Abstract
Calcifying epithelial odontogenic tumors (CEOTs) are rare neoplasms derived from dental tissue with the unique characteristic of calcifying amyloid-like material.
Objectives
To establish primary CEOT epithelial-derived cell populations, investigate the expression of enamel matrix proteins (EMPs), and identify potential ameloblastin (AMBN) and patched I (PTCHI) gene alterations.
Materials and Methods
A 28-year-old patient with a lesion of the posterior maxilla, radiographically characterized by a radiolucency with well-defined borders containing mixed radiopacities, agreed to participate with informed consent. The patient's biopsy confirmed the diagnosis of CEOT, and a small representative tumor fragment was ascertained for cell culture. Explant cultures were established and used to establish primary cell populations. These were analyzed for morphology, cell proliferation, mineralization activity, expression of epithelial-associated markers (qRT-PCR and immunocytochemistry), and gene mutations of AMBN or PTCHI. DNA was extracted from tumor cells and gene coding and exon–intron boundaries overlapping fragments amplified. PCR products were bidirectional DNA sequenced and compared against reference sequence.
Results
A CEOT cell population was established and proliferated in culture and could be maintained for several passages. Expression of EMPs, cytokeratin 14 and 17, and patched (PTCHI), as well as ALP activity, was detected. These cells also had the ability to mineralize, similar to the primary tumor. Two AMBN alterations were identified in the sample: c.1323G>A/A441A (rs7680880) and c.1344*+111delA. Two single-nucleotide polymorphisms were identified in the PTCHI gene.
Conclusions
Our data support the establishment of a CEOT-derived cell population, which expresses known epithelial-associated proteins.
Keywords: ameloblastin, calcifying epithelial odontogenic tumor, enamel matrix proteins, odontogenic tumors, patched
Introduction
Calcifying epithelial odontogenic tumor (CEOT) is a locally aggressive, benign neoplasm arising from the epithelial remnants of dental tissue (1). It was originally described by the Danish pathologist Jens J. Pindborg in 1955 as an unusual type of ameloblastoma. The histopathologic definition, according to World Health Organization classification (2005), describes the tumor as a locally invasive epithelial neoplasm characterized by the development of intra-epithelial structures of an amyloid-like nature, which may become calcified and liberated as the cells break down. The tumor appears as sheets of polyhedral epithelial cells with well-defined borders and distinct bridges. CEOT often exhibits mineralization within radiolucent lesions (2). A unique feature of these tumors is the production of amyloid-like material, which calcifies in a concentric pattern in the form of Liesegang rings. The underlying nature of the amyloid-like material is still unresolved. When stained with Congo red, the amyloid-like material appears as a green bifringement under polarized light (3).
CEOTs are rare, accounting for only 1% of all odontogenic tumors (4). The tumor may appear as a slow-growing, painless mass; however, depending on the tumor size, growth pattern, or location near neurovascular structures, some pain may be experienced (2, 5). Clinically, two tumor sites have been reported, intra-osseously (central or within the bone) and extraosseously (peripheral) (1). CEOT is commonly located in the molar and premolar region of the mandible. It is equally prevalent among both genders of 30–50 years of age. CEOT is associated with both erupted and unerupted teeth, and it may extend to important anatomical structures (6). Tumor size ranges from 1 to 4 cm. Grossly, CEOT color varies from greyish-white or yellow to tan pink. Surgical enucleation of all tissue associated with the lesion is the common treatment. However, these treatment modalities should be specific for each lesion because histological and radiographical features vary (1). The rate of recurrence reported varies widely with Franklin and Pindborg having reported a rate of 14% (7). Of the extraosseous cases reported by Philipsen so far, none have shown signs of recurrence after surgical enucleation (1). Kamath and Abraham recommend long-term periodic follow-up as recurrence occurred 3 years later in their case (8). Kawano et al. reported repeated local recurrence, in which the CEOTs underwent malignant transformation and developed metastatic tumors to the lung (9).
