Abstract
The medial prefrontal cortex (mPFC) is known to regulate higher order processes like cognitive flexibility. Accumulating behavioral evidence suggests that endocannabinoid (eCB) signaling regulates neuronal architecture within the PFC, as well as certain forms of cognitive flexibility; however, all of these studies have been performed in male rodents and it is currently unknown whether the eCB system performs a similar role in females. To this extent, dendritic morphology of layer II/III neurons in the infra- and prelimbic regions of the mPFC was analyzed and cognitive ability and flexibility in a fixed-platform Morris water maze task was assessed in adult female CB1 receptor knockout (CB1KO) mice. Similar to data generated in male mice, female mice exhibited no difference in acquisition relative to wildtype (WT); however, during reversal learning, CB1KO females spent more time in the original training quadrant and took significantly longer to learn the location of the new platform relative to WT. Within the mPFC, female mice had reduced length and complexity of layer II/III neurons within the prelimbic, but not infralimbic region of the PFC. Taken together, these findings indicate that the role of eCB signaling in cognitive flexibility is independent of sex and disrupted CB1 receptor signaling results in compromised structure and function of the PFC, at least within the prelimbic division.
Keywords: sex difference, CB1 receptor knockout, female, endocannabinoid, cognitive flexibility, Morris Water Maze, prelimbic, prefrontal cortex
1. Introduction
Cognitive flexibility allows an organism to modify their choices or behavior in response to changing environmental contingencies as well as inhibit previously appropriate responses. It is an adaptive, higher order process that is, at least partially, regulated by the medial prefrontal cortex (mPFC), which contributes to distinguishing aversive stimuli and determining the controllability of that stimuli [1]. Thus, impaired cognitive flexibility can be observed as an individuals’ inability to adjust strategies when the conditions change and demand alternate solutions. Behavioral evidence for a regulatory role of the mPFC in cognitive flexibility derives from lesioning or inactivation studies in rodents, which results in impairments in an array of behavioral tasks that require this form of flexibility, such as fear extinction and attentional set shifting [1].
The endocannabinoid (eCB) system, which interacts with THC, the main psychoactive constituent of cannabis, has been studied under many contexts, and has been shown to regulate a variety of processes including emotionality, synaptic and structural plasticity, and multiple forms of learning/memory processes [see review, 2]. The eCB system possesses two G-protein coupled cannabinoid receptors: CB1 and CB2. Within the brain, CB1 receptors are primarily located on axon terminals where they act to negatively regulate neurotransmitter release and mediate several forms of synaptic plasticity, such as long term depression [3]. eCB signaling is widely distributed throughout corticolimbic neural circuits, particularly within structures such as the hippocampus, amygdala, and PFC [4]. Within the mPFC, there is a growing body of research that has demonstrated that eCB signaling can regulate the neuronal activity, structural plasticity and functional output of this structure [5]. In fact, the eCB system is implicated in the regulation of several PFC-mediated processes including termination of the stress response [6], anxiety [7], fear extinction [8], attentional set shifting [9] and reversal learning in the Morris water maze [10; MWM] in male rodents. Moreover, impairments in cognitive flexibility following suppression of CB1 receptor signaling appear similar to those observed following lesions to the rodent mPFC [11]. Consistent with this, adult male CB1 receptor knock out (CB1KO) mice have reduced dendritic arbors in layer II/III of the prelimbic cortex [12; PL].
One caveat to much research on the eCB system, including all of the aforementioned studies, is the fact that they were all conducted on male rodents. Given that there are noted sex differences in molecular components of the eCB system [13–15], and that many manipulations (e.g. stress exposure) which modulate PFC function are sex-dependent [16], the question remains as to whether eCB signaling in the regulation of PFC structure and function is modulated by sex. Therefore, we examined the impact of genetic CB1 receptor deficiency in adult female mice on dendritic morphology of neurons in both the PL and infralimbic (IL) regions of the mPFC as well as cognitive performance in the MWM task.
2. Methods
2.1 Subjects
All animal procedures were undertaken with approval of the Rockefeller University IACUC. Adult female C57BL/6 mice were bred in house and weaned and group housed (n=5 per cage) at 21 days old. Testing began at approximately 10 weeks of age. Food and water were available ad libitum for the duration of the experiment. Mice used in this study were generated and backcrossed to a C57/Bl6J background [17] and were provided by the National Institute of Mental Health. Mice and their wild-type littermates were bred as previously described [18]. All female mice remained intact and estrous cycle was not monitored.
