wild-type littermates every 3 days for 2 months. Body weight was measured (A) and motor performances were evaluated by rotarod test (B) and beam test (C) (mean ± SEM, n = 8–9, *P < 0.05, **P < 0.01, ***P < 0.001). Mice brains were then analyzed histologically and biochemically. (D) Representative immunohistochemical images: the cortex and olfactory bulb were stained with anti-pαS, and the thalamus was probed with anti-αS. Insets are shown to show at higher magnification for the cortex. (E) Representative images of immunoblotting (cortex). The intensities of αS in the FA fraction were quantified (mean ± SEM, n = 6, **P < 0.01). FA, formic acid; APN, adiponectin.
APN downregulates the purine monophosphate in a mouse model of α-synucleinopathies: one possible mechanism
The mechanism through which APN ameliorates αS pathology is obscure. To determine whether the antineurodegenerative properties of APN were associated with certain metabolic changes in vivo, brain extracts of αS tg mice were analyzed by CE/MS. The results showed that among the various metabolites affected by APN, there were significant decreases in purine monophosphates, including guanosine monophosphate (GMP) and inosine monophosphate (IMP) (Fig.4A). As both GMP and IMP specifically stimulated aggregation of αS under cell-free conditions (Fig.4C and D), it is possible that a decrease in these purine monophosphates might partly contribute to suppression of aggregation of αS by APN. Recently, it has been shown that progression of PD is inversely correlated with serum urate levels and the frequency of gout.32 Although the present results were not directly linked to this important issue, our results did augment the notion that metabolism and/or catabolism of nucleic acids may play an important role for the regulation of the aggregation of αS in the pathogenesis of α-synucleinopathies.
Figure 4.
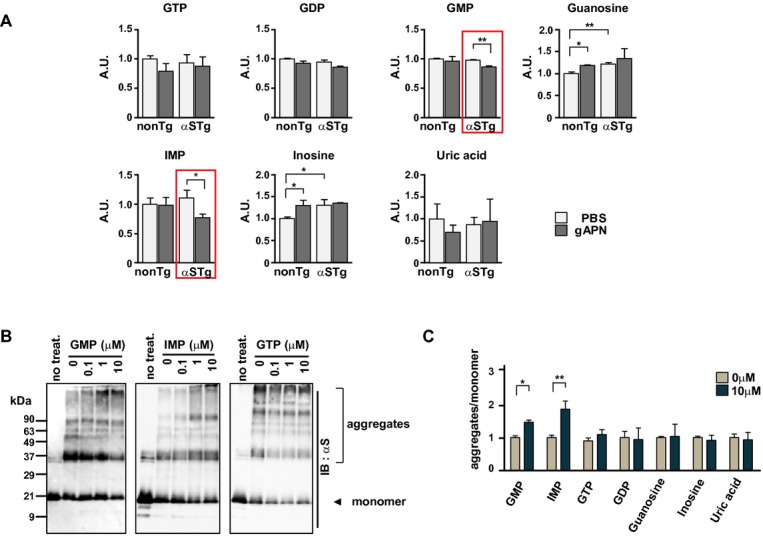
Alteration of purine monophosphates by APN in a mouse model of α-synucleinopathies. Brain extracts of αS tg mice were analyzed by CE/MS (A). Each value represents relative expression compared to values from non-tg control mice treated with PBS, respectively (shown as A.U., mean ± SD, n = 3, *P < 0.05, **P < 0.01). The levels of GMP and IMP were significantly decreased by APN in brain extracts of αS tg mice (red rectangles). Under cell-free conditions (B), high temperature–induced aggregation of rec. αS (0.1 μmol/L) was further stimulated by GMP and IMP but not by GTP (0.1–10 μmol/L each). (C) Relative ratios of high molecular aggregates of αS (more than 90 kD) to monomer αS (19 kD) in the presence of GMP, IMP, GTP, guanosine, inosine, and uric acid (10 μmol/L each) (mean ± SE, n = 3, *P < 0.05, **P < 0.01). CE/MS, capillary electrophoresis/mass spectrometry; GMP, guanosine monophosphate; IMP, inosine monophosphate.