Several studies have investigated the CEOT pathogenesis; however, it remains controversial. Mutations in the ameloblastin (AMBN) gene have been reported in CEOT. AMBN, an enamel matrix protein (EMP), plays an important role in the differentiation of ameloblast cells and epithelium-mesenchyme signaling during odontogenesis (5). Perdigao et al. have reported an AMBN mutation in a CEOT tumor may be relevant to the tumor formation (5). This report prompted us to investigate AMBN and other enamel genes that may be responsible for tumor development.
Mutations in the hedgehog (HH) pathway, which is known to function in the development of teeth among several other developmental processes, have also been reported in odontogenic tumors. Recently, Peacock et al. suggested a mutation in the HH signaling pathway genes may be involved in the development of CEOT. Patched (PTCH) is a 12-pass transmembrane glycoprotein repressing receptor of the HH pathway (10, 11). When HH ligands (Sonic, Indian, Desert) bind to PTCH, smoothened (SMO) inhibition is released and the GLI family of transcription factors induced target genes (11, 12). Gene mutations in PTCH1 have been identified in CEOT (10).
Studies developing characterizing and novel molecular-based therapeutics for CEOTs have been hindered because of lack of an established cell population or cell line. This study is designed to establish a primary CEOT-derived cell population that would be useful in the advancement of basic research regarding development of this type of tumor and the testing of pharmacologic therapeutic agents. Further identification of CEOT tumor cell populations and any alternations in the ameloblast enriched EMP AMBN or the HH-related PTCH1 will provide data for the research of molecular and genetic mechanism of CEOT as well as identifying a possible new target for CEOT treatment.
Materials and methods
Tissue specimen and establishment of cell populations
A 28-year-old patient with a lesion of the posterior maxilla agreed to participate in this study with appropriate informed consent. The study was independently reviewed and approved by the University of Alabama at Birmingham Institutional Review Board. The patient's biopsy confirmed the diagnosis of CEOT, and a small representative tumor fragment was ascertained for cell culture. The tissue was dissected to expose epithelial cords, and primary cell populations were established by explant culture in DMEM with 10% FBS plus antibiotics as previously described.(13) Low-passage (3–6) cell stocks were sustained in liquid nitrogen at −80°C.
Alkaline phosphatase (ALP) activity
A tumor piece and established primary cells grown in 4-well chamber slides and incubated overnight were stained for ALP using the Invitrogen NBT/BCIP Reagent kit (Invitrogen, Carlsbad, CA, USA).
Determination of cell growth rate
Cell proliferation was measured using a MTS assay (Cell Titer96, Promega, Madison, WI, USA). Cultured CEOT cells were plated on a 96-well plate and harvested for cell viability measurements on days 3, 5, 7, and 9. The absorbance at 490 nm was measured (Bio-Tek's Kcjunior, BioTek, Winooski, VT, USA). Growth rates were plotted according to the 490-nm absorbance.
Immunofluorescence and Immunocytochemistry
Commercially available antibodies directed against pan keratin (Ventana, Tucson, AZ, USA), cytokeratin 17 (CK17, Cell Signaling, Danvers, MA, USA), cytokeratin 14 (CK14, Abcam, Cambridge, MA, USA), amelogenin (AMGN, Sigma, St Lois, MO, USA), and enamelin (ENAM, Santa Cruz, Santa Cruz, CA, USA) were used for this study. Primary antibodies for odontogenic ameloblast-associated protein (ODAM; aa 127–172, a gift from Dr. Kestler) (14), AMBN (15), and amelotin (AMTN) (a gift from Dr. Ganss) were also used. Cells were grown for 3–5 days in 4-well chamber slides and fixed with 4% formaldehyde, blocked with 10% BSA, and incubated with primary antibodies for 1–2 h at room temperature or overnight at 4°C. For immunofluorescence, samples were incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Invitrogen) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for nucleus localization. For immunocytochemistry, samples were treated with a secondary antibody for approximately 30 min, and peroxidase stained tissue color was developed by SuperPicTureTM Polymer Conjugate Broad Spectrum Kit (Invitrogen). Negative controls were incubation with normal serum and no primary antibody. Samples were imaged with a Nikon Eclipse TE2000-E inverted microscope (Nikon Instruments, Melville, NY, USA).
