Abstract
The ability to exert self-control in the face of appetitive, alluring cues is a critical component of healthy development. The development of behavioral measures that use disease-relevant stimuli can greatly improve our understanding of cue-specific impairments in self-control. To produce such a tool relevant to the study of eating and weight disorders, we modified the traditional go/no-go task to include food and non-food targets. To confirm that performance on this new task was consistent with other go/no-go tasks, it was given to147 healthy, normal weight volunteers between the ages of 5 and 30. High-resolution photos of food or toys were used as the target and nontarget stimuli. Consistent with expectations, overall improvements in accuracy were seen from childhood to adulthood. Participants responded more quickly and made more commission errors to food cues compared to nonfood cues (F(1,140) = 21.76, P<0.001), although no behavioral differences were seen between lowand high-calorie food cues for this non-obese, healthy developmental sample. This novel food-specific go/no-go task may be used to track the development of self-control in the context of food cues and to evaluate deviations or deficits in the development of this ability in individuals at risk for eating problem behaviors and disorders.
Keywords: Cognitive control, Eating behavior, Impulsivity
1. Introduction
Self-control, or resistance to temptation, has been studied in the context of social, developmental and cognitive psychology. This ability can be operationally defined as the capacity to accomplish goal directed behavior in the face of salient, competing inputs and actions (Casey et al., 2005). A prominent component of self-control is the ability to suppress inappropriate behaviors in favor of appropriate ones, often termed impulse control (Casey et al., 1997; Rothbart et al., 2001; Spinrad et al., 2007), and can be measured by a number of self-report assessments and behavioral tasks (Mobbs et al., 2008; Somerville et al., 2011; Grose-Fifer et al., 2013). The classic go/no-go task measures the ability to maintain behavioral control in the face of interfering stimuli, by measuring an individual’s speed and accuracy when instructed to respond to a frequent target (go trial) and withhold response to a rare nontarget (no-go trial). Modifications of the classic go/no-go task (Durston et al., 2003; Hare et al., 2005, 2008; Tottenham et al., 2011) have been successfully used to measure motivationally-driven behavior using different subsets of salient cues, such as emotional faces, in examining emotion regulation across the developmental spectrum (Casey et al., 1997; Casey et al., 2007; Casey et al., 2011). In clinical populations, such as individuals diagnosed with an eating disorder, clinically-relevant stimulus sets (i.e. appetitive food cues) may be particularly useful to measure self-control in the context of the disorder. Impulsivity has been associated with food intake among healthy-weight individuals (Lindroos et al., 1997; Hays et al., 2002; Guerrieri et al., 2007; Guerrieri et al., 2008; Savage et al., 2009). While viewing advertising logos for food, healthy-weight children show enhanced recruitment of brain regions associated with impulse control relative to obese children (Bruce et al., 2013). Individuals with bulimia nervosa and binge-eating disorder (Nasser et al., 2004) display greater impulsivity scores on self-report measures compared to healthy controls, and, using fMRI, patients with bulimia fail to engage self-regulatory circuits to the same degree as healthy controls (Marsh 2009). Clinically, patients with anorexia nervosa display a remarkable ability to control their food intake behavior (Mayer et al., 2012) and demonstrate enhanced ability to delay monetary (e.g. non-food) rewards (Steinglass, 2012)
When studying eating disorders, it is important to be able to distinguish behavioral differences in food-specific self-control from characteristic developmental differences in overall impulsivity. Eating disorders often first manifest during adolescence, but not uncommonly persist into adulthood, whereas impulse control, gradually improves from childhood to early adulthood. Additionally, subjective sensitivity to appetitive cues in our environment greatly influences our ability to exert self-control. Sensitivity to environmental cues has been shown to differ across development and within populations and can drive differences seen in self-control, where overall impulsivity measures are unable to capture this variability.
