Abstract
The lack of standardized reporting of the magnitude of ischemia on noninvasive imaging contributes to variability in translating the severity of ischemia across stress imaging modalities. We identified the risk of coronary artery disease (CAD) death or myocardial infarction (MI) associated with ≥10% ischemic myocardium on stress nuclear imaging as the risk threshold for stress echocardiography and cardiac magnetic resonance. A narrative review revealed that ≥10% ischemic myocardium on stress nuclear imaging was associated with a median rate of CAD death or MI of 4.9%/year (interquartile range: 3.75% to 5.3%). For stress echocardiography, ≥3 newly dysfunctional segments portend a median rate of CAD death or MI of 4.5%/year (interquartile range: 3.8% to 5.9%). Although imprecisely delineated, moderate-severe ischemia on cardiac magnetic resonance may be indicated by ≥4 of 32 stress perfusion defects or ≥3 dobutamine-induced dysfunctional segments. Risk-based thresholds can define equivalent amounts of ischemia across the stress imaging modalities, which will help to translate a common understanding of patient risk on which to guide subsequent management decisions.
Keywords: cardiac imaging, ischemia, prognosis
Stress imaging is commonly used to evaluate suspected myocardial ischemia in patients with symptoms suggestive of stable ischemic heart disease (SIHD). The evidence to support the use of several stress imaging modalities is substantial and has been synthesized in recent appropriate use criteria and clinical practice guidelines (1–3). The published evidence base for stress nuclear imaging and echo-cardiography as effective tools for diagnosis of coronary artery disease (CAD) and risk stratification is extensive, and there is growing evidence supporting the role of stress cardiac magnetic resonance (CMR).
However, the optimal evaluation and treatment algorithm following stress imaging has not been clearly defined. Although diagnostic coronary angiography is commonly preceded by stress testing, nearly two-thirds of patients manifest no obstructive CAD at the time of cardiac catheterization (4,5). Before elective percutaneous coronary intervention (PCI), less than one-half of patients have had a stress test in the previous 90 days (6). These data illustrate the lack of accuracy and consistency in clinical practice in the appropriate use of stress imaging to guide the management of patients with SIHD (1,7,8).
One noteworthy gap in the current evidence base is the absence of established comparable categories of the magnitude of ischemia across noninvasive imaging modalities. The lack of standardized grading and inconsistency in reporting of the extent and severity of ischemia in clinical practice may contribute to the wide variability in management decisions and high rates of nonobstructive CAD on diagnostic angiography (5). At a recent Joint Commission/American Medical Association Quality Summit, the variable reporting of the extent and severity of ischemia was identified as contributory to the overuse of elective PCI (9). Recent guidance documents support the requirement of moderate-severe ischemia before elective PCI (10).
For this report, experts in the field of stress cardiac imaging were enlisted to propose a consensus of comparable definitions for moderate-severe ischemia for stress nuclear imaging (myocardial perfusion single-photon emission computed tomography and positron emission tomography), echocardiography, and CMR (wall motion or perfusion). The cut points for moderate-severe ischemia were established using the selected, published evidence for each modality correlating stress imaging results with risk of CAD death or myocardial infarction (MI). The aim of this review was to propose a definition for equivalent amounts of ischemia across the stress imaging modalities for patients with SIHD who have preserved left ventricular function, which will help to translate a common understanding of patient risk on which to guide subsequent management decisions.
Targeting Moderate-Severe Ischemia
Most SIHD revascularization strategy trials have included patients with ischemia on stress testing or typical angina with at least 1 coronary stenosis amenable to revascularization, although only a subset of enrolled patients reported stress test results (11,12). The entry criteria for the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial ischemia requirements included a minimum of 1 segment with a perfusion defect or typical ischemic ST-segment changes with exercise (11), resulting in a representation of patients ranging from those with mild to moderate ischemia on imaging (13). Given the potential representation of patients with less extensive and severe ischemia in the COURAGE trial, a lingering question is whether SIHD trial outcomes would have been different if the COURAGE or National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI)-sponsored BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes) trials enrolled a larger proportion of higher-risk patients with moderate or severe ischemia (11,12). There is limited evidence to guide treatment for these higher-risk patients.
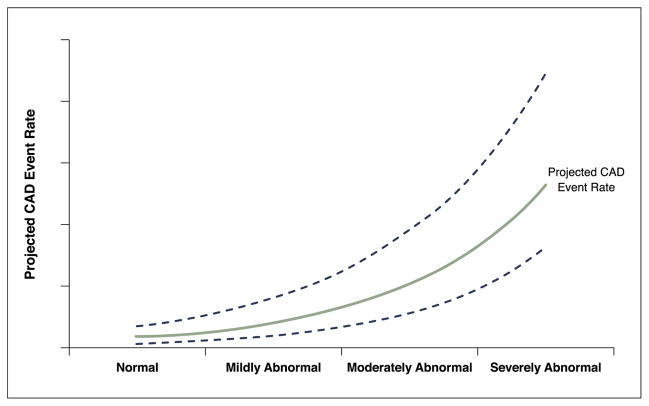
Figure 1 shows the projected relationship between the underlying abnormal stress imaging findings and projected CAD events. For stress imaging, the extent of ischemia is directly related to the rate of subsequent CAD events. Yearly CAD event rates generally range from ~1% for normal stress imaging findings to as high as 10% for severely abnormal studies. Several observational studies have also shown that the degree of relative risk reduction with treatment is related to the amount of ischemia observed on noninvasive imaging (14–18).
Figure 1. Projected CAD Event Rates.
A theoretical plot of the relationship between abnormal stress imaging findings and projected CAD events is shown. The lines include the average projected CAD event rate and 95% confidence intervals. As the stress imaging abnormalities become more extensive and severe, the projected CAD event rate increases. Conversely, for subsets with normal or mildly abnormal studies, the event rates are low. CAD = coronary artery disease.