Quantification real-time PCR (qRT-PCR)
Total RNA was isolated from a CEOT cell population using the RNA STAT-60 kit (TEL-TEST, INC, Friendswood, Texas, USA) according to manufacturer's directions. The isolated total RNA was then reverse transcribed into cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA). The ABI Prism 7500 sequence detection system (Applied Biosystems) was used to perform quantitative real-time PCR (qRT-PCR) analysis of the relative transcriptional levels of selected genes. Reactions were performed using the RT2 SYBR Green/Rox qPCR master mix (SABiosciences, Frederick, MD, USA) according to manufacturer's instructions. All the primers for the gene expression profile were obtained from RT2 qPCR primer arrays (SABiosciences). Transcripts evaluated in this study were as follows: AMBN, AMGN, ATMN, ENAM, ODAM, PTCH1, and CK14. Cycle threshold (Ct) values for transcription levels were obtained and normalized to the housekeeping gene GAPDH to determine the ΔCT value.
Vital mineralization staining
Xylenol orange (XO) staining was performed to analyze for mineralization in live cell cultures. Xylenol orange powder (Sigma) was dissolved in distilled water and filtered to make the 20 mm stock solution, which was stored at 4°C. XO was added to culture medium at a concentration of 20 μm for 4 h and removed. Staining of mineralization with XO is seen as a red color under the fluorescent microscope using a TRITC red filter (Chroma Technology, Rockingham, VT, USA).
DNA extraction
Total genomic DNA was extracted from cells cultured from a CEOT using Wizard genomic DNA extraction kit (Promega) following manufacturer's instructions.
PCR and direct DNA sequencing
The coding and exon–intron boundaries of the AMBN and PTCH1 were amplified using gene specific primers by PCR. PCR product was checked on agarose gel, and 5 μl PCR products were purified by ExoSap enzyme. Bi-directional sequencing of the PCR product was performed using Bigdye 3.0 by ABI 3730 sequencer. Sequencing results were compared against reference AMBN sequence (NC_000004. 10;71492590-71507594) and PTCH1 reference sequence (NC 000009;98205264-98279247) using SeqScape2.5 (Applied Biosystems). The alterations were also checked against available databases to determine whether the alterations are known single-nucleotide polymorphisms (SNPs).
Results
Histopathology of CEOT
A CEOT tumor from a 28-year-old patient with a lesion of the posterior maxilla radiographic ally characterized by well-defined borders and containing mixed areas of radiopacities within a radiolucent background was obtained (Fig. 1A and B). The patient's biopsy confirmed the diagnosis of CEOT, and a small representative tumor fragment was ascertained for cell culture.
Figure 1.
