Summary
Background
Gastrointestinal tract duplication is a rare malformation associated with the presence of additional segment of the fetal gut. The aim of this study was to retrospectively review clinical features and imaging findings in intraoperatively confirmed cases of gastrointestinal tract duplication in children.
Material/Methods
The analysis included own material from the years 2002–2012. The analyzed group included 14 children, among them 8 boys and 6 girls. The youngest patient was diagnosed at the age of three weeks, and the oldest at 12 years of age.
Results
The duplication cysts were identified in the esophagus (n=2), stomach (n=5), duodenum (n=1), terminal ileum (n=5), and rectum (n=1). In four cases, the duplication coexisted with other anomalies, such as patent urachus, Meckel’s diverticulum, mesenteric cyst, and accessory pancreas. Clinical manifestation of gastrointestinal duplication cysts was variable, and some of them were detected accidently. Thin- or thick-walled cystic structures adjacent to the wall of neighboring gastrointestinal segment were documented on diagnostic imaging.
Conclusions
Ultrasound and computed tomography are the methods of choice in the evaluation of gastrointestinal duplication cysts. Apart from the diagnosis of the duplication cyst, an important issue is the detection of concomitant developmental pathologies, including pancreatic heterotopy.
MeSH Keywords: Congenital Abnormalities - surgery, Diagnosis, Differential, Diagnostic Imaging
Background
Gastrointestinal tract duplication is a rare malformation associated with the presence of additional segment of the gut. However, there is no coherent theory explaining the variability of morphological forms of duplication, its location and range of coexisting abnormalities [1]. The incidence of the malformation is 1 in 5–10 thousand of live births [2]. Usually the malformation is diagnosed in children. However approximately 30% of esophageal duplications are discovered in adults [3,4]. Although the duplication may appear in every part of the gastrointerstitial tract, it is most frequently observed in the terminal part of the ileum. The second most common location is esophagus (15% of malformations in this group) [1,3]. Stomach duplications (approx. 7% of cases) and rectum duplications (5%) are less commonly observed [5]. Among stomach duplications a complete pyloric duplication is extremely rare.
To make the diagnosis of GI tract duplication, one must reveal three typical features of malformation: 1) connection with GI tract (presence of a common wall built of serous and muscle membrane), 2) presence of mucous membrane lined with epithelium typical for gastrointerstitial tract, and 3) presence of smooth muscle tissue [6]. There are, however, cases of duplications containing ciliated airway epithelial cells or ectopic pancreatic tissue [7]. Despite their common wall with GI tract, the duplications usually fail to demonstrate a communication with the lumen of the digestive tract [5]. The duplication cysts might be spherical – cystic (type I) or elongated – tubular (type II) in shape.
The most important diagnostic methods of detection of digestive tract duplications include: computed tomography (CT), ultrasound (US) and magnetic resonance imaging (MRI). The majority of GI tract duplications in CT meet the criteria of benign, cystic masses. These are flat, spherical or tubular lesions, which do not infiltrate the adjacent organs. A wall of duplication, showing usually some contrast enhancement, can be easily discriminated from adjacent tissues. On the contrary, the content of duplication is homogenous, with a density of 0 to 20 HU, and no contrast enhancement [8]. In ultrasound imaging duplications of GI tract present as cystic structures with homogenous, amorphic content. Duplication should be suspected in the presence of an internal echogenic layer and outer hypoechogenic, muscular layers [9]. In diagnosing esophagus duplication, endoscopic ultrasonography (EUS) of the upper part of the alimentary tract might be helpful. EUS enables confirmation of muscular layer continuity in the esophagus and its duplication [10]. In MRI, a duplication cyst has a low signal in T1-weighted images and a very low signal in T2-weighted images. Scintigraphy is a method rarely used in GI tract duplication diagnostics. Some duplications lined with gastric mucosa can be detected using technetium-99m scintigraphy with a carrier targeting parietal cells [11]. Increased technetium uptake is observed in Meckel’s diverticulum, which might contain some ectopic tissue, which – in the majority of cases (80%) – derives from the stomach [11]. Scintigraphy has a sensitivity of 85–90% in the diagnostics of GI tract duplications. Falsely negative results are caused by necrotic lesions, increased marker wash-out and projecting into the urinary bladder [12].
The aim of this study was to retrospectively review clinical features and imaging findings in intraoperatively confirmed cases of gastrointestinal tract duplication in children.
Material and Methods
A retrospective analysis included medical records of pediatric patients with GI tract duplication who were diagnosed and managed in our facility in the last 10 years. The analyzed group included 14 children, among them 8 boys and 6 girls. The youngest patient was diagnosed at the age of three weeks, and the oldest at 12 years of age.