Challenges in Uniformly Quantifying Ischemia Across the Stress Imaging Modalities
A major challenge in defining comparable risk-based thresholds for moderate-severe ischemia across modalities is that there are notable differences in the various imaging techniques regarding what is assessed, interpreted, and quantified. Although it is recommended that all stress imaging techniques use the standardized 17-segment model for quantification of wall motion and perfusion (19), the 17th segment (i.e., apical cap) is not separately assessed on stress echocardiography or CMR myocardial perfusion imaging. There are also differences in the numerical scale used to classify the severity of segmental perfusion and wall motion abnormalities, with various methods and differences in the ability to distinguish the subendocardial and subepicardial layers of the myocardium. Nuclear imaging has several validated quantitative software programs for defining the percentage ischemic myocardium, including the use of percentage total perfusion defect as used in the COURAGE trial (20). Given that other modalities interpret ischemic wall motion and perfusion findings semiquantitatively, this may also introduce differences in the quantification of the extent and severity of stress-induced abnormalities. These differences can result in considerable variability in the estimation of the proportion of ischemic myocardium.
There are additional differences in the ischemic cascade that provide challenges to the development of comparable definitions of ischemia. Perfusion abnormalities have larger extent, occur earlier in the ischemic cascade, and are more often associated with intermediate stenosis, whereas wall motion abnormalities occur later in the ischemic cascade and are more often associated with more severe stenosis (21). Given the differences in view orientations across the stress imaging modalities, thresholds that focus on comparing specific segments (i.e., anterior, lateral) would be problematic. These conceptual differences may promote a variable relationship between a threshold for moderate-severe ischemia and a given associated CAD event rate.
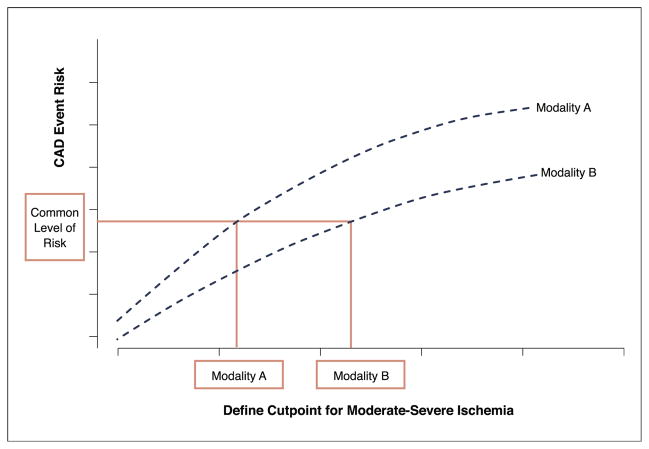
Thus, it remains unlikely that absolute thresholds of percentage ischemic myocardium could be defined similarly across the stress imaging modalities, resulting in the need for an alternative approach. Therefore, we sought to identify those parameters on each imaging modality that portend similarly elevated risk levels and to use these to develop comparable thresholds to guide future care decisions (Fig. 2).
Figure 2. Risk-Based Comparisons.
A theoretical approach to comparing levels of moderate-severe ischemia across the stress imaging modalities is used to define similar CAD event rates. Abbreviation as in Figure 1.
There is an important consideration that risk alone should not be the sole determinant of defining the potential for therapeutic risk reduction. The purpose of the current review is to identify comparable risk groups across stress imaging modalities. For some patient subsets, information on the extent and severity of ischemia must be balanced with correlative information on the burden of comorbidity and CAD as well as the presence of underlying left ventricular dysfunction. As such, for our review, we have limited the focus of our discussion to patients with preserved systolic function.
Because of variable reporting of prognosis by the severity of ischemia, we could not perform a systematic review. In some cases, data were reported only as survival curves with variable follow-up and without inclusion of the number at risk or average duration of follow-up, included revascularization as an endpoint, reported hazard ratios only, or defined ischemia as a dichotomous variable (yes/no). For these reasons, we could not systematically collect clinical outcome data for moderate-severe ischemia and the current review should be considered a narrative that highlights selected published evidence with available rates of CAD death or MI in moderate-severe ischemia. Contributing authors also identified additional data on prognosis with moderate-severe ischemia for the 3 stress imaging modalities. Importantly, we did not use standardized search terms or independently judge study quality; this review represents the opinions of the investigators and is not a systematized synthesis of all available evidence.
The search strategies in MEDLINE included coronary disease prognosis and the specific imaging modality (stress myocardial perfusion imaging, echocardiography, CMR wall motion or perfusion imaging). Selected event rates were derived from the published literature by initially focusing on the rate of CAD death or MI in the nuclear cardiology literature. We selected a variety of published registry reports with available event data and follow-up duration in the subset of patients with moderate-severe ischemia. The event data were obtained from crude rates or reported survival rates. There are several reports on prognosis from selected registries that report similar event rates by moderate-severe ischemia. However, we did not include event data from duplicate patient series.
Published Evidence of Elevated Risk Associated With Moderate-Severe Ischemia
For stress nuclear imaging data, we examined the extensive prognostic evidence reported from multiple sites, for different radioisotopes, and for positron emission tomography and single-photon emission computed tomography to define CAD event risk (22). In the nuclear cardiology literature, a threshold of ≥10% ischemic myocardium has been applied to denote high-risk status (14–16,22–26). Initial prognostic reports used a summed difference score (summed stress score – summed rest score) to provide an estimate of the overall magnitude of ischemia, combining both extent of ischemia (i.e., number of myocardial segments) and severity (i.e., depth of the defect) (26–29). Recent reports have relied on percentage myocardium as a summary metric to enhance clinical understanding of the complexity of findings of ischemia. The percentage ischemic myocardium may be measured using semiquantitative ([summed difference score/68 (maximal segmental score = 4)] · 100 = percentage ischemic myocardium) or computer-based quantitative techniques (percentage ischemic myocardium) (22). There may be variability when using quantitative or semiquantitative approaches for interpretation such that a threshold of 10% from 1 method may be slightly higher or lower using an alternative technique. However, in the nuclear cardiology literature, a threshold of ≥10% of the myocardium has been reported across a number of prognostic series to denote moderate-severe ischemia (14–16,22–26). For clinical practice purposes, the risk associated with a threshold of ~10% ischemic myocardium has been reported and forms the target cut point for our cross-modality estimation of the risk of CAD death or MI (1,16,22,27,30).
