Abstract
Foxp3+ regulatory T (Treg) cells are a crucial immunosuppressive population of CD4+ T cells, yet the homeostatic processes and survival programs that maintain the Treg cell pool are poorly understood. Here we report that peripheral Treg cells markedly alter their proliferative and apoptotic rates to rapidly restore numerical deficit through an interleukin 2–dependent and costimulation-dependent process. By contrast, excess Treg cells are removed by attrition, dependent on the Bim-initiated Bak- and Bax-dependent intrinsic apoptotic pathway. The antiapoptotic proteins Bcl-xL and Bcl-2 were dispensable for survival of Treg cells, whereas Mcl-1 was critical for survival of Treg cells, and the loss of this antiapoptotic protein caused fatal autoimmunity. Together, these data define the active processes by which Treg cells maintain homeostasis via critical survival pathways.
The expression of the transcription factor Foxp3 in T cells results in radical transcriptional rewiring1 and the consequent functional differentiation of these cells into Treg cells. The most profound effect is the switch from a proimmunity potential to a protolerance function that is essential for preventing fatal systemic autoimmunity2. In addition to this archetypal characteristic, the transcriptional rewiring also alters the basic cellular properties of Treg cells, including differential reliance on cytokines3,4 and T cell receptor (TCR) signaling5 for homeostasis as well as an unusual anergic and apoptotic behavior in vitro. The size of the Treg cell population is critical for immunological balance; even relatively minor modulation alters immunity6. Abnormally low numbers of Treg cells have been observed in multiple autoimmune and inflammatory conditions7, whereas high numbers of Treg cells in the aged are thought to contribute to the partial immunosuppressed state8,9. In addition, Treg cells are postulated to impede effective immunity against cancer10, with expansion of Treg cells after radiotherapy reported to limit lymphoma clearance11. Given the growing interest in manipulating Treg cell numbers in clinical settings, there is a pressing need to understand the cellular and molecular biology of Treg cell homeostasis.
Apoptotic cell death is a major regulator of hematopoietic cell homeostasis12. In vertebrates, two distinct, but ultimately converging, pathways control apoptosis. The ‘extrinsic’ or ‘death receptor’ pathway is initiated by ligation of cell-surface death domain–containing members of the TNF-receptor family. The ‘intrinsic’ or ‘mitochondrial’ pathway is initiated by cellular stressors that alter the balance between proapoptotic and antiapoptotic members of the Bcl-2 family of proteins, culminating in the activation of Bax and Bak, and subsequent release of apoptogenic factors from mitochondria13. The two pathways converge upon the activation of ‘executioner’ caspases that demolish the cell. In the intrinsic apoptosis pathway, activation of Bax or Bak is the ‘point of no return’ and thus requires tight regulation. The prosurvival proteins Bcl-2, Bcl-xL, Mcl-1, A1 and Bcl-w restrain the activation of Bax and Bak, whereas proapoptotic proteins containing only the Bcl-2 homology domain BH3 (the so-called BH3-only proteins: Bim, Puma, Bid, Bmf, Bad, Noxa, Bik and Hrk) inhibit the prosurvival members and are essential for initiation of apoptosis signaling14. The relative importance of each of these proteins varies among different cell types or cytotoxic stimuli and therefore has to be evaluated on a case-by-case basis.
In this study we took advantage of the location of Foxp3 on the X chromosome and the effect of X inactivation on a diphtheria toxin (DT) receptor knock-in construct to study the dynamics of Treg cell responses to homeostatic perturbation in a highly controlled manner. We found that modulation of the Treg cell population created a TCR costimulation-dependent and IL-2–dependent feedback loop, which increased proliferation of Treg cells and diminished apoptosis to drive rapid restoration of Treg cell numbers. Furthermore, we found that the Bak- and Bax-dependent intrinsic apoptotic pathway naturally limited the Treg cell population, with Treg cell accumulation observed in absence of Bak and Bax. Prosurvival Bcl-2 family member Mcl-1 safeguarded the survival of Treg cells; deletion of Mcl-1 caused a rapid loss of Treg cells and onset of fatal autoimmunity. Mcl-1 expression is regulated by interleukin 2 (IL-2), which increased Mcl1 transcription during the Treg expansion phase after in vivo depletion. Finally, the BH3-only protein, Bim, is the primary antagonist of Mcl-1 in Treg cells, as conditional deletion of Bim led to accumulation of excess Treg cells, as observed with loss of Bak and Bax.