Results
Table 1 contains a summary of demographic data, clinical presentation, imaging findings and intraoperative diagnoses. The duplication cysts were identified in every part of the digestive tract: in the esophagus (n=2), stomach (n=5), duodenum (n=2), terminal ileum (n=5), and rectum (n=1). In four cases, the duplication coexisted with other anomalies, such as patent urachus, Meckel’s diverticulum, mesenteric cyst, accessory pancreas, and ASO II heart defect. An atypical case was found in Patient 5, where the stomach duplication cyst included an ectopic, pancreatic tissue, which had a connective duct communicating with main pancreatic duct.
Table 1.
Demographic data, US and CT image, and intraoperative findings in the analyzed group of patients with gastrointestinal duplication cysts.
| No. | Initials/sex /age | Location of the duplication | Clinical manifestation/reason for hospitalization | Cyst presentation and other pathological lesions in US and CT scan | Intraoperative diagnosis |
|---|---|---|---|---|---|
| 1. | K.P./M/3 months | Mediastinal enterogenous cyst | Widened mediastinum in chest X-ray, ASO II heart defect | Fluid compartment, cyst wall thickness 1–2 mm, no contrast enhancement | Mediastinal enterogenous cyst |
| 2. | B.K./M/3 years | Esophagus | Neck tumor | Thick content (40 HU) compartment, contrast-enhancing cyst wall of 3 mm in thickness | Tubular esophageal duplication (common mucous layer of the cyst and esophagus in the middle part) |
| 3. | D.M./M/9 months | Stomach | Underweight, suspicion of food allergy | Fluid compartment with septa, cyst wall thickness 1–2 mm, no contrast enhancement | Duplication of gastric posterior wall near fundus, small bowel duplication |
| 4. | J.J./M/3 weeks | Stomach | Vomiting after feeding | Thick content (40 HU) compartment, cyst wall thickness 1–2 mm, no contrast enhancement | Stomach duplication cyst lined with gastric mucosa |
| 5. | E.S./F/12 years | Stomach | Acute pancreatitis | Thick content (24 HU) compartment, contrast enhancing cyst wall, 7 mm thick, perimural nodular lesions, tail pancreatitis, accessory pancreas, fluid in omental bursa | Duplication cyst of the prepyloric part of stomach lined with polypomatous, hypertrophic mucosa with heterotopic foci of pancreas, accessory pancreas |
| 6. | J.K./F/5 months | Stomach | Abdominal pain, sepsis | Fluid compartment, cyst wall, 2 mm thick, no contrast enhancement, inflammatory infiltration around the cyst, spleen infarct, kidney microabscesses, fluid in peritoneal cavity and in pleural cavities | Stomach duplication cyst lined with gastric mucosa, adjacent inflammatory infiltration |
| 7. | J.A./F/10 years | Pylorus and duodenum | Acute pancreatitis | Fluid compartment, contrast enhancing cyst wall, 4–9 mm thick | Cystic duplication of pylorus and proximal part of duodenum, inflammatory reaction in pancreatic head |
| 8. | J.P./F/2 months | Duodenum | Diagnostics of patent urachus | Fluid compartment, cyst wall, max. 3 mm thick, no contrast enhancement, small, additional cystic structures | Duodenum duplication, patent urachus, Meckel’s diverticulum containing pancreatic tissue, mesenteric cyst, mesenteric lymphadenopathy |
| 9. | F.L./M/2 years | Ileum | GI tract intussusception | Fluid compartment, contrast enhancing cyst wall, max. 2 mm thick intestine intussusception | Terminal ileal duplication cyst, adjacent inflammatory, interstitial lesions |
| 10. | J.W./F/3 months | Ileum | Colic, anxiety | Fluid compartment, cyst wall thickness 1–2 mm, no contrast enhancement | Terminal ileal duplication cyst, lined with cylindrical epithelium |
| 11. | N.M./F/6 months | Ileum | Routine prenatal US exam | Fluid compartment, cyst wall thickness 2–3 mm, no contrast enhancement | Ileal duplication cyst |
| 12. | M.G./M/4 months | Ileum | Chronic constipations | Fluid compartment, contrast enhancing cyst wall, max. 4 mm thick | Terminal ileal duplication cyst |
| 13. | K.S./M/6 months | Ileum | Accidental diagnosis in US exam | Fluid compartment, cyst wall thickness of 2–3 mm, no contrast enhancement | Terminal ileal duplication cyst |
| 14. | F.B./M/5 months | Rectum | Abdominal distension meteorism and problems with defecation | Fluid compartment, contrast enhancing cyst wall, max. 7 mm thick, fluid in lesser pelvis, bowel obstruction | Rectal duplication cyst, lined with cylindrical, intestinal epithelium |
In all children a diagnosis of duplication was made preoperatively and appropriate surgical management was carried out. There were no surgery complications observed.
