Abstract
Borderline personality disorder (BPD) is a prevalent and difficult to treat psychiatric condition characterized by abrupt mood swings, intense anger and depression, unstable interpersonal relationships, impulsive self-destructive behavior and a suicide rate of approximately 10%. Possible underlying molecular dysregulations in BPD have not been well explored. Protein kinase C (PKC) and brain-derived neurotrophic factor (BDNF) have both been implicated in affective disorders, but their role in BPD has not been examined. Platelets were isolated from blood obtained from 24 medication-free BPD patients and 18 healthy control subjects. PKC-α, phosphorylated-PKC-α (p-PKCα), PKC-β II, and BDNF were measured in platelet homogenates by immunoblotting. In the males, platelet BDNF and PKC-α levels were lower in patients than controls. p-PKC-α and PKC-βII were lower at trend levels. In the entire sample, platelet p-PKC α and PKC-α activity were lower, at a trend level, in patients compared to controls. This is the first report to our knowledge of PKC and BDNF activity in BPD and calls for replication. These findings are consistent with altered PKC and BDNF activity in a range of neuropsychiatric conditions including bipolar disorder, depression and suicide.
Keywords: PKC, BDNF, Neurotrophic Factors, Second Messengers, Personality Disorders, Borderline Personality Disorder
1. Introduction
Borderline personality disorder (BPD) is a prevalent and enduring psychiatric condition which affects 2 to 5.9% of the population (American Psychiatric Association, 1994; Grant et al., 2008) and 20% of psychiatric inpatients. It is characterized by abrupt mood swings, intense anger and depression, unstable interpersonal relationships, impulsive self-destructive behavior and a suicide rate of approximately 10% (Skodol et al., 2002). The disorder is difficult to treat and its response to pharmacotherapy is modest (Koenigsberg et al., 2007). Possible underlying neurochemical dysregulations in BPD have not been well explored. Protein kinase C (PKC) and brain-derived neurotrophic factor (BDNF) both play important roles in an array of neural processes including neutrotransmission, intracellular signal transduction, regulation of neuronal plasticity and gene expression (DiazGranados and Zarate, 2008; Numakawa et al., 2010). Moreover dysregulations of these substances have been implicated in bipolar disorder and depression. Since BPD is characterized by mood dysregulation, has a high comorbidity with depression (Koenigsberg et al., 1999), and has been hypothesized to be a form of bipolar disorder (Perugi et al., 2003; Smith et al., 2004), study of these systems in BPD can help us to better understand the mechanisms of mood dysregulation in BPD and its relationship to both depression and bipolar disorder. There are no reports in the literature of PKC or BDNF levels in BPD patients to our knowledge.
1.1 PKC
PKC isoenzymes play an important regulatory role in the intracellular phosphoinosotide second messenger system. Isoforms of PKC regulate pre- and post-synaptic neurotransmission, integrate activity from multiple external neurotransmitter systems, influence neuronal plasticity, and affect gene expression (DiazGranados and Zarate, 2008; Gould and Manji, 2002). PKC is found in the cell in an autoinhibited and an activated form. The activation is regulated by G-proteins which initiate a cascade of processes leading to the translocation of PKC from cytosol to the plasma membrane where it becomes activated and phosphorylated (p-PKC) (Rosse et al., 2010).
PKC dysregulation has been implicated in major depression and bipolar disorder. In patients with major depression, PKC binding to [3H]phorbol dibutyrate (PDBu) in the platelet cytosolic fraction was increased relative to healthy controls, and there was no difference in membranal levels (Pandey et al., 1998). The functional implication of the elevated cytosolic PKC is unclear, but it could indicate an amplified responsiveness to activation by diacylglycerol (DAG) in response to a G-protein receptor signal. A subsequent study examining the isoforms PKC-α, PKC-βI, PKC-βII, and PKC-δ in platelets of unipolar depressives reported no difference in either cytosolic or membranal fractions compared to healthy controls (Pandey et al., 2002) The reason for the discrepant findings between these two studies is unclear, but a possibility suggested by the authors is that PDBu may bind to sites additional to PKC. In fibroblasts cultured from skin biopsies of melancholic depressives there is decreased phosphorylation of cAMP regulatory element binding protein (CREB) in response to activation of PKC compared to non-melancholic depressives or healthy controls (Akin et al., 2005). From a pharmacologic perspective, preclinical studies have shown that chronic administration of antidepressants (fluoxetine and desipramine) decrease PKC activity in the cortex and hippocampus of rats (Mann et al., 1995). In teenaged suicide victims PKC in prefrontal (Brodmann Area 8 (BA8) and BA9) brain tissue is reduced in membranal and cytosolic fractions compared to age matched controls who died of causes other than suicide (Pandey et al., 1997). In a subsequent study of prefrontal and hippocampal postmortem tissue from teenage suicide victims Pandey et al found decreased membrane and cytosolic fraction PKC activity, decreased levels in both fractions of the isoenzymes PKC-α, PKC-βI, PKC-βII, PKC-γ, and of their respective m-RNA’s (Pandey et al., 2004). Hrdina and coworkers (Hrdina et al., 1998) reported no difference in PKC-α, PKC-β, PKC-γ and PKC-ε levels in BA9 and BA10 between adult depressed suicide victims and controls. A study of adult suicide victims with histories of depression reported increased PKC binding to [3H]PDBu in the cytosolic fraction in frontal and hippocampal regions (Coull et al., 2000), but this finding could be a artifact of [3H]PDBu binding non-specifically to other proteins than PKC. A postmortem adult study of tissue from BA9 in persons with major depression found decreased levels of PKC-βl and PKC-ε as well as PKC-βll in the melancholic subsample (Shelton et al., 2009). These findings are consistent with the Pandey et al 1997 and 2004 studies.