Others have modified the traditional go/no-go task to examine impulse control using food cues (Batterink et al., 2010; Mobbs et al., 2011; Jasinska et al., 2012; Loeber et al., 2012; Meule et al., 2012; Loeber et al., 2013); however, the tasks vary in design, and results are inconsistent across studies. Some using vegetables (go target) and desserts (no-go non-target) have found positive associations between commission errors to high-calorie food (dessert) cues and body mass index (BMI) (Batterink et al., 2010), however, the lack of a neutral control condition make it difficult to establish whether the association was due to lower inhibitory control in general or was food-mediated. Others have used food and nonfood words in hungry healthy (Loeber et al., 2013) and obese (Mobbs et al., 2011; Loeber et al., 2012) populations to show diminished inhibitory control in response to food-associated cues. The use of words as targets and nontarget, however, greatly limit the application of these tasks. For example, when food cues are words rather than pictures, developmental differences in reading ability and abstract thought will influence performance. Such tasks would be difficult to use early in development when changes in eating habits begin to emerge. Recently, variants of the go/no-go task using pictures have found rate of commission errors to be associated with aspects of unhealthy eating (specifically, emotional eating) (Jasinska et al., 2012), as well as faster reactions times on a modified go/no-go-’XY’ attention task when food pictures were presented behind the “go” targets (Meule et al., 2012). These findings are confounded, however, by a lack of neutral comparison condition or homogeneity of food stimuli (only high-calorie desserts), respectively. Without these comparisons, it is difficult to determine the extent to which the decrease in inhibitory control is driven by food and more specifically types of food that are commonly associated with unhealthy eating behaviors.
While these studies exemplify how psychological tasks can be modified to measure impulses relevant to eating behavior, the following study attempts to address potential limitations of existing food go/no-go tasks. We introduce an upgraded food go/no-go task that uses pictures of low- and high-calorie food stimuli and interesting nonfood stimuli to test the specific influence of appetitive food cues on self-control. Based on previous studies (Casey et al., 1997; Durston et al., 2002; Somerville et al., 2011), we hypothesized that overall impulse control would increase across age groups and that participants would exhibit more behavioral interference to the appetitive food cues compared to the neutral nonfood cues, especially to the high-calorie food cues.
2. Methods
2.1. Participants
Participants were 147 normal-weight, healthy volunteers (96 females) between the ages of 5 and 30 years. Height was measured in centimeters to the nearest 0.1cm using a wall-mounted stadiometer (Detecto 3PHTROD-WM), and weight was measured in light clothing on either a beam balance scale (Detecto) or a digital medical scale (HealthOMeter 349KLX) to the nearest 0.1kg. Participants were asked to remove their shoes for both measurements. BMI was calculated as weight (kg) divided by height (m) squared. Potential participants were screened with a brief clinical interview conducted by an MD- or PhD-level clinician. Healthy individuals with no significant medical illness, neurologic history of or active Axis I psychiatric disorder, and BMI less than 30 kg/m2 were included. Participants with known Attention Deficit Hyperactivity Disorders or learning disability were excluded due to known performance differences on standard go/no-go tasks. This study was approved by the New York State Psychiatric Institute/Columbia University Department of Psychiatry Institutional Review Board. Participants were recruited through street fairs and study flyers posted throughout the New York-Presbyterian Hospitals of Columbia University Medical Center and Weill Cornell Medical Center. Prior to study participation, all child and adolescent participants assented and their parents and adult participants provided written informed consent.
2.2. Stimuli
The set of stimuli consisted of 30 color images of common high- (8) and low-calorie (7) foods and common toys (15) (see Supplemental Figure 1). Prior to the experiment, an independent test group rated each image on valence (e.g. How pretty is this image? How familiar is this image?), and arousal (e.g. How exciting is this image?) on a 7-point likert scale. Intra-class correlation coefficients and Cronbach’s alpha were also used as measures of inter-rater reliability in generating the final stimulus set. These data are reported in Supplemental Table 1.