Clinical manifestation of GI tract duplication cyst
The duplication cysts located in the chest (n=2) were revealed incidentally. In the first case, the cyst was detected while performing diagnostic examinations of a cyst located in the neck (Figure 1A, 1B). In the second patient the diagnosis was made during assessment of ASO II heart defect, where after detection of widened mediastinum in chest X-ray, a computed tomography examination was performed (Figure 2).
Figure 1.
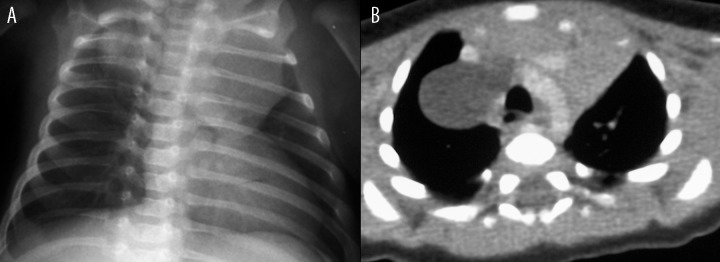
Patient K.P., diagnosed due to ASOII congenital heart defect. (A) Chest radiogram: a widened upper mediastinum on the right side. (B) Contrast-enhanced CT: an esophageal duplication cyst in the upper mediastinum on the right side, adjacent to the esophagus, trachea, large vessels, and thymus.
Figure 2.
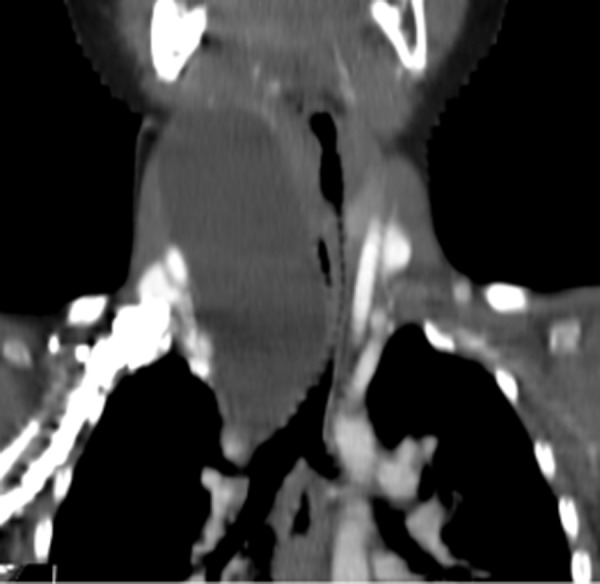
Patient B.K., 3-year-old boy with growing cyst on the neck. Contrast-enhanced CT, MPR in coronal plane: tubular esophageal duplication in the neck and mediastinum, clinically asymptomatic compression of the trachea.
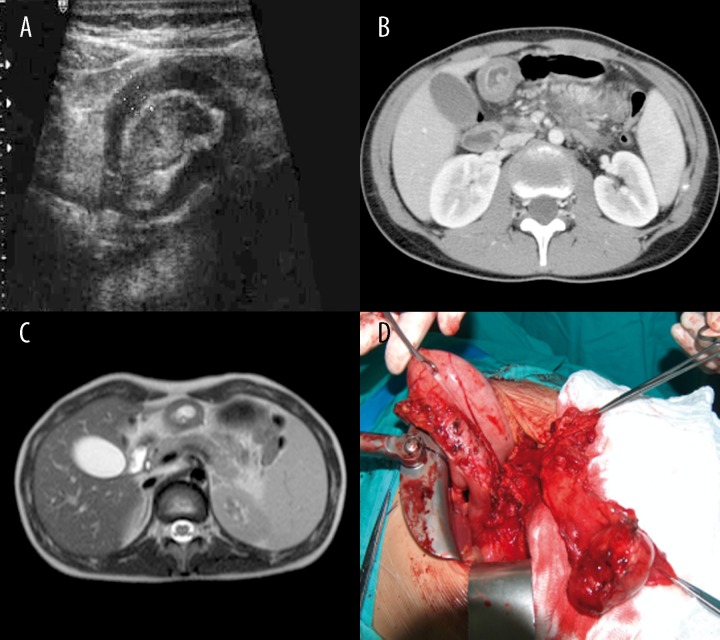
The duplication cysts of the stomach showed variable clinical manifestations: starting from underweight suggesting food allergy (Patient 3, Figure 3A, 3B), through vomiting after feeding (Patient 4, Figure 4), ending with symptoms of acute pancreatitis and peritonitis.
Figure 3.