In manic bipolar patients the ratio in platelets of membrane-bound to cytosolic PKC was found to be elevated compared to healthy volunteers and patients with schizophrenia (Friedman et al., 1993). PKC translocation to membrane in response to serotonin was also increased in the manic patients compared to the other groups. Treatment with lithium reduced the elevated membrane/cytosolic PKC ratio and the serotonin-induced translocation to membrane. Wang et al (Wang and Friedman, 1996) examined PKC activity in post-mortem brain tissue in patients with bipolar disorder and reported increased membranal PKC activity, increased levels of cytosolic PKC-α and membranal PKC-γ and PKC-ξ; and decreased cytosolic PKC-ε relative to controls. A study comparing platelet PKC activity between manic bipolar patients, patients with acute major depression, patients with schizophrenia and controls also found increased membrane to cytosolic PKC activity and serotonin stimulation of PKC translocation to membrane in the bipolars compared to the other groups (Wang et al., 1999). In contrast, Pandey et al (Pandey et al., 2002) found in a group of manic, depressed and euthymic medication-free bipolar patients a decrease in membranal and cytosolic fraction PKC activity and in the expression of PKC-α, PKC-βI, PKC-βII and and PKC-δ1 isoforms. While the difference between this and the other studies could be explained by the inclusion of depressive and euthymic bipolar patients in the sample, Pandey et al found no significant differences between these groups.
Chronic lithium treatment decreased membranal PKC activity and the membrane-associated PKC-α and PKC-ε in hippocampal tissue in rats (Manji and Lenox, 1999). Similar effects of chronic lithium administration on the PKC system have been demonstrated in rat brain, rat platelets, human cultured neuronal cells and human platelets (Manji and Lenox, 1999). Chronic administration of valproic acid, an anti-manic medication, structurally different from lithium, has been shown to have the same effects as lithium on the PKC isoenzymes (Gould and Manji, 2002; Zarate and Manji, 2009). Recent studies have suggested that the PKC inhibitor tamoxifen may have anti-manic effects (Zarate and Manji, 2009). Thus, there is growing evidence that medications which decrease PKC activity are effective treatment for the manic phase of bipolar illness.
At present there is considerable variability among studies of platelet and brain levels of PKC in unipolar and bipolar disorders. The reasons for these differences remain unclear, but differences in phenotype (e.g. suicidal vs. non-suicidal; melancholic vs. nonmelancholic,depression), small sample sizes, differences in brain regions sampled, exposure to psychotropic medications and methodological assay differences may all play a role. Nevertheless, taken together these studies provide considerable evidence for PKC dysregulations in depression, bipolar disorder and suicide.
1.2 BDNF
Brain-derived neurotrophic factor (BDNF) and its receptor, TrkB are widely expressed in the brain and participate in a range of intracellular signaling processes, neuronal protection, axonal and dendritic morphology and synaptic plasticity (Numakawa et al., 2010). The BDNF gene codes for precursor protein BDNF (preproBDNF) which is cleaved to form proBDNF in the endoplasmic reticulum. ProBDNF has recently been shown to be biologically active and binds to the p75NTR receptor (Hashimoto, 2010; Yang et al., 2009). ProBDNF is cleaved by intra or extracellular proteases to form BDNF (Hashimoto, 2010). BDNF expression is also regulated in part by PKC phosphorylation of CREB.