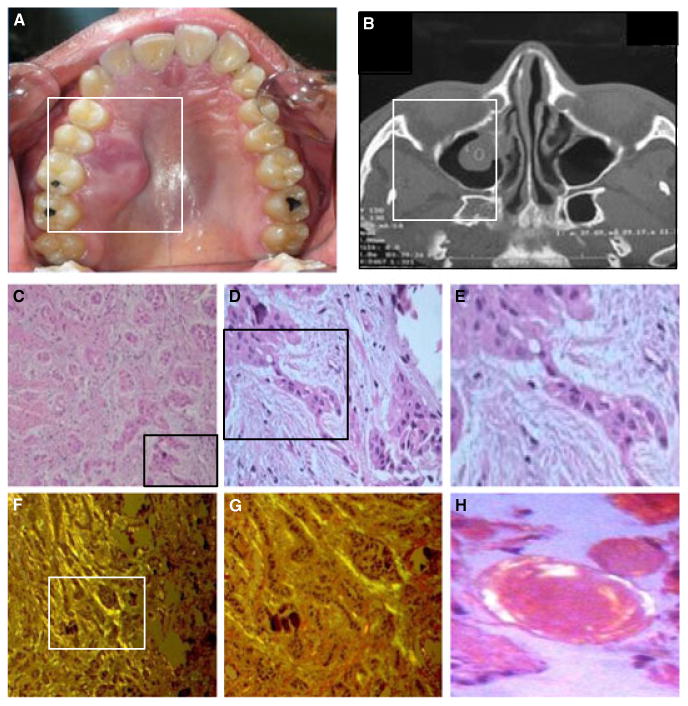
Clinical and histological features of the primary CEOT tumor. (A) Clinical aspect of the tumor showing marked expansion of cortical bone and extension to the palate. (B) Radiographic presentation of the maxillary lesion showing an expansive mass without perforation. (C-E) H and E of CEOT show cords and nests of odontogenic epithelium (C) (original magnification ×10) and epithelial cords showing areas of hyperchromatic nuclei and pleomorphism (D) (magnification × 20) and (E) (magnification × 40). (F-G) Congo red staining of tumor section showing green fluorescence of mineralization (original magnification × 20 and × 40). (H) H and E staining of amyloid deposits in the section (magnification × 100).
The tumor was histologically composed of numerous cords, strands, and islands of polygonal epithelial cells in a fibrous connective tissue stroma (Fig. 1C–E). These epithelial cells showed intercellular bridges in focal areas, with prominent hyperchromatic and pleomorphic nuclei. Areas of an amorphous, eosinophilic, hyalinized material consistent with amyloid extracellular material were observed within the stroma. Numerous foci of calcified material in a concentric pattern (Liesegang rings) were also evident. Under polarized light characteristic of CEOT, the amyloid-like material exhibited green bifringement when stained with Congo red (Fig. 1F–G).
Establishment of CEOT cell population
Tumor tissue shown in Fig. 2A was used to establish explant cultures. The tumor exhibited characteristics of mineralization shown by ALP staining (Fig. 2B). The tumor tissue was minced, dissociated to free epithelial cords, and used to establish the explant cultures. Primary cells were established by passaging the initial outgrowths from isolated epithelium (Fig. 2C–D). The established cell populations appear as squamous epithelia (Fig. 2D–G). To begin initial characterization of the cell population, we examined ALP activity as a marker of mineralization potential. We observed strong ALP staining the majority of the cells (Fig. 2E–F). Similar to the primary specimen, the cell population showed green bifringement staining with Congo red under polarized light (Fig. 2G–H). This cell population was maintained in cell culture under low passages. The growth rate increased until day 7 and decrease thereafter, demonstrating our ability to maintain cells in culture (Fig. 2I).
Figure 2.

Establishment and characterization of CEOT primary cell population. (A) Photomicrograph of a portion of the tumor. (B) In situ histochemical ALP staining of CEOT tumor piece. (C) Tumor explant culture showing cell outgrowth. (D) Phase contrast micrograph of CEOT cell population (magnification ×10). (E-F) ALP staining of CEOT cells (magnification × 10 (E) and × 40 (F)). Congo red staining under non-polarized light of the CEOT cells (G) and polarized light (H) showing green areas of mineralization (magnification × 10). (I) Growth chart of CEOT cell population over 9 days of culture.