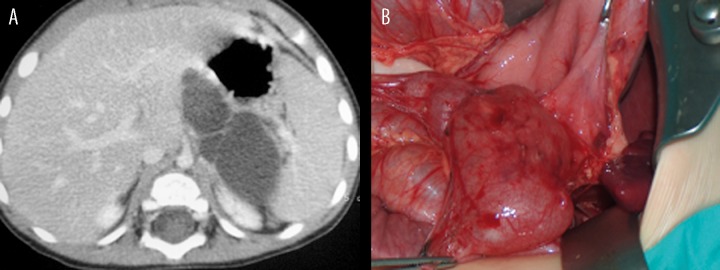
Patient D.M., clinically: underweight. (A) Contrast-enhanced CT: tubular gastric duplication cyst with septa on the posterior wall of the stomach and in the fundal region. (B) Intraoperative image of the cyst.
Figure 4.
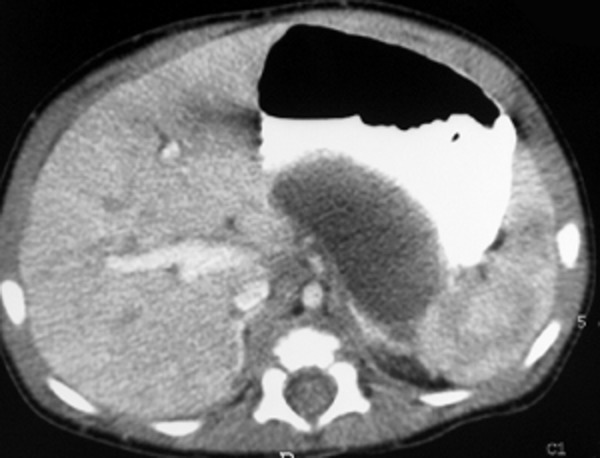
Patient J.J., history of vomiting after feeding. Contrast-enhanced CT: tubular gastric duplication cyst on the posterior wall of the stomach, above the pancreas.
The duplications of the ileum, all located near the ileocaecal valve, were asymptomatic, or led to bowel obstruction or subileus of the GI tract. The only case of rectum duplication had a clinical manifestation of abdominal distension meteorism and problems with defecation, and in a per rectum examination a flexible tumor, modelling the posterior wall of rectum was detected.
Duplication cysts in imaging studies
In diagnostic imaging, ultrasound examination (n=13), computed tomography (n=12), MRI (n=2), and small-bowel follow-through examination (n=1) were performed. In ultrasound, all duplication cysts presented as fluid-filled structures, usually surrounded by a wall 4 mm thick. Some thicker walls (7–9 mm) were found in two cases of stomach duplications and in one case of rectum duplication. A maximal cyst dimension was within the range of 20–94 mm (mean of 45 mm). A liquid content of cysts (<10 HU) was revealed in 7 out of 10 CT examinations, and in the remaining three (two gastric duplication cysts and one esophageal duplication cyst) some thicker matter (24–40 HU) was observed. In the majority of cases enhancement in the cyst wall was observed after contrast agent administration; a particularly strong enhancement was observed in the cyst containing the ectopic foci of pancreatic tissue.
In two children with esophageal duplication, the diagnosis was made based on CT examination. In every child with stomach duplication, ultrasonography was performed, which confirmed the presence of duplication cyst in 4 out of 5 patients, as well as CT scan, which revealed the malformation in all patients.
In Patient 6, a CT scan revealed some significant lesions, which were not previously observed in ultrasound examination: features of disseminated inflammatory process with some spleen infarctions and microabscesses present in both kidneys. In Patient 3, a small-bowel follow-through examination was performed, revealing a significant obstruction in contrast agent propagation at the level of the corpus of the stomach, poorly filled with contrast medium. Additionally, in Patient 5, MRI cholangiography was carried out in order to perform some more accurate assessment of the coexisting malformation of an accessory pancreas and an additional duct communicating with the pancreatic duct (Figure 5A–D). It is worth noticing that in Patient 4, neither ultrasound imaging, nor CT scan revealed the additional malformation – a small duplication cyst of the small intestine.
Figure 5.
Patient E.S., clinical signs of acute pancreatitis. (A) Sonographic image of thick-walled cyst in the prepyloric region of the stomach, lined with polypomatous hypertrophic mucosa. (B) Contrast-enhanced CT: thick-walled duplication cyst of the prepyloric region of the stomach with heterotopic foci of pancreatic tissue; accessory pancreas between the pancreas and stomach. (C) MRI image of the duplication cyst. (D) Intraoperative image.
The presence of duodenum duplication cyst in Patient 8 was confirmed in ultrasound and CT scans. Additionally, in ultrasound imaging, some extra, pathological fluid compartments with a diameter of less than 2 cm were found, which were later verified intraoperatively to be: a Meckel’s diverticulum, a mesenteric cyst and a patent urachus.