BDNF has been implicated in a number of psychiatric disorders. Post-mortem studies of suicide victims have demonstrated reduced levels of BDNF (Karege et al., 2005b) and of BDNF mRNA (Dwivedi et al., 2003) in brain tissue. BDNF passes the blood-brain barrier bi-directionally and is stored peripherally in platelets. Serum BDNF levels are found to be higher than plasma levels because clotting releases BDNF stored in platelets (Serra-Millas et al., 2011). A meta-analysis of studies of serum BDNF levels in depressed patients provides strong support for decreased levels in depression and for increases with antidepressant treatment (Sen et al., 2008). Three recent studies demonstrating decreased platelet BDNF in depression (Lee and Kim, 2009; Pandey et al., 2010; Serra-Millas et al., 2011) indicate that the decreased serum levels are not simply due to the failure of platelets to release accumulated BDNF into serum. Two non-pharmacological treatments for depression, electroconvulsive therapy (ECT) and repetitive trans-cranial magnetic stimulation (rTMS) have both been shown in animal models to increase brain BDNF levels (Hashimoto, 2010). These observations provide strong support for involvement of BDNF in depression. A recent meta-analysis documents decreased serum and plasma BDNF in the manic and depressive, but not euthymic phases of bipolar disorder (Fernandes et al., 2011). Medications which treat bipolar disorder, lithium, valproic acid and lamotrigine have all been shown to increase BDNF levels (Hashimoto, 2010). Studies in some populations have shown an association between the val allele of the BDNF val66met polymorphism and bipolar disorder with a possible particular connection to the rapid cycling variant (Post, 2007).
We report here on our studies of platelet PKC isoenzymes and BDNF levels in BPD patients relative to those in healthy volunteers. Based on the findings described above for mood disordered patients we expected decreased levels of BDNF in BPD patients. The literature, however, is not consistent enough to support specific predictions for the PKC isoforms
2. Methods
2.1 Subjects
Subjects were male and female and over 18 years of age. The BPD patients in our sample met DSM-IV criteria for BPD and in addition the DSM-IV BPD criterion for affective instability (criterion 6.) and two or more of the following BPD criteria: (2) a pattern of unstable and intense interpersonal relationships, (3) identity disturbance, and (7) chronic feelings of emptiness. These criteria were selected to define the affectively unstable subgroup because they are associated with the biological trait of affective instability as measured by a depressive reactivity in BPD patients to a phisostigmine challenge (Steinberg et al., 1997). In addition the BPD patients did not meet criteria for any current DSM-IV axis I disorder or past history of substance dependence, schizophrenia, schizoaffective disorder, or other psychotic disorders, bipolar I and bipolar II disorder, obsessive-compulsive disorder, eating disorders, posttraumatic stress disorder (PTSD), panic disorder, or cognitive disorders. The presence of a substance use disorder in the 6 months prior to the study was also an exclusion criterion. Patients who had taken antipsychotic medication within the last month or antidepressants within the last 3 weeks (fluoxetine within 5 weeks) were excluded. The healthy volunteers did not meet criteria for any DSM-IV axis-I or axis-II disorder. Subjects with significant medical illnesses were excluded. Subjects were recruited by newspaper advertisements and by referral from the psychiatry outpatient and inpatient departments of the James J Peters VA Medical Center and Mount Sinai Medical Center and all provided informed consent.
Research diagnoses were made by means of semistructured interviews, the Schedule for Afffective Disorders and Schizophrenia (SADS) and the Structured Interview for DSM-IV Personality (SIDP), administered by experienced interviewers (masters or doctoral level clinical psychologists). Diagnoses follow DSM-IV criteria. We have achieved an interrater reliability kappa of .81 for diagnosing BPD. Ratings of mood were obtained with the Hamilton Depression Rating Scale (HDRS) and the the Affective Lability Scale (ALS; (Harvey et al., 1989) a 54-item self-report measure of affective instability that has been validated in the BPD population (Koenigsberg et al., 2002; Koenigsberg et al., 2001). Impulsivity was measured using the Barrett Impulsivity Scale, version 11 (BIS11).
2.2 Sample Collection and Processing
Samples of 50cc of venous blood were drawn from supine subjects and collected into a tube containing anticoagulant acid citrate dextrose. The blood was centrifuged immediately at 210 g for 10 min at 4°C to obtain platelet-rich plasma, which was centrifuged at 4000 g for 10 min at 4°C to obtain the platelet pellet. The plasma was removed and the platelets were gently resuspended in 2 mL Tyrodes buffer (10 mM HEPES, 0.4 mM NaH2PO4, 137 mM NaCl, 5.5 mM glucose, 2.8 mM KCl 1 mM MgCl, 12 mM NaHCO3). The platelets were then centrifuged a second time. The buffer was removed and the pellet was frozen immediately and stored at −80C until analyzed. Platelets were separated and stored at −80C until the assays were conducted. We obtained two samples separated by an interval of one to two months (samples were obtained from female subjects at the same phase of their menstrual cycles) from a subset of 17 subjects to examine test-retest reliability.
