Abstract
A Disintegrin And Metalloproteinase (ADAM)-10 plays critical roles in neuronal migration and distribution. Recently, ADAM10 deletion was shown to disrupt myelopoiesis. We found that inducible deletion of ADAM10 using Mx1-driven Cre recombinase for a period of three weeks resulted in mast cell hyperplasia in the skin, intestine and spleen. Mast cells express surface ADAM10 in vitro and in vivo, at high levels compared to other immune cells tested. ADAM10 is important for mast cell migration, since ADAM10-deficiency reduced c-Kit-mediated migration. As with some mast cell proteases, ADAM10 expression could be altered by the cytokine microenvironment, being inhibited by IL-10 or TGFβ1, but not by several other T cell-derived cytokines. Collectively these data show that the ADAM10 protease is an important factor in mast cell migration and tissue distribution, and can be manipulated by environmental cues.
Keywords: mast cell, ADAM-10, cytokine, stem cell factor, migration
Introduction
Mast cells are sentinels of the innate immune system that rapidly respond to pathogens and mediate allergic inflammation. They do this primarily through antigen (Ag)-induced cross-linking of IgE bound to the high-affinity IgE receptor, FcεRI [1; 2; 3]. The resulting signals induce mast cells to release myriad early and late phase mediators. These include proteases, histamine, and arachidonic acid metabolites released in the first few minutes after activation. Cytokines are predominantly produced hours later, though TNF and IL-4 can be stored in cytoplasmic granules [1; 2; 3]. These factors perform a variety of immunomodulatory effects, but mainly are inflammatory in nature [1; 2; 3].
Mast cells migrate and proliferate in response to many signals, with stem cell factor (SCF) being among the most potent [4]. By interacting with the transmembrane receptor c-Kit, SCF attracts mast cells into tissues, where it promotes proliferation, differentiation, and survival [5; 6]. Mast cell hyperplasia occurs in allergic disease. Moreover, the majority of transformed or malignant mast cells express a single form of mutated c-Kit [7]. Further, many tumors secrete SCF, attracting mast cells to the tumor site, where they appear to promote metastasis [8]. Thus, understanding how SCF controls mast cell survival and distribution is critical to many diseases.
Mast cells express many proteases, of which the chymase and tryptase members have been intensively studied [9]. These enzymes have both pro- and anti-inflammatory functions, such as neutrophil recruitment in arthritic joints [10] and TNF degradation [11]. Hence, understanding the role of these individual enzymes can provide insight into mast cell function. ADAM10 is a member of the ADAM proteinase family, whose members cleave many transmembrane proteins through ectodomain shedding or regulated intramembrane proteolysis (RIP) [12; 13; 14]. In an example of the former, TNF is released from the cell membrane by ADAM17 [12]. In a common example of RIP, Delta-like 1 (DL1) binding to Notch2 results in Notch2 cleavage by ADAM10, followed by γ-secretase activity that allows Notch2 Intracellular Domain (N2ICD) to translocate to the nucleus and elicit transcription [15]).
ADAM10 has been implicated in the proper distribution, development, and function of neurons, and is important for signaling pathways including N-Cadherins, Ephrin, Notch, and γ-Protocadherins [13]. However, ADAM10 cleaves many substrates involved in immunity, including Notch 1, Notch 2, DL1, CD23, TNF, and IL-6R [14; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26]. ADAM10-dependent Notch signaling has been demonstrated in recent years to be crucially important for the proper differentiation and distribution of T cells and B cells [17; 19; 24].
The critical nature of ADAM10 was demonstrated when gene-deleted mice were found to die at day 9.5 of embryogenesis [21]. This lethal phenotype led investigators to develop mice exhibiting cell-specific ADAM10 deletion using the Cre recombinase/loxP system [19; 24]. Using an inducible Mx1-Cre transgenic mouse, Yoda et al., recently showed that ADAM10 deletion resulted in myeloproliferative disease (MPD) with overt splenomegaly and increased granulocytes [27]. Using this model, we noted an overt increase in tissue mast cell numbers within 3 weeks of gene deletion. We demonstrate that mast cells abundantly express ADAM10 on their cell surface. The increase in tissue mast cells may be due to direct effects of ADAM10, since its deletion suppressed Kit-mediated migration, while enhancing survival and proliferation. These data demonstrate that this ADAM family member plays important roles in mast cell migrations and distribution, perhaps contributing to mast cell-associated inflammatory diseases.
Methods
Animals
C57BL/6, Mx1CreTg C57BL/6, and ADAM10fl/fl C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at a minimum of 6 wk old, with approval from the Virginia Commonwealth University Institutional Animal Care and Use Committee. STAT3 −/− mice and controls were kindly provided by John O’Shea (NIAMS/NIH, Bethesda, MD). Mx1CreTg mice were bred with ADAM10fl/fl mice, and through successive breeding resulted in mice that were Mx1CreTg ADAM10fl/fl (hereafter referred to as ADAM10 KO mice), or Mx1CreTg ADAM10+/+ (hereafter referred to as WT mice) as a negative control. All mice used were on a C57BL/6 background.
Genotyping was accomplished by genomic PCR, performed according to Jackson Laboratory protocols for Mx1Cre expression and ADAM10 expression utilizing primers of as follows: Mx1Cre 5’ GCGGAGCCAGCACTATTTA 3’ and 5’ CCGGCATCAACGTTTTCTTTT 3’, and ADAM10 5’ GAGAGGAAAGAAAGTGGCAGA 3’ and 5’ AGTGGGTGGGTTAATGAGCA 3’.
To induce ADAM10 deletion, Mx1CreTg mice were injected with 250µg of poly-inosine: poly-cytosine (poly-IC) intraperitoneally (i.p) every other day for 5 days, for a total of 3 injections. Mice were allowed to rest for a minimum of 9 days after the final injection of poly-IC prior to harvesting bone marrow to derive mast cell cultures; in all experiments involving histology or peritoneal lavage the mice were allowed to rest a minimum of 16 days. ADAM10 protein loss was confirmed via flow cytometry.
Mouse Mast Cell Cultures
Mouse bone marrow-derived mast cells (BMMCs) were derived by culture in complete RPMI (cRPMI) 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate, and 1 mM HEPES (all from Biofluids, Rockville, MD), supplemented with IL-3–containing supernatant from WEHI-3 cells and SCF-containing supernatant from BHK-MKL cells. The final concentration of IL-3 and SCF were adjusted to 1 ng/ml and 10 ng/ml, respectively, as measured by ELISA. BMMC were used between 3–9 weeks of culture, when mast cell purity was greater than 95% based on c-Kit or FcεRI staining.
Cytokines and Reagents
All cytokines, including IL-3, IL-4, IL-5, IL-6, IL-10, IL-12, IL-17 TGF-β, IFN-β, and SCF were purchased from PeproTech (Rocky Hill, NJ). Normal goat IgG and rat-anti mouse ADAM10 primary and secondary antibodies were purchased from R&D (Minneapolis, MN). PE-Goat anti-rat IgG was purchased from Southern Biotech (Birmingham, AL). Collagen IV was purchased from BD Biosciences (Bedford, MA). Antibodies recognizing mouse B220, FcεRI, F4/80, Mac1, Gr1, c-Kit, CD3, CD4, and CD8 were purchased from BD Pharmingen (San Diego, CA). Propidium iodide was purchased from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin (BSA) was purchased from Gemini Bio Products (West Sacramento, CA).
siRNA Depletion of ADAM10
BMMC (3×106 cells/group) were transfected with pooled scrambled control siRNA or pooled ADAM10 siRNA (Thermo Fisher Scientific, Waltham, MA) using the AMAXA Cell Line Nucleofector Kit V buffer and the human monocyte setting on an AMAXA Nucleofector II device (Lonza, Basel, Switzerland). Efficacy of gene suppression was measured via flow cytometry; only BMMC populations with 50% or greater ADAM10 negative cells were utilized in experiments.