Discussion
As far as we know, this study is the first to show that APN may negatively regulate the pathogenesis of α-synucleinopathies in an animal model. The protective roles of APN have recently been implicated in a wide range of diseases, including cancer,33 osteoporosis,34 and chronic pulmonary obstructive disease.35 Notably, the protective actions of APN may occur beyond the brain–blood barrier, as APN has been shown to be protective against cerebral ischemia36 and depression37 in the rodent nervous system. Taken together, these results suggest that α-synucleinopathies can be regarded as systemic diseases in terms of APN protection. In this context, it is reasonable to speculate that decreases in systemic APN in pathological conditions such as diabetes and other lifestyle disorders may result in a loss of protective function of APN, leading to deterioration of a variety of systemic diseases, including α-synucleinopathies.
Our study shows that intranasal treatment with a short peptide derived from APN (e.g., gAPN) may suppress disease progression in mice models of α-synucleinopathies. Given that APN is systemically involved in protection in various tissues and organs, it is likely that reinforcement of APN in human brain would not be associated with severe side effects. Thus, our noninvasive treatment using gAPN could be a candidate for therapy for α-synucleinopathies. Basic understanding of APN has markedly progressed in diabetes, with the recognition that expression and activity of APN are enhanced by stimulation of peroxisome proliferator-activated receptor γ.11,12 Furthermore, osmotin, a plant protein that is a ligand for the yeast homolog of AdipoR (PHO36), has been shown to activate AdipoR signaling in C2C12 myocytes.11 Moreover, it was also recently shown that both the features of diabetes and shortened life span observed in genetically obese db/db mice fed a high-fat diet were significantly improved by treatment with small molecules that act as AdipoR agonists.38 It is possible that these developments in diabetes might also be applicable in therapy for neurodegenerative diseases. Thus, future studies are warranted to examine the therapeutic potential of APN for α-synucleinopathies and possibly for other neurodegenerative diseases.
Acknowledgments
We thank Tomo Urano (University of Tokyo) for his continuous encouragement. This work was supported in part by a grant-in-aid for Science Research for Young Scientists B25830044 (to K. S.); a Cell Innovation Project (to S. I.); and funding for Innovative Areas “Brain Environment 24111555” (to M. H.), Basic Science Research B 25290019 (to M. H.), and Challenging Exploratory Research 25640030 (to M. H.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and grant from Japan Foudation for Neuroscience and Mental Health (to M. H.); by a NIBIO grant (to S. I.); by NIH Grants AG18440, AG022074, AG10435, and ES016731 (to E. M.); and by NIH Grants AG033082 and NS065874 (to A. R. L.).
Authorship
K. S., M. W., H. A., and M. H. analyzed autopsy brains. K. S., M. F., S. S., A. K., and E. R. analyzed transgenic mice. K. S., M. F., T. T, M. W., Y. T., J. W., A. R. L., E. M., S. I., and M. H. designed and analyzed the data. K. S., M. W., E. M., S. I, A. R. L., and M. H. supervised and wrote the study. All authors interpreted the data.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Additional characterization of APN in human brains in cases of α-synucleinopathies. (A) Immunostaining of APN in human brains in cases of α-synucleinopathies. Pale bodies (upper panels, thin arrows) and Lewy neurites (lower panels, thick arrows) derived from the substantia nigra of PD brains were identified by H&E staining and immunohistochemistry using anti-pαS, respectively. Double immunofluorescence using anti-C-terminal APN and anti-pαS failed to detect immunoreactivities of APN in pale bodies or in Lewy neurites. (B) Immunoblot analysis of APN in human brains in cases of α-synucleinopathies. 293T cells were transfected with pCEP4-APN or pCEP4 alone and harvested after 48 h. The transfected cells and brain samples (from the cingulate cortex of DLB brains) were dissolved with lysis buffer (1% Nonidet P-40, 50 mmol/L HEPES, 150 mmol/L NaCl, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 100 mmol/L sodium fluoride-containing protease inhibitor mixture), and total extracts were analyzed by immunoblotting using anti-C-terminal APN. The immunoreactivity of the APN monomer (30 kDa) was augmented by heating (95°C, 5 min) under reducing conditions. Transfection of the APN expression vector was performed as previously described.16 (C) Comparison of expression levels of APN between α-synucleinopathies and controls. Immunoreactivities of the APN monomer (30 kDa) in SDS-soluble extracts of DLB brains (cingulate cortex) were significantly higher than those in extracts from non-DLB control brains (cingulate cortex). However, there were no differences in AdipoR1 and AdipoR226 between DLB and control brains, and immunoreactivities of APN, AdipoR1, and AdipoR2 were not observed in FA fractions, in contrast to the extensive aggregation of αS in DLB brains. Intensities of APN were quantified relative to those of actin (mean ± SEM, n = 7, **P < 0.01, n.d., not detected).