The PKC isoenzymes. BDNF and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels were obtained using the following procedure. The platelet pellet was homogenized in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 2 mM EGTA, 5 mM EDTA, 0.2% Triton X-100, 1 mM dithiothreitol, protease inhibitor cocktail and phosphatase inhibitor cocktails (Sigma). Homogenates were centrifuged at 14 000×g for 10 min to remove undissolved debris. The concentration of protein was determined using Pierce BCA protein assay kit.
Quantitation of PKC Isozymes and BDNF by Western Blot
The linearity of the protein concentration for immunoblotting of PKC isozymes was ascertained by resolution of selected concentrations of protein. Subsequent PKC isozyme immunoblotting was performed using protein concentrations demonstrated to be within the linear range for Western blotting using previously described methods (Yuan et al., 2001). Briefly, equal amount of samples mixed in gel loading buffer (50 mM Tris-HCl [pH 6.8], 4% -mercaptoethanol, 1% sodium dodecylsulfate (SDS), 40% glycerol, and bromphenol blue) were denatured at 95C for 5 min and then kept on ice. The samples were loaded onto 10% Tris-glycine gel and run with the SureLock gel apparatus (BioRad, Hercules, CA) at 120 volts. An aliquot of pooled ‘standard’ platelet sample was run on one lane of each gel, to allow normalization of the data against the pooled standards, in order to minimize variability between blots. The proteins were subsequently transferred electrophoretically to nitrocellulose membrane using the iBlot transfer system (Invitrogen). Membranes were washed with TBST buffer (10 mM Trisbase, 0.15 M NaCl, and 0.05% Tween 20) for 10 min. The blots were blocked by incubating with 5% nonfat milk in TBST. The blots were then incubated with primary antibodies overnight at 4°C (PKCa, phospho-PKCa, PKCb2, PKCe, phospho-PKCe antibodies were purchased from Upstate Biotechnologies; BDNF, Pro-BDNF antibodies were from Santa Cruz Biotechnologies; beta-actin, GAPDH antibodies were from Abcam). The dilution of antibodies ranged from 1:200 to 1:2000 depending on the antibody used. Membranes were washed with TBST and incubated with horseradish peroxidase-linked secondary antibody (anti-rabbit or anti-mouse IgG, GE/Amershan) for 1h at room temperature. Membranes were extensively washed with TBST and the immunocomplexes were detected with an Amersham ECL or ECL plus kit. Membranes were stripped using stripping solution (Chemicon International, Temecula, CA) for reprobing. The specific bands were quantified using the SynGene Image Analysis System, and the optical density of each band of the PKC isozymes and BDNF was corrected by the optical density of the corresponding GAPDH band. The values are represented as a percentage of the control.
2.3 Data Analysis
Isoenzyme and BDNF values were normalized to the housekeeping protein GAPDH. General linear models were used to compare groups. Because the BPD and control subjects differed in mean age, age was entered as a covariate in the GLM models. In subsequent analyses, Hamilton Depression Rating Scale (HAMD) score was entered as an additional covariate to control for the effect of depressive symptoms. Ttests were employed to test for group differences in age and behavioral measures. For the predicted group difference in BDNF, significance was tested at p<0.05,(two-tailed). A Bonferroni corrected p-value (p=0.008, two-tailed) was used in exploratory tests of the PKC isoforms and proBDNF.
3. Results
The sample consisted of 24 BPD patients (18 male) and 18 healthy controls (11 male). See Table 1 for the sample characteristics. Three controls with spurious values for GAPDH were not included. The patients were older than the controls. Although the patients had higher Hamilton depression scores than the controls, their level of depression was modest. The patients and controls did not differ in ALS scores or BIS11 scores.
Table I.