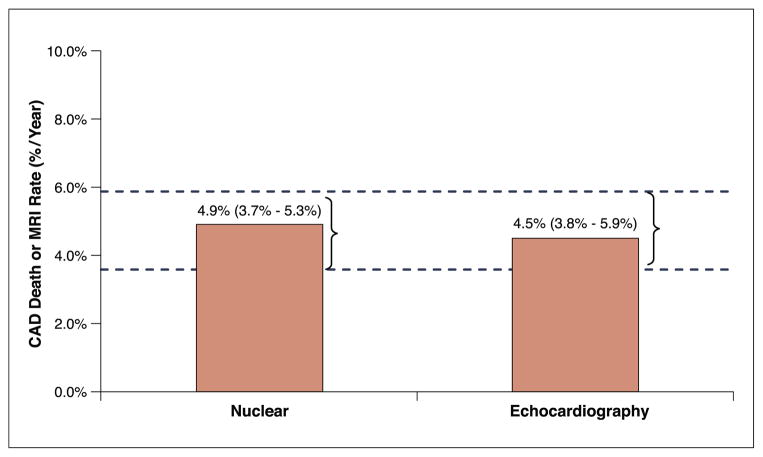
A review of the published literature reveals that the median rate of CAD death or MI for patients with ≥10% ischemic myocardium was ~4.9%/year (interquartile range: ~3.7% to 5.3%/year) (Fig. 3) (16,27,30–34). For stress nuclear imaging, the range of CAD event rates was from as low as 2.3%/year (32) to as high as 6.9%/year (34). These findings of a ~5% annual CAD event risk are consistent with recent SIHD trials reporting a similar rate of yearly endpoints (11,12).
Figure 3. Risk of CAD Death or MI for Moderate-Severe Ischemia.
The median rates of CAD death or MI (%/year) on the basis of moderate-severe ischemia on stress nuclear myocardial perfusion imaging and stress echocardiography are shown. This narrative review highlights selected published evidence of rates of CAD death or MI for stress nuclear imaging and echocardiography; the dashed lines show the interquartile range of the CAD event rates (per year). The expected rate of CAD death or MI across all of the stress imaging modalities is ~4% to 6%/year. There are limitations to our median estimate, including that this was not a systematic review because of differential sample size, variable length of follow-up, and the use of mortality only or revascularization as an endpoint. MI = myocardial infarction; other abbreviation as in Figure 1.
Although not derived from the rigors of a clinical trial setting, observational evidence suggests that the threshold of ≥10% ischemic myocardium may be used as a benchmark from which to define treatment effectiveness (14–16). Observational evidence indicates that medical therapy alone is associated with a reduced risk of death as compared with revascularization for patients with less extensive and severe ischemia (i.e., <10% of the myocardium); conversely, patients with ≥10% ischemic myocardium had a reduced risk of CAD and all-cause death with coronary revascularization as compared with medical therapy (14,15). Selection bias is operational in observational evidence whereby patients with significant comorbidity would be less likely to undergo revascularization despite their ischemic risk, thus rendering the comparison of medical therapy and revascularization for patients with ≥10% ischemia as hypothesis generating. Importantly, in a secondary analysis of 468 patients in the COURAGE trial with site-interpreted moderate-severe ischemia, randomization to PCI with optimal medical therapy (OMT) did not result in improved death or MI-free survival when compared with OMT alone (p = 0.72) (13).
The published research on stress echocardiography commonly indicates that the wall motion score index includes a very high risk threshold of >1.5 or >1.7 (17,35–39) with an annual CAD mortality rate of >7% and a significantly elevated hazard for death of 6 (36,37). This high-risk wall motion score index is rarely observed in clinical practice. We sought to define clinically useful criteria at a lower level to define a threshold of moderate ischemia. Applying a threshold of 3 or more ischemic segments, the hazard for CAD events was elevated ~4-fold when compared with patients with normal stress echo-cardiographic results (35,40,41). The available evidence using a definition of ≥3 newly dysfunctional segments revealed an interquartile range from 3.8% to 5.9% (median 4.5%) for annual risk of CAD death or MI (Fig. 3) (17,35,39,41–49). For stress echocardiography, the CAD event rates ranged from as low as 2.8%/year (47) to as high as 9.4%/year (45). This result overlaps with the findings of moderate-severe ischemia with stress nuclear imaging.
A review of the published literature on stress CMR does not reveal consistent documentation of higher-risk versus lower-risk ischemia but more often a dichotomous reporting of normal/abnormal or ischemia (yes/no). However, in one report, a subset of very-high-risk patients with >5 of 16 segments with perfusion defects (including ischemic and fixed) had a risk of a CAD event of ~14%/year (50). Similarly, a subset of high-risk patients with 2 to 3 vessel perfusion defects had a hazard ratio that was elevated 4.5-fold to 7.0-fold that of patients without perfusion defects (51). There are also several patient series reporting that patients with events had an average of 4 of 16 segments with stress perfusion defects or dysfunctional segments as compared with <2 abnormal segments for event-free survivors (50,52,53). Moreover, the unadjusted hazard for CAD death or MI was elevated ~1.2 for every segment with an ischemic perfusion defect when compared with patients with a normal stress perfusion study who have an observed CAD event rate of ~1%/year, suggesting that patients with >3 ischemic perfusion defects would have an annual risk of CAD death or MI of ~5% (52,53). A similar relative hazard per ischemic defect or dysfunctional segment was reported from a larger series of 908 patients undergoing stress perfusion CMR (54).
