Abstract
There is increasing evidence of the potential for radiation therapy to generate antitumor immune responses. The mechanisms of this immune-activating potential include actions on tumor cells such as immunogenic cell death and phenotypic change. Radiation modulates tumor cell surface expression of cell death receptors, tumor-associated antigens and adhesion molecules. This process of immunomodulation sensitizes tumor cells to immune-mediated killing. Radiation also affects immune compartments, including antigen-presenting cells, cytotoxic T lymphocytes and humoral immunity, leading to specific antitumor immune responses. Recognizing the importance of immunity as a potentiator of response to radiation leads to rational augmentation of antitumor immunity by combining radiation and immunotherapy. Targeted immunotherapy manipulates the immune system in a way that best synergizes with radiation. This article discusses the ability of radiation monotherapy to induce antitumor immunity, with a focus on the effect of radiation on antigen-presenting cells and cytotoxic T lymphocytes. We define two important responses generated by tumor cells, immunogenic cell death and immunomodulation, both of which are radiation dose-dependent. In conclusion, we describe the translation of several combination therapies from the preclinical to the clinical setting and identify opportunities for further exploration.
INTRODUCTION
Radiation therapy (RT) is an integral component of cancer care. A recently published article in the Journal of Clinical Oncology reported that the demand for RT during the initial course of cancer treatment is expected to increase by 22% (from 470,000 patients receiving RT in 2010 to 575,000 in 2020) as a result of the aging and diversification of the U.S. population (1). Radiation therapy is delivered as low or high dose. In addition, external beam radiation therapy (EBRT), commonly used in the clinic, can be delivered as standard fractionation of 1.8–2.0 Gy daily for 5–9 weeks or a large dose with less numbers of fractions (hypofractionated doses) or as small doses given in multiple fractionation setting (hyperfractionated doses). Irrespective of the quality and/or delivery of radiation, immune cells are the most radiation-sensitive cells in the body. Immunosuppressive effects after whole-body irradiation, as well as possible immune activation during tumor therapy, have been observed with the use of high-dose ionizing radiation. Conversely, the effects of low-dose ionizing radiation on the immune system are controversial and seem to vary greatly among individuals and species (2).
Traditionally, RT is employed to destroy tumor cells or to alter tumor/stroma architecture with either curative or palliative intent. However, it is often the case that not all tumor cells in a given mass receive a lethal dose of radiation due to dose constraints mandated by the need to limit damage to normal tissue. Nevertheless, even sublethal doses of radiation can generate potent immune responses by altering tumor cells in a variety of ways (3, 4). Combining radiation therapy with active immunotherapy allows one to exploit two broad areas: 1. radiation-induced tumor cell death as a potential source of tumor antigens for immunotherapy; and 2. postirradiation tumor cell modulation that allows more efficient immune cell access and increased sensitivity to T-cell killing (3, 4). These tumor-specific T cells could arise endogenously or can be induced from active vaccination strategies. Many clinical trials exploring the use of radiation and vaccines in the treatment of cancer are currently underway.
Preclinical studies often employ EBRT. Preclinical work with modalities of RT other than EBRT provide evidence that RT delivery techniques, such as bone-seeking radionuclides, radiolabeled monoclonal antibodies (mAb) and brachytherapy synergize with immunotherapy similarly to EBRT (4). Studies of high-dose spatially fractionated GRID radiation therapy (SFGRT) also offer evidence of an immunostimulatory capacity that may complement immunotherapy (5). Here, we review the immunogenic nature of radiation in preclinical models as well as in the clinic. We also provide a rationale for combining RT with immunotherapeutic approaches.
RADIATION MONOTHERAPY INDUCES ANTITUMOR IMMUNE RESPONSE
RT has played a central role in cancer therapy since the first successful radiation treatment of basal cell carcinoma in 1899 (6), yet new findings continue to emerge regarding the multiple mechanisms by which RT induces tumor cell killing. Increased understanding of radiation therapy's action on tumor cells and components of the tumor microenvironment has in turn highlighted the importance of the immune system, as evidenced by Lee et al., who reported that in a mouse model, RT requires the presence of CD8+ T cells for post-RT tumor control (Table 1) (7).
TABLE 1.
Radiation As Monotherapy
| Immune effect | Disease | Comments | Ref. |
|---|---|---|---|
| Radiation as monotherapy | |||
| DC/T cell | Melanoma | OVA-specific DCs and T cells in OVA-expressing B16-F0 mouse model | (11) |
| T cell | Melanoma | Increased IFN-γ+ tumor-specific splenocytes in OVA-B16-F0 mouse model | (14) |
| Antibody | Prostate cancer | De novo antibody to prostate antigens | (12) |
| T cell | Colorectal/prostate cancer | Survivin-specific CD8+ T cells | (13) |
| DC/T cell | Melanoma | Radiation-induced T-cell priming and DC maturation | (7) |
| Radiation combined with immunotherapy | |||
| T cell | Prostate cancer | Listeria monocytogenes-based PSA vaccine and EBRT | (39) |
| Antibody/NK cell | Lung cancer | TLR9 agonist and EBRT | (38) |
| T cell | Breast cancer | Anti-CD137, anti-PD1 and EBRT | (37) |
| T cell | Colon cancer | Yttrium-90 anti-CEA mAb and CEA/TRICOM | (52) |
| T cell | Prostate/breast/lung cancer | Samarium-153 | (58) |
| T cell | Colon cancer | EBRT and anti-CEA mAb and CEA/TRICOM | (34) |
Recently reported studies have introduced the concept that RT may lead to the conversion of tumor into an in situ vaccine. The coincidence of proper host factors and appropriate tumor makeup can lead to immune-mediated rejection of nonirradiated metastatic lesions after irradiation of the primary lesion in a process known as the abscopal effect (8–10). Evidence for RT-generated immune responses will be discussed below. Multiple immune compartments are affected by RT, including T-cell priming, dendritic cell (DC) maturation and B-cell antibody production (Table 1) (7, 11–14).