Characterization of CEOT
To initially confirm the epithelial origins of the established cell population, we examined the expression of epithelial cell markers, pan keratin, CK14, and CK17. The pan keratin antibody broadly recognizes a number of human cytokeratins that are components of the epithelial cell cytoskeleton. CK17 also expressed by epithelial cells has been shown to be a component of other epithelial tumors such as keratocystic odontogenic tumors and oral squamous cell carcinoma (16, 17). CK14 is an additional marker of epithelial cells. Furthermore, the CK14 promoter has been used to create conditional knockouts in epithelial cells of dental ameloblast lineage (18). The CEOT cell population stained uniformly positive for all three keratins (Fig. 3A–C) with no detectable staining seen in the control (3D). Any potentially negative cells within the CEOT population would be identified by nuclear staining using DAPI (2A-C). CK14 expression was confirmed at the mRNA level by qRT-PCR (Fig. 3D). In addition, we examined EMP expression that has been associated with dental epithelium and other odontogenic tumors (5, 19). AMGN, AMTN, ENAM, and ODAM transcript levels were detected by qRT-PCR (Fig. 3D). However, their levels of expression varied with AMBN having the lowest level. Diffuse cytoplasmic staining was exhibited for AMBN, AMGN, AMTN, ENAM, and ODAM (Fig. 3E–J), while AMBN and AMGN also demonstrated strong nuclear staining. PTCH1, which has been previously associated with CEOT, was detected in the CEOT cell population at the most abundant level of expression (Fig. 3E, J) (10). The CEOT cell population with H and E staining showed characteristic hyperchromatic and pleomorphic nuclei features (3M). Also these tumors are described as having larger giant cells within the epithelial cords that are evident in the established cell population (arrowhead, 3M).
Figure 3.
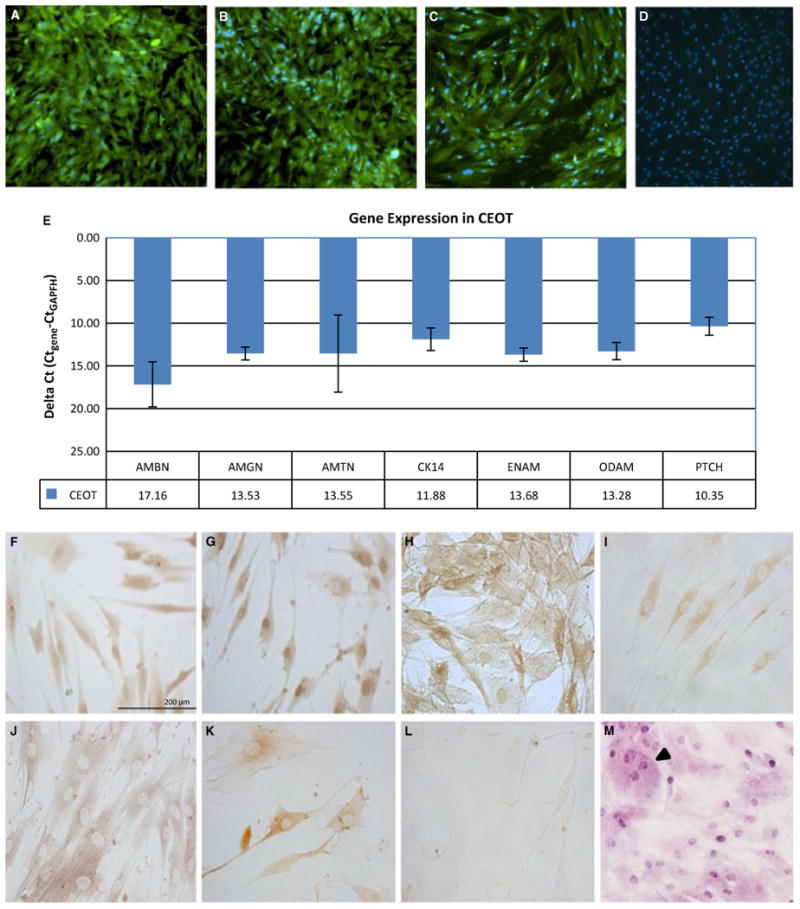
Gene and protein expression profile of the CEOT cell population. (A-C) Immunofluorescence cytochemistry (green stain) of (A) pan keratin, (B) CK17, and (C) CK14 (original magnification ×10) with nuclear counterstained using DAPI (blue). (D) Negative control for keratin antibodies using no primary antibody cells stained with DAPI. (E) Gene expression as determined by qRT-PCR. Housekeeping gene GAPDH was used to normalize data. Immunocytochemistry analysis of (F) AMBN, (G) AMGN, (H) AMTN, (I) ENAM, (J) ODAM, (K) PTCH, and (L) the negative control with no primary antibody. (M) H and E staining of the CEOT cells showing presence of giant cells (arrowhead) and hyperchromatic nuclei (scale bar = 720 μm).