In every child with ileal duplication the malformations were detected in ultrasound examination, which – in two cases – were later confirmed in CT scan (Patient 12, Figure 6A, 6B). In one patient, the duplication cyst detected previously in ultrasonography, was not observed in computed tomography.
Figure 6.
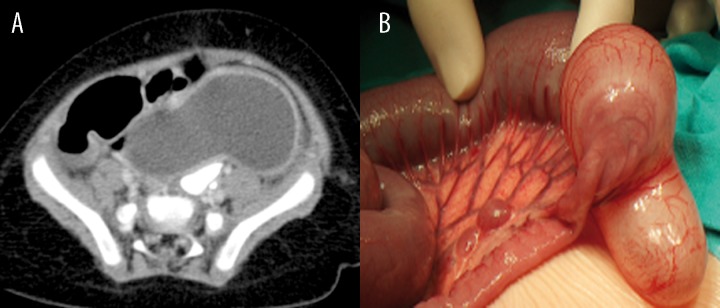
Patient M.G., history of constipation. (A) Contrast-enhanced CT: tubular ileal duplication cyst located close to the ileocaecal valve. (B) Intraoperative image.
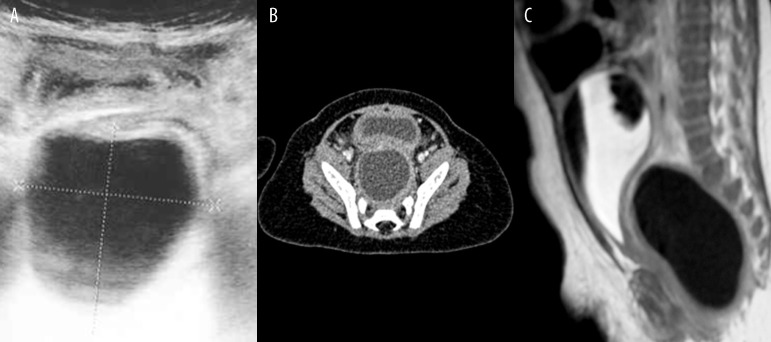
The diagnosis of rectum duplication was made based on ultrasound scan, which was later verified in computed tomography. Next, an MRI examination was performed, which aimed to verify an exact topography of the duplication, as well as to exclude any coexisting pathologies of the spine and spinal cord or a potential communication between the cyst and the spinal canal (Patient 14, Figure 7A–C).
Figure 7.
Patient F.B., history of abdominal distension meteorism and problems with defecation. (A) Abdominal sonography: tubular cyst in the presacral space. (B) Contrast-enhanced CT: tubular rectal duplication cyst located between the rectum and sacrum. (C) MRI image of the duplication cyst.
Discussion
The gastrointestinal tract duplications are relatively rare congenital malformations. In our own material, including 10 years of observation, they were diagnosed in 14 children. The duplications occur most commonly in the small intestine. Although the majority of duplications diagnosed in that part of gut stay in connection with the intestinal wall, they may later migrate in the fetal development towards the mesentery [13]. In our material, the lesions of the intestine were found in 36% of children with gastrointerstitial tract duplications. The same incidence rate was observed in case of stomach duplications. There was only one case of duodenum duplication and one case of rectum duplication. The low incidence rate of rectum duplication stays in accordance with data available in the literature: only a few dozen of corresponding cases were described in the literature [14]. Duplication cysts of the digestive tract result in variable clinical manifestations. Some of them might be asymptomatic – as it was in 4 out of 14 of our analyzed patients. In diagnostic imaging, the duplication of the GI tract takes a form of cystic structure. In case of bleeding into a cyst or cyst infection, the content of the cyst becomes heterogeneous, with a density higher than in fluids; it may lead to a false diagnosis of a solid lesion.
Esophageal duplication cysts account for 15–20% of all masses found in the mediastinum and may be observed in every part of the mediastinum. Apart from esophagus duplications, the majority of lesions found in this region are congenital, benign bronchial cysts, celomatic cysts, thymic cysts, meningocele, matured cystic teratomas, and lymphangiomas [15–17]. On the other hand, a large number of mediastinal tumors may undergo cystic degeneration – especially after chemo- or radiotherapy – becoming impossible to distinguish from congenital cysts [17,18]. Other fluid-containing lesions, which may be observed in mediastinum, comprise abscesses and false cysts appearing in the course of acute pancreatitis. The intramural duplication cysts of esophagus require differentiation with abscesses, but also with hematomas, lipomas and myomas [3].