RESULTS
Treg cells exhibit IL-2–dependent niche-filling behavior
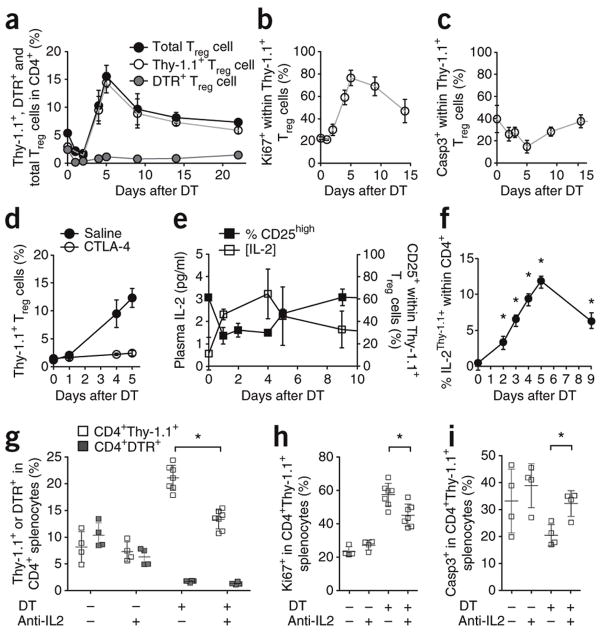
To determine the homeostatic characteristics of Treg cells, we compared the proliferative behavior of Foxp3+ Treg cells in 5-bromodeoxyuridine (BrdU) labeling experiments. In contrast to prior in vitro studies that characterized Treg cells as semianergic, quiescent cells, we found that Treg cells proliferated at a substantially faster rate than conventional T cells (CD4+ or CD8+) in vivo, with ~50% of the population having undergone proliferation within a 10-d window in unmanipulated hosts during homeostatic conditions (Supplementary Fig. 1). As these results suggest a highly dynamic yet stable population, we developed a 50% depletion system to examine responses to such perturbations in the Treg cell niche using two different Foxp3 constructs. The first was Thy1 (Thy1.1 variant) and the second was human HBEGF (diphtheria toxin receptor; DTR), each knocked into the Foxp3 locus on the X chromosome. Female mice heterozygous for the Foxp3Thy1.1 and Foxp3DTR alleles (Foxp3Thy1.1/DTR mice) have two distinct populations of Treg cells due to random X inactivation of Foxp3 alleles. Half express the marker Thy1.1, and the other half express DTR. Non-Treg cells express neither marker. Upon injection of DT, the DTR+ Treg cells will be rapidly eliminated and the response of the DTR−Thy1.1+ compartment to this 50% drop in total Treg cell numbers can be tracked. An additional advantage of this system is that the use of Thy1.1 to mark untouched Treg cells circumvents the difficulties in measuring apoptosis caused by the cleavage of Foxp3 by activated caspases (Supplementary Fig. 2). DT addition efficiently eliminated DTR+ Treg cells, but the overall proportion of Foxp3+ cells was rapidly restored by the expansion of DTR−Thy1.1+ Treg cells (Fig. 1a). The six fold increase in the number of Thy1.1+ Treg cells by day 5 caused an initial overshoot of ~200% in total Treg cells, followed by a slow decline to basal levels (Fig. 1a and Supplementary Fig. 3). During the niche-filling process, proliferation rate of Thy1.1+ Treg cells increased, with the percentage of cells expressing the cell cycle protein Ki67 increasing from ~20% to ~70% (Fig. 1b). At the same time, the apoptosis rate of Thy1.1+ Treg cells decreased from ~40% to ~20% active caspase-3+ (Fig. 1c). We found no evidence of any substantial contribution to the peripheral homeostasis of Treg cells by recent thymic emigrants (tracked using a Rag2-GFP transgene or comparison to thymectomized mice; Supplementary Fig. 3h–j) or peripheral conversion of conventional T cells into Treg cells, as this mechanism would have resulted in equal contribution by DTR+ and Thy1.1+ Treg cells (the injected DT is cleared within hours). Therefore, we conclude that the expansion of existing Treg cells must be the major driver of niche-filling in the 50% depletion system.
Figure 1.