2.3. Behavioral paradigm
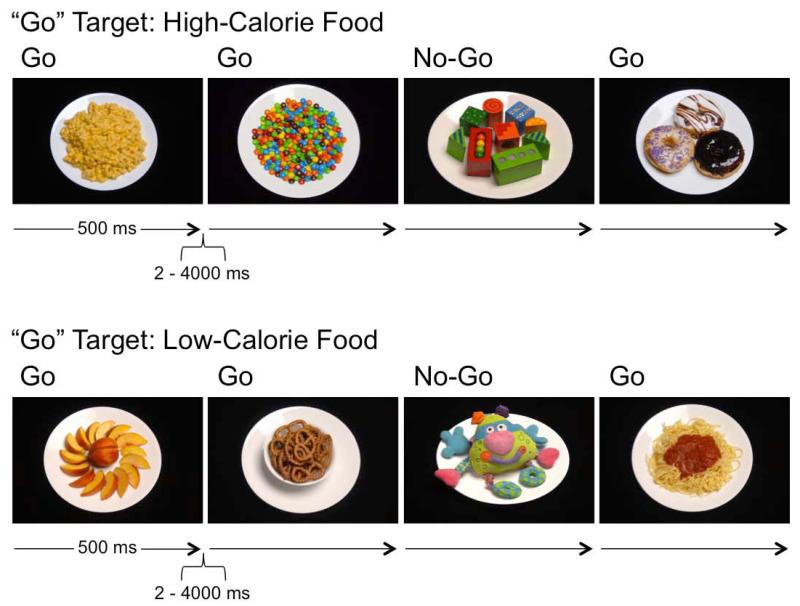
The go/no-go task was programmed and administered using E-Prime 2.0 presentation software (Psychology Software Tools, Inc. Pittsburgh, PA). The target (“go”) stimuli appeared in 75% of the trials, in order to develop a prepotent response pattern, and nontargets appeared 25% of the time. The task was administered across four runs with each cue type serving as both a target and nontarget. Because of the potential variability across individuals of different ages in consistently identifying foods as high- or low-calorie, participants were not asked to distinguish between high- and low-calorie foods. Rather, food images were grouped by calorie level (high or low) and presented in separate runs. That is, participants were presented with the general instruction: “Press the spacebar when you see food. Don’t press for any other pictures, only food. Go as fast as you can without making any mistakes.” Within a given run, the food images presented were either all high calorie or all low calorie. Stimuli were presented for 500ms, with a variable, inter-trial interval of 2000-4000ms (Figure 1). The order of the four conditions of high-calorie food “go” with toy “no-go”, low-calorie food “go” with toy “no-go”, toy “go” with high-calorie food “no-go”, and toy “go” with low-calorie food “no-go”, were counter-balanced across participants. Participants were presented with a three-minute practice session to ensure they understood and could follow the instructions, followed by 192 trials (12 minutes).
Figure 1.
Schematic of Food Go/No-Go Task. Examples of food “go”, nonfood “no-go” trials. Here, participants were instructed to press the spacebar only to food (top: high-calorie; bottom: low-calorie).
2.4. Statistical analysis
Age and BMI scores were compared across the three age groups of 5-12 (n=39), 13-17 (n=49) and 18-30 (n=59) year olds using an analysis of variance (ANOVA, SPSS version 20, IBM Corporation).
Three separate ANOVAs were used to compare each primary outcome variable using between-subject factors of age group (children, adolescents, adults) and gender (male, female), and within-subject factors of cue type (toy, low-calorie food or high-calorie food). A Bonferroni correction for multiple comparisons was employed for the three ANOVAs (P < 0.016). Primary outcome variables included: 1) overall reaction time (RT) in milliseconds during correct “go” trials, 2) rate of omission errors (missed “go” trials), and 3) false alarm rate (rate at which participants erroneously press to a no-go stimulus). Within each subject, RTs greater or less than 3 standard deviations of their mean were excluded from the analyses. The two runs in which toys were the “go” targets were averaged to derive mean RTs and rate of omission errors for the toy condition for each participant. Similarly, the two runs in which toys were the “no-go” targets were averaged to derive mean false alarm rates to toys for each participant. Post-hoc t-tests were conducted to further interrogate main effects and Bonferroni corrected for multiple comparisons.