Mineralization analysis
Xylenol orange incorporates into newly mineralized tissue and can be used as a vital indicator of mineralization potential (20). In the CEOT cell cultures, no detectable mineralization was observed at 7 days in culture but became observed through 28 days of culture (Fig. 4). Production of a mineralized amyloid material is consistent with the properties of the primary tumor.
Figure 4.

Xylenol orange vital staining for mineralization of the CEOT cells in long-term culture. Primary cell cultures were stained at day 7 (A, C) and day 28 (B, D). Cells imaged under fluorescence (C-D) (scale bar = 1000 μm) demonstrate positive (red) mineralization in the terminal 28-day cultures.
DNA sequencing analysis
The association of AMBN gene mutations with epithelial odontogenic tumors identified by Perdigao et al. prompted us to investigate any potential AMBN genetic alterations within our CEOT sample (5). Figure 5A–B shows two identified AMBN gene alterations. Also the implication of HH pathway in the pathogenesis of CEOT by Peacock et al. encouraged us to investigate any potential alterations within PTCH1 (10). Figure 5C–D illustrates two SNPs identified with the PTCH sequencing analysis.
Figure 5.
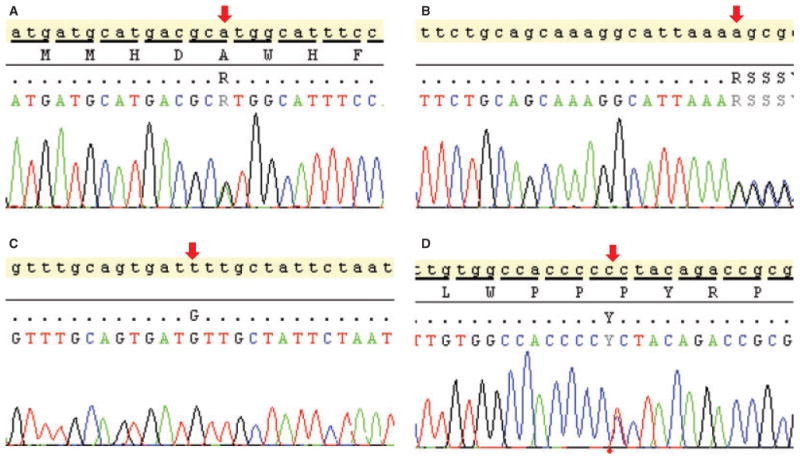
Genetic alterations in primary CEOT sample. Two AMBN DNA sequence variations and two PTCH SNPs were identified in this study. (A) c.1323G>A, p.A441A (rs7680880) in exon 13 and (B) c.1334 + 111delA in the 3′ untranslated region (UTR) of AMBN. (C) c.395-36T>G (D) and c.3944C>T, p.P1315L identified alterations in PTCH1.
Discussion
The establishment of a primary CEOT population would be useful in understanding the fundamental characteristics and phenotypic profile of CEOTs for the development of potential novel therapeutics. This study establishes a primary CEOT population for the first time and explores its basic characteristics. CEOTs are reported to be epithelial-derived tumors. The established cell population expressed various cytokeratins, including CK17 and CK14, thus verifying the epithelial origin of the cells. EMPs, such as AMBN, AMGN, and ENAM, are secreted by ameloblasts during enamel formation and have been shown to be expressed by odontogenic tumors (11, 13, 19). Our investigation confirms expression of these EMPs at both the mRNA and protein levels. These results suggest a dental origin for the CEOT cell population.