Homeostatic expansion of Treg cells is driven by increased production of IL-2. (a) Percentages of DTR+ Treg cells Thy1.1+ Treg cells and total Treg cells in Foxp3Thy1.1/DTR females depleted of Foxp3DTR+ Treg cells on day 0. Blood leukocytes were assessed on indicated days (n=4,3,7,3,4,4,4,4 mice). (b,c) Proliferation rate (b; percentage Ki67+) and apoptosis rate (c; percentage activated caspase 3+) of Thy1.1+ Treg cells, after DT treatment of Foxp3Thy1.1/DTR females on day 0. (d) Percentages of Thy1.1+ Treg cells in peripheral blood after DT treatment of saline-treated (n=3,3,3 mice/group) or CTLA4-Ig–treated Foxp3Thy1.1/DTR female mice (n=3,3,3 mice/group). (e) Plasma IL-2 (left axis, n=27,3,3,12,14 mice/group) and surface CD25 expression on Thy1.1+ Treg cells (right axis, n=4,3,7,3,4,4 mice/group) from days 0 to 15 for female Foxp3Thy1.1/DTR mice depleted of Foxp3DTR+ Treg cells (f) Proportion of Thy1.1+ (reporter for IL-2) cells within the CD4+Foxp3− population after DT injection of Foxp3+/DTR.IL2-Thy1.1 mice. (g) Foxp3Thy1.1/DTR mice were depleted of DTR+ Treg cells and injected daily with an IL-2 blocking antibody (a-IL2) or an immunoglobulin isotype-matched control antibody. Splenocytes were analyzed on day 6 for the percentages of DTR+ and Thy1.1+ Treg cells, with or without Treg cell depletion (DT treatment) and with or without anti–IL-2 treatment (n=4,4,7,7 mice/group). (h,i) Proliferation rate (h; percentage Ki67+) and apoptosis rate (i; activated caspase-3+) of Thy1.1+ regulatory T cells, with or without DT treatment and with or without anti-IL-2 treatment (n=4). (a–i) Mean ± s.d., *P < 0.05, t-test. Data are representative of 3(a–f) and 2 (g–i) independent experiments.
We observed an increase in effector-memory CD44hiCD62LloCD4+ T cells after partial depletion of Treg cells (Supplementary Fig. 3); therefore, we determined whether Treg cell niche-filling depended upon activation of T cells. Costimulatory blockade with a soluble CTLA4-immunoglobulin fusion protein (CTLA4-Ig) to prevent activation of T cells negated restoration of Treg cell numbers after partial depletion (Fig. 1d), consistent with a requirement for activation of conventional T cells (although this experiment does not rule out a Treg cell–intrinsic requirement for costimulation). To test the role of dendritic cells (DCs) in this process, Itgax-Cre (CD11c-Cre) transgenic mice were crossed to Rosa26-flstopfl-DTR (stop sequence flanked by LoxP-sites) knock-in mice to allow depletion of DCs via injection of DT. Depletion of dendritic cells (DCs) in Foxp3DTR/+ CD11c-Cre Rosa26-flstopfl-DTR mice simultaneous with partial depletion of Treg cells did not alter niche-filling kinetics (Supplementary Fig. 4), indicating that residual DCs or an alternate costimulation source is sufficient. Synchronous with Treg cell niche-filling was an increase in plasma IL-2 levels and downregulation of the high-affinity subunit of the IL-2 receptor, CD25, by Treg cells (Fig. 1e). The primary shift in CD25 expression was from CD25hi to CD25int, a change associated with increased proliferation (Supplementary Fig. 4), identifying IL-2 as a potential mediator of feedback from activation of conventional T cells to Treg cells. To determine the source of IL-2 in this context, we generated mice with a bacterial artificial chromosome (BAC) transgenic Thy1 (Thy1.1 variant) reporter of IL-2 production (unpublished data, RJ Luther and CT Weaver and crossed it onto the Foxp3DTR/+ background to track IL-2 production during 50% Treg cell depletion. The numbers of IL-2–expressing conventional CD4+ T cells paralleled Treg cell expansion kinetics (Fig. 1f). Accordingly, antibody-mediated IL-2 blockade in Foxp3Thy1.1/DTR mice during the 50% Treg cell depletion impaired niche-filling by partially inhibiting the greater proliferation rate and by completely blocking the decrease in apoptosis in the remaining Treg cells (Fig. 1g–i). By contrast, short-term neutralization of IL-2 had little effect in the mice not treated with DT (data not shown), which may suggest that the low to undetectable baseline amounts of IL-2 have little effect on the default proliferative or apoptotic characteristics of Treg cells, whereas the greater amount of IL-2 after perturbation drives substantial changes. The partial nature of the anti–IL-2 treatment may be due to the inability of the blocking antibody to completely inhibit this paracrine factor or may indicate that other factors can drive proliferation of Treg cells during homeostatic expansion (Fig. 1h). Nevertheless, IL-2 was directly responsible for the altered apoptotic rate in Treg cells (Fig. 1i), inhibition of which substantially blunts Treg cell homeostatic expansion (Fig. 1g). Collectively, these data demonstrate that Treg cells actively maintained homeostasis by swiftly responding to partial insufficiency via a feedback loop involving activation of conventional T cells, greater IL-2 production and altered Treg cell proliferation and apoptosis.