3. Results
Data from 146 participants (39 children, 49 adolescents and 58 adults) were included in the analyses. Data from one adult were excluded due to a misunderstanding of the instructions, where he only responded to the presence of fruits. Demographic information for each age group is presented in Table 1. There was a main effect of age group in raw BMI scores (F(2,121) = 25.49, P < 0.001), where post-hoc t-tests revealed that children significantly differed from both adolescents (t(73) = 5.65, P < 0.001) and adults (t(80) = 7.11, P < 0.001). There was no difference in BMI between the adult and adolescent groups. BMI percentiles, based on CDC BMI-for-age growth charts for males and females (Kuczmarski et al., 2002), were calculated for participants between the ages of 5 and 20 for whom we had BMI measures (n = 94) to control for age differences. The resulting percentiles did not correlate with task performance nor did they show any age effects for this healthy non-obese and non-disordered sample.
Table 1.
Demographics
| Children | Adolescents | Adults | |||
|---|---|---|---|---|---|
|
|
|||||
| N=39 | N=49 | N=58 | F | p< | |
| Age (yrs) | 9.31 (±1.98) | 15.1 (±1.39) | 22.52 (±3.61) | 305.75 | 0.001 |
| BMI (kg/m2) | 18.05* (±3.04) | 22.29 (±3.35) | 22.26 (±2.32) | 25.49 | 0.001 |
| BMI (Percentiles) | 58.7 (±32.5) | 65.0 (±26.3) | 52.7 (±24.1)** | 1.34 | p>.26 |
| Gender (% female) | 69% | 51% | 76% | ||
Mean BMI of adolescents and adults significantly greater than mean BMI of children.
Ages 17-20 (N=19)
Next, a 3 (condition: toys, low-calorie food, high-calorie food) × 3 (group: children, adolescents, adults) × 2 (gender: male, female) mixed general linear model was performed for each of the following primary outcome variables: mean reaction time (RT), rate of omission errors, and false alarm rate.
3.1. Mean reaction time
There were main effects of condition (F(1,140) = 31.48, P < 0.001), and age group (F(2,140) = 13.37, P < 0.001) for mean RT (see Table 2). The main effect of condition showed that participants were quicker to respond to both low-calorie (t(145) = −5.98, P < 0.001) and high-calorie (t(145) = −5.79, P < 0.001) foods relative to toys, but no difference in RT between low- and high-calorie food go trials (t(145) = 0.10, P = 0.9). The main effect of age group showed that children were slower than both adolescents (t(86) = 4.99, P < 0.001) and adults (t(95) = 3.93, P < 0.001), but there was no difference in RT between adolescents and adults (t(105) = −1.65, P = 0.1). There were no other significant main effects or interactions.
Table 2.