Figure S2. Effects of APN on B103 neuroblastoma cells expressing αS. (A and B) Effects of APN on signaling molecules. Cells expressing αS and vector-transfected cells were incubated under serum-free conditions for 2 h, followed by treatment with APN (5 µg/mL) under serum-free conditions for the indicated times. (A) Expression and phosphorylation of signaling molecules, including P38, AMPK, and GSK-3, were assessed by immunoblotting with antibodies against pP38, AMPK, pAMPK, pGSK-3 (all from Cell Signaling), P38, and GSK-3 (all from BD). (B) Phosphorylation of GSK-3 was inhibited in the presence of SB203580 (1 µmol/L), but not with compound C (1 µmol/L), indicating that GSK-3 may be situated downstream of p38, but not of AMPK. (C) APN protects against neurotoxicities by chemical reagents. Cells expressing αS were incubated with various neurotoxic reagents, including tunicamycin (1 µg/mL), rotenone (100 nmol/L), and lactacystin (1 µmol/L), under serum-free conditions in the presence or absence of APN (5 µg/mL) for 48 h. Cell viability was evaluated by measurement of LDH release16 (mean ± SEM, n = 4–6, *P < 0.05). (D and E) Effects of APN on proteasome and lysosome. Cells expressing αS and vector-transfected cells were incubated with APN (5 µg/mL) for 18 h under serum-free conditions. Proteasome (D) and lysosome (E) activities were measured as described in the Supplemental Methods. APN weakly but significantly increased proteasome activity, but had little effect on lysosome activity (mean ± SEM, n = 5–6, *P < 0.05). (F and G) Effects of APN on mitochondria. Cells expressing αS and vector-transfected cells were incubated with APN (5 µg/mL) for 18 h under serum-free conditions. (F) Mitochondrial activity was measured by mitochondrial complex I activity assay as described in the Supplemental Methods (mean ± SEM, n = 6). Cells expressing αS cells were incubated with APN for 3 h under serum-free conditions. (G) mRNA level of peroxisome proliferator-activated receptor γ coactivator α (PGC1-α) and cytochrome c was evaluated by qPCR (mean ± SEM, n = 4).
Figure S3. Preparatory studies for intranasal delivery of gAPN into mice brain. (A) gAPN treatment suppresses aggregation of αS in a cell model of α-synucleinopathies. To determine whether gAPN inhibits aggregation of αS, cells expressing human αS were incubated under serum-free conditions with rec. gAPN (5 µg/mL) or rec. full-length APN (5 µg/mL) for 18 h. Cells were harvested and cell extracts were fractionated and immunoblotted using anti-αS. The intensities of αS were quantified relative to those of actin (mean ± SEM, n = 4, *P < 0.05). (B) gAPN treatment stimulated phosphorylation of signaling molecules in a cell model of α-synucleinopathies. To determine whether gAPN stimulates the phosphorylation of signaling molecules such as APN, cells expressing αS were incubated under serum-free conditions for 2 h, followed by treatment with gAPN (5 µg/mL) or APN (5 µg/mL) under serum-free conditions for 30 min. Expression and phosphorylation of signaling molecules, including P38 and AMPK, were assessed by immunoblotting with antibodies against P38, pP38, AMPK, pAMPK, and actin. (C and D) Intranasally injected gAPN reaches various regions of mice brains. αS tg or wild-type mice were intranasally treated with gAPN (C) (1 mg/mL, 10 µL PBS), FLAG-gAPN (D) (1 mg/mL, 10 µL PBS), or PBS alone. Mice brains were analyzed by immunohistochemistry using anti-C-terminal APN (C) and immunoblotting using anti-FLAG antibody and anti-actin (D). Representative figures of olfactory bulb and cortex are shown in (C). Insets are shown to show at higher magnification for the olfactory bulb and cortex. Brain extracts (D) were prepared from olfactory bulb, frontal cortex, striatum, hind cortex, hippocampus, thalamus, hypothalamus, and brainstem, and evaluated by immunoblotting using anti-FLAG antibody and antiactin.
Figure S4. Full scans of immunoblot data. Uncropped image of (A). A representative immunoblots of TBS, SDS, and FA-extractable fractions using anti-αS antibody. All the intensities of αS using TBS, SDS, and FA-extractable fraction were quantified similarly as2(A).19 *Nonspecific bands.
Supplementary Methods.