Sample Characteristics
| BPD Patients |
Healthy Controls |
Statistic | |
|---|---|---|---|
| N | 24 | 18 | |
| Age (SD) | 37.9 (9.2) | 29.9 (7.4) | t(40) = 3.05* |
| Gender (Male/Female) | 18/6 | 11/7 | X2 = 0.93 |
| HAMD (SD) | 8.6 (4.4) | 4.9 (2.4) | t(36) = 3.04* |
| ALS Total (SD) | 16.5 (10.2) | 17.2 (9.9) | t(40) = 0.22 |
| BIS11 (SD) | 14.5 (10.3) | 12.3 (9.6) | t(39) = 0.71 |
| Ethnicity | |||
| Black | 12 | 4 | |
| Caucasian (non-Hispanic) | 8 | 10 | |
| Hispanic | 3 | 0 | |
| Other | 1 | 4 |
p < .05
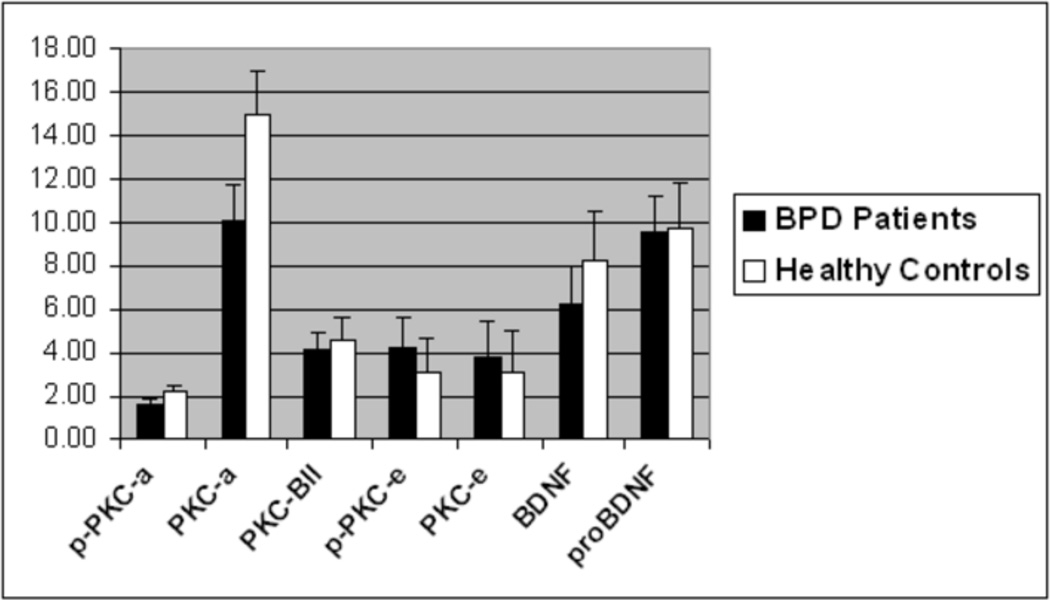
The mean values for the platelet isoenzymes and BDNF for each group are presented in Figure 1 and Table 2. For the sample as a whole, there was a trend for both p-PKC-α and PKC-α to be lower in patients than controls. For the males platelet BDNF levels were lower in patients than in controls and PKC-α was reduced in BPD’s compared to HC’s. There was a trend for p-PKC-α and PKC-βII to be lower in male patients than controls as well.
Figure 1.
PKC Isoenzyme and BDNF Levels in Entire Sample
p-PKCa – phosphorylated PKC-alpha; PKCa – PKC-alpha; PKC-BII – PKC-beta II; p-PKCe – phosphorylated PKC-epsilon, BDNF – Brain-Derived Neurotrophic Factor; pro- BDNF – BDNF precursor. Values are normalized to the housekeeping protein GAPDH.
Table 2. PKC Isoenzyme Measures and BDNF Levels.