Approximately 60% of esophageal duplications are located in the lower, third part of the esophagus [19]. The esophageal duplications are most frequently observed in the posterior mediastinum on the right – adjacent to the esophagus or intramurally [3]. A complete esophageal duplication is very rare and usually accompanied by stomach duplication [5]. A duplicated part of the esophagus usually has thick walls, composed of smooth muscles and lined with epithelium typically found in the GI tract, i.e. mucous membrane of the esophagus and stomach. This may result in ulcers, bleeding, perforations and inflammations [1,3,20]. There are also esophageal duplication cysts lined with airway epithelium [19] – which are described as “foregut cyst”. This term refers to esophageal and bronchogenic cysts [3]. In newborns and young children, a compression of the esophagus, bronchus or lung caused by the duplication cyst may result in swallowing difficulties, vomiting, body mass loss, respiratory insufficiency, caugh attacks and inflammatory lesions of the lungs [3,5]. However, esophageal duplications are relatively often asymptomatic [3]. In one of our patients (patient 2), who was finally diagnosed with esophageal duplication involving almost the whole length of the organ, a growing neck tumor was the only observed sign. Despite the tracheal stenosis (to 4 mm) detected in CT scan, there were no respiratory problems observed. It is worth noticing that in the available literature we have not found any case report of such a large esophageal malformation.
The differential diagnosis of GI tract duplication cysts located in the abdomen should include all abdominal pathologies presenting with bubbles in the belly [21]. Apart from duplication cysts, that group includes: omental and mesenteric cysts, Meckel’s diverticulum, lymphangiomas, mesothelial cysts and false cysts, not derived from pancreas (natural evolution stages of hematomas or mesenteric and omental abscesses). The mesothelial cysts present as single, thin-walled, fluid compartments, with no capsule detectable in CT or MRI scans [21]. The presence of Meckel’s diverticulum is observed in 2% of population. In contrast to duplication cyst, it is located in the free wall of the ileum [11]. Apart from the above mentioned lesions, the presence of cystic mesothelioma, smooth muscle sarcoma with degenerative lesions and cystic teratoma were reported within the omentum and mesentery [21]. The most important aim of imaging diagnostics is to confirm that the lesion is fluid in nature and to establish the organ it derives from.
The stomach duplication cysts may differ in shape and location – there were submucosal, intramuscular and extra-gastric lesions described [5]. The duplication usually takes a form of closed, spherical or oval cyst with no communication with the stomach lumen, located in the greater gastric curvature. Clinical manifestation of stomach duplication depends on the size of duplication cyst, its location and communication with digestive tract [5]. The most common clinical symptoms include abdominal pain and vomiting. However, in some patients, the malformation remains asymptomatic. According to the literature, the stomach duplication cysts are most frequently lined with mucosa typical for the stomach. However, there were some lesions described, which contained airway epithelium and pancreatic cells [22]. In one of children analyzed by us (patient 5) we found an ectopic pancreatic tissue and additional duct communicating with the pancreatic duct within the duplication cyst. Literature data suggest that the presence of ectopic gastric mucosa within the duplicated pylorus favors ulcerations and occurrence of clinical symptoms of ulcerative disease [5].
Duplication cysts of the intestine are usually located at the dorsal or mesenteric side of maternal segment of digestive tract. Intestine duplications may result in intussusception of the adjacent part of the gut [23]. Duodenum duplications usually do not communicate with duodenal lumen and are located along its I and II part, at the mesenteric side. Such a location of duplication may lead to bowel obstruction or symptoms produced by bile ducts or pancreas [24]. Therefore it requires differentiation with congenital cyst, derived from the above mentioned organs.
Duplications of the ileum are usually diagnosed in patients under 2 years of age and have a form of spherical or tubular structures, with the length ranging from a few centimeters to the length of the whole gut [11]. A duplicated segment of intestine may contain ectopic tissue of the pancreas or stomach; the presence of the last-mentioned tissue may predispose to bleeding and perforations [11,13]. Walecka et al. [25] reported three cases of enterogenous ileal cyst. However, the lesion was detectable in ultrasound examination in one child only, and in the remaining two cases, proper visualization was impossible due to the accumulation of bowel gas [25]. In comparison, in our analyzed material, the ileal duplication cysts were diagnosed in all five children subjected to ultrasound examination. Additionally, the ultrasound proved to be very valuable in case of a 3-month-old infant, where it revealed additional pathological fluid compartments, not visualized with CT.
Apart from the above mentioned classification of duplication cysts into cystic and tubular type, in case of rectum duplication cysts, there are also other types described: with fistualization, diverticular and extrophic. [26]. The last type is more frequently observed in girls. The majority of cases of rectum duplications are localized in the retrorectal and presacral region. There are also some single cases of duplications located in the prerectal space [14]. Clinical manifestation of rectal duplications is variable and depends on its size, location, presence of fistualization, infection, or ectopic tissues – deriving from the stomach or pancreas [27]. The most common clinical symptoms of rectum duplication include: constipation, rectal hemorrhage, rectal prolapse, urinary tract infections, obstructive uropathies, hemorrhoids, fistulas – communicating with rectum or perirectal, genital tract fistulas, vaginal vestibule fistulas and perirectal abscesses [7,26,28,29]. In adults there were also cases of neoplastic degeneration as a result of rectum duplication [29].