Summary of Main Effects
| Reaction Time (ms) | Omission Error Rate | False Alarm Rate | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean (SD) | F | Mean (SD) | F | Mean (SD) | F | |
| Development | ||||||
| 13.37*** | 14.89*** | 42.77*** | ||||
| Children | 549 (98) | 0.04 (0.05) | 0.46 (0.21) | |||
| Adolescents | 466 (57) | 0.02 (0.03) | 0.29 (0.18) | |||
| Adults | 485 (62) | 0.01(0.01) | 0.13 (0.09) | |||
| Task Condition | ||||||
| 31.48*** | 0.14 | 21.76*** | ||||
| Low-Calorie | 484 (84) | 0.02 (0.04) | 0.32 (0.24) | |||
| High-Calorie | 485 (83) | 0.02 (0.04) | 0.30 (0.25) | |||
| Toys | 507 (84) | 0.02 (0.04) | 0.23 (0.21) | |||
| Gender | ||||||
| Male | 484 (92) | 0.59 | 0.03 (0.04) | 7.98** | 0.33 (0.22) | 6.01* |
| Female | 502 (71) | 0.02 (0.03) | 0.24(0.20) | |||
P < 0.05
P < 0.01
P < 0.001
3.2. Mean omission errors
There were main effects of age group (F(2,140) = 14.89, P < 0.001, Table 2) and gender (F(1,140) = 7.97, P < 0.005) for mean accuracy on go trials with greater accuracy in adults relative to children (t(95) = 4.98, P < 0.001) and adolescents (t(105) = 4.173, P < 0.001). There was only a trend for differences between children and adolescents (t(86) = 1.981, P = 0.051). Females performed better than males (t(144) = 2.656, P < 0.01). There were no other significant main effects or interactions.
3.3. Mean false alarm rate
There were main effects of condition (F(1,140)=21.76, P < 0.001), age group (F(2,140) = 42.77, P < 0.001), and gender (F(1,140) = 6.01, P < 0.015) with more false alarms to both low- (t(145) = 6.73, P < 0.001) and high-calorie (t(145) = 5.14, P < 0.001) foods than nonfoods (see Table 2). As was the case in RT, there was no difference in false alarm rates between the two categories of food stimuli (t(145) = −1.45, P = 0.15). The main effect of age group showed that adults had fewer false alarms on no-go trials than adolescents (t(105) = 6.10, P < 0.001) or children (t(95) = 10.610, P < 0.001) and adolescents had fewer false alarms than the children (t(86) = 4.13, P < 0.001). Females made fewer false alarms than males (t(144) = 2.482, P < 0.015). There were no other significant main effects or interactions.
4. Discussion
The current study introduces a task that utilizes both appetitive and neutral cues to examine behavioral inhibition in the face of rewarding food cues relative to nonfood cues. Our food-specific go/no-go task manifests the developmentally-expected age differences in task performance and demonstrates an effect of stimulus type, namely food versus toy targets, on behavior.
Reaction times were expectedly slower in children compared to adolescents and adults, consistent with their stage of brain and motor development. However, across all age groups, reaction times to food cues were consistently faster suggesting increased salience of food relative to nonfood cues. Interestingly, the difference in reaction times between foods and toys remained essentially constant across groups, suggesting that sensitivity to food cues (relative to nonfood items) develops early and is maintained across development. Though one might expect the children to be more motivationally driven by the toys (or at least equally driven by foods and toys), this early emerging sensitivity to food cues speaks to the role of food as a potent primary reinforcer throughout development. It is notable that the study sample was within a normal weight range. It is possible that responses to food relative to nonfood items would be different across the weight and eating disordered spectrum.
False alarm rates, which serve as an index of impulse control, differed across age groups, suggesting differences in overall impulsivity. These findings are consistent with previous studies that have shown that cognitive control is a process that continues to develop into adulthood (Somerville et al., 2011). Our findings indicate that salient food cues interfere with behavioral inhibition more than nonfood cues. False alarm rates were higher on trials when food was the “no-go” nontarget, consistent with the interpretation that these cues were more salient. Thus, the ability to regulate impulsive responses is altered in the context of food cues.
In our group of healthy, normal weight individuals, calorie level (i.e. high or low) did not appear to alter behavioral performance. There were no differences in false alarm rates or reaction times between high-calorie and low-calorie food cues. This result is not entirely surprising, in that our sample consisted of healthy normal-weight individuals who were screened for any aberrant eating behaviors prior to participating. Our results suggest that in young, healthy, non-eating disordered, non-obese individuals, high- and low-calorie foods elicit similar behavioral responses and may reflect as much of a preference for low-calorie as high-calorie foods. Future studies could explore this finding by targeting populations with known predisposition to high-calorie/high-fat foods, such as individuals with Prader-Willi Syndrome or carriers of the MCR4 and FTO risk alleles (Cecil et al., 2008; Wardle et al., 2009) or in patients with eating disorders (e.g. strong aversions to high-fat/high-calorie foods, such as in anorexia nervosa).