Mean values and standard deviations are shown for each measure. Statistical tests include age as a covariate
| BPD | HC | F | df | p | |||
|---|---|---|---|---|---|---|---|
| Mean ± STD | Mean ± STD | ||||||
| Males & Females | |||||||
| p-PKC-α | 1.66 | 1.01 | 2.17 | 1.77 | 3.765 | 1,39 | 0.06 |
| PKC-α | 10.06 | 7.17 | 14.62 | 9.51 | 3.011 | 1,39 | 0.09 |
| PKC-βII | 4.17 | 3.65 | 4.49 | 4.28 | 0.518 | 1,39 | ns |
| p-PKC-ε | 4.22 | 7.77 | 3.11 | 3.92 | 0.083 | 1,39 | ns |
| PKC-ε | 3.90 | 9.92 | 2.96 | 5.43 | 0.171 | 1,39 | ns |
| BDNF | 6.29 | 9.57 | 7.96 | 8.84 | 0.299 | 1,39 | ns |
| proBDNF | 9.09 | 7.04 | 10.23 | 9.43 | 0.176 | 1,39 | ns |
| Males | |||||||
| p-PKC-α | 1.68 | 1.07 | 2.36 | 2.22 | 5.213 | 1,26 | 0.03 |
| PKC-α | 8.71 | 3.54 | 16.21 | 10.95 | 12.116 | 1,26 | 0.002 |
| PKC-βII | 4.06 | 3.20 | 5.45 | 5.27 | 3.814 | 1,26 | 0.06 |
| p-PKC-ε | 3.94 | 7.58 | 3.77 | 4.61 | 0.353 | 1,26 | ns |
| PKC-ε | 4.80 | 11.39 | 3.78 | 6.89 | 0.401 | 1,26 | ns |
| BDNF | 4.60 | 2.66 | 8.62 | 10.40 | 7.141 | 1,26 | 0.01 |
| proBDNF | 9.02 | 8.12 | 8.48 | 8.82 | 0.083 | 1,26 | ns |
| Females | |||||||
| p-PKC-α | 1.59 | 0.88 | 1.87 | 0.69 | 0.263 | 1,10 | ns |
| PKC-α | 14.14 | 12.94 | 12.12 | 6.69 | 0.320 | 1,10 | ns |
| PKC-βII | 4.49 | 5.13 | 2.98 | 1.06 | 0.902 | 1,10 | ns |
| p-PKC-ε | 5.17 | 9.26 | 1.66 | 0.66 | 1..572 | 1,10 | ns |
| PKC-ε | 1.21 | 0.28 | 1.66 | 1.12 | 0.999 | 1,10 | ns |
| BDNF | 11.34 | 18.89 | 6.93 | 6.27 | 0.657 | 1,10 | ns |
| proBDNF | 9.30 | 2.65 | 12.98 | 10.38 | 0.793 | 1,10 | ns |
We carried out two further analyses to test whether these differences could be accounted for by depression or depressive symptoms in the patient group. First, we removed from the sample all patients with a history of major depression (8 males, 1 female). Among the males the finding of reduced BDNF in BPD’s remained significant (F(1,18)= 5.46, p =0.03), PKC-α and p-PKC-α remained significant at the uncorrected p<0.05 level (respective F-values: F(1,18)= 4.87, p =0.04; F(1,18)= 7.84, p = 0.01).. For the combined male and female group, the finding of a decrease in p-PKC-α in patients relative to controls remained at trend level (F(1,30)=3.140, p = 0.09). In a second analysis, we entered HAMD score as a covariate. For the entire sample, the p-PKC-α finding did not survive covarying for HAMD (F(1,1,34)= 1.162, ns). However, among the males, the reduced levels of PKC-α and BDNF in patients remained significant (respective F’s: F(1,1,23)= 9.15, p = .006, and F(1,1,23)= 5.07, p = .03).
4. Discussion
The purpose of the present study was to compare platelet PKC isoenzyme and BDNF levels in BPD patient’s with those of controls. We found that male BPD patients have reduced levels of PKC-α, and BDNF, relative to controls and a trend toward lower levels of p-PKC-α and PKC-βII.
The patients were more depressed than the controls, although their mean Hamilton depression score of 8.4 indicates only a relatively mild degree of depression. Since PKC and BDNF are reduced in patients with depression, we carried out analyses to determine whether the presence of depression or depressive symptoms in the patients could account for the finding of reduced PKC and BDNF. To address the possibility of a trait effect of major depressive disorder, we reanalyzed the data eliminating all patients with a prior history of major depression. The decreased platelet PKC-α, and BDNF in the BPD males relative to controls remained significant. We next entered HAMD score as a covariate in the analysis to control for a possible state effect of depressive symptoms. The finding of reduced PKC-α, and BDNF in the male BPD subjects survived this analysis as well.
The present study identifies differences in PKC-α, BDNF and possibly p-PKC-α and PKC-βII activity in platelets of BPD patients compared to controls and thus highlights these proteins as potential biomarkers for personality as well as mood disorders with prominent mood dysregualtions. A limitation is that PKC and BDNF are measured in peripheral tissue and may not provide information about molecular mechanisms in the brain underlying affective dysregulations in BPD. While this does not detract from the value of these peripheral measures as biomarkers, a body of converging evidence suggests that platelet PKC and BDNF may in fact mirror central nervous system (CNS) processes (Manji and Lenox, 1999). For example, PKC levels in post-mortem brain tissue of suicide victims, and in fibroblasts and platelets of depressed patients are all reduced in most studies (Akin et al., 2005; Pandey et al., 1998; Pandey et al., 1997; Pandey et al., 2004). In addition PKC activity in rat brain tissue, rat platelets, human neuronal cells in culture and human platelets are all similarly modulated by lithium (Manji and Lenox, 1999). BDNF crosses the blood-brain barrier and is stored in platelets. Serum and plasma levels of BDNF correlate with CNS levels (Fernandes et al., 2011; Karege et al., 2005a; Pan et al., 1998).