2.3 Morris Water Maze
WT (n=8) and CB1KO (n=9) female mice were trained in the MWM, which had a diameter of 110 cm. It was filled to a depth of two feet with water rendered opaque white to prevent animals from being able to see the location of the platform. The MWM paradigm used in the current study was based on Varvel and Lichtman’s [10] previous study assessing cognitive performance in the MWM in male CB1KO mice. Briefly, acquisition training consisted of 4 trials per day for four consecutive days and intertrial intervals of 10 min. Four points around the circumference of the maze were arbitrarily designated as N, S, E, and W, which served as a reference for experimenters when releasing the mice into the pool. Extra maze cues were present around the room to provide spatially oriented cues. Within each acquisition session (i.e., 4 trials in a day), mice were randomly released from each of the four points, facing the pool wall. Once the mouse located the hidden platform, the mouse remained on the platform for 30 s before removal from the tank. If a mouse failed to locate the platform within 90 s, it was guided to the platform and remained there for 30 s before removal from the pool. Following 4 acquisition sessions, the mice were given a 1-day reversal learning task (4 trials with 10 min intertrial interval), which maintained the same procedures as acquisition training, except the hidden platform was moved to the opposite quadrant from which it had originally been placed (for acquisition training). Noldus Ethovision (Leesburg VA USA) was used to analyze escape latency and time spent in specific quadrants of the MWM.
2.3 Morphological Analyses
Naïve WT (n=6) and CB1KO (n=6) female mice (approximately 10 weeks of age) were rapidly decapitated within the first 1/3 of their light cycle. Naïve animals were used to avoid any additional effects of behavioral training or associated stress of the MWM on prefrontal cortical dendritic morphology. Brain tissue was harvested and washed in distilled water and processed for Golgi staining according to manufacturer instructions of the rapid Golgi kit (FD Neurotech). Tissue was sliced coronally at 150 μm thickness, mounted on slides and coverslipped. Using a Nikon Eclipse microscope (60×) and Neurolucida software (MicroBrightField), neurons were 3D reconstructed. Dendritic morphology of pyramidal neurons located in layer II/III of the PL and IL of the mPFC were examined as previous work has demonstrated that these neurons possess the capacity to undergo dendritic remodeling and are accompanied by changes in cognitive ability [19]. Boundaries between PL and IL were determined as previously described [20; see Figure 1A]. Within each region, apical dendrites of 6 neurons from each animal were reconstructed by an experimenter blind to condition (Figure 1B). Dendritic length and branch points were quantified (using the centre of the soma as a reference point) and averaged for each animal. Pyramidal cells within the two regions were defined as having a cell body immediately lateral to layer I (which is considerably absent of cells), the presence of a basilar dendritic tree, and a distinct apical dendrite projecting toward the pial surface. Inclusion of cells for analysis were based on criteria previously published [12]: 1) cell bodies within the midsection of the tissue to minimize dendritic truncation; 2) relative isolation of the cell body from neighboring impregnated neurons; 3) cell bodies exist between 150 and 250 μm from the pial surface to prevent artifacts due to unrepresentative sampling from neurons of varying distance from the midline; and 4) the presence of intact primary, secondary, and tertiary dendrites (as evidenced by well-defined endings) to at least 50 μm from the cell body.
Figure 1.
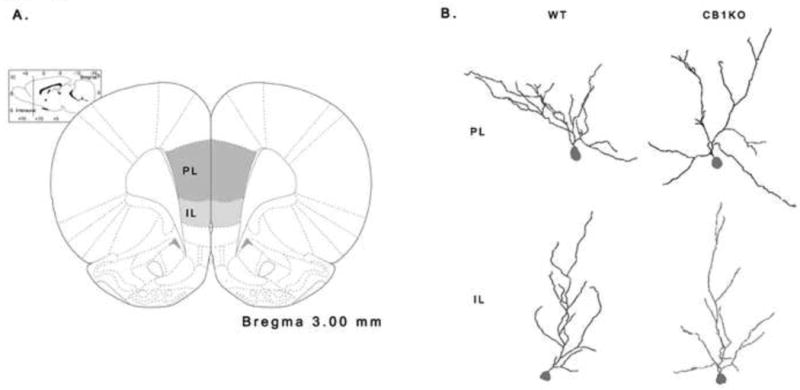
(A) Schematic diagram indicating the prelimbic (PL) and infralimbic (IL) regions of the prefrontal cortex in which layer II/III neurons were sampled. (B) Representative dendritic reconstructions of wild-type (WT) and CB1 receptor knockout (CB1KO) females within the PL and IL, respectively.