Ultrasonography is usually the first diagnostic step in the preoperative assessment of rectal duplication cyst, while the differential diagnostics encompasses CT, MRI, as well as rectal contrast enema and fistulography [2,6,14,26]. In differential diagnostics of rectum duplications the following conditions need to be considered: first of all sacrococcygeal tumors, as well as anterior meningocele and meningomyelocele of lumbo-sacral part of the spine [6]. Next, the differential diagnostics should also include hydrocolpos, hydrometrocolpos, hydrosalpinx, leiomyosarcoma, dermoid cysts, cysts of rectal glands [14,28,29] and Currarino syndrome [6].
Surgery is the principal method of management in case of symptomatic duplications of the gastrointerstitial tract. Segmental resection is a sufficient way of treatment in case of small lesions, enabling final diagnosis and remission of clinical symptoms [12]. In asymptomatic patients, questionable nature of the observed lesion should constitute an indication for surgical treatment [3]. Some duplications might be multilocular, which makes radical surgery significantly harder. In cases where a radical resection of malformation is impossible, or where opening of the lumen of the GI tract is contraindicated, a complete ablation of the mucous membrane lining the lesion is necessary. When the resection of tubular duplication is impossible, creating a connection between the lumina of the neighboring parts of gut might be a solution [30]. In case of infection of the duplication, a multistage approach might be necessary [27].
Conclusions
Clinical manifestations of gastrointestinal duplication cysts are variable. Imaging studies most frequently reveal thin- or thick-walled cystic structures, adjacent to the wall of the neighboring gastrointestinal segment. Ultrasound and computed tomography are the methods of choice in the evaluation of gastrointestinal duplication cysts. It is important to diagnose not only the duplication cyst, but also potential concomitant developmental pathologies, including pancreatic heterotopy.
References
- 1.Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics. 1993;13:1063–80. doi: 10.1148/radiographics.13.5.8210590. [DOI] [PubMed] [Google Scholar]
- 2.Hartin CW, Lau ST, Escobar MA, et al. Laparoscopic excision of a newborn rectal duplication cyst. J Pediatr Surg. 2008;43:1572–74. doi: 10.1016/j.jpedsurg.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlman JE, Fishman EK, Wang KP, et al. Esophageal duplication cyst: CT and transesophageal needle aspiration. Am J Roentgenol. 1985;145:531–32. doi: 10.2214/ajr.145.3.531. [DOI] [PubMed] [Google Scholar]
- 4.Salo JA, Ala-Kulju KV. Congenital esophageal cysts in adults. Ann Thorac Surg. 1987;44:135–38. doi: 10.1016/s0003-4975(10)62023-1. [DOI] [PubMed] [Google Scholar]
- 5.Berrocal T, Torres I, Gutierrez J, et al. Congenital anomalies of the upper gastrointestinal tract. Radiographics. 1999;19:855–72. doi: 10.1148/radiographics.19.4.g99jl05855. [DOI] [PubMed] [Google Scholar]
- 6.Jayarani K, Kumar G. It is not just constipation – duplication cyst and spinal anomaly. Eur J Radiol Extra. 2007;64:35–38. [Google Scholar]
- 7.Connaughton JC, Poletti L, Broderick T, et al. Rectal duplication cyst with a large perineal hernia presenting as recurrent perineal abscesses. Surgery. 1998;124:926–28. [PubMed] [Google Scholar]
- 8.Kuhlman JE, Fishman EK, Wang KP, et al. Mediastinal cysts: diagnosis by CT and needle aspiration. Am J Roentgenol. 1988;150:75–78. doi: 10.2214/ajr.150.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Segal SR, Sherman NH, Rosenberg HK, et al. Ultrasonographic features of gastrointestinal duplications. J Ultrasound Med. 1994;13:863–70. doi: 10.7863/jum.1994.13.11.863. [DOI] [PubMed] [Google Scholar]
- 10.Bhutani MS, Hoffman BJ, Reed C. Endosonographic diagnosis of an esophageal duplication cyst. Endoscopy. 1996;28:396–97. doi: 10.1055/s-2007-1005489. [DOI] [PubMed] [Google Scholar]