References
- Mattson MP. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res. 2000;886:47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9:5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6:551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda E, Barichella M, Cassani E, et al. Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurology. 2012;78:1507–1511. doi: 10.1212/WNL.0b013e3182553cc9. [DOI] [PubMed] [Google Scholar]
- Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson's disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136:374–384. doi: 10.1093/brain/aws009. [DOI] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Une K, Takei YA, Tomita N, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer's disease. Eur J Neurol. 2011;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- Cassani E, Cancello R, Cavanna F, et al. Serum adiponectin levels in advanced-stage Parkinson's disease patients. Parkinsons Dis. 2011;2011:624764. doi: 10.4061/2011/624764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Takayama M, Abe Y, Hirose N. Adipokines and aging. J Atheroscler Thromb. 2011;18:545–550. doi: 10.5551/jat.7039. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2 + ) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Jung TW, Lee JY, Shim WS, et al. Adiponectin protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Biochem Biophys Res Commun. 2006;343:564–570. doi: 10.1016/j.bbrc.2006.02.186. [DOI] [PubMed] [Google Scholar]
- Chan KH, Lam KS, Cheng OY, et al. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS ONE. 2012;7:e52354. doi: 10.1371/journal.pone.0052354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waragai M, Wei J, Fujita M, et al. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson's disease. Biochem Biophys Res Commun. 2006;345:967–972. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Wei J, Fujita M, Nakai M, et al. Protective role of endogenous gangliosides for lysosomal pathology in a cellular model of synucleinopathies. Am J Pathol. 2009;174:1891–1909. doi: 10.2353/ajpath.2009.080680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem. 2011;286:44913–44920. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Sugama S, Sekiyama K, et al. A beta-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nat Commun. 2010;1:110. doi: 10.1038/ncomms1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Natsume H, Iwata S, et al. Improved nasal absorption of drugs using poly-L-arginine: effects of concentration and molecular weight of poly-L-arginine on the nasal absorption of fluorescein isothiocyanate-dextran in rats. Eur J Pharm Biopharm. 2001;52:21–30. doi: 10.1016/s0939-6411(01)00149-7. [DOI] [PubMed] [Google Scholar]
- Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011;49:2376. doi: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, et al. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Desplats P, Sigurdson C, et al. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Corti O, Brice A. Mitochondrial dysfunctions in Parkinson's disease. Rev Neurol (Paris) 2013;170(5):339–343. doi: 10.1016/j.neurol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomark Med. 2010;4:701–712. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2703–2713. doi: 10.1210/jc.2011-0047. [DOI] [PubMed] [Google Scholar]
- Miller M, Cho JY, Pham A, et al. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182:684–691. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- Chen B, Liao WQ, Xu N, et al. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009;1273:129–137. doi: 10.1016/j.brainres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Guo M, Zhang D, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;109:12248–12253. doi: 10.1073/pnas.1202835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional characterization of APN in human brains in cases of α-synucleinopathies. (A) Immunostaining of APN in human brains in cases of α-synucleinopathies. Pale bodies (upper panels, thin arrows) and Lewy neurites (lower panels, thick arrows) derived from the substantia nigra of PD brains were identified by H&E staining and immunohistochemistry using anti-pαS, respectively. Double immunofluorescence using anti-C-terminal APN and anti-pαS failed to detect immunoreactivities of APN in pale bodies or in Lewy neurites. (B) Immunoblot analysis of APN in human brains in cases of α-synucleinopathies. 293T cells were transfected with pCEP4-APN or pCEP4 alone and harvested after 48 h. The transfected cells and brain samples (from the cingulate cortex of DLB brains) were dissolved with lysis buffer (1% Nonidet P-40, 50 mmol/L HEPES, 150 mmol/L NaCl, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 100 mmol/L sodium fluoride-containing protease inhibitor mixture), and total extracts were analyzed by immunoblotting using anti-C-terminal APN. The immunoreactivity of the APN monomer (30 kDa) was augmented by heating (95°C, 5 min) under reducing conditions. Transfection of the APN expression vector was performed as previously described.16 (C) Comparison of expression levels of APN between α-synucleinopathies and controls. Immunoreactivities of the APN monomer (30 kDa) in SDS-soluble extracts of DLB brains (cingulate cortex) were significantly higher than those in extracts from non-DLB control brains (cingulate cortex). However, there were no differences in AdipoR1 and AdipoR226 between DLB and control brains, and immunoreactivities of APN, AdipoR1, and AdipoR2 were not observed in FA fractions, in contrast to the extensive aggregation of αS in DLB brains. Intensities of APN were quantified relative to those of actin (mean ± SEM, n = 7, **P < 0.01, n.d., not detected).