Flow Cytometric Analysis
Surface expression of proteins was measured by flow cytometry on a BD FACScalibur. For ADAM10 staining, 1–2×105 cells were pelleted, washed in FACS buffer (PBS, 3% FBS, 0.1% sodium azide), then blocked with 10 µL Goat IgG at 50 µg/ml in FACS buffer and incubated at 4°C for 10 minutes. 10 µL of 20 µg/ml rat IgG or rat anti-mouse ADAM10 was added to the cells and incubated at 4°C for 30 minutes. Cells were washed in FACS buffer, resuspended in 10 µL of 2 µg/ml PE-goat anti-rat IgG diluted in FACS buffer and then incubated at 4°C for 30 minutes. Cells were washed and resuspended in FACS buffer for analysis. For directly-labeled antibody staining, cell pellets were incubated in 10 µL 2.4G2 rat anti-mouse FcγRII/III culture supernatant with PE anti-mouse ADAM10 and/or 10 µg/ml FITC-labeled B220, FcεRI, F4/80, Mac1, Gr1, c-Kit, CD3, CD4, or CD8 at 4°C for 30 minutes.
Measurement of ADAM10 Levels in Peritoneal Cells
C57BL/6 mice were injected i.p. with 3 ml PBS, agitated, and then PBS was recovered. Cells were assessed by flow cytometric analysis as described above.
Effects of Cytokines on ADAM10 Expression In Vitro
Unless otherwise noted, BMMC were cultured in cRPMI supplemented with 10 ng/ml IL-3 +/− 50 ng/ml of IL-10, TGF-β1, IL-17, IL-4, IFN-γ, IL-6, IL-5, or IL-12 for 3 days. ADAM10 expression was calculated as fold of control measured by flow cytometry.
mRNA Measurement
Cells were harvested and total RNA was extracted with TRIzol reagent (Life Technologies, Grand Island, NY). cDNA was synthesized using the Verso cDNA Kit (Thermo Scientific, Waltham, MA) following the manufacturer’s protocol using oligo dT primers provided in the kit. cDNA was quantified using the Thermo Scientific NanoDrop™ 1000 UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA) according to manufacturer’s recommended protocol. qPCR analysis was performed with the ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR® Green detection using a relative standard curve method. Each reaction was performed according to the manufacturer’s protocol using 100ng of sample cDNA, 12.5µl of 2X Absolute QPCR SYBR® Green Fluoroscein Mix (Themo Scientific, Waltham, MA) and ADAM10 or GAPDH (housekeeping gene) primers in a final reaction volume of 50µl. Amplification conditions for all reactions consisted of a heat-activation step at 95°C for 15 minute followed by 40 cycles of 95°C for 15s, 53°C for 30s and 72°C for 34s. Fluorescence data was collected during the extension step of the reaction. The instrument was set to run in 9600 emulation mode, with auto ramping. Resulting data were analyzed with ABI’s SDS v1.2 software package using a manual Ct of 0.20 and the auto baseline setting.
For each gene amplified, a standard curve from serial dilutions of a known concentration of cDNA was achieved. The standard curve consisted of eight 2-fold dilutions of a 400ng/reaction cDNA sample taken from unstimulated B6×129 wild-type cells. The standard curve dynamic range used in this study was selected such that a reliable standard curve encompassing most unknown Ct values was generated. For each gene being analyzed, the input (concentration) of each unknown sample was calculated by comparing its Ct value with the standard curve using the formula 10^[(Ct – b)/m] where b is the y-intercept of the standard curve and m is the slope of the standard curve. ADAM10 relative expression values were normalized by dividing by the amount of GAPDH detected in the same sample.
Migration Assays
8 µm polycarbonate 24 well transwell inserts from Corning were coated with 1 µg/cm2 mouse collagen IV resuspended in 0.05 M HCl. Plates were incubated for 1 hour at room temperature to allow the collagen to solidify. BMMC were resuspended at 2×106 cells/ml and starved in FBS-free cRPMI for 2 hours. A chemotactic gradient was created by adding 700 µL of FBS-free cRPMI with IL-3 at 0.5 ng/ml +/− 100–200 ng/ml SCF in the bottom well and 200 µL of the previously starved BMMC supplemented with 0.5 ng/ml IL-3 in the upper well. Cells were incubated for 16 hours at 37° C and live cells that had migrated to the bottom chamber were enumerated via timed counting by flow cytometry, using propidium-iodide (PI) exclusion staining. Fold of negative control (media lacking SCF) for each population was calculated for all groups. An alternative protocol was performed in the same manner, modified to exclude the use of collagen IV, and including FBS-free cRPMI supplemented with 10mg/ml bovine serum albumin (BSA) as the coating and migration media.
To assess IgE-induced migration, ADAM10 siRNA-depleted or control scrambled siRNA-treated BMMC pre-incubated +/− IgE were resuspended at 2×106 cells/ml in FBS-free cRPMI/BSA for 2 hours. A chemotactic gradient was created by adding 700 µL FBS-free cRPMI/BSA with IL-3 at 10 ng/ml in the bottom well +/− 50 ng/ml dinitophenyl-coupled human serum albumin (DNP-HSA), and 200 µL of the previously starved BMMC supplemented with 10 ng/ml IL-3 in the upper well. Live cell numbers were determined by timed flow cytometry counting as above.
Histology
Tissue and organ samples were prepared via surgical excision after euthanasia. Following peritoneal lavage performed as above and collection of femurs for bone marrow cultures, tissue and organs were removed and fixed in either Carnoy’s solution or formalin. Samples were stained with hematoxylin/eosin or pinacyanol erythrosinate (Histo-Scientific Research Laborities in Mount Jackson, Virginia). Mast cells were enumerated based on color and morphology after pinacyanol erythrosinate staining, using a minimum of 5 random 40× fields (20× fields for spleen samples). Sections were imaged on a Nikon E-600 compound microscope using a 20× dry or 100× oil immersion objective. Images were processed using Adobe Photoshop®.
Proliferation Assay
BMMC were cultured at 1×105 cells/ml in cRPMI with 1 ng/ml IL-3 and either 10, 50, 100, or 200 ng/ml SCF for 4 days. A positive control of 10 ng/ml IL-3 + SCF was done as well. After 4 days, cells were mixed and then counted via a Biorad automatic cell counter in triplicate.
Survival Assay
BMMC were cultured at 5×105 cells/ml in CRPMI with 2, 10, 50, or 250 ng/ml SCF for a period of 3 days. A positive control for death was also performed, with those cells receiving no growth factors. After 3 days, cells were triturated, aliquoted into 200 µL FACS tubes, and analyzed via PI exclusion staining. Measurements were taken in triplicate on a FASCalibur.