4.1. Limitations
While our task was modeled on well-established go/no-go tasks and included appetitive food and nonfood cues, we did not validate this task against a standard go/no-go task without appetitive stimuli. It is notable, however, that performance on this task is consistent with performance seen in other go/no-go tasks (i.e. overall accuracy and reaction times) (Durston et al., 2002; Hare et al., 2005; Somerville et al., 2011; Casey et al., 2007), which speaks to the validity of the task as a go/no-go paradigm within a normal population. Additionally, given the variability in behavioral responses (e.g. false alarm rates), particularly in the younger age group, a larger sample might have shown age by condition interactions. A third limitation could be in our choice of high- and low-calorie foods. It is possible, that our high- and low-calorie foods were not sufficiently distinct, thus our lack of behavioral difference to calorie level was not because high- and low-calorie foods are equally appetitive to normal weight individuals, but because our normal weight individuals could not correctly identify high- and low-calorie foods. Separating the runs into distinct high-calorie targets and low-calorie targets should have minimized the effect that difficulty in calorie identification might have had on performance.
Though the current study uses calorie content as a method of classifying food cues, recent studies have suggested the use of “palatability” over calorie content as a superior classifier (Houben et al., 2012). Namely, that behavioral biases to high-calorie foods may be due more to the palatability of the food than the actual caloric content. Due to the potential subjectivity of this measure across our large age range, however, caloric content was still used. High- and low-calorie categories, it could be argued, offered a somewhat more “objective” distinction between our food groups. Nonetheless, these limitations exist and should be considered in the context of the findings. Similarly, while the stimulus set used was matched on arousal and overall appeal of the items, other visual characteristics like color and visual complexity could potentially bias the appetitive nature of certain images (Meule & Blechert, 2012) and these measures should be considered in future applications of the task.
4.2. Conclusions
Self-control, or lack there of, has been linked to a myriad of behavioral measures and health outcomes. The development of age-appropriate and clinically relevant tasks that measure impulse control in the face of alluring cues is key in understanding the core components of self-control with reliability and specificity. Clinically, impulsivity in the context of food-specific cues is an important aspect of the study of eating and weight disorders, including obesity – a global epidemic. We have successfully developed a task that shows clear developmental differences among groups, suggesting that our task may be used to study developmental trajectories in eating behavior and the development of eating disorders among a broad range of ages. This food go/no-go task may be used to learn more about different populations including individuals with anorexia nervosa, bulimia nervosa, binge eating disorder and obesity.
Supplementary Material
Supplemental Figure 1. Food and Nonfood Stimuli. Thumbnails of each of the stimuli used in the task. Stimuli were rated and matched on valence and arousal prior to the current study, using an independent test group. Ratings are reported in Supplemental Table 1.