CD8+ T cells, important mediators of antitumor immunity, can eliminate tumor cells through surface molecule interaction, including Fas/TRAIL and/or perforin/granzyme-mediated pathways (15). Lymphocytes are highly radiosensitive, in a maturation dependent fashion (16). However, several studies have shown that RT can promote productive antitumor T-cell responses despite the radiosensitivity of lymphocytes. Mouse studies have shown that local tumor irradiation (15 Gy) is associated with an increase in T cells, within draining lymph nodes (DLNs) that secrete IFN-γ upon tumor-specific peptide stimulation (Table 1) (11). Similarly, in a B16-OVA murine melanoma model, anti-OVA T-cell response, as measured by IFN-γ production, increased after RT (7.5–15 Gy), and this increase was associated with a delay in tumor growth (Table 1) (14). Clinical studies also report RT-induced CD8+ T cell activity. Schaue et al. analyzed the relationship between cytotoxic T cells, regulatory T cells (Tregs) and RT by analyzing survivin. This molecule, which is highly expressed in tumor cells, increases cell survival and proliferation and causes resistance to RT. In a study of colorectal cancer patients receiving chemoradiation (45 Gy total), 9/13 patients had increased survivin-specific CD8+ T cells in peripheral blood as a result of treatment (Table 1) (13). The apparent disconnect between T-cell radiosensitivity and the induction of productive T-cell responses after RT is likely due to the focal effect of RT versus the systemic nature of immune populations (i.e., T cells outside the radiation field are not negatively affected), suggesting that interaction between immune cells and tumor cells post-RT is essential for systemic immunity.
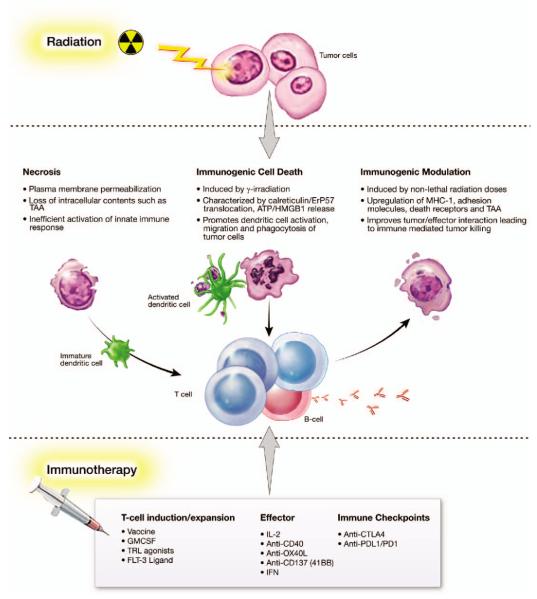
Generation of tumor-specific CD8+ T cells generally requires the maturation of DCs capable of antigen uptake and presentation (Table 1) (11). RT can induce multiple types of tumor cell death by highly regulated processes, including necrosis, apoptosis and immunogenic cell death (ICD) (Fig. 1) (17). RT-induced tumor cell death releases large numbers of tumor-associated antigens (TAAs) that are then available for uptake and presentation by DCs (18, 19). Lee et al. studied the effects of RT on DC maturation and migration and noted that 5 days after RT (20 Gy), DLNs showed an increase in tumor-specific DCs, which are associated with elevated MHC class II, a marker of maturation (7). A B16-OVA murine melanoma model showed that after a single dose of RT (15 Gy), antigen-presenting cells from DLNs exhibited a threefold greater ability to activate tumor-specific T cells (Table 1) (11). Tumor irradiation is believed to promote intratumoral DC maturation as well, with RT facilitating tumor cell death that leads to the release of TAAs available for DC uptake and presentation. DCs are able to migrate to DLNs and exhibit strong CD8+ T-cell priming capabilities (Fig. 1).
FIG. 1.
Molecular determinants of radiation effects. Immune-relevant characteristics of radiation-induced necrosis, ICD and IM, as well as real and potential immunotherapeutic interventions.
In addition to cell-mediated cytotoxicity as a mechanism of tumor cell killing, antitumor antibody production has been observed in a significant proportion of cancer patients treated with RT (Table 1) (12). Nesslinger et al. studied pre-and post-RT serum samples from 73 men with nonmetastatic prostate cancer and 50 cancer-free controls. When evaluated by Western blot, samples revealed treatment-associated autoantibody (prostate) responses in nearly 14% of prostate cancer patients compared with 6% of controls (Table 1) (12). Although 28/29 patients undergoing EBRT had previous hormonal therapy, the results are suggestive of RT-mediated autoantibody production. Also of note, pre-and post-treatment serum samples from patients who received brachytherapy with no previous hormonal therapy showed new emergence of seroreactivity in 30% of patients (12).
As discussed above, tumor irradiation can beneficially affect several immune compartments. The mechanisms by which RT induces immunostimulation have been reviewed by Burnette et al. (20). The effects of RT involve a delicate balance between immunostimulation and immunosuppression. For example, Schaue et al. treated mice with a single dose of up to 15 Gy of radiation and found that tumor control and levels of tumor-reactive T cells increased with the dose of radiation. However, at the highest dose this effect was offset by an increase in Tregs. Fractionated treatment of 7.5 Gy/fraction appeared to be optimal for induction of antitumor immunity (14). Investigators continue to study the optimal dose and schedule of RT to maximize immune response (21). Unfortunately, the abscopal effect occurs rarely, and only a small percentage of cancer patients treated with RT exhibit an immune response sufficient to affect progression-free survival or overall survival. Numerous excellent reviews discuss the potential for augmenting the post-RT immune response through combination with immunotherapy (Fig. 1) (4, 20, 22).