Statistical Analysis
Data were analyzed using GraphPad Prism 6 software to determine p values by Student’s t Test.
Results
ADAM10 KO mice have altered mast cell distribution
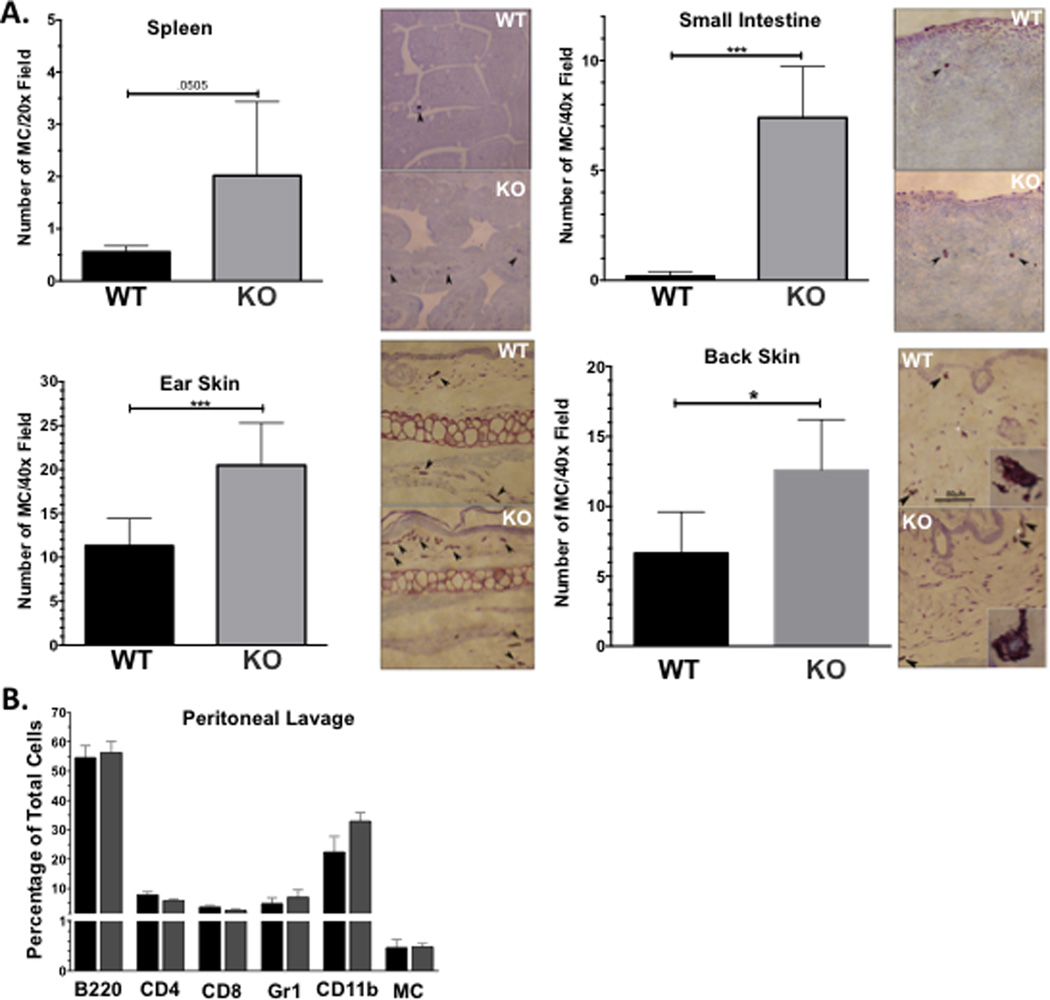
We elicited ADAM10 deletion in Mx1-cre/ADAM10-floxed mice by poly-IC injection. Control ADAM10-floxed mice lacking Mx1-cre also received poly-IC. Three weeks post-injection, we noted severe splenomegaly that was consistent with the work of Yoda and co-workers [27] (not shown). We evaluated tissue samples from the spleen, proximal small intestine, ear skin, and back skin. Mast cells were enumerated after histochemical staining with pinacyanol erythrosinate (Figure 1A). Splenic tissue from ADAM10 KO mice trended toward increased mast cells numbers (p=.0505), while intestine and skin had overtly higher mast cell counts. We measured the relative abundance of immune cell lineages in the peritoneal cavity of ADAM10 KO mice. ADAM10 was undetectable on the surface of all peritoneal lineages tested from ADAM10 KO mice (not shown). Loss of ADAM10 did not affect the total number of peritoneal cells, nor the fractions expressing B220, CD4, CD8, Gr1, CD11b, or c-Kit/FcεRI as measured by flow cytometry (Figure 1B). These data demonstrate that systemic ADAM10 deficiency has myriad consequences, including mastocytosis in the intestine and skin.
Figure 1. ADAM10 KO mice show aberrant distribution of mast cells in several tissues.
Data shown are from mice harvested 3 weeks after Poly I:C injection. (A) Mast cell numbers were determined by histological analysis using pinacyanol erythrosinate as described. Example photomicrographs are shown. Back skin data includes analysis of unaffected and inflamed skin lesion samples. White asterisk indicates the individual mast cell shown in the inset. Final magnification was 200× for all images, except insets, which were 1000×. *p <.05; **p<.01; ***p <.001. (B) Flow cytometry analysis of peritoneal lavage cells.
ADAM10 Is Expressed by Mast Cells
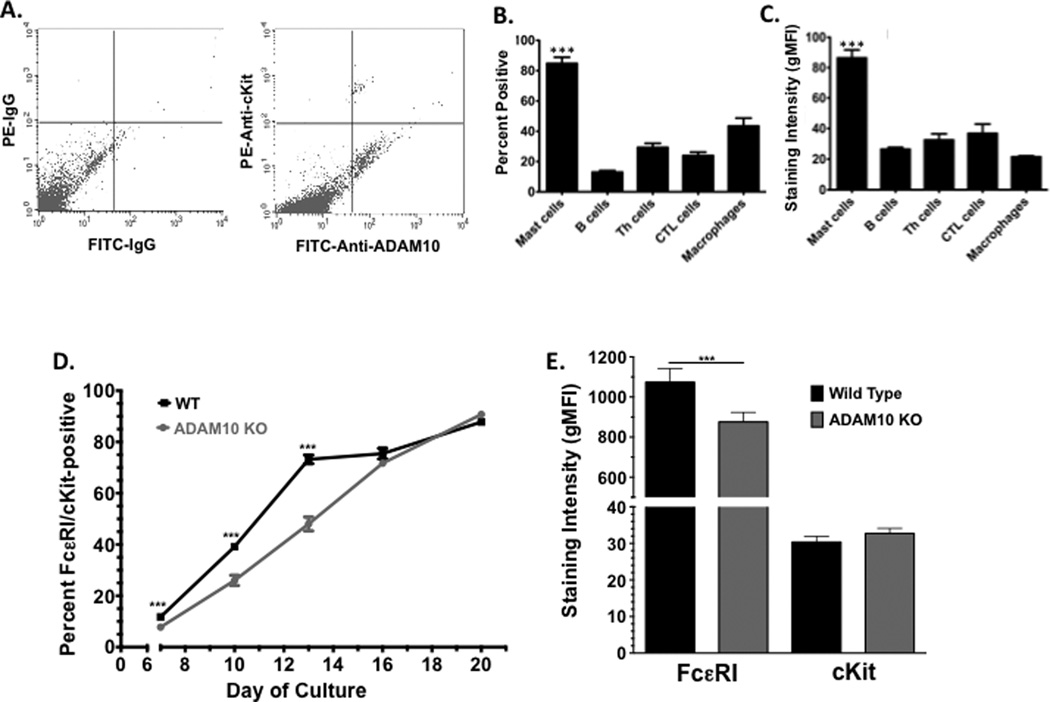
The increased mast cell numbers in ADAM10-deficient mice could be secondary to inflammation caused by the gene deletion. To ascertain direct effects of ADAM10, we first measured its expression on mast cells. ADAM10 protein expression has been noted on the HMC-1 human mast cell line, and ADAM10 mRNA was detected in mast cells cultured from human fibrotic lung tissue [28; 29]. To assess expression among mouse mast cells in vivo, peritoneal lavage cells were employed (Figure 2A). We measured surface ADAM10 on several immune cell types via lineage markers with flow cytometry, which corroborated that many lineages express surface ADAM10, including mast cells (Figure 2B) (31,32). A clear majority (~85%) of peritoneal mast cells were surface ADAM10-positive. This was significantly greater than all other populations examined, which had minor ADAM10-positive subpopulations, ranging from 10–45%. These included B cells (B220+), Th cells (CD4+), CTL (CD8+), and macrophages (CD11bhi) (Figure 2B). In addition, peritoneal mast cells expressed ADAM10 at levels that were 2–3 times higher than all other cell types examined, suggesting that ADAM10 is expressed at relatively high levels in mast cells (Figure 2C).