Peripheral Treg cell number is constrained by apoptosis
The cellular dynamics of Treg cells after ablation highlighted the potential importance of Treg cell apoptosis during two phases, namely during the expansion phase, when apoptosis was decreased, and during the contraction phase, when apoptosis mediated the decline in numbers. Conversely, proliferation rates when there was a surplus of Treg cells did not drop below baseline at homeostasis (Fig. 1b), and we found no evidence for ‘deconversion’ of excess Treg cells into conventional T cells when we used a fate-mapping tracker in Foxp3Cre/DTR Rosa26-flstopfl-YFP(stop sequence flanked by LoxP-sites) mice (Supplementary Fig. 3k). Despite this (indirect) evidence for modulation of apoptosis being crucial for homeostasis of Treg cells, defects in the ‘death receptor’ pathway have not been associated with increases in Treg cell numbers15,16. By contrast, the marked expansion of Treg cells observed in mice lacking the proapoptotic proteins Bax and Bak, or those lacking the BH3-only protein Bim, implicates the intrinsic pathway of apoptosis in regulating numbers of Treg cells4,17, although these studies could not distinguish greater conversion into the Treg cell lineage because of defective thymocyte deletion from elevated peripheral homeostasis. To analyze the impact of the intrinsic pathway of apoptosis specifically on peripheral Treg cell homeostasis, we generated Foxp3Cre Bak1−/− Baxfl/fl mice. Owing to the redundancy of Bak and Bax18, this resulted in a Treg cell–specific knockout of the entire intrinsic apoptotic pathway. These mice exhibited normal thymic development of Treg cells (Supplementary Fig. 5), but peripheral accumulation of Foxp3+ Treg cells to twice the normal numbers (Fig. 2). This accumulation was not due to excess proliferation (turnover was lower; Fig. 2a). These data indicate that the intrinsic pathway of apoptosis is a critical regulator of peripheral Treg cell homeostasis.
Figure 2.

The intrinsic apoptosis pathway is required to restrain Treg cell numbers to homeostatic levels. (a) Representative flow profiles (TCRβ versus Foxp3 gated on CD4+ cells, Ki67 histograms gated on Foxp3+ CD4+ cells) for wild-type, Foxp3CreBak−/−Baxfl/fl mice and control littermates at 6–8 weeks of age. Numbers in plots indicate (top) the percentage of Foxp3+TCRβ+ regulatory T cells and (bottom) the fraction of proliferating Foxp3+CD4+ cells (Ki67+). (b) Average percentages and absolute numbers (mean ± s.d.) of splenic Foxp3+ Treg cells in wt, Foxp3CreBak−/−Baxfl/fl mice and control littermates at 6–8 weeks of age (n=3,3,5,3 mice/group). (a–b) Data from one experiment representative of three are shown. Mean ± s.d., *P < 0.05, t-test.
Treg cells require Mcl-1 for survival
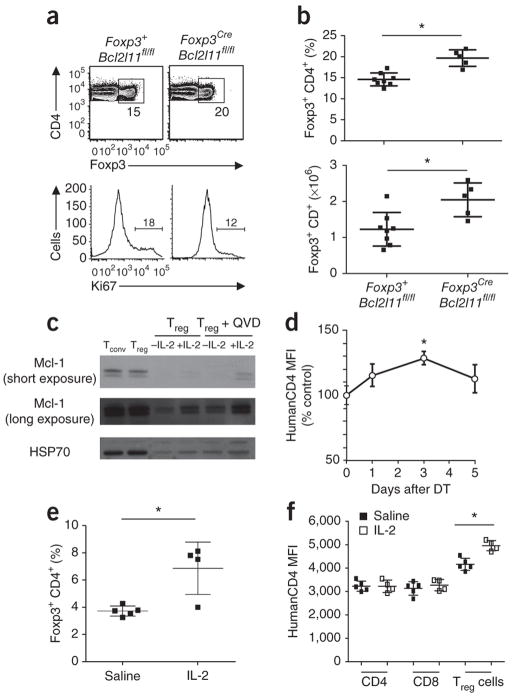
Bak and Bax activation is tightly regulated by prosurvival members of the Bcl-2 family, of which Bcl-2, Bcl-xL and Mcl-1 have been reported to be expressed in Treg cells1. The leading prosurvival candidate for maintaining Treg cell survival was Bcl-2, because of the dynamic expression of Bcl-2 observed in Treg cells19–21 and the Treg cell accumulation that occurs in mice with forced Bcl-2 overexpression21. However, lethally irradiated mice reconstituted with a 50:50 mixture of C57BL/6.Ly5.1:Bcl2−/− hematopoietic precursors exhibited a normal proportion of Treg cells derived from the Bcl2-deficient compartment, demonstrating that Bcl-2 is dispensable for their survival (Fig. 3a,b). We could not analyze the second candidate, Bcl-xL, using conventional knockouts (and hematopoietic reconstitution), as it is required for cell survival at the CD4+CD8+ thymocyte stage22,23. We therefore created mice with Treg cell–specific ablation of Bcl-xL by crossing a Foxp3Cre strain24 with Bcl2l1fl mice25. The resulting Foxp3CreBcl2l1fl/fl mice had normal numbers of Treg cells in both the thymus and periphery with no obvious immunological or pathological phenotype, demonstrating no role for this anti-apoptotic protein in homeostasis of Treg cells (Fig. 3c,d).