A number of limitations should be noted. Our sample was selected to include borderline patients specifically meeting borderline affective instability criteria. Exclusion criteria were current co-morbid Axis I disorders and a history of several potentially confounding Axis I disorders. Males were more prevalent than females. Thus, caution must be taken in generalizing the findings of this study to a more general clinical borderline population. Surprisingly, the the patients and controls did not differ in ALS or BIS11 scores. Finally, body mass index and exercise level were not controlled.
This is the first study, to our knowledge, to examine PKC and BDNF in BPD. Decreased PKC activity and PKC isoforms have been reported in some (Akin et al., 2005; Pandey et al., 1997; Pandey et al., 2004; Shelton et al., 2009), but not all (Coull et al., 2000; Hrdina et al., 1998; Pandey et al., 1998; Pandey et al., 2002) studies of depressed or suicidal patients. Reports of PKC activity in bipolar disorder are mixed, with some studies documenting increased activity (Friedman et al., 1993; Wang and Friedman, 1996; Wang et al., 1999) and others reporting decreased activity (Pandey et al., 2002). The divergence in findings among these studies may reflect differences in methodology as well as in tissue type sampled. However, the observation that three structurally distinct medications that treat mania (lithium, valproic acid and tamoxifen) all decrease PKC activity provides additional support for an association between increased functional PKC activity and bipolar illness (DiazGranados and Zarate, 2008).
Platelet BDNF levels were reduced in men with borderline personality disorder relative to male controls. Since BDNF crosses the blood brain barrier, is stored in platelets and is released during clotting processes, care must be taken in comparing platelet, serum and plasma findings. Platelet and serum levels have been shown to strongly correlate, while serum and plasma levels may differ as a function of the amount of BDNF released from platelets during the clotting process (Serra-Millas et al., 2011). In depression, platelet and serum BDNF levels have been consistently shown to be reduced (Lee and Kim, 2009; Pandey et al., 2010; Sen et al., 2008; Serra-Millas et al., 2011). Studies of plasma BDNF have shown both decreased and increased levels, however this may be the result of differences in assay methods employed (Serra-Millas et al., 2011). Serum and platelet levels of BDNF are decreased in bipolar disorder patients relative to healthy controls (Fernandes et al., 2011). Thus our finding of decreased platelet BDNF in BPD is consistent with findings in both depression and bipolar disorder.
The present study suggests that platelet PKC-α and BDNF are decreased in borderline personality disorder, extending the potential pathophysiological role of these substances beyond the affective disorders to borderline personality and possibly other disorders characterized by affective instability. Further work is needed to determine whether they may be of value as surrogate laboratory markers for treatment response in BPD. The finding of decreased platelet PKC activity in BPD raises interesting questions about the use of the mood stabilizers, lithium and valproic acid, in the treatment of BPD. These agents have been employed to address the mood instability and impulsive aggression seen in the disorder, but their effectiveness is only modest. One possible explanation of this is that to the extent that these agents lower already reduced PKC activity, they could have counterproductive effects on an underlying pathophysiology of the disorder. Further work is called for to replicate the findings of this study, to study a larger sample of female patients, and to examine the effects of treatment on platelet PKC and BDNF levels in borderline personality disorder.
ACKNOWLEDGEMENTS
This work was supported by the James J Peters Veterans Affairs Medical Center and a grant from the National Center for Research Resources, National Institutes for Health for the Mount Sinai General Clinical Research Center (5MO1 RR00071).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented at the 66th Annual Meeting of the Society of Biological Psychiatry, San Francisico, May 2011.
CONFLICT OF INTEREST
The authors have no financial conflicts of interest with regard to the content of this work.