Figure 2. ADAM10 is expressed on mast cells in vivo and in vitro.
(A–C) Peritoneal lavage was harvested from C57BL/6 mice, and cells were stained as described in Materials and Methods to identify mast cells (c-Kit/FcεRI-positive), B cells (B220-positive), Th cells (CD4-positive), cytotoxic T cells (CTL; CD8-positive), and macrophages (CD11b-positive. Gr1-negative). Example plot of ADAM10 surface expression among c-Kit+ mast cells is shown in (A). Percentage of each cell population expressing ADAM10 and the geometric mean fluorescence intensity (gMFI) are shown in (B and C). Data shown are from 6–16 samples representative of three independent experiments. *** p<.001 when comparing mast cells to all other groups. (D and E) Mast cells were cultured from bone marrow as described in Materials and Methods, and the percentage of cells dually positive for c-Kit and FcεRI was measured by flow cytometry. Staining intensity was measured on day 21. Data shown are from 10–15 samples per point.
ADAM10-deficient (KO) bone marrow-derived mast cells (BMMC) were cultured from Mx1-Cre-expressing mice, as described in Materials and Methods. By monitoring the fraction of FcεRI/c-Kit-positive mast cells throughout 21 days of in vitro development, we noted a modest delay in mast cell maturation among the ADAM10 KO cultures (Figure 2D). This lag was transient, as wild type and ADAM10 KO cultures had similarly high percentages of mast cells by day 21. We also noted that ADAM10 KO BMMC tended to have a slight but statistically significant reduction in FcεRI staining intensity, while c-Kit expression was not appreciably different (Figure 2E). Cell morphology was not noticeably different after 3 weeks of culture. These data suggested that ADAM10 is expressed by mast cells and participates in their early differentiation, but functional mast cells can be cultured in the absence of this protease.
ADAM10 Depletion alters c-Kit-mediated migration, proliferation, and survival
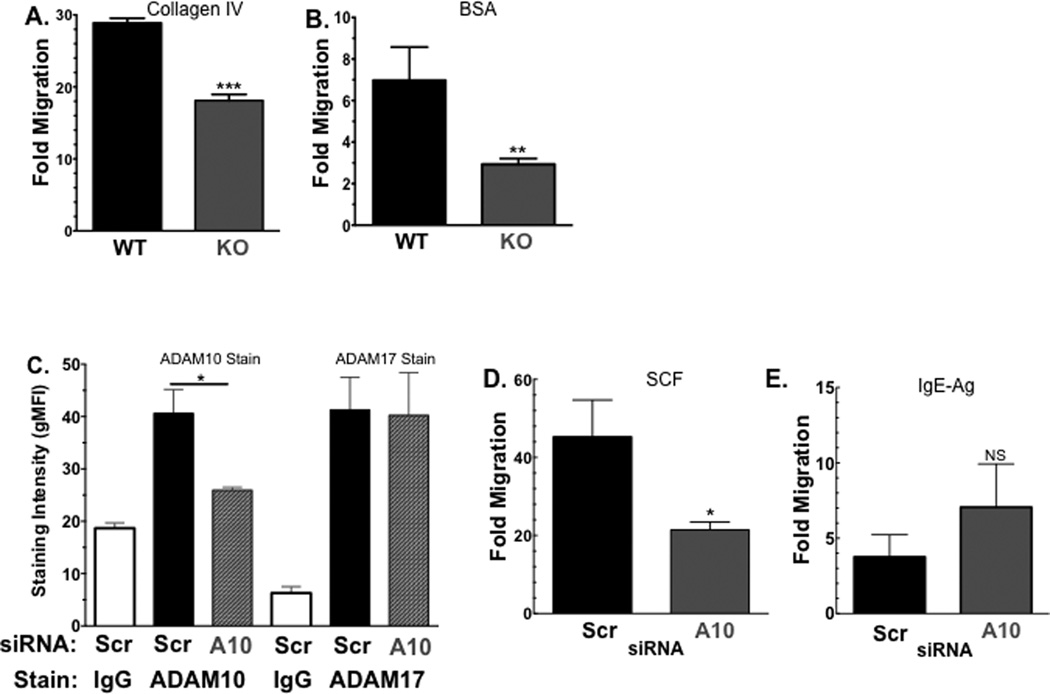
If ADAM10 participates in mast cell function, it may have a role in c-Kit-mediated effects, which include proliferation, survival, and migration. For example, the related protease ADAM17 is known to regulate cleavage of both c-Kit and its ligand, SCF [28; 30]. Since ADAM10 cleaves many substrates involved in adhesion and migration, we hypothesized that ADAM10 deficiency could reduce BMMC migration through the known ADAM10 substrate, collagen IV [14], an integral part of the basal lamina. Using collagen IV-coated transwells, we showed that ADAM10 KO BMMC had significantly less SCF-induced migration than their WT counterparts (Figure 3A). This defect was not restricted to collagen IV. When transwell membranes were coated in media containing bovine serum albumin (BSA) in place of collagen IV, ADAM10 KO BMMC also demonstrated reduced migration towards SCF (Figure 3B).
Figure 3. ADAM10 suppresses SCF-induced migration.
(A and B) WT and ADAM10 KO BMMC were assessed for migration through collagen IV-coated transwells (A) or BSA-coated transwells (B) in response to SCF, as described. (C–E) WT BMMC transfected with ADAM10-targeting or scrambled control siRNA were assessed for surface ADAM10 and ADAM17 expression by flow cytometry in (C). These cells were then assessed for migration through collagen IV-coated transwells in response to SCF (D) or IgE+antigen (E) as described. Data shown are means and SEM of at least 8 samples. *p <.05; **p<.01; ***p <.001.