TUMOR IRRADIATION INDUCES IMMUNOGENIC CELL DEATH AND IMMUNOGENIC MODULATION
New RT technologies avoid damage to normal tissue while focusing heterogeneous high-dose radiation on areas of high risk for local failure (23). The impact of dose on RT-induced immune response is still controversial, but it can be hypothesized that a heterogeneous dose may lead to a similarly heterogeneous response by tumor cells. RT-treated tumor cells undergo several types of well defined cell death, including necrosis, mitotic catastrophe and immunogenic cell death (ICD) (17). However, the immunomodulatory effects of radiation do not end with cell death. Cells that survive fractionated RT show alterations in several pathways, including immune response (23). Besides inducing antitumor immunity through direct killing of tumor cells, radiation can enhance susceptibility to immune-mediated killing in tumor cells that survive RT. Below we will further describe ICD and immunogenic modulation (IM) and their role in antitumor immunity.
Zitvogel and Kroemer et al. have shown that certain chemotherapeutic regimens in combination with RT may trigger cancer cell death while stimulating endogenous immune responses against the tumor through ICD (24–29), which requires the transfer of tumor-derived antigen to immune cells that then stimulate a tumor-specific immune response (Fig. 1) (24). ICD is a cascade in which dying tumor cells release immunogenic factors that are received and processed by DCs, which in turn present antigen to activated cytotoxic T lymphocytes (CTLs). Another factor in ICD is increased ectopic expression of calreticulin on tumor cells. Interaction between calreticulin and DCs is considered an absolute requirement for ICD (Fig. 1) (30, 31). As noted above, the relationship between dose level of radiation and induction of ICD is being explored preclinically. In one study, ICD in murine colon carcinoma cells occurred in vivo after a single dose of 75 Gy of radiation (31), while indications of ICD were observed after exposure to 10 Gy of radiation in an EL-4 lymphoma cell line (32).
Nonlethal doses of radiation also appear to have an impact on immune recognition of tumor cells (Figs. 1, 2, 3 and 5). Dose heterogeneity and the need to limit toxicity to normal tissue mean that some tumor cells will not receive adequate irradiation to induce cell death. There is increasing evidence that the phenotype of tumor cells that receive sublethal doses of radiation is altered via IM, which increases their susceptibility to immune-mediated attack (4). Molecules reported to be altered by sublethal irradiation include TAAs, MHC class I, Fas/CD95 and intercellular adhesion molecule 1 (ICAM-1) (33–35). These cell surface molecules are known to play a role in CTL-mediated antitumor response. Reits et al. (35) further examined the response of MHC class I cell surface expression and intracellular peptide pool to irradiation and found that from 1 Gy up to 25 Gy induced upregulation of MHC class I expression in a dose-dependent manner, and that the response was maintained for up to 3 days. Irradiation also appeared to accelerate degradation of existing proteins, leading to an increased intracellular peptide pool for MHC class I loading. Irradiation was also shown to activate the mammalian target of rapamycin, resulting in increased protein synthesis and availability of peptide for MHC class I loading (35).
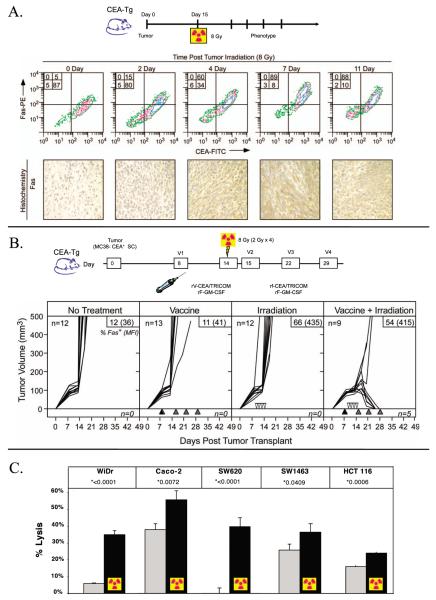
FIG. 2.
Phenotypic changes in tumor cells postirradiation increase sensitivity to T-cell lysis. Panel A: Expression of upregulated Fas on tumor cells postirradiation is maintained for >11 days. C57BL/6 mice were injected with MC38-CEA+ tumor cells subcutaneously, 14 days later, tumors were subjected to EBRT (8 Gy). Tumors were surgically removed at several time points after RT and analyzed for Fas expression by flow cytometry and immunohistochemistry. Panel B: Irradiation of tumor cells in vivo enhances efficacy of vaccine therapy. CEA-transgenic mice were injected with MC38-CEA+ tumor cells subcutaneously. As indicated, mice received no treatment; were vaccinated with rV-CEA/TRICOM on day 8 (closed triangles) followed by boosting with rF-CEA/TRICOM on days 15, 22 and 29 (gray triangles); subjected to fractionated EBRT (2 Gy) in situ on days 11, 12, 13 and 14 (open inverted triangles); or were vaccinated on day 8 (closed triangles) and subjected to fractionated EBRT. A subset of mice from each treatment group had tumors surgically removed at day 21 post-tumor transplant. Tumors were then stained with CEA and Fas antibodies. Inset panels: % Fas+ cells (mean fluorescence intensity). Taken from ref. (34). Panel C: Irradiation increases human tumor cell sensitivity to antigen-specific CTL killing. CEA+ human tumor cells were mock irradiated (gray bar) or irradiated with 10 Gy (black bar) and re-cultured for 72 h, then coincubated with HLA-A2-restricted CEA-specific CTLs. All cell lines were both CEA+ and HLA-A2+. Taken from ref. (33).
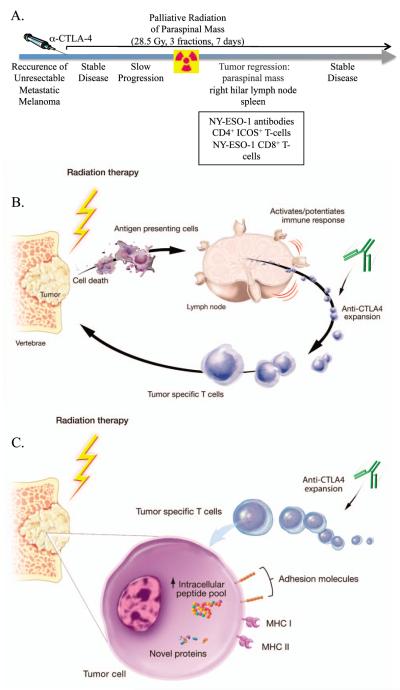
FIG. 3.