Specific data with regard to prognosis associated with dobutamine-induced dysfunctional segments have been reported. The relative hazard for events was elevated 7.1-fold for 3 to 5 dobutamine-induced wall motion abnormalities when compared with a ~1% event rate for patients with normal stress CMR (51). Similarly, a relative hazard of 1.2-fold per dysfunctional segment at stress was reported, which would equate an annual CAD event rate of ~5% for ≥3 abnormal segments (52,53).
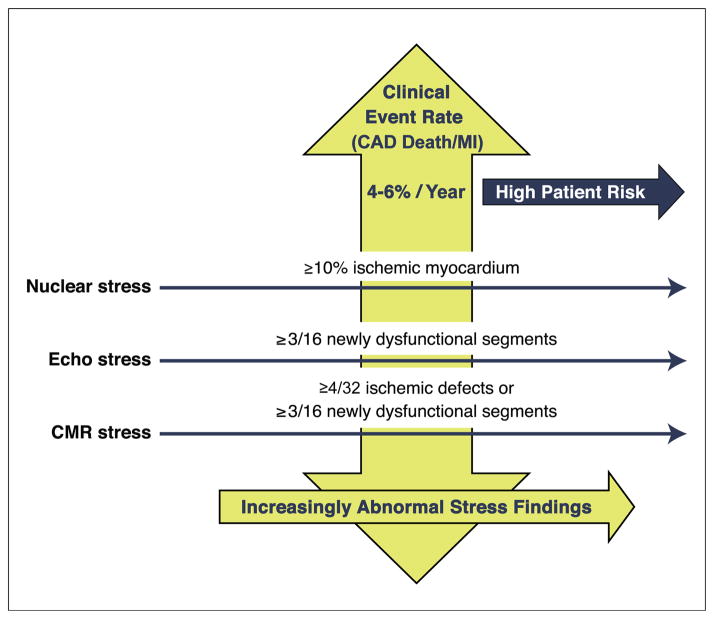
Thus, a reasonable definition for moderate-severe ischemia with stress CMR (annual rate of CAD death or MI of ~5%) may be ≥4 of 32 stress perfusion defects (≥2 of 16 segments) or ≥3 dobutamine-induced dysfunctional segments (of 16 segments), with CAD event rates similar to those of stress nuclear imaging and echocardiography.
Although differences exist in interpretation of ischemia across the modalities, the overall prognostic findings reveal that qualitative or quantitative measures can be defined that identify overlap in the annual rates of CAD death or MI of ~5%/year (Fig. 4). It remains likely that the threshold requirement of moderate-severe ischemia may allow for greater harmonization in the estimated risk of CAD events in patients with SIHD.
Figure 4. Definitions of Moderate-Severe Ischemia.
Comparable multimodality estimates of moderate-severe ischemia using risk-based thresholds of CAD death or MI rates of 4% to 6%/year. CMR = cardiac magnetic resonance; other abbreviations as in Figures 1 and 3.
Consideration of Ancillary High-Risk Markers
We considered revising the criteria for moderate-severe ischemia by including additional high-risk qualifiers using left ventricular function or volume measurements in addition to other modality-specific measures (such as strain, scarring, edema, or transient ischemic dilation of the left ventricle). There is added value when multiple parameters are combined during the same scan. For example, the presence or absence of myocardial scarring or edema assessed during the same scan adds to the information derived from perfusion and wall motion analyses (55). Data on the impact of combining these markers on prognosis and therapeutic decision making and outcome, however, are lacking. Our proposed criteria do not distinguish exercise and pharmacological stress and do not incorporate patient clinical risk or functional capabilities that would affect the anticipated risk of CAD events.
There was considerable discussion about the use of imaging-based criteria alone without inclusion of ancillary markers. One argument was put forth that simpler is better. The Duke Treadmill Score integrates 3 parameters into an estimation of 5-year CAD mortality (56). Although additional exercise parameters have been reported in the peer-reviewed literature, the novelty of the Duke Treadmill Score is its parsimony and ease of use. Ultimately, it was believed that the use of a simple criterion would be more reliably applied in clinical practice and the data regarding prognosis are less robust when additional factors are included. Ideally, this report could serve as the starting point for the establishment of stress imaging criteria to be applied clinically. This would then form the basis for further revisions that may refine and integrate important clinical and stress parameters that improve the precision of CAD risk estimates and the generalizability of the findings to varying patient subsets. Importantly, clinicians should use our thresholds as an initial guide to estimated risk. Inclusion of additional clinical, stress testing, or imaging parameters may further refine estimates for the individual patient.
Study limitations
The extent to which we cannot consistently identify the prognosis of moderate-severe ischemia from the published literature exemplifies the limitations of our current knowledge. The development of more quantitative methods for determining perfusion defect or dysfunctional segmental analysis could help define risk and guide decisions about treatment. Moreover, the development of easily defined categories of risk may further promote quality-based treatment and performance metrics for laboratory standards. The prognostic significance of noninvasive testing differs on the basis of the population in which it is applied. For example, the risk in younger or exercising patients differs from that of older or functionally impaired patients. The prevalence of prior MI or revascularization can also alter the CAD event risk estimates. We attempted to narrow the width of expected CAD event rates by focusing on patients with SIHD who had moderate-severe ischemia and preserved left ventricular function. The sample size and length of follow-up would further contribute variability to the CAD risk estimates. The smaller sample sizes for stress echocardiography and CMR (e.g., median sample sizes of 1,737 and 503, respectively) may add variability to the CAD event rates when compared with the larger stress nuclear series (median sample size: 5,845).