To rule out potential effects of ADAM10 deletion on mast cell diffrentiation or on ADAM17 expression, we conducted migration assays using BMMC transfected with ADAM10-targeting siRNA. As shown in Figure 3C, siRNA directed against ADAM10 significantly reduced ADAM10 expression compared to a non-targeting (“scrambled”) siRNA, without altering ADAM17 expression. ADAM10 depletion with siRNA correlated with reduced SCF-mediated migration through collagen IV-coated transwells. (Figure 3D). Finally, we noted that antigen-induced migration among cells pre-coated with IgE was not affected by ADAM10 depletion, demonstrating that ADAM10-deficient mast cells are capable of migration, and that the role of ADAM10 is restricted to some mast cell stimuli. These data support the hypothesis that ADAM10 is required for SCF-induced mast cell migration.
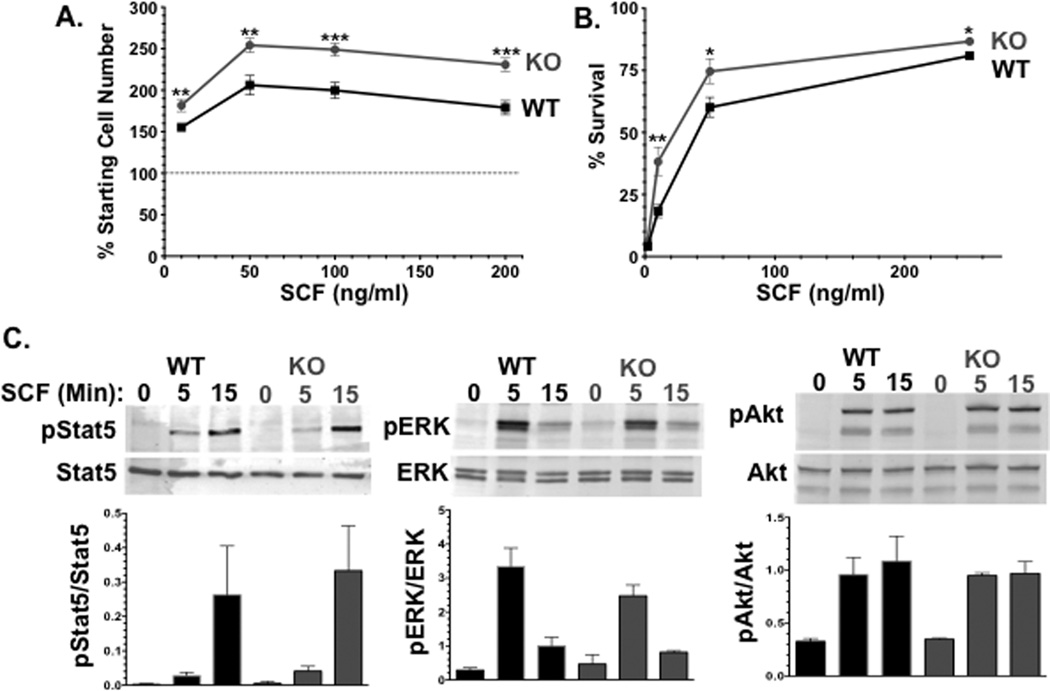
We also tested ADAM10-deficient BMMC for SCF-induced proliferation and survival, to rule out deficient migration as a result of poor survival. As shown in Figures 4A and B, loss of ADAM10 yielded modest but significantly greater proliferation and survival responses to SCF. This enhancement did not coincide with greater expression or a reduced internalization rate of c-Kit among ADAM10 KO BMMC (Figure 2E and data not shown). The mechanism by which ADAM10 deficiency alters c-Kit signaling was assessed by western blotting for known signaling proteins activated by this receptor. We have recently found Stat5 to be required for SCF-induced migration [31], while ERK and Akt are well-known proliferation and survival factors. However, we noted no difference in SCF-induced phosphorylation of Stat5, ERK, or Akt when comparing wild type and ADAM10-deficient BMMC. Each of these proteins was also expressed at levels comparable to control cultures. The means by which ADAM10 contributes to c-Kit function remains unclear.
Figure 4. ADAM10 deficiency enhances SCF-induced proliferation and survival.
(A) WT and ADAM10 KO BMMC were treated with 1ng/ml IL-3 and varying amounts of SCF as indicated for 4 days. Live cell numbers were measured by cell counting. (B) Survival of WT and ADAM10 KO BMMC was measured using various amounts of SCF as indicated over the course of 3 days. Data shown in A and B means and SEM of 15–18 samples from 2 independent experiments. *p <.05; **p<.01; ***p <.001.
Cytokine-mediated Control of ADAM10 Expression
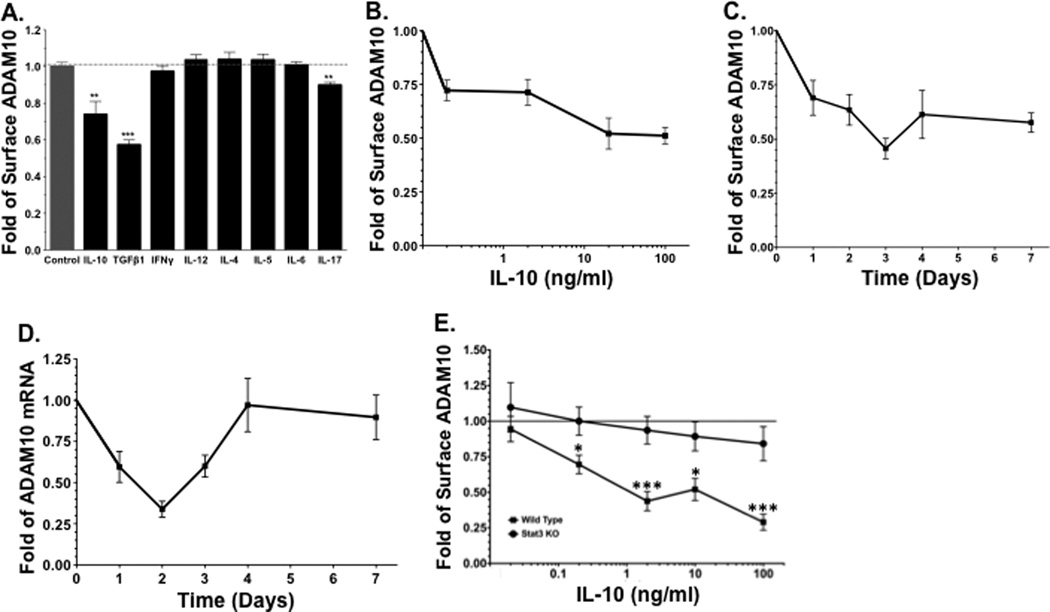
Mast cell protease expression and migration can be altered by cytokines in the microenvironment. For example, TGFβ1 and IL-10 increase mMCP-1 expression [32; 33]. TGFβ1 is a mast cell chemoattractant [34; 35; 36], but it also blocks IL-4-induced migration [37]. Likewise, IL-10 suppresses mast cell migration [38]. We sought the effects of these and other cytokines representing the functional responses of Treg, Th1, Th2, and Th17 cells on ADAM10 expression. BMMC were cultured for 3 days in media containing IL-3 and one of several cytokines, including IL-10, TGF-β1, IL-12, IFN-γ, IL-4, IL-5, IL-6, or IL-17, after which surface ADAM10 expression was measured by flow cytometry. IL-10 and TGFβ1 both decreased ADAM10 surface levels (Figure 5A), while other cytokines had little or no effect.