Combination therapy with anti-CTLA-4 and palliative radiation. Panel A: Patient presented with metastatic melanoma, received anti-CTLA-4, progressed and received palliative RT for a paraspinal mass. Improvement was seen in irradiated mass as well as metastatic lesion outside the radiation field. Induction of antigen-specific T- and B-cell responses were also seen. Adapted from ref. (43). Panel B: Proposed mechanism of action for anti-CTLA-4 and RT-induced primary immune responses. Panel C: Further expansion of RT-induced tumor-specific T cells.
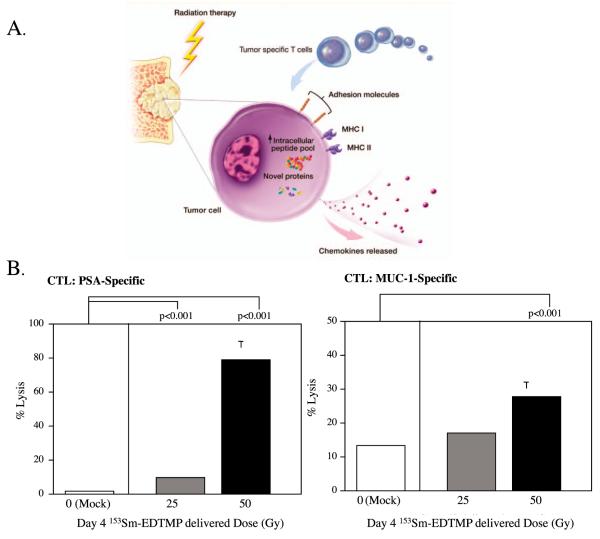
FIG. 5.
Combination therapy with vaccine and palliative radionuclide. Panel A: Irradiation modulates tumor-cell phenotype and increases immune recognition. Irradiation can cause: 1. upregulation of chemokines and adhesion molecules that signal T cells to traffic to areas of tumor; 2. upregulation of MHC molecules and TAAs, facilitating T-cell recognition of tumor; and 3. upregulation of Fas and downregulation of Tregs, facilitating tumor-specific CTL killing. Taken from ref. (3). Panel B: Effect of 153Sm-EDTMP on sensitivity of human prostate cancer cells to antigen-specific CTL killing. LNCaP cells were exposed to 0, 25 or 50 Gy of 153Sm-EDTMP. Cells were harvested 72 h after exposure and incubated with PSA- or MUC-1-specific T cells. Taken from ref. (58).
In a murine adenocarcinoma cell line transfected to express carcinoembryonic antigen (CEA), (MC38-CEA+), Chakraborty et al. showed that irradiation (20 Gy) enhanced Fas expression at the molecular, phenotypic and functional levels (36). Fas was maximally upregulated at doses of 10–20 Gy and was upregulated threefold at the molecular level by RT-PCR. ICAM-1 expression was also induced after 10–20 Gy. These phenotypic findings were confirmed in vivo (Fig. 2). Immunohistochemistry of MC38-CEA+ tumors harvested 72 h after 8 Gy EBRT showed upregulation of Fas. Radiation-sensitized MC38-CEA+ cells exposed to antigen-specific CTLs were killed by the Fas/FasL pathway. After irradiation (20 Gy), MC38-CEA+ cells underwent markedly increased CTL lysis, an effect abrogated by the presence of anti-FasL mAb (36). In a subsequent in vivo study using C57BL/6 mice with MC38-CEA+ tumors, the combination of a CEA vaccine and RT achieved dramatic cures, while neither therapy alone was sufficient to inhibit tumor growth (Fig. 2). This synergy was dependent on Fas/FasL since dominant-negative Fas tumor cells were not susceptible to combination therapy (34).
Garnett et al. then studied 23 human carcinoma lines for postirradiation phenotypic changes in TAAs, MHC class I, adhesion molecules and Fas (33). Cell lines were irradiated in vitro (10 or 20 Gy) and examined after 72 h. Of the 23 cell lines tested, 91% showed upregulation of one or more immune-relevant surface markers. The functional significance of these phenotypic changes was confirmed by CTL killing. Each of 5 HLA-A2+ colon cancer cell lines tested showed enhanced killing by CEA-specific HLA-A2-restricted CTLs after irradiation (10 Gy), compared to nonirradiated cells (Fig. 2) (33).
The functional consequences of the IM demonstrated in the studies described above include improved CTL killing in vitro and synergy with immunotherapy in vivo (33, 34). RT of human tumors could induce a continuum of responses ranging from necrosis, to classic and nonclassic ICD, to IM (Fig. 1), providing a rationale for the use of immunotherapy to augment the immunosensitivity of post-RT tumors.
COMBINING RADIATION AND IMMUNOTHERAPY: PRECLINICAL TO CLINICAL RESULTS
Multiple preclinical studies have demonstrated the potential for RT in combination with various immunotherapy modalities. Targeted inhibition or activation of selected molecules can be achieved with mAbs. As an example, anti-CD137 combined with RT and the checkpoint inhibitor anti-PD1 mediated tumor rejection in a murine model of mammary carcinoma (Table 1) (37). Toll-like receptor (TLR) agonists simulate the structure of pathogen-associated molecular patterns (PAMPs), which are highly conserved molecular patterns common to the cell surface and nuclear components of pathogens. The association of PAMPs with TLRs activates adaptive immune responses. TLR9 agonist combined with RT initiated a systemic antitumor humoral response in a murine lung adenocarcinoma model. The combination therapy reduced the number of pulmonary metastases and improved median survival from 38 days in the untreated group to 78 days in the combination treatment group (Table 1) (38). Hannan et al. examined the combination of RT and a Listeria monocytogenes-based prostate-specific antigen (PSA) vaccine for the treatment of prostate cancer in a murine model. The vaccine plus RT led to a significant delay in tumor growth compared to other cohorts, and complete tumor regression was seen in 60% of mice treated with vaccine plus RT (39). Similar results have been seen with a vaccinia-based vaccine combined with RT (Table 1) (34).