2.4 Data Analyses
Dendritic length, branch points, and savings ratios were analyzed using independent t-tests with genotype as the independent factor. Escape latency in the acquisition and reversal Learning sessions were analyzed using a repeated measures analysis of variance (ANOVA) with genotype as the independent factor and day/trial as the within subjects factor. Significance for all tests was established at p ≤ 0.05. All data presented in the figures are listed as mean values ± standard error.
For acquisition training, the 4 daily escape latencies were averaged. Individual escape latencies were analyzed for the reversal learning phase. To further assess any impairment in cognitive flexibility, time spent in the original training quadrant was measured during reversal learning. Lastly, a modified savings ratio was calculated in which the escape latency of the first trial was divided by the sum of the first and fourth escape latencies [10, 21]. Thus, a ratio of 0.5 indicates no difference in escape latency between the first and last trials of reversal learning. A ratio greater than 0.5 indicates the platform was located faster during the fourth trial compared to the first trial.
3. Results
Analyses of dendritic morphology within the PL of the mPFC revealed that CB1KO females had significantly shorter apical length (t(69) = 2.36, p ≤ 0.05; Fig. 2A) and less branch points (t(69) = 3.01, p ≤ 0.005; Fig. 2C) than WT females. However, there were no significant differences in apical length (t(74) = 0.10, p = 0.92; see Fig. 2B) and branch points (t(74) = 0.65, p = 0.52; Fig. 2D) in the IL.
Figure 2.
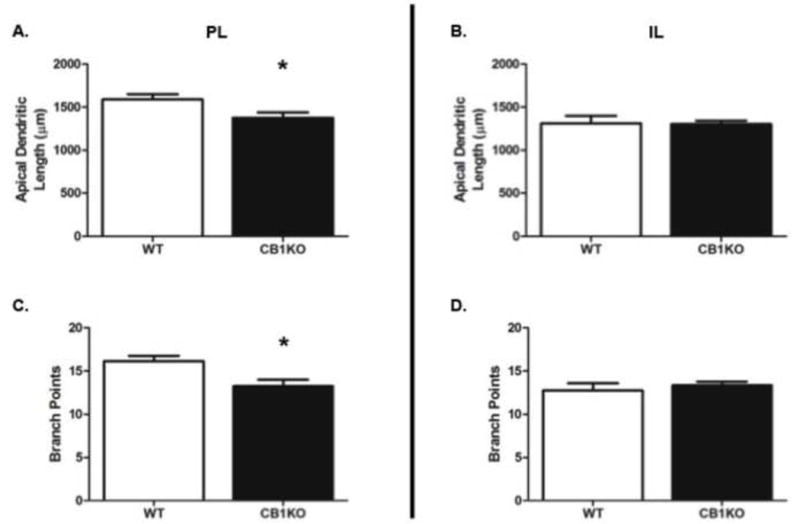
CB1 receptor knockout (CB1KO) female mice have shorter and less complex neurons in the prelimbic, (PL) but not infralimbic (IL) region of the medial prefrontal cortex relative to wild type (WT). (A) Mean apical dendrite length of PL neurons; (B) Mean apical dendrite length of IL neurons; (C) Mean number of dendritic branch points on PL neurons; (D) Mean number of dendritic branch points on IL neurons. * indicates p ≤ 0.05.
During the acquisition phase of the MWM task, no significant interaction was found between day and genotype (F(3,48) = 0.87, p = 0.47; Fig. 2A) and no main effect of genotype on escape latency (F(1,48) =1.09, p = 0.31). However, a main effect of day emerged in which all mice had significantly lower mean escape latencies by the fourth day of training, indicating that the mice learned the task regardless of genotype (F(3, 48) = 19.46, p ≤ 0.0001).
Analyses of escape latency during reversal learning revealed no significant interaction between trial and genotype (F(3, 45) = 1.13, p ≥ 0.05; Fig. 3B) or main effect of genotype (F(1, 45) = 0.95, p ≥ 0.05). A main effect of time emerged in which all animals had significantly lower mean escape latencies by the 4th trial of reversal learning (F(3,45) = 3.95, p ≤ 0.05), indicating that learning for the new location of the platform occurred. However, WT females had a significantly higher savings than CB1KO mice (t(15) = 2.42, p ≤ 0.05; Fig. 3C), meaning the WT animals exhibited significantly greater improvement in escape latency across trials relative to the CB1KO mice. There was no significant interaction between trial and genotype for time spent in the original training quadrant (F(3, 48) = 1.58, p = 0.21). However, a main effect of time (F(3,48) = 5.002, p < 0.005; not shown) emerged such that the average time spent in the original training quadrant was significantly lowered across the four reversal learning trials, indicating that some learning had occurred. Lastly, CB1KO females perseverated significantly longer in the original training quadrant than WT females across the four trials (F(1,48) = 8.79, p < 0.005; Fig. 3D).