Figure 5. TGFβ1 and IL-10 alter ADAM10 expression on mast cells.
ADAM10 levels were measured by flow cytometry except for D, which was measured by qPCR. (A) BMMC were cultured for 3 days in the presence of IL-3 +/− the indicated cytokines at 50 ng/ml; (B) BMMC were cultured for 3 days in 10 ng/ml IL-3 +/− IL-10 at the indicated concentrations prior to surface staining for ADAM10 expression. (C) BMMC were cultured for the indicated times in IL-3 +/− IL-10 at 10 ng/ml prior to surface staining for ADAM10 expression. (D) BMMC were cultured for the indicated times in IL-3 +/− IL-10 at 10 ng/ml prior to qPCR analysis for ADAM10 expression. (E) Wild type (WT) or STAT3-deficient (KO) BMMC were cultured for 3 days in 10 ng/ml IL-3 +/− IL-10 at the indicated concentrations prior to surface staining for ADAM10 expression. Data shown are representative or means of 2 or more experiments with 6 or more samples each. *p <.05; ***p < .001.
To further characterize the nature of ADAM10 regulation by IL-10, we determined the concentration and time dependence of IL-10 effects on mast cell ADAM10 expression. Increasing the IL-10 concentration reduced ADAM10 surface expression by approximately 50% at 100ng/ml IL-10, with half-maximal inhibition at approximately 0.2ng/ml (Figure 5B). Surface ADAM10 decreased over the course of 4 days in response to IL-10, and remained suppressed for 7 days (Figure 5C). In order to determine if changes in ADAM10 surface levels were matched by altered mRNA expression, we measured ADAM10 expression by qPCR over the 7-day time course. Reduced ADAM10 surface expression was initially matched by decreased ADAM10 mRNA, but mRNA levels rebounded after day 2 (Figure 5D). Since surface protein was reduced for at least 3 days longer than mRNA, we interpreted this to mean that post-transcriptional regulation of ADAM10 must occur. Many IL-10 effects, especially those requiring mRNA alterations, involve the transcription factor STAT3. Hence, we assessed the effects of IL-10 on wild type and STAT3 KO BMMC. As shown in Figure 5E, IL-10 downregulation of ADAM10 surface expression was completely STAT3-dependent, even when high concentrations of IL-10 were present. These data demonstrate that surface ADAM10 expression can be regulated by the microenvironment, including cytokines known to regulate mast cell proteases and migration.
Discussion
While ADAM10 is well known for its role in development, cancer, and Alzheimer’s disease, a number of papers reporting its immunologic functions have arisen in recent years [12; 13; 14; 39]. ADAM10, effective in site 2 cleavage in Notch1 and 2 signaling, and is important for T cell and marginal zone B cell development [15; 19; 24]. Several papers have shown ADAM10 to regulate B cell Ig production, including its role as the primary sheddase of CD23, a negative regulator of IgE production [17; 19; 23; 26]. Important to our work, Mathews et al. showed that B cell-restricted ADAM10 deficiency decreased antigen-specific IgE, eosinophila and IL-5 production in an IgE/mast cell-dependent airway hyperresponsiveness (AHR) model [22]. These studies suggested a pro-inflammatory role for ADAM10, and prompted us to investigate its role in mast cell function.
Perhaps the most striking finding in this study was that ADAM10 deletion in vivo for three weeks yielded tissue mastocytosis. Whether this was due to an intrinsic defect in ADAM10 KO mast cells or to the general inflammatory conditions induced by ADAM10 deficiency needs to be further investigated. Our finding that ADAM10 KO mast cells are less migratory than wild type cells may seem in conflict with increased cell numbers among the ADAM10 KO mice. However, a failure of mast cell emigration coupled with increased proliferative responses (Figure 4) to inflammatory signals in the skin and spleen could partly explain increased mast cell numbers in these tissues. We were surprised by the lack of change in peritoneal mast cells, given that other tissues showed hyperplasia. However, there is precedence for varied responses to mast cell proliferative stimuli in vivo. For example, Lantz et al., showed that 21 days of subcutaneous SCF injections dramatically increased mast cell numbers not only in the skin but also in the forestomach. By contrast, mast cells in the intestinal mucosa and submucosa increased perhaps two-fold, while those in the muscularis did not change [40]. These data provide evidence of ADAM10’s overall importance in mast cell distribution.
ADAM10 expression on mast cells explanted from human fibrotic lung tissue and on HMC-1, a human cell line, has been reported [28; 29]. Our work provides a specific exploration of the role of ADAM10 with regard to mast cell function. The relative abundance of surface ADAM10 on nearly all mast cells, compared to other immune cell types (Figure 2), implied that this protease functions in mast cell biology, as our work demonstrates. Studies of ADAM10 depletion via cre/lox technology or siRNA yielded mast cells with reduced SCF-mediated migration through transwells pre-coated with the known ADAM10 substrate collagen IV, or coated with BSA. These data did not appear to be due to off-target effects, since the related protease ADAM17 was not altered by siRNA treatment. We also found that ADAM10 KO BMMC migrate normally to IgE/antigen stimulation, demonstrating that loss of ADAM10 does not cause a global defect in migratory ability.
In addition to suppressing SCF-induced migration, ADAM10 deficiency led to a slight increase in proliferation and survival. The mechanism by which these effects are induced is not clear. ADAM17 is known to cleave c-Kit [28]. However, we did not observe significant changes in surface c-Kit expression (Figure 2) or on c-Kit internalization during SCF signaling (not shown) among ADAM10 KO BMMC, indicating that this is not the means by which ADAM10 controls mast cell function. We also found no difference in expression or SCF-induced activation of Stat5, ERK, or Akt. Of the possible mechanisms by which ADAM10 could regulate mast cell function and distribution, the ADAM10 substrates Notch 1, Notch 2, and CD44 are worthy candidates. Notch 1 has been shown to promote IgE-mediated cytokine production in mast cells and to confer antigen-presenting capabilities to BMMC [41; 42; 43]. Notch2 is also involved in mast cell cytokine production [42], differentiation [44], and the proper distribution of intestinal mast cells and eradication of Strongyloides venezuelensis [44]. CD44, which binds hyaluronic acid and undergoes ectodomain shedding by ADAM10, is important for mast cell distribution [45; 46]. These data may be particularly relevant, as human mast cells can associate with bronchial smooth muscle cells in vitro via CD44 and collagen I interactions [47]. How ADAM1-participates in cKit signaling is a focus of our current work.
We and others have shown that TGFβ1- and IL-10 serve as autocrine and paracrine regulators of the mast cell response [37; 48; 49; 50; 51; 52; 53; 54; 55; 56; 57; 58; 59; 60; 61; 62; 63], including protease expression [32; 33; 64; 65] and migration [34; 35; 36]. The suppressive effects of TGFβ1 and IL-10 we have previously reported require approximately 3 days to manifest [31; 50; 58; 66], fitting the kinetics of ADAM10 downregulation. We were surprised by the return of normal ADAM10 mRNA expression after 4 days of IL-10 culture, since surface protein remained suppressed for at least one week (Figure 5). These results indicate some kind of post-transcriptional regulation. As one means of investigating how IL-10 controls mast cell function, we have begun seeking IL-10-induced microRNAs (miRs), which can diminish protein expression without decreasing corresponding mRNA levels. To date we have identified several IL-10-induced miRs, but have not yet defined their mRNA targets.