The three combinations of radiation and immunotherapy described below exemplify the translation of preclinical findings into successful clinical applications.
Radiation and the Immune Checkpoint Inhibitor Anti-CTLA-4
CTLs are the target of immune checkpoint inhibitors such as anti-CTL-4, anti-PD1 and anti-PDL1 blocking antibodies (Fig. 1) (40). CTLA-4, which is expressed by activated T cells, regulates the immune response by maintaining homeostasis and preventing autoimmunity (20). Current evidence suggests that anti-CTLA-4 mAb facilitates T-cell proliferation and activation and abrogates the suppressive function of Tregs, but does not reduce the frequency of Tregs (41). Ipilimumab, an anti-CTLA-4 mAb, was approved by the U.S. Food and Drug Administration (FDA) in 2011 after two randomized phase III trials showed significant increases in median survival in patients with melanoma. Preclinical studies provided evidence of the efficacy of combining radiation and anti-CTLA-4 mAb. A synergistic effect was seen in mice implanted with TSA mouse breast carcinoma cells in 2 sites: a primary site that was irradiated and a secondary site outside the radiation field. Irradiation alone (8 Gy × 3 or 6 Gy × 5 fractions on consecutive days) and anti-CTLA-4 mAb alone delayed tumor growth at the primary site, but only the combination of anti-CTLA-4 mAb and fractionated RT achieved complete regression at the primary site. Furthermore, only the combination therapy significantly delayed tumor growth at the secondary site. The combination regimen was also associated with a significant increase in CD4+ and CD8+tumor-infiltrating lymphocytes in the secondary tumor (42).
Several clinical studies subsequently confirmed these preclinical findings. Hiniker et al. reported a patient with a history of metastatic melanoma who progressed 4 years after initial diagnosis and was treated with standard-of-care chemotherapy and surgical resection of a pulmonary metastasis. One year after the patient enrolled in a study of ipilimumab pharmacokinetics, a CT scan showed disease progression requiring palliative radiation (28.5 Gy) to a paraspinal mass. Ten months after RT, a CT scan showed stable disease and regression of lesions not targeted by RT. Additionally, activated CD4+ T cells increased post-RT, suggesting immune-mediated disease suppression (Fig. 3) (43). Postow et al. have since reported a second case of abscopal effect induced by combination therapy with RT and anti-CTLA-4 mAb (8). Stamell et al. also reported on the abscopal effect in a patient with metastatic melanoma. Disease progressed after chemoradiation and the patient was treated with ipilimumab for systemic disease and intracranial stereotactic radiosurgery for palliation of a metastatic lesion. Thereafter, the patient achieved complete remission and was shown to have elevated anti-MAGEA3 titer and a new response to the cancer antigen PASD1 compared to pre-ipilimumab serology (44). These studies offer further evidence that local RT can elicit a systemic response and cause tumor regression at a site distant from the irradiated field (4).
Accumulating evidence suggests that the abscopal effect is immune-mediated and can be induced through combination RT and immunotherapy (9, 45). Demaria et al. studied the abscopal effect in a murine model. Fms-like tyrosine kinase receptor 3 ligand (FLT3-L) is a growth factor that stimulates DC production. It was hypothesized that expanding the DC compartment with FLT3-L in combination with RT would enhance antitumor immunity. Mice bearing syngeneic mammary carcinoma (67NR) in both flanks were treated with RT (2–6 Gy) to the primary tumor and given systemic FLT3-L daily for 10 days post-RT. Only the combination therapy was able to delay growth in the nonirradiated tumor. The effect was abrogated in the absence of T cells (45). Similar results were seen in a preclinical study of combined vaccine and RT. CEA-transgenic mice were implanted with MC38-CEA+ tumors at a primary site and MC38-CEA− tumors at a secondary site. The vaccine consisted of a prime composed of recombinant vaccinia containing the human CEA gene and the costimulatory molecules B7.1, ICAM-1, and LFA-3 (rV-CEA/TRICOM) and a boost containing recombinant fowlpox (rF-CEA/TRICOM). Mice received rV-CEA/TRICOM on day 8 and RT on day 14 and a boost with rF-CEA/ TRICOM on days 15, 22 and 29. The combination therapy induced significant regression of the nonirradiated tumor and increased levels of tumor-infiltrating lymphocytes. Significantly, a study by Hodge et al. demonstrated that combining RT and immunotherapy can induce the abscopal effect in antigen-disparate tumors. In mice treated with the combination therapy, T-cell specificity extended beyond the expected generation of CEA-specific T cells to T cells specific for the murine endogenous retrovirus gp70 and proapoptotic protein p53 (9).
Antigen cascade has also been demonstrated clinically. Patients treated with EBRT, GM-CSF and IL2 and a vaccinia-based vaccine encoding PSA, developed T cell responses to XAGE-1, PAGE-4 and MUC-1—TAAs not expressed by the vaccine (46). Antigen cascade has important implications for our understanding of radiation biology. Radiation induces specific antitumor immunity. However, the antitumor immune response releases new TAAs that are then recognized, allowing an immune response targeted against distant metastatic lesions that may be differentially expressing TAAs due to inherent genetic instability.