Acknowledgements
The research reported in this article was supported by funding from the Diabetes and Endocrinology Research Center 5P30 DK063608-10 and R56 DK097399, and in part by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health T32GM007739. We would like to thank Eve Vagg of the Digital Communications Department at the New York State Psychiatric Institute for her amazing photography, Janet Schebendach for her food photography skills, Natasha Mehta for her input, and our study participants, for making this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batterink L, Yokum S, Stice E. Body mass correlated inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52(4):1696–703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Lepping RJ, Bruce JM, Cherry JBC, Martin LE, Davis AM, Brooks WM, Savage CR. Brain responses to food logos in obese and healthy weight children. The Journal of Pediatrics. 2013;162(4):759–764. doi: 10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164(11):1729–36. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, Wilson NL, Teslovich T, Glover G, Zayas V, Mischel W, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences U S A. 2011;108(36):14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have learned about cognitive development? Trends in Cognitive Sciences. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Cohen JD, Noll DC, Giedd J, Castellanos X, Haxby J, Forman SD, Dahl RE, Rapoport JL. A pediatric functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–47. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. New England Journal of Medicine. 2008;359(24):2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- Durston S, Tottenham N, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–8. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Grose-Fifer J, Rodrigues A, Hoover S, Zottoli T. Attentional capture by emotional faces in adolescence. Advances in Cognitive Psychology. 2013;9:81–91. doi: 10.2478/v10053-008-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. The interaction between impulsivity and a varied food environment: its influence on food intake and overweight. International Journal of Obesity. 2008;32(4):708–14. doi: 10.1038/sj.ijo.0803770. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49(1):66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 years. American Journal of Clinical Nutrition. 2002;75:476–483. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- Houben K, Roefs A, Jansen A. Guilty pleasures II: Restrained eaters’ implicit preference for high, moderate and low-caloric food. Eating Behaviors. 2012;13(3):275–277. doi: 10.1016/j.eatbeh.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Yasuda M, Burant CF, Gregor N, Khatri S, Sweet M, Falk EB. Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite. 2012;59(3):738–47. doi: 10.1016/j.appet.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Statistics. 2000;11(246):2002. [PubMed] [Google Scholar]
- Lindroos A, Lissner L, Mathiassen ME, Karlsson J, Sullivan M, Bengtsson C, Sjöström L. Dietary intake in relation to restrained eating, disinhibition and hunger in obese and nonobese Swedish women. Obesity Research. 1997;5:175–182. doi: 10.1002/j.1550-8528.1997.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Herpertz S, Kiefer F, Herpertz SC. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite. 2013;71:32–9. doi: 10.1016/j.appet.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, Wiers RW, Mann K, Kiefer F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes. 2012;36(10):1334–9. doi: 10.1038/ijo.2011.184. [DOI] [PubMed] [Google Scholar]
- Mayer LES, Schebenbach J, Bodell LP, Shingleton RM, Walsh BT. Eating behavior in anorexia nervosa: Before and after treatment. International Journal of Eating Disorders. 2012;45(2):290–3. doi: 10.1002/eat.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Blechert J. A picture database for the study of eating and appetite. Obesity Facts. 2012;5(Suppl. 2):20. [Google Scholar]
- Meule A, Lukito S, Vögele C, Kübler A. Enhanced behavioral inhibition in restrained eaters. Eating Behaviors. 2011;12(2):152–5. doi: 10.1016/j.eatbeh.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Meule A, Lutz A, Vögele C, Kübler A. Women with elevated food addiction symptoms show accelerated reactions, but no impaired inhibitory control, in response to pictures of high-calorie food-cues. Eating Behaviors. 2012;13:423–8. doi: 10.1016/j.eatbeh.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57:263–71. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Van der Linden M, d’Acremont M, Perroud A. Cognitive deficits and biases for food and body in bulimia: investigation using an affective shifting task. Eating Behaviors. 2008;9(4):455–61. doi: 10.1016/j.eatbeh.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43(3):303–7. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eating Behaviors. 2006;7(4):315–22. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the Children’s Behavior Questionnaire. Child Development. 2001;72(5):1394–408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Savage JS, Hoffman L, Birch LL. Dieting, restraint and disinhibition predict women’s weight change over 6 yrs. American Journal of Clinical Nutrition. 2009;90:33–40. doi: 10.3945/ajcn.2008.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20(2):236–41. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–34. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinrad RL, Eisenberg N, Gaertner BM. Measures of effortful regulation for children. Infant Mental Health Journal. 2007;28:606–626. doi: 10.1002/imhj.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. International Journal of Obesity. 2009;33(1):42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Food and Nonfood Stimuli. Thumbnails of each of the stimuli used in the task. Stimuli were rated and matched on valence and arousal prior to the current study, using an independent test group. Ratings are reported in Supplemental Table 1.