There are also challenges in the reproducibility of physician-interpreted moderate-severe ischemia (57). Reproducibility is affected by reader expertise, equipment, image quality, and other factors. We anticipate greater variability in the reproducibility of moderate-severe ischemia when a subjective interpretation is used.
Historically, varied analytical approaches have been applied in prognostic series to handle the subsets of patients who undergo coronary revascularization during follow-up. Patients who undergo early revascularization (e.g., ≤90 days) are excluded from analysis in some cases but are censored at the time of the procedure in other cases (28,43). The rationale for the censoring of patients who undergo early revascularization is that the procedure is believed to affect CAD survival. In this case, the CAD event rates reflect a medical strategy. More recent series do not use this method of censoring for revascularization because recent trials failed to show a clinical benefit from coronary revascularization when compared with OMT (11,12).
Importantly, these factors influence the precision of our estimate. However, we believe that the attempt to impose rigor in the field of stress CAD imaging can prompt a greater emphasis on standardized approaches to image interpretation. We propose comparable risk-based definitions for moderate-severe ischemia that may help to translate a common understanding of patient risk on which to guide subsequent management decisions. Our definitions are formed on the basis of a number of assumptions, including that the stress imaging protocols, interpretation, and reporting are consistent with imaging society standards.
Future Comparative Trial Evidence
In 2012, the NIH/NHLBI-sponsored ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial began enrolling patients with moderate-severe ischemia on stress nuclear imaging, echocardiography, or CMR. The ISCHEMIA eligibility criteria for moderate-severe ischemia are detailed in Table 1 and correspond to those discussed in this report. The primary aim of the ISCHEMIA trial is to test the hypothesis that among patients with moderate-severe ischemia on stress imaging, a routine early invasive strategy with coronary angiography followed by optimal revascularization plus OMT is superior to an initial conservative strategy of OMT alone, with angiography and revascularization reserved for those who fail to respond to medical therapy. The primary endpoint is a composite of incident cardiovascular death or MI over ~4 years of follow-up. The ISCHEMIA trial will aid in identifying patients who may benefit from coronary angiography and revascularization, with the trial results affecting the lives of the nearly 10 million patients who undergo stress imaging each year.
Table 1.
National Institutes of Health/National Heart, Lung, and Blood Institute–Sponsored ISCHEMIA Trial Criteria for Moderate-Severe Ischemia
| Nuclear Perfusion | Echocardiographic Wall Motion | Cardiac Magnetic Resonance Perfusion or Wall Motion |
|---|---|---|
| ≥10% ischemic myocardium | ≥3 of 16 segments with stress-induced hypokinesis or akinesis* | ≥4 of 32 stress perfusion defects (≥2 of 16 segments) or ≥3 dobutamine-induced dysfunctional segments (out of 16 segments) |
The one exception to the characterization of ischemic wall motion change is that when segments change from akinetic to dyskinetic during stress in the absence of demonstration of a biphasic response, this is considered a nonspecific response rather than an ischemic response.
Current evidence indicates that substantial equipoise exists with regard to the decision to refer a patient for coronary angiography, despite the common belief to the contrary in the cardiology community. On the basis of 9 published reports from 51 sites (N = 5,833), the rate of referral from stress nuclear imaging to coronary angiography in patients with moderate-severe ischemia was only 35% to 65% (58–66). Contemporary data from the NIH/NHLBI SPARC (Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in CAD), a 40-center multimodality registry of 3,019 patients enrolled between 2006 and 2008, revealed that only 42% of patients with moderate-severe ischemia were referred for coronary angiography (63).
Conclusions
On the basis of a selected review of the published literature, comparable CAD event rates (rate of CAD death or MI of ~5%/year) for patients with SIHD who have moderate-severe ischemia can be identified for those undergoing stress nuclear imaging, echocardiography, or CMR. An example of each of the imaging modalities using the proposed definition for moderate-severe ischemia is illustrated in Figure 5 (see Online Videos 1, 2, 3, and 4 for stress echocardiography example). Defining comparable risk-based thresholds for moderate-severe ischemia across the different stress imaging modalities will help to translate a common understanding of patient risk and guide management decisions. These risk-based thresholds should be applied only when standardized protocols and interpretation are used during stress imaging. Definitive guidance on the therapeutic effectiveness of an angiographic-guided strategy for patients with moderate-severe ischemia will be derived from ongoing clinical trials, such as the ISCHEMIA trial.
Figure 5. Case Examples.