Figure 3.
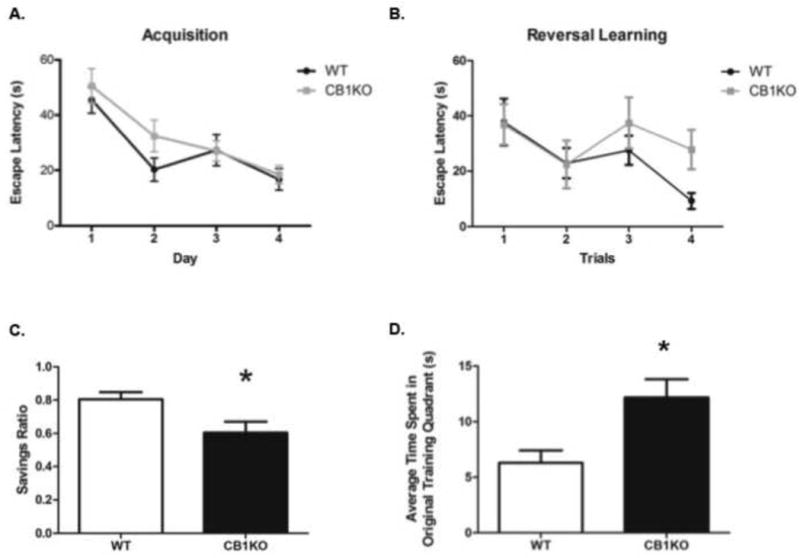
CB1 receptor knockout female mice exhibit impairments during reversal learning in the Morris water maze task. (A) Mean escape latency during the 4-day Acquisition phase; (B) Mean escape latency during the 4-trial reversal learning phase; (C) Mean savings ratio during Reversal learning. (D) Mean time spent in the original training quadrant during the Reversal learning phase. * indicates p ≤ 0.05.
4. Discussion
In this study, genetic deletion of CB1 receptors failed to result in any significant differences in escape latency for acquisition and reversal learning phases; however, during the reversal learning task, CB1KO females exhibited a greater degree of perseveration by spending significantly more time in the original training quadrant and had smaller savings ratios than WT mice. Adult female CB1KO mice also had significantly shorter and less complex pyramidal neurons in the PL, but not the IL of the mPFC. Taken together, our data indicates that CB1KO female mice have shorter and less complex dendrites of neurons in the PL and corresponding impairments in cognitive flexibility, suggestive of compromised structure and function of the PFC.
Consistent with this relationship between dendritic morphology and performance in a PFC-dependent task in females, Leuner and Gould [22] recently established a relationship between dendritic complexity in layer II/III neurons of the mPFC and greater ability to learn the new location of a hidden platform during reversal learning in the MWM as a function of parity experience in female rats. Thus, within the PFC, structural plasticity is tightly linked to behavioural function; less complex and shorter dendritic arborization of neurons in the PFC is associated with deficits in cognitive flexibility, while greater dendritic complexity and length is associated with greater cognitive flexibility [23]. This is also consistent with relationships between prefrontal cortical dendritic morphology and tasks of cognitive flexibility that have been documented in male rodents [19]. The current data presented here is consistent with this theory as genetic CB1 receptor deletion resulted in shorter and less complex neurons in the PL of the mPFC relative to WT female mice and this deficit in eCB signaling also significantly impaired the ability to find the new location of the hidden platform.