Figure 3.
Regulatory T cell survival is independent of Bcl-2 and Bcl-xL. (a) C57BL/6.Ly5.1 (Ly5.1) chimeras reconstituted with a 50:50 mixture of hematopoietic precursors from wild-type Ly5.1 and either Bcl2+/+ or Bcl2−/− mice, analyzed 8–12 weeks later. Gates show the percentage of Ly5.2+CD4+Foxp3+ cells recovered from the thymus or spleen. (b) Average percentages (mean ± s.d.) of Ly5.2+CD4+Foxp3+ cells recovered from the thymus and spleen of the same mixed hematopoietic chimeras reconstituted with precursors from either wild-type (Bcl2+/+) or Bcl2−/− mice (n= 4,6 mice/group). (a,b) Data from one experiment representative of three are shown. (c) Representative flow profiles of CD4 versus Foxp3 gated on CD4+ cells from Foxp3CreBcl2l1wt/wt and Foxp3CreBcl2l1fl/fl mice. Gates show the percentage of CD4+Foxp3+ cells recovered from the thymus or spleen. (d) Average percentages (mean ± s.d.) of CD4+Foxp3+ Treg cells in the thymus and spleen of Foxp3CreBcl2l1wt/wt and Foxp3CreBcl2l1fl/fl siblings at 6–8 weeks of age (n = 6,9 respectively). (c,d) Data pooled from three independent experiments are shown.
To investigate a potential role of Mcl-1 in regulating Treg cell apoptosis, we used a huCD4 reporter of Mcl-1 expression. The Mcl1fl allele we used was designed to bring a human CD4 reporter in-frame after Cre recombinase–mediated excision26. When we crossed Mcl1fl/+ mice to Cd127Cre knock-in mice27, expression of Cre recombinase in early lymphoid progenitors resulted in recombination of the Mcl1fl allele in the entire lineage, allowing for tracking of Mcl1 transcription in all T cell subsets using the huCD4 reporter, while maintaining their survival with the wild-type Mcl1 allele (that is, the genotype becomes Mcl1huCD4/+ in the T cell lineage). This strategy allows the relative quantification of Mcl-1 expression in all T cell subsets with a greater dynamic range than previous profiling28. During thymic development, Mcl-1 reporter expression peaked at the DP stage, before an ~80% decrease in conventional CD4+CD8− thymocytes (Fig. 4a). In contrast to conventional CD4+CD8− cells, Foxp3+CD4+CD8− cells maintained elevated Mcl-1 reporter expression (Fig. 4a), and likewise peripheral Treg cells expressed the Mcl-1 reporter at ~50% higher levels than conventional T cells (Fig. 4b,c). To assess the function of Mcl-1 in Treg cells, while circumventing the impact of its loss on the early thymocyte stage29, we created mice with Treg cell–specific deletion of Mcl1. In contrast to the redundancy of Bcl-2 and Bcl-xL in survival of Treg cells, we found that Mcl-1 was essential. Foxp3CreMcl1fl/fl mice succumbed to a fatal immunopathology, surviving to only 4–8 weeks of age (Fig. 4d,e). Pathology was associated with immunological dysregulation, inflammatory infiltrate, hyper-IgE (100 times normal levels), elevated amounts of antibodies to dsDNA, abnormally high proliferation of CD8+ T cells, greater activation of CD4+ T cells and spontaneous differentiation into TH1, TH2 and TH17 effector cells (Fig. 4f–i, Supplementary Fig. 6 and data not shown), all hallmarks of the Foxp3-deficient scurfy phenotype.
Figure 4.