Innate Immunity and Spatially Fractionated GRID Radiotherapy (SFGRT)
As previously described, RT enhances the immune response. Antitumor effects are achieved in part by the release of TAAs from dying tumor cells and by modification of the tumor microenvironment (Table 1) (11, 45, 47–52). Interestingly, focal high-dose RT can cause regression of nonirradiated tumors through the abscopal effect. In traditional stereotactic radiosurgery and stereotactic body radiotherapy, coverage of the targeted tumor volume by the maximum prescribed radiation dose is not always achievable, due to heterogeneity within the tumor (as much as 50%) and adjacent structures (such as bowel), which may or may not generate a systemic immune response. In contrast, spatially fractionated GRID radiotherapy (SFGRT) only partially covers the tumor volume with the prescribed dose of radiation (53). In early applications of SFGRT, 2-dimensional grid fields were used, typically with orthovoltage beams allowing spatially alternated dose distribution. By this technique, multiple focused beams may not only generate intratumoral bystander effects along with a systemic abscopal effect, but may also stimulate a robust immune response. This traditional approach to SFGRT was used mostly as salvage therapy for bulky tumors (53). In a clinical study of 308 patients treated with high-dose GRID RT, single doses of 12–20 Gy (median 15 Gy) were well tolerated and produced minimal changes in normal tissue, even when combined with full-dose conventional RT. Tumor responses were impressive, considering that all patients had bulky tumors ranging from 30 × 25 to 8 × 8 cm. A complete clinical response was observed in 15% of patients and a partial response in 57% of patients. Overall, dramatic clinical responses have been reported with GRID RT in several types of cancers (54–57). While these studies demonstrated effective clinical responses, no studies undertaken during clinical trials have confirmed that bystander/abscopal effects may mediate immune stimulation.
A recent preclinical study using SFGRT demonstrated that both bystander and abscopal effects can be elicited in mice bearing A549 lung adenocarcinoma xenograft contralateral tumors (unpublished data). Both the SFGRT-treated (15 Gy) and untreated tumors showed diminished growth over a 90-day period postirradiation. Notably, booster doses of 2 Gy fractions to the untreated tumor significantly enhanced regression of both the treated and untreated tumors, suggesting that the SFGRT-treated tumor may have released systemic inhibitory factors, such as cytokines, that inhibited the growth of the nonirradiated tumor. Although the mice in this study were immunocompromised, it is possible that factors secreted by immune cells played a role in the observed abscopal effects.
To further understand the mechanisms of immune activation in response to high-dose SFGRT, syngeneic mouse contralateral tumors were developed using Lewis lung carcinoma cells. SFGRT was modified as 3D-GRID and administered in a scheme similar to that described above to compare the effects of high-dose RT directed to the total tumor volume versus RT directed to partial tumor volumes (10, 20 and 50%). Maximal regression was seen in tumors treated with RT (20 Gy) directed to 10% of tumor volume and in nonirradiated tumors, compared to tumors treated with RT directed to total tumor volume or other percentages of volume. Mice treated at 10 and 50% of tumor volume showed the highest levels of IL2 and IFN-gamma. Also in these mice, levels of TNF-alpha and TRAIL significantly increased, while levels of IL4, IL10 and keratinocyte chemoattractant decreased. Increased endothelial cell death was observed when HUVEC cells were treated with serum obtained from the 10% volume-treated mice. Infiltration of CD3+ T cells increased in mice treated at 10, 20 and 50% of tumor volume. Together, these observations (unpublished) strongly suggest that high-dose RT directed to partial tumor volumes elicits more robust immune stimulation than RT to total tumor volume (Fig. 4) [can also be viewed as YouTube video: http://youtu.be/KvQ8z91J6A8].
FIG. 4.
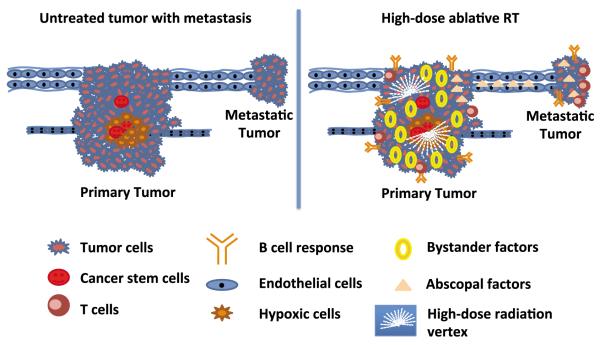
Impact of high-dose ablative RT on tumor microenvironment. High-dose ablative RT given in lattice (2 vertices) to the tumor induces bystander/abscopal effect, endothelial-cell death coupled with immune activation. The underlying radiobiological mechanisms for improved outcome with high-dose hypofractionated RT may be multifactorial, including differential effects on tumor endothelium and cancer stem cells. Complex immunologic pathways may be linked to high-dose radiation-induced mechanisms. All of these pathways may be affected by bystander/abscopal factors released by the tumor after high dose spatially fractionated RT. Taken from ref. (65).
Components of the tumor microenvironment include epithelial and endothelial compartments, hypoxic areas, and a cancer stem cell niche. Irradiating partial tumor volume with 2 focused high-dose vertices using lattice RT (3D form of SFGRT) induces high-dose-irradiated cells to secrete factors such as TNF-alpha and TRAIL and immune-related cytokines that target the killing of the nonirradiated epithelial tumor compartment intratumorally as well as in distant tumors. High-dose-irradiated cells also activate aSMase to generate ceramide to kill tumor endothelium intratumorally as well as in distant tumors. This leads to activation of T and B cells to synergistically enhance tumor regression. High-dose radiation vertices also help to potentiate the effect of conventional fractionated RT and chemotherapeutic drugs, without adding toxicity above that expected with standard chemoradiation when implemented in a clinical setting.
153Sm-EDTMP in Combination with Therapeutic Vaccines
Alternative methods of delivering RT include radiolabeled mAb and bone-seeking radiopharmaceuticals. Both modalities have been shown to induce IM and enhance immune-mediated antitumor effects (Table 1) (52, 58). In a study combining the anti-CEA radiolabeled mAb yttrium-90 (Y-90 COL1), a recombinant poxviral vaccine was combined with Y-90 COL1 in a murine colon adenocarcinoma model. Y-90 COL1 induced upregulation of Fas/CD95, and combination therapy led to a survival advantage compared to either vaccine or Y-90 COL1 monotherapy (Table 1) (52).