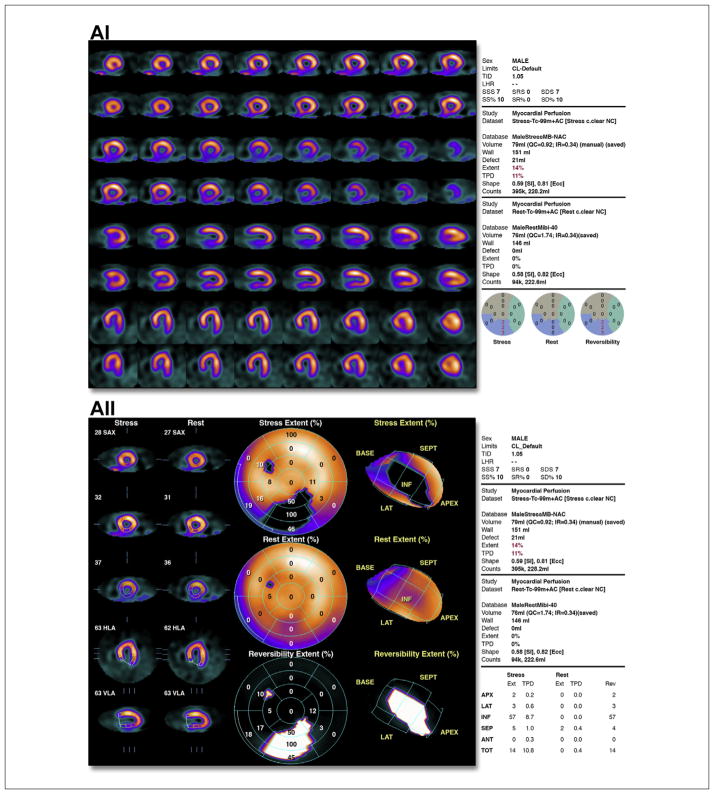
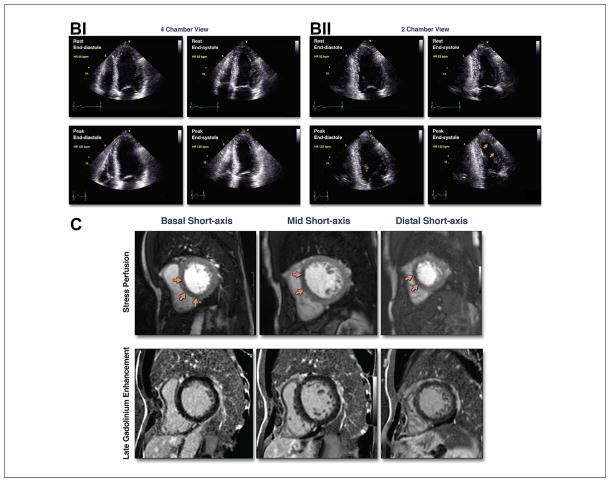
(A) (i) Illustrative case of moderate ischemia with stress myocardial perfusion SPECT. Regadenoson stress (upper rows)/rest (lower rows) Tc-99m sestamibi SPECT images show moderate reduction of perfusion in the distal, mid, and basal inferior wall. Semiquantitative visual analysis (lower right polar maps) reveals 3 abnormal segments at stress with a total score of 7 and normal rest scores. The summed difference score is 7, representing 10% of the myocardium. (ii) Quantitative analysis of the case shown in i. Stress polar maps (middle column) reveal a perfusion defect (black area) on stress images (top) and normal rest images (middle). The TPD at stress is 11% and at rest is zero, indicating an ischemic TPD of 11%. (B) Illustrative case of moderate ischemia with stress echocardiography. Apical views from an exercise stress echocardiogram show moderate ischemia. Regional wall motion is normal at rest. At peak stress, wall motion abnormalities (severe hypokinesis) are observed in 3 segments: mid anterior, apical anterior, and apical lateral segments (arrows). See Online Videos 1, 2, 3, and 4. (C) Illustrative case of moderate ischemia with stress cardiac magnetic resonance perfusion imaging. The top row shows stress perfusion imaging, and the bottom row shows late gadolinium enhancement imaging of infarction. In both rows, basal short-axis locations are on the left, mid short-axis locations are in the middle, and distal short-axis locations are on the right. Note the spatial extent of the stress perfusion defect involving the basal anteroseptal (subendocardial) and inferoseptal (subendocardial and subepicardial), mid anteroseptal (subendocardial and subepicardial), and distal septal (subendocardial and subepicardial) walls. There are 7 subsegments of 32 demonstrated abnormal stress perfusion defects. None of these subsegments demonstrated evidence of infarction by late gadolinium enhancement imaging. This case illustrates a patient with moderate ischemia without infarction in the left anterior descending territory. SPECT = single-photon emission computed tomography; TPD = total perfusion deficit.
Moreover, we put forth a simple criterion that we believe could be easily applied in clinical practice. We also identified limitations to the development of comparable definitions. We hope that this report will serve as a starting point and that further revisions will ensue to improve the precision of the CAD risk estimate and the generalizability of the findings to varying patient subsets.
Supplementary Material
Acknowledgments
The authors thank Dr. Raymond Gibbons for his careful review and comments on this manuscript.
Dr. Shaw has received research support from Astellas and Bracco Diagnostics Inc. Dr. Friedrich is a board member, advisor, and shareholder for Circle Cardiovascular Imaging. Dr. Senior is a member of the speaker’s bureau for and has received honoraria from Bracco Diagnostics Inc. and Philips Healthcare. Dr. Min is a member of the medical advisory board and speaker’s bureau for and has received research support from GE Healthcare; is a member of the medical advisory board for Arineta Ltd.; has received research support from Philips Healthcare; and holds equity interest in TC3 and MDDX. Dr. Arai has received research support from Siemens. Dr. Iskandrian is a member of the advisory committee for Rapidscan Pharma. Dr. Bateman is a member of the advisory committee for and receives research support from Astellas, Lantheus Medical Imaging, GE Healthcare, and Spectrum Dynamics. Dr. Miller is a consultant for Astellas. Dr. Nagel has received research support from Philips Healthcare and Bayer HealthCare. Dr. Borges-Neto is a member of the speaker’s bureau and advisory board for and has received grant support from Astellas and is a member of the advisory board for and has received grant support from GE Healthcare. Dr. Hochman has received a consulting fee and modest honoraria from GlaxoSmithKline.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- MI
myocardial infarction
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- OMT
optimal medical therapy
- PCI
percutaneous coronary intervention
- SIHD
stable ischemic heart disease
APPENDIX
For supplemental videos and legend, please see the online version of this article.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography. J Am Coll Cardiol. 2008;51:1127–47. doi: 10.1016/j.jacc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and inhospital mortality in the american college of cardiology-national cardiovascular data registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin GA, Dudley RA, Lucas FL, et al. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300:1765–73. doi: 10.1001/jama.300.15.1765. [DOI] [PubMed] [Google Scholar]
- 7.Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 Chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50:2264–74. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article. J Am Coll Cardiol. 2003;41:159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 9.Proceedings From the National Summit on Overuse; [Accessed May 2, 2014]. Available at: www.ama-assn.org/ama/pub/news/news/2013/2013-07-10-strategies-to-minimize-overuse.page. [Google Scholar]