Spontaneous fatal immunopathology after Treg cell–specific deletion of Mcl-1. (a) HuCD4 reporter for Mcl-1 expression was measured in lymphocyte subsets in Cd127CreMcl1wt/fl-huCD4 female mice, 6–8 weeks of age. Average huCD4 reporter MFI in CD4−CD8− (DN), CD4+CD8+ (DP) and single positive (SP) thymocytes, the latter subdivided into CD4−CD8+ SP, conventional CD4+CD8− SP and CD4+CD8− Foxp3+ SP (n = 3 mice/group). (b) Average huCD4 reporter MFI in splenic CD19+ B cells, naïve conventional CD4+ (CD4+ nTc), activated conventional CD4+ (CD4+ actTc), Foxp3+ Treg cells, naive conventional CD8+ (CD8+ nTc) and activated conventional CD8+ T cells (CD8+ actTc) (n = 3 mice/group). (c) Representative histogram of huCD4 reporter MFI in naive conventional CD4+ (CD4+ nTc), Foxp3+ Treg cells and naive conventional CD8+ (CD8+ nTc), with control huCD4 staining in wild-type Foxp3+ Treg cells. (a–c) Data from one experiment representative of three. (d) Weights of male Foxp3CreMcl1wt/wt, Foxp3CreMcl1wt/fl and Foxp3CreMcl1fl/fl littermates at 6–8 weeks of age (n=11,20,16 mice/group). (e) Survival curve for male Foxp3CreMcl1wt/wt, Foxp3CreMcl1wt/fl and Foxp3CreMcl1fl/fl littermates (n=16,18,18 mice/group)). (f) Plasma IgE levels in male Foxp3CreMcl1wt/wt and Foxp3CreMcl1fl/fl littermates at 4–8 weeks of age (n=6,12 mice/group). (g,h) Average disease score (g) and representative histology (h; scale bar, 200 μm) of the lungs and small intestine of male Foxp3CreMcl1wt/wt and Foxp3CreMcl1fl/fl littermates at 4–8 weeks of age (n=11, 9 mice/group). (i) Average percentage of CD44+CD62Llow activated cells within CD4+ and CD8+ splenic T cells in Foxp3CreMcl1wt/wt, Foxp3CreMcl1wt/fl and Foxp3CreMcl1fl/fl littermates at 6–8 weeks of age (n=12,9,11,10,4,d8 mice/group). (d–i) Data pooled from 3 experiments. Mean ± s.d., * P < 0.05, t-test.
In young Foxp3CreMcl1fl/fl mice, thymic Treg cell development was relatively undisturbed and initially there was only a ~60% decrease in peripheral Treg cells (Supplementary Fig. 6). However, unlike the Foxp3Thy1.1/DTR model, this Treg cell deficit could not be corrected by peripheral expansion in Foxp3CreMcl1fl/fl mice, with an additional decrease in Treg cell numbers observed (Fig. 5a). Loss of Mcl-1–deficient Treg cells was even more extreme in a competitive environment (Supplementary Fig. 7). Nevertheless, the deficit-sensing mechanism appeared intact, as the remaining Treg cells in Foxp3CreMcl1fl/fl mice demonstrated a compensatory increase in proliferation (Fig. 5b). We observed no evidence of an outgrowth of Treg cells with intact Mcl1, nor Mcl1 excision in Foxp3− cells, using the huCD4 reporter expressed only when Mcl1 is excised (Fig. 5c). To measure the kinetics of Treg cell loss after Mcl1 ablation, we generated mixed hematopoietic chimeras with 50% Ly5.1 congenically labeled bone-marrow and 50% Ly5.2 labeled bone-marrow bearing a tamoxifen-inducible CreERT2 and loxP-flanked (floxed) alleles of either Mcl-1 or Bcl2l1. After reconstitution, we induced inducible knockout in a competitive context by oral gavage with tamoxifen. Punctual deletion of Mcl1, but not Bcl2l1, in this system caused Treg cell numbers to collapse within 2 d (Fig. 5d,e), revealing an acute necessity for Mcl-1 in survival of Treg cells.
Figure 5.

Mcl-1 is required for Treg cell survival. (a) Average percentages of Foxp3+ Treg cells within splenic CD4+ T cells in male Foxp3CreMcl1wt/wt, Foxp3CreMcl1wt/fl and Foxp3CreMcl1fl/fl littermates at 6–8 weeks of age (n=14,12,11 mice/group). (b) Average percentages of Ki67+ cells within splenic Foxp3+ Treg cells in Foxp3CreMcl1wt/wt, Foxp3CreMcl1wt/fl and Foxp3CreMcl1fl/fl littermates at 6–8 weeks of age (n=14,12,11 mice/group). (a,b) Data pooled from 3 experiments. c) Expression of the huCD4 reporter for excision of Mcl1 in Foxp3+ and Foxp3− cells in male Foxp3CreMcl1fl/fl mice (n=3,4,3 mice/group). Data from one experiment representative of three. (d) Ly5.1 versus Foxp3 expression on lymph node cells from mixed chimeras with Mcl1fl/fl or Bcl2l1fl/fl Ly5.2 donor compartments analyzed 3 d after treatment with tamoxifen or vehicle control. Gates display the fraction of Treg cells arising from (top) wildtype Ly5.1+ and (bottom) Mcl1fl/fl or Bcl2l1fl/fl Ly5.1− cells. Plots are representative of 3 experiments, each with n = 3 mice/group. (e) Ratios of Ly5.1+ to Ly5.1− CD4+Foxp3+ lymph node cells in mixed chimeras with the indicated Ly5.2+ donor compartment 3 d after treatment with tamoxifen or vehicle (n=3,3,4,6 mice/group). Data from one experiment representative of three is shown. Mean ± s.d., *P < 0.05, t-test.