Samarium-153 (153Sm-EDTMP; Quadramet®, Cytogen Corp.) is a radiopharmaceutical FDA approved for palliation of pain in patients with confirmed osteoblastic metastatic bone lesions that enhance on a radionuclide scan. 153Sm-EDTMP has high affinity for newly deposited bone, and targets well to osteoblastic bone lesions with a high rate of bone turnover, 153Sm-EDTMP is a β-emitter with a half-life of 1.95 days. A phase III randomized placebo-controlled trial confirmed 153Sm-EDTMP as an option for pain relief in castration-resistant prostate cancer metastatic to bone (59).
Chakraborty et al. studied 153Sm-EDTMP-induced IM in an in vitro model. Human tumor cell lines (4 prostate, 2 breast and 4 lung) were exposed to various concentrations of 153Sm-EDTMP and analyzed by flow cytometry for upregulation of selected cell surface markers. Concentrations of 153Sm-EDTMP were chosen to mimic palliative doses used clinically. Fas/CD95 was upregulated in 100% of cell lines, CEA (90%), MUC-1 (60%), MHC class I (50%) and ICAM-1 (40%) were also upregulated consistently across multiple tumor types. The observed upregulation of death receptors, TAAs and adhesion molecules proved to be functionally important. The human prostate cancer cell line LNCaP was used in a CTL assay. Dramatic improvement in killing by CTLs was observed in LNCaP cells exposed to 153Sm-EDTMP compared to untreated LNCaP cells (Table 1) (58). These findings identify bone-seeking radionuclides as a potential source of radiation for combination with immunotherapy. An ongoing phase II multicenter trial is based on the preclinical work discussed above (Fig. 5). The trial randomizes patients with castration-resistant prostate cancer with bone metastases to receive either 153Sm-EDTMP or 153Sm-EDTMP with PROSTVAC, a recombinant poxviral-based vaccine targeting PSA. The primary end point is progression-free survival at 4 months, with secondary end points of overall survival and immunologic changes. Early results at 4 months showed improved progression-free survival in the combination arm compared to the 153Sm-EDTMP-alone arm, warranting continued accrual to the study (60). These promising results suggest the potential for 153Sm-EDTMP to evolve from a palliative agent to a therapeutic agent through combination with immunotherapy.
FUTURE Perspectives
The synergy of RT and immunotherapy underlies a novel strategy with the potential to better target local irradiated/viable tumor cells, and to provide better control of distant systemic disease. Systemic metastatic disease requires systemic therapy, which has traditionally been chemotherapy. However, the resultant systemic immunosuppression makes chemotherapy less desirable for combination with immunotherapeutic agents. While RT has traditionally been considered only as local therapy, it is now clear that it has systemic effects, many of which lead to improvements in antitumor immunity. Furthermore, the absence of systemic immunosuppression makes RT an attractive adjuvant for combination with immunotherapy. Radiation has been shown in select circumstances to also decrease the efficacy of immunotherapy by causing immunosuppression and/or lymphopenia, indicating that careful attention to timing and sequencing of RT with immunotherapy may be important. In addition, learning how to exploit radiation-induced changes to tumor-cell antigens and how to induce effective immune responses to these cumulatively immunogenic stimuli will be an ongoing challenge. As knowledge of the synergistic effects of RT and immunotherapy increases, the translational use of this strategy for a variety of cancers will become more feasible and more available to patients.
Immunotherapeutics available for combination with RT are currently limited to a few FDA-approved agents, including vaccines (Provenge®, Dendreon; Gardisil®, Merck), cytokines (GM-CSF, IL-2, IFN-gamma), and select mAbs. Of particular interest for combination with RT is the recently approved mAb anti-CTLA-4 (Yervoy®, Bristol-Myers Squibb) (Fig. 3). Emerging mAbs such as anti-CD40, anti-OX40L and anti-41BBL have shown significant activity as single agents and will soon be incorporated into prospective radiation clinical trials. There is also growing interest in mAbs directed at PD-1, a receptor expressed on T, B and NK cells. PD-1 induces T-cell inactivation and apoptosis when bound to its ligand, PDL-1. The PD-1/PDL-1 pathway may be an important mechanism for tumor escape from immune recognition. Tumor cells express PD-1, thus promoting T-cell tolerance to tumor cells. Blockade of this receptor is associated with restoration of T-cell activity. Rosenblatt et al. employed an anti-PD-1 antibody in combination with a DC/myeloma fusion vaccine that uniformly expresses PDL-1. This combination reversed the vaccine-mediated upregulation of PD-1 expression and decreased Treg expansion, resulting in enhanced tumor killing (61). These results suggest a potentially greater role for IM in combination with RT.
Emerging evidence suggests that exosomes and microvesicles secreted by a multitude of cells including tumor cells and DCs, play a major role in immune-mediated tumor control. Tumor-cell exosomes have been shown to have anti-immune effects and to be a possible marker of disease progression (62). However, tumor-cell exosomes from irradiated cells have been shown to be immunogenic in in vitro experiments, as they promote CTL cross-priming through DCs (63). These findings could lead to new treatment options.
Substantial preclinical evidence has indicated a synergistic relationship between RT and immunotherapy. Anecdotal and prospective clinical data also support the efficacy of this treatment regimen. Additional clinical trials are needed to determine if adding active immunotherapy to definitive RT can affect clinical outcomes, as most of the studies reviewed here have had immunologic response as their primary end point. Learning how best to exploit radiation-induced immunogenic modulation in cancer patients with the addition of active immunotherapy is an exciting frontier in cancer research, with the potential to greatly improve patient care in the future (64).