- 10.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol. 2012;59:857–81. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 12.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw LJ, Weintraub WS, Maron DJ, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164:243–50. doi: 10.1016/j.ahj.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 15.Hachamovitch R, Rozanski A, Hayes SW, et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol. 2006;13:768–78. doi: 10.1016/j.nuclcard.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–24. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 17.Yao SS, Bangalore S, Chaudhry FA. Prognostic implications of stress echocardiography and impact on patient outcomes. J Am Soc Echocardiogr. 2010;23:832–9. doi: 10.1016/j.echo.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Califf RM, Armstrong PW, Carver JR, D’Agostino RB, Strauss WE. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. J Am Coll Cardiol. 1996;27:1007–19. doi: 10.1016/0735-1097(96)87733-3. [DOI] [PubMed] [Google Scholar]
- 19.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 21.Leong-Poi H, Rim SJ, Le DE, Fisher NG, Wei K, Kaul S. Perfusion versus function: the ischemic cascade in demand ischemia: implications of single-vessel versus multivessel stenosis. Circulation. 2002;105:987–92. doi: 10.1161/hc0802.104326. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LJ, Hage FG, Berman DS, Hachamovitch R, Iskandrian A. Prognosis in the era of comparative effectiveness research: where is nuclear cardiology now and where should it be? J Nucl Cardiol. 2012;19:1026–43. doi: 10.1007/s12350-012-9593-y. [DOI] [PubMed] [Google Scholar]
- 23.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. A prognostic score for prediction of cardiac mortality risk after adenosine stress myocardial perfusion scintigraphy. J Am Coll Cardiol. 2005;45:722–9. doi: 10.1016/j.jacc.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LJ, Min JK, Hachamovitch R, Hendel RC, Borges-Neto S, Berman DS. Nomograms for estimating coronary artery disease prognosis with gated stress myocardial perfusion spect. J Nucl Cardiol. 2012;19:43–52. doi: 10.1007/s12350-011-9468-7. [DOI] [PubMed] [Google Scholar]
- 25.Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Berman DS, Abidov A, Kang X, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion spect interpretation. J Nucl Cardiol. 2004;11:414–23. doi: 10.1016/j.nuclcard.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Shaw LJ, Cerqueira MD, Brooks MM, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19:658–69. doi: 10.1007/s12350-012-9548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 29.Berman DS, Kang X, Van Train KF, et al. Comparative prognostic value of automatic quantitative analysis versus semiquantitative visual analysis of exercise myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1998;32:1987–95. doi: 10.1016/s0735-1097(98)00501-4. [DOI] [PubMed] [Google Scholar]
- 30.Dorbala S, Di Carli MF, Beanlands RS, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61:176–84. doi: 10.1016/j.jacc.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima K, Nishimura T. Cardiovascular events in Japan. Lessons from the J-ACCESS multicenter prognostic study using myocardial perfusion imaging. Circ J. 2012;76:1313–21. doi: 10.1253/circj.cj-12-0260. [DOI] [PubMed] [Google Scholar]
- 32.Slim HB, Nair SU, Arora S, Heller GV. Does location matter? Prognostic value of single-photon emission computed tomography myocardial perfusion imaging by vascular territory. J Nucl Cardiol. 2012;19:458–64. doi: 10.1007/s12350-011-9486-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee DS, Verocai F, Husain M, et al. Cardiovascular outcomes are predicted by exercise-stress myocardial perfusion imaging. Am Heart J. 2011;161:900–7. doi: 10.1016/j.ahj.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Lertsburapa K, Ahlberg AW, Bateman TM, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium-82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2008;15:745–53. doi: 10.1007/BF03007355. [DOI] [PubMed] [Google Scholar]
- 35.Yao SS, Qureshi E, Sherrid MV, Chaudhry FA. Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease. J Am Coll Cardiol. 2003;42:1084–90. doi: 10.1016/s0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 36.Chaowalit N, Arruda AL, McCully RB, Bailey KR, Pellikka PA. Dobutamine stress echocardiography in patients with diabetes mellitus: Enhanced prognostic prediction using a simple risk score. J Am Coll Cardiol. 2006;47:1029–36. doi: 10.1016/j.jacc.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Hoque A, Maaieh M, Longaker RA, Stoddard MF. Exercise echocardiography and thallium-201 single-photon emission computed tomography stress test for 5- and 10-year prognosis of mortality and specific cardiac events. J Am Soc Echocardiogr. 2002;15:1326–34. doi: 10.1067/mje.2002.126109. [DOI] [PubMed] [Google Scholar]
- 38.Bernheim AM, Kittipovanonth M, Takahashi PY, Gharacholou SM, Scott CG, Pellikka PA. Does the prognostic value of dobutamine stress echocardiography differ among different age groups? Am Heart J. 2011;161:740–5. doi: 10.1016/j.ahj.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 39.van der Sijde JN, Boiten HJ, Sozzi FB, Elhendy A, van Domburg RT, Schinkel AF. Long-term prognostic value of dobutamine stress echocardiography in diabetic patients with limited exercise capability. Diabetes Care. 2012;35:634–9. doi: 10.2337/dc11-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao SS, Wever-Pinzon O, Zhang X, Bangalore S, Chaudhry FA. Prognostic value of stress echocardiogram in patients with angiographically significant coronary artery disease. Am J Cardiol. 2012;109:153–8. doi: 10.1016/j.amjcard.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCully RB, Roger VL, Mahoney DW, et al. Outcome after abnormal exercise echocardiography for patients with good exercise capacity: prognostic importance of the extent and severity of exercise-related left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1345–52. doi: 10.1016/s0735-1097(02)01778-3. [DOI] [PubMed] [Google Scholar]