Figure S2. Effects of APN on B103 neuroblastoma cells expressing αS. (A and B) Effects of APN on signaling molecules. Cells expressing αS and vector-transfected cells were incubated under serum-free conditions for 2 h, followed by treatment with APN (5 µg/mL) under serum-free conditions for the indicated times. (A) Expression and phosphorylation of signaling molecules, including P38, AMPK, and GSK-3, were assessed by immunoblotting with antibodies against pP38, AMPK, pAMPK, pGSK-3 (all from Cell Signaling), P38, and GSK-3 (all from BD). (B) Phosphorylation of GSK-3 was inhibited in the presence of SB203580 (1 µmol/L), but not with compound C (1 µmol/L), indicating that GSK-3 may be situated downstream of p38, but not of AMPK. (C) APN protects against neurotoxicities by chemical reagents. Cells expressing αS were incubated with various neurotoxic reagents, including tunicamycin (1 µg/mL), rotenone (100 nmol/L), and lactacystin (1 µmol/L), under serum-free conditions in the presence or absence of APN (5 µg/mL) for 48 h. Cell viability was evaluated by measurement of LDH release16 (mean ± SEM, n = 4–6, *P < 0.05). (D and E) Effects of APN on proteasome and lysosome. Cells expressing αS and vector-transfected cells were incubated with APN (5 µg/mL) for 18 h under serum-free conditions. Proteasome (D) and lysosome (E) activities were measured as described in the Supplemental Methods. APN weakly but significantly increased proteasome activity, but had little effect on lysosome activity (mean ± SEM, n = 5–6, *P < 0.05). (F and G) Effects of APN on mitochondria. Cells expressing αS and vector-transfected cells were incubated with APN (5 µg/mL) for 18 h under serum-free conditions. (F) Mitochondrial activity was measured by mitochondrial complex I activity assay as described in the Supplemental Methods (mean ± SEM, n = 6). Cells expressing αS cells were incubated with APN for 3 h under serum-free conditions. (G) mRNA level of peroxisome proliferator-activated receptor γ coactivator α (PGC1-α) and cytochrome c was evaluated by qPCR (mean ± SEM, n = 4).
Figure S3. Preparatory studies for intranasal delivery of gAPN into mice brain. (A) gAPN treatment suppresses aggregation of αS in a cell model of α-synucleinopathies. To determine whether gAPN inhibits aggregation of αS, cells expressing human αS were incubated under serum-free conditions with rec. gAPN (5 µg/mL) or rec. full-length APN (5 µg/mL) for 18 h. Cells were harvested and cell extracts were fractionated and immunoblotted using anti-αS. The intensities of αS were quantified relative to those of actin (mean ± SEM, n = 4, *P < 0.05). (B) gAPN treatment stimulated phosphorylation of signaling molecules in a cell model of α-synucleinopathies. To determine whether gAPN stimulates the phosphorylation of signaling molecules such as APN, cells expressing αS were incubated under serum-free conditions for 2 h, followed by treatment with gAPN (5 µg/mL) or APN (5 µg/mL) under serum-free conditions for 30 min. Expression and phosphorylation of signaling molecules, including P38 and AMPK, were assessed by immunoblotting with antibodies against P38, pP38, AMPK, pAMPK, and actin. (C and D) Intranasally injected gAPN reaches various regions of mice brains. αS tg or wild-type mice were intranasally treated with gAPN (C) (1 mg/mL, 10 µL PBS), FLAG-gAPN (D) (1 mg/mL, 10 µL PBS), or PBS alone. Mice brains were analyzed by immunohistochemistry using anti-C-terminal APN (C) and immunoblotting using anti-FLAG antibody and anti-actin (D). Representative figures of olfactory bulb and cortex are shown in (C). Insets are shown to show at higher magnification for the olfactory bulb and cortex. Brain extracts (D) were prepared from olfactory bulb, frontal cortex, striatum, hind cortex, hippocampus, thalamus, hypothalamus, and brainstem, and evaluated by immunoblotting using anti-FLAG antibody and antiactin.
Figure S4. Full scans of immunoblot data. Uncropped image of (A). A representative immunoblots of TBS, SDS, and FA-extractable fractions using anti-αS antibody. All the intensities of αS using TBS, SDS, and FA-extractable fraction were quantified similarly as2(A).19 *Nonspecific bands.
Supplementary Methods.