It was also interesting to note the importance of Stat3 in IL-10-mediated suppression of ADAM10. While the mechanism for this effect is not known, we located 2 potential Stat3 binding sites in the ADAM10 promoter (GenBank CT025701), located at 72 and 431 nucleotides prior to the transcriptional start site (starting at residues 102927 and 103286, respectively). We postulate that ADAM10 blockade is one of several anti-inflammatory effects of TGFβ1 and IL-10, designed to limit mast cell-mediated pathology. Suppressing ADAM10 could alter mast cell migration, and have implications for parasite immunity, contact hypersensitivity, and asthma.
Our results show that ADAM10 has an important role in mast cell migration. The mechanisms by which this is accomplished and the potential for targeting ADAM10 in mast cell-associated diseases warrant further study.
Highlights.
ADAM10 deficiency results in skin and intestinal mast cell hyperplasia.
Mast cells express ADAM10 at high levels compared to other immune cells.
ADAM10 deficient mast cells are defective in SCF-, but not IgE-mediated migration.
ADAM10 expression can be suppressed by TGFβ1 and by IL-10. IL-10 employs Stat3 for this suppressive effect.
Acknowledgements
Supported by grants from the National Institutes of Health (1R01AI59638 to JJR; U19AI077435 to JJR and DHC).
Abbreviations
- ADAM10
A Disintegrin and Metalloproteinase 10
- BMMC
bone marrow-derived mast cell
- FcεRI
Fc epsilon receptor I
- mMCP
mouse mast cell protease
- MIP
macrophage inflammatory protein
- SCF
stem cell factor/c-Kit ligand
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-7. [DOI] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halova I, Draberova L, Draber P. Mast cell chemotaxis - chemoattractants and signaling pathways. Front Immunol. 2012;3:119. doi: 10.3389/fimmu.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107:51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26:387–405. v. doi: 10.1016/j.iac.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, Feng ZH. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piliponsky AM, Chen CC, Rios EJ, Treuting PM, Lahiri A, Abrink M, Pejler G, Tsai M, Galli SJ. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol. 2012;181:875–886. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruessmeyer J, Ludwig A. The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Semin Cell Dev Biol. 2009;20:164–174. doi: 10.1016/j.semcdb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Reiss K, Saftig P. The"a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Gibb DR, Saleem SJ, Chaimowitz NS, Mathews J, Conrad DH. The emergence of ADAM10 as a regulator of lymphocyte development and autoimmunity. Mol Immunol. 2011;48:1319–1327. doi: 10.1016/j.molimm.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armanious H, Gelebart P, Anand M, Belch A, Lai R. Constitutive activation of metalloproteinase ADAM10 in mantle cell lymphoma promotes cell growth and activates the TNFalpha/NFkappaB pathway. Blood. 2011;117:6237–6246. doi: 10.1182/blood-2010-10-313940. [DOI] [PubMed] [Google Scholar]
- 17.Chaimowitz NS, Martin RK, Cichy J, Gibb DR, Patil P, Kang DJ, Farnsworth J, Butcher EC, McCright B, Conrad DH. A disintegrin and metalloproteinase 10 regulates antibody production and maintenance of lymphoid architecture. J Immunol. 2011;187:5114–5122. doi: 10.4049/jimmunol.1102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad DH, Gibb DR, Sturgill J. Regulation of the IgE response. F1000 Biol Rep. 2011;2 doi: 10.3410/B2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibb DR, El Shikh M, Kang DJ, Rowe WJ, El Sayed R, Cichy J, Yagita H, Tew JG, Dempsey PJ, Crawford HC, Conrad DH. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J Exp Med. 2011;207:623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibb DR, Saleem SJ, Kang DJ, Subler MA, Conrad DH. ADAM10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of Notch. J Immunol. 2011;186:4244–4252. doi: 10.4049/jimmunol.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 22.Mathews JA, Ford J, Norton S, Kang D, Dellinger A, Gibb DR, Ford AQ, Massay H, Kepley CL, Scherle P, Keegan AD, Conrad DH. A potential new target for asthma therapy: a disintegrin and metalloprotease 10 (ADAM10) involvement in murine experimental asthma. Allergy. 2011;66:1193–1200. doi: 10.1111/j.1398-9995.2011.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews JA, Gibb DR, Chen BH, Scherle P, Conrad DH. CD23 Sheddase A disintegrin and metalloproteinase 10 (ADAM10) is also required for CD23 sorting into B cell-derived exosomes. J Biol Chem. 2010;285:37531–37541. doi: 10.1074/jbc.M110.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol. 2008;20:1181–1187. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- 25.van Tetering G, van Diest P, Verlaan I, van der Wall E, Kopan R, Vooijs M. Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J Biol Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weskamp G, Ford JW, Sturgill J, Martin S, Docherty AJ, Swendeman S, Broadway N, Hartmann D, Saftig P, Umland S, Sehara-Fujisawa A, Black RA, Ludwig A, Becherer JD, Conrad DH, Blobel CP. ADAM10 is a principal 'sheddase' of the low-affinity immunoglobulin E receptor CD23. Nat Immunol. 2006;7:1293–1298. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- 27.Yoda M, Kimura T, Tohmonda T, Uchikawa S, Koba T, Takito J, Morioka H, Matsumoto M, Link DC, Chiba K, Okada Y, Toyama Y, Horiuchi K. Dual functions of cell-autonomous and noncell- autonomous ADAM10 activity in granulopoiesis. Blood. 2011;118:6939–6942. doi: 10.1182/blood-2011-06-357210. [DOI] [PubMed] [Google Scholar]
- 28.Cruz AC, Frank BT, Edwards ST, Dazin PF, Peschon JJ, Fang KC. Tumor necrosis factor-alpha-converting enzyme controls surface expression of c-Kit and survival of embryonic stem cell-derived mast cells. J Biol Chem. 2004;279:5612–5620. doi: 10.1074/jbc.M312323200. [DOI] [PubMed] [Google Scholar]
- 29.Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, Wolters PJ, Hoopes C, Dolganov GM, Fang KC. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol. 2005;206:279–290. doi: 10.1002/path.1780. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi N, Horiuchi K, Becherer JD, Toyama Y, Besmer P, Blobel CP. Different ADAMs have distinct influences on Kit ligand processing: phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. J Cell Sci. 2007;120:943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- 31.Fernando J, Faber TW, Pullen NA, Falanga YT, Kolawole EM, Oskeritzian CA, Barnstein BO, Bandara G, Li G, Schwartz LB, Spiegel S, Straus DB, Conrad DH, Bunting KD, Ryan JJ. Genotype-Dependent Effects of TGF-beta1 on Mast Cell Function: Targeting the Stat5 Pathway. J Immunol. 2013;191:4505–4513. doi: 10.4049/jimmunol.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992;149:2123–2129. [PubMed] [Google Scholar]
- 33.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–3486. [PubMed] [Google Scholar]
- 34.Gruber BL, Marchese MJ, Kew RR. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994;152:5860–5867. [PubMed] [Google Scholar]