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH). This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program). The authors thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
REFERENCES
- 1.Smith BD, Haffty BG, Wilson LD, Smith GL, Patel AN, Buchholz TA. The future of radiation oncology in the United States from 2010 to 2020: will supply keep pace with demand? J Clin Oncol. 2010;28:5160–5. doi: 10.1200/JCO.2010.31.2520. [DOI] [PubMed] [Google Scholar]
- 2.Manda K, Glasow A, Paape D, Hildebrandt G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol. 2012;2:102. doi: 10.3389/fonc.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology. 2008;22:1064–70. discussion 75:80–1, 84. [PMC free article] [PubMed] [Google Scholar]
- 4.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters ME, Shareef MM, Gupta S, Zagurovskaya-Sultanov M, Kadhim M, Mohiuddin M, et al. Potential utilization of bystander/abscopal-mediated signal transduction events in the treatment of solid tumors. Curr Signal TransductTherapy. 2007;2:129–43. [Google Scholar]
- 6.Mould RF. Invited review: the early years of radiotherapy with emphasis on X-ray and radium apparatus. Br J Radiol. 1995;68:567–82. doi: 10.1259/0007-1285-68-810-567. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:2035. doi: 10.1056/NEJMc1203984. author reply 35–6. [DOI] [PubMed] [Google Scholar]
- 9.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84:879–80. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 12.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 13.Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14:4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–64. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 16.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 17.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonteneau JF, Larsson M, Bhardwaj N. Interactions between dead cells and dendritic cells in the induction of antiviral CTL responses. Curr Opin Immunol. 2002;14:471–7. doi: 10.1016/s0952-7915(02)00358-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 20.Burnette B, Fu YX, Weichselbaum RR. The confluence of radiotherapy and immunotherapy. Front Oncol. 2012;2:143. doi: 10.3389/fonc.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39:323–39. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makinde AY, John-Aryankalayil M, Palayoor ST, Cerna D, Coleman CN. Radiation Survivors: Understanding and exploiting the phenotype following fractionated radiation therapy. Mol Cancer Res. 2013;11:5–12. doi: 10.1158/1541-7786.MCR-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannani D, Sistigu A, Kepp O, Galluzzi L, Kroemer G, Zitvogel L. Prerequisites for the antitumor vaccine-like effect of chemotherapy and radiotherapy. Cancer J. 2011;17:351–8. doi: 10.1097/PPO.0b013e3182325d4d. [DOI] [PubMed] [Google Scholar]
- 25.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8:3723–8. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 26.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–9. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 27.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–58. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 28.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 29.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 30.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 31.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 32.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 33.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 35.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 37.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–74. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Liu L, Yu D, Kandimalla ER, Sun HB, Agrawal S, et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PLoS One. 2012;7:e38111. doi: 10.1371/journal.pone.0038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61:2227–38. doi: 10.1007/s00262-012-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan S, Burt DJ, Ralph C. Thistlethwaite FC, Hawkins RE, Elkord E. Tremelimumab (anti-CTLA4) mediates immune responses mainly by direct activation of T effector cells rather than by affecting T regulatory cells. Clin Immunol. 2011;138:85–96. doi: 10.1016/j.clim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–5. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 47.Mitsudo K, Koizumi T, Iida M, Iwai T, Oguri S, Yamamoto N, et al. Thermochemoradiation therapy using superselective intra-arterial infusion via superficial temporal and occipital arteries for oral cancer with N3 cervical lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;83:e639–45. doi: 10.1016/j.ijrobp.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 48.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenfield JP, Cobb WS, Lyden D. Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas. J Clin Invest. 2010;120:663–7. doi: 10.1172/JCI42345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horikoshi S, Miura T, Kajitani M, Serpone N. Microwave discharge electrodeless lamps (MDEL). III. A novel tungsten-triggered MDEL device emitting VUV and UVC radiation for use in wastewater treatment. Photochem Photobiol Sci. 2008;7:303–10. doi: 10.1039/b715774f. [DOI] [PubMed] [Google Scholar]
- 52.Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008;57:1173–83. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohiuddin M, Curtis DL, Grizos WT, Komarnicky L. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation. A pilot study. Cancer. 1990;66:114–8. doi: 10.1002/1097-0142(19900701)66:1<114::aid-cncr2820660121>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 54.Mohiuddin M, Fujita M, Regine WF, Meigooni AS. Overcoming the barrier of radiation-resistance in advanced cancer by using high dose spatially fractionated radation. Int J Radiat Oncol Biol Phys. 1997;39(Suppl):329. http://inis.iaea.org/search/search.aspx?orig_q=RN:35007090. [Google Scholar]
- 55.Mohiuddin M, Fujita M, Regine WF, Megooni AS, Ibbott GS, Ahmed MM. High-dose spatially-fractionated radiation (GRID): a new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys. 1999;45:721–7. doi: 10.1016/s0360-3016(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 56.Huhn JL, Regine WF, Valentino JP, Meigooni AS, Kudrimoti M, Mohiuddin M. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat. 2006;5:607–12. doi: 10.1177/153303460600500608. [DOI] [PubMed] [Google Scholar]
- 57.Penagaricano JA, Moros EG, Ratanatharathorn V, Yan Y, Corry P. Evaluation of spatially fractionated radiotherapy (grid) and definitive chemoradiotherapy with curative intent for locally advanced squamous cell carcinoma of the head and neck: Initial response rates and toxicity. Int J Radiat Oncol Biol Phys. 2009;76:1369–75. doi: 10.1016/j.ijrobp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008;14:4241–9. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higano CS, Quick DP, Bushnell D, Sartor O. Safety analysis of repeated high doses of samarium-153 lexidronam in men with hormone-naive prostate cancer metastatic to bone. Clin Genitourin Cancer. 2008;6:40–5. doi: 10.3816/CGC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 60.Heery CR, Madan RA, Bilusic M, Kim JW, Singh NK, Rauckhorst M, et al. Interim analysis of a phase II randomized clinical trial of samarium-153 (Sm-153) with or without PSA-TRICOM vaccine in metastatic castration-resistant prostate cancer after docetaxel. http://meetinglibrary.asco.org/content/94849-114.
- 61.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–18. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 64.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta S, Wu X, Carlson DJ, Kolesnick MD, Mohiuddin M, Pollack A, et al. Radiobiological concepts of high-dose hypofractionated radiation therapy. In: Pollack A, Ahmed MM, editors. Hypofractionation: Scientific concepts and clinical experiences. LumiText Publishing; Ellicot City, MD: 2011. [Google Scholar]