- 11.Łukasik E, Rusek-Zychma M, Łukasik M, et al. Trudności diagnostyczne rozpoznania uchyłka Meckela i zdwojenia jelita cienkiego u dzieci z niedokrwistością i nawracającymi bólami brzucha – opis przypadków. Pediatria Współczesna Gastroenterologia, Hepatologia i Żywienie Dziecka. 2002;4:177–80. [in Polish] [Google Scholar]
- 12.Brown RL, Azizkhan RG. Gastrointestinal bleeding in infants and children: Meckel’s diverticulum and intestinal duplication. Semin Pediatr Surg. 1999;8:202–9. doi: 10.1016/s1055-8586(99)70027-2. [DOI] [PubMed] [Google Scholar]
- 13.Ros PR, Olmsted WW, Moser RP, et al. Mesenteric and omental cysts: histologic classification with imaging correlation. Radiology. 1987;164:327–32. doi: 10.1148/radiology.164.2.3299483. [DOI] [PubMed] [Google Scholar]
- 14.Amjadi K, Poenaru D, Soboleski D, et al. Anterior rectal duplication: a diagnostic challenge. J Pediatr Surg. 2000;35:613–14. doi: 10.1053/jpsu.2000.0350613. [DOI] [PubMed] [Google Scholar]
- 15.Glazer HS, Siegel MJ, Sagel SS. Low-attenuation mediastinal masses on CT. Am J Roentgenol. 1989;152:1173–77. doi: 10.2214/ajr.152.6.1173. [DOI] [PubMed] [Google Scholar]
- 16.Graeber GM, Thompson LD, Cohen DJ, et al. Cystic lesion of the thymus. An occasionally malignant cervical and/or anterior mediastinal mass. J Thorac Cardiovasc Surg. 1984;87:295–300. [PubMed] [Google Scholar]
- 17.Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics. 2002;22:S79–93. doi: 10.1148/radiographics.22.suppl_1.g02oc09s79. [DOI] [PubMed] [Google Scholar]
- 18.Lewis CR, Manoharan A. Benign thymic cysts in Hodgkin’s disease: report of a case and review of published cases. Thorax. 1987;42:633–34. doi: 10.1136/thx.42.8.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst. Case report. Am J Gastroenterol. 1980;73:329–32. [PubMed] [Google Scholar]
- 20.LeBlanc J, Guttentag AR, Shepard JA, et al. Imaging of mediastinal foregut cysts. Can Assoc Radiol J. 1994;45:381–86. [PubMed] [Google Scholar]
- 21.Stoupis C, Ros PR, Abbitt PL, et al. Bubbles in the belly: imaging of cystic mesenteric or omental masses. Radiographics. 1994;14:729–37. doi: 10.1148/radiographics.14.4.7938764. [DOI] [PubMed] [Google Scholar]
- 22.Nissan S. Duplications of the stomach. Am J Surg. 1960;100:59–63. doi: 10.1016/0002-9610(60)90539-0. [DOI] [PubMed] [Google Scholar]
- 23.Kohut I, Broen B, Mandat K, et al. Torbiel enterogenna kątnicy przyczyną przewlekłego wgłobienia jelit u szesnastoletniego chłopca. Chirurgia Pol. 2007;9:237–43. [in Polish] [Google Scholar]
- 24.Inouye WY, Farrell C, Fitts WT, et al. Duodenal duplication: case report and literature review. Ann Surg. 1965;162:910–16. doi: 10.1097/00000658-196511000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walecka A, Gawrych E, Sawicki M, et al. Sprawność metod obrazowych w diagnozowaniu wybranych wrodzonych wad rozwojowych okresu noworodkowego oceniona w materiale własnym. Annales Academiae Medicae Stetinensis. 2009;55:28–35. [in Polish] [PubMed] [Google Scholar]
- 26.Rajah S, Ramanujam TM, Anas SR, et al. Duplication of the rectum: report of four cases and review of the literature. Pediatr Surg Int. 1998;13:373–76. doi: 10.1007/s003830050343. [DOI] [PubMed] [Google Scholar]
- 27.Park WH, Choi SO, Park KK. Cystic rectal duplication: a rare cause of neonatal bladder-outlet obstruction and hydronephrosis. Pediatr Surg Int. 2001;17:221–23. doi: 10.1007/s003830000461. [DOI] [PubMed] [Google Scholar]
- 28.Altinli E, Balkan T, Uras C, et al. Rectal duplication as an unusual cause of chronic perianal fistula in an adult: report of a case. Surg Today. 2004;34:796–98. doi: 10.1007/s00595-004-2802-9. [DOI] [PubMed] [Google Scholar]
- 29.Knudtson J, Jackson R, Grewal H. Rectal duplication. J Pediatr Surg. 2003;38:1119–20. doi: 10.1016/s0022-3468(03)00248-3. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez-Resendiz A, Asz J, Medina-Vega FA, et al. Anterior colorectal duplication presenting as rectal prolapse. Pediatr Surg Int. 2007;23:919–21. doi: 10.1007/s00383-007-1908-8. [DOI] [PubMed] [Google Scholar]