Regulation of Mcl-1 in Treg cells by Bim and IL-2
The importance of Mcl-1 expression for Treg cell survival suggested that regulation of Mcl-1 may be important in setting the homeostatic balance of Treg cells. Several of the proapoptotic BH3-only members of the Bcl-2 family can overcome the prosurvival function of Mcl-1 and thereby initiate apoptosis30; however, elevated numbers of Treg cells have been observed only in Bim-deficient mice4,17,19,21. In these mice with germ-line deletion of Bcl2l11, this effect has been ascribed to additional T cells entering the Treg cell lineage because of defective negative selection17, and secondary effects owing to low-grade inflammation31. To circumvent these issues, we generated mice bearing a floxed Bcl2l11 allele and crossed them with Foxp3Cre mice to create a Treg cell–specific deletion of Bcl2l11, where Bim is lost only after Treg cell development. The Foxp3CreBcl2l11fl/fl mice exhibited normal thymic differentiation, with substantial peripheral expansion of Foxp3+ Treg cells (Fig. 6a,b). Notably, the scale of peripheral Treg cell expansion expansion in Foxp3Cre Bcl2l11fl/fl mice was not as great as that observed in Foxp3Cre Bak−/− Baxfl/fl mice (Fig. 2a,b), indicating that, although Bim is the primary initiator of homeostatic Treg cell apoptosis, additional BH3-only proteins (for example, Puma32) may have additional roles. In addition to the negative regulation of Mcl-1 by Bim, the IL-2–dependent decrease in apoptosis during niche-filling (Fig. 1i) suggested that IL-2 might act as a positive regulator of Mcl-1. In vitro we observed that stimulation of Treg cells with IL-2 increased the amount of Mcl-1 protein (Fig. 6c). We therefore crossed the Cd127CreMcl1huCD4/+ reporter system (described above) to the Foxp3DTR/+ partial depletion system, to determine whether the greater availability of IL-2 during expansion of Treg cells (Fig. 1) was linked to in vivo changes in expression of Mcl-1. We observed a rapid increase in Mcl-1 reporter expression in Treg cells after partial depletion (Fig. 6d), coinciding with the IL-2–dependent decrease in apoptosis. To directly test the ability of IL-2 to induce expression of Mcl-1 in vivo, we injected Mcl-1 reporter mice (Cd127CreMcl1huCD4/+) with complexes of IL-2 and antibody to IL-2 (IL-2–anti-IL-2), which (as previously reported33) caused a rapid expansion of Foxp3+ Treg cells, with a twofold increase in the peripheral blood by day 2 (Fig. 6e). Accompanying this increase in Foxp3+ Treg cells was an increase in huCD4 expression (reporter for Mcl-1 transcription) in Treg cells, whereas conventional CD4+ and CD8+ T cells exhibited no change in huCD4 expression (Fig. 6f). Together, these results demonstrate that IL-2 regulates expression of Mcl-1 in vitro and in vivo, indicating a direct pathway in Treg cells from greater availability of IL-2 availability to greater expression of Mcl-1 (and perhaps lower Bim expression; data not shown), less apoptosis and subsequent peripheral expansion (Supplementary Fig. 8).
Figure 6.
Regulation of Mcl-1 in Treg cells by Bim and IL-2. (a) Representative flow cytometric profiles for CD4 vs Foxp3 gated on CD4+ cells (gates indicate fraction of Treg cells within CD4+ lymph node cells) and Ki67 histograms gated on Foxp3+CD4+ cells (gate indicates the fraction of proliferating Treg cells) for Foxp3wtBcl2l11fl/fl and Foxp3CreBcl2l11fl/fl littermates at 6–8 weeks of age. (b) Average percentages and absolute number of CD4+Foxp3+ Treg cells from pooled lymph nodes of Foxp3wtBcl2l11fl/fl and Foxp3CreBcl2l11fl/fl littermates (n=8,5 mice/group). Data from one experiment representative of three are shown. (c) Immunoblot analysis of Mcl-1 expression in ex vivo isolated conventional T cells (Tconv) or Treg cells, or Treg cells cultured overnight with or without IL-2, in the absence or presence of QVD-OPH (a broad spectrum caspase inhibitor used to prevent apoptosis in the absence of IL-2). Data from one experiment representative of three are shown. (d) Cd127CreMcl1wt/fl-huCD4Foxp3wt/DTR females were depleted of Foxp3DTR+ Treg cells on day 0 and huCD4 (Mcl1 reporter) expression was measured in Foxp3+ Treg cells during the expansion phase. MFI was normalized to Treg cells from Cd127CreMcl1wt/fl-huCD4Foxp3wt mice (n=4 mice/group). Data from one experiment representative of two are shown. (e) Cd127CreMcl1wt/fl-huCD4 mice were injected with IL-2-anti-IL-2 antibody complexes or saline and on day 2 were measured for CD4+Foxp3+ Treg cell expansion in the peripheral blood and f, expression of huCD4 reporter for Mcl1 expression (n=5,4 mice/group). Mean ± s.d., * P < 0.05, t-test.