Another important maintained characteristic of the CEOT cells is their ability to produce a mineralized matrix in vitro. High ALP expression is commonly associated with mineralization potential of matrix-producing cells. The majority of the cells in culture demonstrated high levels of ALP staining can correlated with their ability to mineralize in culture similar to the primary tumor tissue. Further supporting their mineralization potential was the incorporation of XO into the cell cultures over time. CEOT is unique from other odontogenic tumors based on the production of an amyloid-like material. Reports indicate the amyloid material contains ODAM (also known as Apin) (21). Histologically, after Congo red staining, the amyloid appears as a green bifringement when viewed under polarized light in the primary tumor and isolated cell population. Our results confirm production of amyloid, as well as expression of ODAM by the cell population.
While the pathogenesis of CEOT remains to be clarified, genetic alterations within the AMBN and PTCH1 gene have been associated with these primary tumors. Of the AMBN alterations identified in this study, one was a missense mutation and the other was a deletion in the 3′-untranslated region both likely not leading to a loss of function. Similarly, the PTCH1 alterations discovered were previously identified SNPs, one producing an amino acid change (P1315L) of unknown functional significance. Interestingly, this polymorphism has been detected in breast cancer showing a significant association with the disease as compared to healthy controls (22). Mutations in other HH pathway proteins, such as SMO, PTCH2, or SUFU, have been associated with the development of odontogenic tumors and may also play a role in CEOTs (23).
Our results demonstrated for the first-time establishment of a stable primary CEOT cell population. The CEOT epithelial-derived cells were able to be maintained in culture, expressed numerous dental associated EMPs, and had the ability mineralize similar to the properties of the primary tumor. This established cell population will be extremely useful to immortalize, creating stable clonally derived cell lines. These cell populations and cell lines will be critical for future studies characterizing this tumor type, determining the pathogenesis of CEOT, and developing novel targeted therapeutics for this rare tumor.
Acknowledgments
We would like to thank the patient for their participation in this study; and Y. Wu and O. Mamaeva for their technical assistance.
Conflict of interest: This study was supported by NIDCR – DART T32DE017601/T90DE022736, the University of Alabama at Birmingham (UAB) School of Dentistry Institute of Oral Health Research, and the UAB Global Center for Craniofacial Oral and Dental Disorders (GC-CODED).
References
- 1.Philipsen HP, Reichart PA. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36:17–26. doi: 10.1016/s1368-8375(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 2.Sedghizadeh PP, Wong D, Shuler CF, Linz V, Kalmar JR, Allen CM. Multifocal calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e30–4. doi: 10.1016/j.tripleo.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 3.Solomon A, Murphy CL, Weaver K, et al. Calcifying epithelial odontogenic (Pindborg) tumor-associated amyloid consists of a novel human protein. J Lab Clin Med. 2003;142:348–55. doi: 10.1016/S0022-2143(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira MG, Chaves AC, Visioli F, et al. Peripheral clear cell variant of calcifying epithelial odontogenic tumor affecting 2 sites: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:407–11. doi: 10.1016/j.tripleo.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Perdigao PF, Carvalho VM, De Marco L, Gomez RS. Mutation of ameloblastin gene in calcifying epithelial odontogenic tumor. Anticancer Res. 2009;29:3065–7. [PubMed] [Google Scholar]