References
- Akin D, Manier DH, Sanders-Bush E, Shelton RC. Signal transduction abnormalities in melancholic depression. Int J Neuropsychopharmacol. 2005;8:5–16. doi: 10.1017/S146114570400478X. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Coull MA, Lowther S, Katona CL, Horton RW. Altered brain protein kinase C in depression: a post-mortem study. European Neuropsychopharmacology. 2000;10:283–288. doi: 10.1016/s0924-977x(00)00084-5. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Zarate CA., Jr A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Current Psychiatry Reports. 2008;10:510–519. doi: 10.1007/s11920-008-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of General Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Gama CS, Maria Cereser K, Yatham LN, Fries GR, Colpo G, de Lucena D, Kunz M, Gomes FA, Kapczinski F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. Journal of Psychiatric Research. 2011 doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biological Psychiatry. 1993;33:520–525. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Signaling networks in the pathophysiology and treatment of mood disorders. Journal of Psychosomatic Research. 2002;53:687–697. doi: 10.1016/s0022-3999(02)00426-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, Smith SM, Dawson DA, Pulay AJ, Pickering RP, Ruan WJ. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2008;69:533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. Journal of Clinical Psychology. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry and Clinical Neurosciences. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- Hrdina P, Faludi G, Li Q, Bendotti C, Tekes K, Sotonyi P, Palkovits M. Growth-associated protein (GAP-43), its mRNA, and protein kinase C (PKC) isoenzymes in brain regions of depressed suicides. Molecular Psychiatry. 1998;3:411–418. doi: 10.1038/sj.mp.4000435. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biological Psychiatry. 2005a;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Research Molecular Brain Research. 2005b;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Anwunah I, New AS, Mitropoulou V, Schopick F, Siever LJ. Relationship between depression and borderline personality disorder. Depression and Anxiety. 1999;10:158–167. doi: 10.1002/(sici)1520-6394(1999)10:4<158::aid-da4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou VJ. Characterizing Affective Instability in Borderline Personality Disorder. American Journal of Psychiatry. 2002 doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, New AS. Are the Interpersonal and Identity Disturbances in the Borderline Personality Disorder Criteria …. Journal of Personality Disorders. 2001 doi: 10.1521/pedi.15.4.358.19181. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Woo-Ming AM, Siever LJ. Psychopharmacological Treatment of Personality Disorders. In: Nathan PE, Gorman JM, editors. A Guide to Treatments that Work. Third Edition ed. New York: Oxford University Press; 2007. [Google Scholar]
- Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Progress in Neuro-psychopharmacol & Biological Psychiatry. 2009;33:849–853. doi: 10.1016/j.pnpbp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biological Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Mann CD, Vu TB, Hrdina PD. Protein kinase C in rat brain cortex and hippocampus: effect of repeated administration of fluoxetine and desipramine. British Journal of Pharmacology. 1995;115:595–600. doi: 10.1111/j.1476-5381.1995.tb14973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histology and Histopathology. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Kumari R, Janicak PG. Protein kinase C in platelets of depressed patients. Biological Psychiatry. 1998;44:909–911. doi: 10.1016/s0006-3223(97)00535-0. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA. Protein kinase C in the postmortem brain of teenage suicide victims. Neuroscience Letters. 1997;228:111–114. doi: 10.1016/s0304-3940(97)00378-9. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Archives of General Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010;34:645–651. doi: 10.1016/j.pnpbp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, SridharaRao J, Ren X, Janicak PG, Sharma R. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology. 2002;26:216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- Perugi G, Toni C, Travierso MC, Akiskal HS. The role of cyclothymia in atypical depression: toward a data-based reconceptualization of the borderline-bipolar II connection. Journal of Affective Disorders. 2003;73:87–98. doi: 10.1016/s0165-0327(02)00329-4. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. Journal of Psychiatric Research. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nature Reviews Molecular Cell Biology. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biological Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Millas M, Lopez-Vilchez I, Navarro V, Galan AM, Escolar G, Penades R, Catalan R, Fananas L, Arias B, Gasto C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology (Berl) 2011;216:1–8. doi: 10.1007/s00213-011-2180-0. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Hal Manier D, Lewis DA. Protein kinases A and C in post-mortem prefrontal cortex from persons with major depression and normal controls. International Journal of Neuropsychopharmacology. 2009;12:1223–1232. doi: 10.1017/S1461145709000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Muir WJ, Blackwood DH. Is borderline personality disorder part of the bipolar spectrum? Harvard Review of Psychiatry. 2004;12:133–139. doi: 10.1080/10673220490472346. [DOI] [PubMed] [Google Scholar]
- Steinberg BJ, Trestman R, Mitropoulou V, Serby M, Silverman J, Coccaro E, Weston S, de Vegvar M, Siever LJ. Depressive response to physostigmine challenge in borderline personality disorder patients. Neuropsychopharmacology. 1997;17:264–273. doi: 10.1016/S0893-133X(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biological Psychiatry. 1996;40:568–575. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- Wang HY, Markowitz P, Levinson D, Undie AS, Friedman E. Increased membrane-associated protein kinase C activity and translocation in blood platelets from bipolar affective disorder patients. Journal of Psychiatric Research. 1999;33:171–179. doi: 10.1016/s0022-3956(98)90057-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nature Neuroscience. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. Journal of Biological Chemistry. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23:569–582. doi: 10.2165/00023210-200923070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]