- 42.Elhendy A, Mahoney DW, Burger KN, McCully RB, Pellikka PA. Prognostic value of exercise echocardiography in patients with classic angina pectoris. Am J Cardiol. 2004;94:559–63. doi: 10.1016/j.amjcard.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Shaw LJ, Vasey C, Sawada S, Rimmerman C, Marwick TH. Impact of gender on risk stratification by exercise and dobutamine stress echocardiography: long-term mortality in 4234 women and 6898 men. Eur Heart J. 2005;26:447–56. doi: 10.1093/eurheartj/ehi102. [DOI] [PubMed] [Google Scholar]
- 44.Innocenti F, Agresti C, Baroncini C, et al. Prognostic value of dobutamine stress echocardiography in diabetic patients. Int J Cardiovasc Imaging. 2010;26:499–507. doi: 10.1007/s10554-010-9598-z. [DOI] [PubMed] [Google Scholar]
- 45.Chelliah R, Anantharam B, Burden L, Alhajiri A, Senior R. Independent and incremental value of stress echocardiography over clinical and stress electrocardiographic parameters for the prediction of hard cardiac events in new-onset suspected angina with no history of coronary artery disease. Eur J Echocardiogr. 2010;11:875–82. doi: 10.1093/ejechocard/jeq086. [DOI] [PubMed] [Google Scholar]
- 46.Innocenti F, Caldi F, Tassinari I, et al. Prognostic value of exercise stress test and dobutamine stress echo in patients with known coronary artery disease. Echocardiography. 2009;26:1–9. doi: 10.1111/j.1540-8175.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- 47.Elhendy A, Mahoney DW, Khandheria BK, et al. Prognostic significance of the location of wall motion abnormalities during exercise echocardiography. J Am Coll Cardiol. 2002;40:1623–9. doi: 10.1016/s0735-1097(02)02338-0. [DOI] [PubMed] [Google Scholar]
- 48.Olmos LI, Dakik H, Gordon R, et al. Long-term prognostic value of exercise echocardiography compared with exercise 201tl, ECG, and clinical variables in patients evaluated for coronary artery disease. Circulation. 1998;98:2679–86. doi: 10.1161/01.cir.98.24.2679. [DOI] [PubMed] [Google Scholar]
- 49.Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103:2566–71. doi: 10.1161/01.cir.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 50.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischaemic cascade. Heart. 2009;95:49–55. doi: 10.1136/hrt.2007.139683. [DOI] [PubMed] [Google Scholar]
- 51.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–33. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 52.Kelle S, Chiribiri A, Vierecke J, et al. Long-term prognostic value of dobutamine stress CMR. J Am Coll Cardiol Img. 2011;4:161–72. doi: 10.1016/j.jcmg.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50:1174–9. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation. 2011;123:1509–18. doi: 10.1161/CIRCULATIONAHA.109.907659. [DOI] [PubMed] [Google Scholar]
- 55.Korosoglou G, Elhmidi Y, Steen H, et al. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1,493 consecutive patients. J Am Coll Cardiol. 2010;56:1225–34. doi: 10.1016/j.jacc.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Mark DB, Shaw L, Harrell FE, Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–53. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 57.Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective a2a agonist regadenoson versus adenosine in myocardial perfusion imaging integrated advance-mpi trial results. J Am Coll Cardiol Img. 2008;1:307–16. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Shaw LJ, Hachamovitch R, Heller GV, et al. Noninvasive strategies for the estimation of cardiac risk in stable chest pain patients. Am J Cardiol. 2000;86:1–7. doi: 10.1016/s0002-9149(00)00819-5. [DOI] [PubMed] [Google Scholar]
- 59.Hachamovitch R, Hayes SW, Friedman JD, et al. Is there a referral bias against catheterization of patients with reduced left ventricular ejection fraction? Influence of ejection fraction and inducible ischemia on post-single-photon emission computed tomography management of patients without a history of coronary artery disease. J Am Coll Cardiol. 2003;42:1286–94. doi: 10.1016/s0735-1097(03)00991-4. [DOI] [PubMed] [Google Scholar]
- 60.Thomas GS, Miyamoto MI, Morello AP, III, et al. Technetium 99m sestamibi myocardial perfusion imaging predicts clinical outcome in the community outpatient setting. J Am Coll Cardiol. 2004;43:213–23. doi: 10.1016/j.jacc.2003.07.041. [DOI] [PubMed] [Google Scholar]
- 61.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Stress myocardial perfusion single-photon emission computed tomography is clinically effective and cost effective in risk stratification of patients with a high likelihood of coronary artery disease (cad) but no known cad. J Am Coll Cardiol. 2004;43:200–8. doi: 10.1016/j.jacc.2003.07.043. [DOI] [PubMed] [Google Scholar]
- 62.Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol. 2003;41:1125–33. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 63.Hachamovitch R, Johnson J, Hlatky M, et al. Short-term referral rates to catheterization after noninvasive cardiac imaging: results from the study of myocardial perfusion and coronary anatomy imaging roles in cad (SPARC) trial 90 day follow-up (abstr) Circulation. 2009;120:S486. [Google Scholar]
- 64.Shaw LJ, Hachamovitch R, Berman DS, et al. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients. J Am Coll Cardiol. 1999;33:661–9. doi: 10.1016/s0735-1097(98)00606-8. [DOI] [PubMed] [Google Scholar]
- 65.Bateman TM, O’Keefe JH, Jr, Dong VM, Barnhart C, Ligon RW. Coronary angiographic rates after stress single-photon emission computed tomographic scintigraphy. J Nucl Cardiol. 1995;2:217–23. doi: 10.1016/s1071-3581(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 66.Nallamothu N, Pancholy SB, Lee KR, Heo J, Iskandrian AS. Impact on exercise single-photon emission computed tomographic thallium imaging on patient management and outcome. J Nucl Cardiol. 1995;2:334–8. doi: 10.1016/s1071-3581(05)80078-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.