- 35.Olsson N, Piek E, Sundstrom M, ten Dijke P, Nilsson G. Transforming growth factor-beta-mediated mast cell migration depends on mitogen-activated protein kinase activity. Cell Signal. 2001;13:483–490. doi: 10.1016/s0898-6568(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 36.Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-beta family. J Leukoc Biol. 2000;67:350–356. doi: 10.1002/jlb.67.3.350. [DOI] [PubMed] [Google Scholar]
- 37.Macey MR, Sturgill JL, Morales JK, Falanga YT, Morales J, Norton SK, Yerram N, Shim H, Fernando J, Gifillan AM, Gomez G, Schwartz L, Oskeritzian C, Spiegel S, Conrad D, Ryan JJ. IL-4 and TGF-beta 1 counterbalance one another while regulating mast cell homeostasis. J Immunol. 2010;184:4688–4695. doi: 10.4049/jimmunol.0903477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietrzak A, Misiak-Tloczek A, Brzezinska-Blaszczyk E. Interleukin (IL)-10 inhibits RANTES-, tumour necrosis factor (TNF)- and nerve growth factor (NGF)-induced mast cell migratory response but is not a mast cell chemoattractant. Immunol Lett. 2009;123:46–51. doi: 10.1016/j.imlet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 40.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 41.Nakano K, Siar CH, Tsujigiwa H, Nagatsuka H, Nagai N, Kawakami T. Notch signaling in benign and malignant ameloblastic neoplasms. Eur J Med Res. 2008;13:476–480. [PubMed] [Google Scholar]
- 42.Nakano N, Nishiyama C, Yagita H, Koyanagi A, Akiba H, Chiba S, Ogawa H, Okumura K. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81. e1. doi: 10.1016/j.jaci.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 43.Nakano N, Nishiyama C, Yagita H, Koyanagi A, Ogawa H, Okumura K. Notch1-mediated signaling induces MHC class II expression through activation of class II transactivator promoter III in mast cells. J Biol Chem. 2011;286:12042–12048. doi: 10.1074/jbc.M110.138966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakata-Yanagimoto M, Sakai T, Miyake Y, Saito TI, Maruyama H, Morishita Y, Nakagami- Yamaguchi E, Kumano K, Yagita H, Fukayama M, Ogawa S, Kurokawa M, Yasutomo K, Chiba S. Notch2 signaling is required for proper mast cell distribution and mucosal immunity in the intestine. Blood. 2011;117:128–134. doi: 10.1182/blood-2010-07-289611. [DOI] [PubMed] [Google Scholar]
- 45.Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takano H, Nakazawa S, Shirata N, Tamba S, Furuta K, Tsuchiya S, Morimoto K, Itano N, Irie A, Ichikawa A, Kimata K, Nakayama K, Sugimoto Y, Tanaka S. Involvement of CD44 in mast cell proliferation during terminal differentiation. Lab Invest. 2009;89:446–455. doi: 10.1038/labinvest.2008.159. [DOI] [PubMed] [Google Scholar]
- 47.Girodet PO, Ozier A, Trian T, Begueret H, Ousova O, Vernejoux JM, Chanez P, Marthan R, Berger P, Tunon de Lara JM. Mast cell adhesion to bronchial smooth muscle in asthma specifically depends on CD51 and CD44 variant 6. Allergy. 2010;65:1004–1012. doi: 10.1111/j.1398-9995.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- 48.Bissonnette EY, Enciso JA, Befus AD. TGF-beta1 inhibits the release of histamine and tumor necrosis factor-alpha from mast cells through an autocrine pathway. Am J Respir Cell Mol Biol. 1997;16:275–282. doi: 10.1165/ajrcmb.16.3.9070612. [DOI] [PubMed] [Google Scholar]
- 49.Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS. Transforming growth factor-beta 1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation. J Immunol. 1989;143:1591–1597. [PubMed] [Google Scholar]
- 50.Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, Kepley CL, Ryan JJ. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashyap M, Bailey DP, Gomez G, Rivera J, Huff TF, Ryan JJ. TGF-beta1 inhibits late-stage mast cell maturation. Exp Hematol. 2005;33:1281–1291. doi: 10.1016/j.exphem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Meade R, Askenase PW, Geba GP, Neddermann K, Jacoby RO, Pasternak RD. Transforming growth factor-beta 1 inhibits murine immediate and delayed type hypersensitivity. J Immunol. 1992;149:521–528. [PubMed] [Google Scholar]
- 53.Norozian F, Kashyap M, Ramirez CD, Patel N, Kepley CL, Barnstein BO, Ryan JJ. TGFbeta1 induces mast cell apoptosis. Exp Hematol. 2006;34:579–587. doi: 10.1016/j.exphem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Toyota N, Hashimoto Y, Matsuo S, Iizuka H. Transforming growth factor beta 1 inhibits IL-3-and IL-4-dependent mouse connective tissue-type mast cell proliferation. Arch Dermatol Res. 1995;287:198–201. doi: 10.1007/BF01262332. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-beta1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–7272. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin TJ, Befus AD. Differential regulation of mast cell function by IL-10 and stem cell factor. J Immunol. 1997;159:4015–4023. [PubMed] [Google Scholar]
- 57.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirmonsef P, Shelburne CP, Yeatman C Fitzhugh, 2nd, Chong HJ, Ryan JJ. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3'-kinase. J Immunol. 1999;163:2530–2539. [PubMed] [Google Scholar]
- 59.Royer B, Varadaradjalou S, Saas P, Gabiot AC, Kantelip B, Feger F, Guillosson JJ, Kantelip JP, Arock M. Autocrine regulation of cord blood-derived human mast cell activation by IL-10. J Allergy Clin Immunol. 2001;108:80–86. doi: 10.1067/mai.2001.115753. [DOI] [PubMed] [Google Scholar]
- 60.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001;31:694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 61.Shelburne CP, Ryan JJ. The role of Th2 cytokines in mast cell homeostasis. Immunol Rev. 2001;179:82–93. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 62.Speiran K, Bailey DP, Fernando J, Macey M, Barnstein B, Kolawole M, Curley D, Watowich SS, Murray PJ, Oskeritzian C, Ryan JJ. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J Leukoc Biol. 2009;85:826–836. doi: 10.1189/jlb.0708448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeatman CF, 2nd, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, Sawyer ST, Shelburne CP, Ryan JJ. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192:1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Funaba M, Ikeda T, Murakami M, Ogawa K, Abe M. Up-regulation of mouse mast cell protease-6 gene by transforming growth factor-beta and activin in mast cell progenitors. Cell Signal. 2005;17:121–128. doi: 10.1016/j.cellsig.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Funaba M, Ikeda T, Murakami M, Ogawa K, Tsuchida K, Sugino H, Abe M. Transcriptional activation of mouse mast cell Protease-7 by activin and transforming growth factor-beta is inhibited by microphthalmia-associated transcription factor. J Biol Chem. 2003;278:52032–52041. doi: 10.1074/jbc.M306991200. [DOI] [PubMed] [Google Scholar]
- 66.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, Bouton LA, Bailey DP, Gharse A, Mirmonsef P, Odom S, Gomez G, Rivera J, Fischer-Stenger K, Ryan JJ. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]