- 6.Pilch BZ. Head and neck surgical pathology. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 7.Franklin CD, Pindborg JJ. The calcifying epithelial odontogenic tumor. A review and analysis of 113 cases. Oral Surg Oral Med Oral Pathol. 1976;42:753–65. doi: 10.1016/0030-4220(76)90098-0. [DOI] [PubMed] [Google Scholar]
- 8.Kamath G, Abraham R. Recurrent CEOT of the maxilla. Dent Res J (Isfahan) 2012;9:233–6. doi: 10.4103/1735-3327.95242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano K, Ono K, Yada N, et al. Malignant calcifying epithelial odontogenic tumor of the mandible: report of a case with pulmonary metastasis showing remarkable response to platinum derivatives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:76–81. doi: 10.1016/j.tripleo.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Peacock ZS, Cox D, Schmidt BL. Involvement of PTCH1 mutations in the calcifying epithelial odontogenic tumor. Oral Oncol. 2010;46:387–92. doi: 10.1016/j.oraloncology.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Ren C, Amm HM, Devilliers P, et al. Targeting the sonic hedgehog pathway in keratocystic odontogenic tumor. J Biol Chem. 2012;287:27117–25. doi: 10.1074/jbc.M112.367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diniz MG, Borges ER, Guimaraes AL, et al. PTCH1 isoforms in odontogenic keratocysts. Oral Oncol. 2009;45:291–5. doi: 10.1016/j.oraloncology.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Ren C, Diniz MG, Piazza C, et al. Differential enamel and osteogenic gene expression profiles in odontogenic tumors. Cells Tissues Organs. 2011;194:296–301. doi: 10.1159/000324759. [DOI] [PubMed] [Google Scholar]
- 14.Kestler DP, Foster JS, Macy SD, Murphy CL, Weiss DT, Solomon A. Expression of odontogenic ameloblast-associated protein (ODAM) in dental and other epithelial neoplasms. Mol Med. 2008;14:318–26. doi: 10.2119/2008-00010.Kestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebsbach PH, Lee SK, Matsuki Y, Kozak CA, Yamada KM, Yamada Y. Full-length sequence, localization, and chromosomal mapping of ameloblastin. A novel tooth-specific gene. J Biol Chem. 1996;271:4431–5. doi: 10.1074/jbc.271.8.4431. [DOI] [PubMed] [Google Scholar]
- 16.Aragaki T, Michi Y, Katsube K, et al. Comprehensive keratin profiling reveals different histopathogenesis of keratocystic odontogenic tumor and orthokeratinized odontogenic cyst. Hum Pathol. 2010;41:1718–25. doi: 10.1016/j.humpath.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Wei KJ, Zhang L, Yang X, et al. Overexpression of cytokeratin 17 protein in oral squamous cell carcinoma in vitro and in vivo. Oral Dis. 2009;15:111–7. doi: 10.1111/j.1601-0825.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Zhou YL, Gonzalez FJ, Snead ML. CCAAT/enhancer-binding protein delta (C/EBPdelta) maintains amelogenin expression in the absence of C/EBPalpha in vivo. J Biol Chem. 2007;282:29882–9. doi: 10.1074/jbc.M702097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC. Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med. 2012;41:272–80. doi: 10.1111/j.1600-0714.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 20.Shu R, McMullen R, Baumann MJ, McCabe LR. Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J Biomed Mater Res A. 2003;67:1196–204. doi: 10.1002/jbm.a.20021. [DOI] [PubMed] [Google Scholar]
- 21.Murphy CL, Kestler DP, Foster JS, et al. Odontogenic ameloblast-associated protein nature of the amyloid found in calcifying epithelial odontogenic tumors and unerupted tooth follicles. Amyloid. 2008;15:89–95. doi: 10.1080/13506120802005965. [DOI] [PubMed] [Google Scholar]
- 22.Chang-Claude J, Dunning A, Schnitzbauer U, et al. The patched polymorphism Pro1315Leu (C3944T) may modulate the association between use of oral contraceptives and breast cancer risk. Int J Cancer. 2003;103:779–83. doi: 10.1002/ijc.10889. [DOI] [PubMed] [Google Scholar]
- 23.Li TJ. The odontogenic keratocyst: a cyst, or a cystic neoplasm? J Dent Res. 2011;90:133–12. doi: 10.1177/0022034510379016. [DOI] [PubMed] [Google Scholar]