Interestingly, previous work indicates the PL is primarily involved in higher-order processing that employ strategy shifts rather than reversal learning tasks [which involve lower-order processes using the same general strategy; 1]. In a task requiring rats to dig in specific sand cups for food reinforcement, acquisition rates were similar. However, when faced with having to discriminate the correct cup based on different criteria (e.g. location of the cup versus odor cues), temporary inactivation of the PL induced impaired learning for the new contingency rule, confirming that the PL is largely involved in cognitive flexibility requiring shifts in strategy [1]. Our findings are consistent with this as no differences in escape latency during the reversal learning trials were observed. However, CB1KO females did exhibit some indications of impaired cognitive flexibility with significantly greater perseverative responses than WT during the reversal learning phase. The CB1KO females’ inability to change search strategy to find the new location of the submerged platform could, at least in part, be attributed to shorter and less complex dendritic morphology of the PL. Future research is warranted as it is also possible that CB1 receptor deletion altered the structure and function of other frontal cortical regions that contribute to the increase in perseverative behaviour observed.
Somewhat surprisingly, no significant differences were observed within the IL of the mPFC. While both regions are believed to contribute to cognitive flexibility, their exact contributions require further clarification; however, it has been proposed that the IL is involved in habit formation and maintenance [see review, 1]. Our findings are consistent with this as the CB1KO females exhibited less adaptability to a new contingency in the MWM task; however, habit formation and maintenance do not appear to be directly challenged in this paradigm.
These data demonstrate that female CB1KO mice exhibit comparable alterations in dendritic morphology of the mPFC and cognitive flexibility as male CB1KO mice. This indicates that eCB regulation of PFC structure and function is not sexually divergent. Having established this similarity, it will be important in future studies to determine whether individual differences in cognitive flexibility correspond to changes in eCB system function. While this relationship certainly requires further study, it is tempting to speculate that the eCB system may be an ideal therapeutic target for a myriad of clinical conditions exhibiting symptoms of impaired cognitive flexibility.
Highlights.
Female, like male CB1KO mice, exhibited reduced cognitive flexibility in a Morris Water Maze task.
Prelimbic, but not infralimbic, neurons of the PFC are shorter and less complex in female CB1KO mice.
Endocannabinoid regulation of cognitive flexibility functions similarly in males and females.
Acknowledgments
The authors wish to thank Drs. Ilia Karatsoreos, Jason Gray and Melinda Miller for their technical expertise and assistance and the following sources of funding: TTYL -Canadian Institutes of Health Research (CIHR; salary) and the Canadian Consortium for the Investigation of Cannabinoids (CCIC; salary); MNH -CIHR (operating); BSM – NIH (R01 MH41256).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ragozzino M. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Parker L. The endocannabinoid system and the brain. Annual Review of Psychology. 2012;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 3.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 4.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin R, Hill M, Gorzalka B. Prefrontocortical endocannabinoid signaling and the regulation of stress and emotionality. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2014.02.006. submitted. [DOI] [PubMed] [Google Scholar]
- 6.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT-Y, Gray JM, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences. 2010;107:9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cerebral Cortex. 2008;18:1292–301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- 8.Marsicano G, Wotjak C, Azad S, Bisogno T, Rammes G, Cascio M, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 9.Klugmann M, Goepfrich A, Friemel C, Schneider M. AAV-Mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Frontiers in Behavioral Neuroscience. 2011;5 doi: 10.3389/fnbeh.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varvel S, Lichtman A. Evaluation of CB1 receptor knockout mice in the Morris water maze. Journal of Pharmacology and Experimental Therapeutics. 2002;301:915–24. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- 11.Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behavioral Brain Research. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 12.Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cerebral Cortex. 2011;21:2056–64. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riebe CJ, Hill MN, Lee TT-Y, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;25:1265–9. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft R, Marusich J, Wiley J. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sciences. 2013;92:476–81. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattore L, Fratta W. How important are sex differences in cannabinoid action? British Journal of Pharmacology. 2010;160:544–8. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shansky R, Hamo C, Hof P, McEwen B, Morrison J. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009;19:2479–84. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer A, Zimmer A, Hohmann A, Herkenham M, Bonner T. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proceedings of the National Academy of Sciences. 1999;96:5780–5. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litvin Y, Phan A, Hill M, Pfaff D, McEwen B. CB1 receptor signaling regulates social anxiety and memory. Genes, Brain and Behavior. 2013;12:479–89. doi: 10.1111/gbb.12045. [DOI] [PubMed] [Google Scholar]
- 19.Liston C, Miller M, Goldwater D, Radley J, Rocher A, Hof P, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varvel S, Anum E, Lichtman A. Disruption of CB1 receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology. 2005;179:863–72. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- 22.Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. Journal of Neuroscience. 2010;30:13499–503. doi: 10.1523/JNEUROSCI.3388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mclaughlin K, Baran S, Conrad C. Chronic stress- and sex- specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology. 2009;40:166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]