DISCUSSION
Far from being the semianergic lineage first described in in vitro experiments, Foxp3+ Treg cells were highly dynamic and responsive in our experiments in vivo. Although entry into the Foxp3+ lineage is a gated event34, peripheral proliferation and apoptosis are the primary determinants of compartment size, with ~50% of the population having undergone proliferation every 10 d under homeostatic conditions. Several explanations present themselves for the necessity of a high turnover of the Treg cell pool. First, chronic proliferation caused by TCR stimulation via self-antigen stimulation35 may necessitate compensatory apoptosis; second, a proapoptotic effect of Foxp3 expression leads to greater basal apoptosis levels in Treg cells36, which may require compensatory proliferation; or third, high turnover itself may be required for sufficient regulatory function37. Assessment of Treg cell responsiveness to perturbations from this basal state requires both swift contraction and a mechanism to leave the remaining cells unaffected. The natural chimeric state of female Foxp3Thy1.1/DTR mice used here fulfills both of these conditions, unlike models which rely on partial antibody-mediated depletion38 or escape of abnormal DT-resistant Treg cell clones39. This approach revealed a much more rapid and dynamic Treg cell response to perturbation than that demonstrated in previous models38,39, characterized by promptly increased proliferation with concomitant decrease in apoptosis. Furthermore, this system revealed an ‘overfilling’ effect followed by the slower attrition of Treg cells via apoptosis to re-establish homeostatic levels. The contrast between rapid expansion in the face of Treg cell deficiency and gradual contraction during Treg cell excess is commensurate with the more severe physiological consequences of suboptimal immune suppression. In the range of induced variation studied here, modulation of the proliferation to apoptosis balance was sufficient to drive homeostatic correction, with any substantial involvement of thymic production, peripheral conversion or ‘de-conversion’ all excluded through experiments under these conditions. While these processes are capable of rapidly restoring Treg cell numbers, an implication of this heavy reliance on remaining Treg cell expansion (as opposed to production of new Treg cells) is that repeated episodes of partial Treg cell deficiency may narrow the diversity of the TCR repertoire in the restored Treg cell pool. The extent of any TCR repertoire restriction is unknown, but may be substantial in aged individuals due to periodic Treg cell contraction and expansion, in addition to the stochastic loss of diversity due to the high baseline rates of Treg cell proliferation and apoptosis.
Dissection of the molecular mediators of Treg cell homeostatic responsiveness revealed how well-known participants in Treg cell biology modify previously unappreciated survival pathways. We found a direct correlation between modulation of Treg cell numbers and production of IL-2 by conventional T cells in a costimulation-dependent manner. Elevated expression of IL-2 by conventional T cells occurs before the attainment of a typical activated cell-surface profile, a phenomenon that may be related to the lower threshold of TCR signaling required for cytokine production40 and which may serve to shorten the time lag of Treg cell expansion in response to depletion. Consistent with previous reports identifying the importance of IL-2 for Treg cells35, blockade of IL-2 blunted the homeostatic rebound after 50% Treg cell depletion, playing an essential role in the transient decrease in Treg cell apoptosis and a significant role in the boost to proliferation. The reliance of the Treg cell homeostatic feedback circuit on an inducible cytokine lies in stark contrast to the homeostatic feedback loops of B cells and non-regulatory T cells mediated by the cytokines, BAFF41 and IL-7 (ref. 42), respectively, which are not made by activated T cells but constitutively produced by stromal cells43,44. Notably, a feature of such static consumption-based homeostatic systems is that only numerical, rather than functional, sufficiency is selected for. In the Treg cell homeostatic system described here, by contrast, the dynamic production of IL-2 in response to Foxp3+ regulatory T cell numbers makes the niche dependent on regulatory T cell function (that is, restraining improper T cell activation) as opposed to merely numerical sufficiency. It is predicted that such a homeostatic model will prove to show greater robustness when challenged by variation in the efficiency of Treg cell suppression, as demonstrated by the increase in Treg cell numbers in several models of impaired function45,46.
A previously unappreciated key feature of the Treg cell homeostatic feedback loops described here is the central role for apoptosis in regulating Treg cell numbers. Expansion of Treg cells during numerical deficit was accompanied by an IL-2–dependent suspension of apoptosis, while contraction during surplus involved apoptotic processes. Accordingly, Treg cell–specific ablation of the intrinsic apoptosis pathway provoked the accumulation of surplus Treg cells. Our approach of systematic assessing pro-survival members of the intrinsic apoptosis pathway revealed that Bcl-2 and Bcl-xL were redundant, in contrast to prior supposition17,19–21, while Mcl-1 was essential for Treg cell survival. Furthermore, Mcl-1 appears to represent a rheostat for controlling the Treg cell homeostatic niche, with positive regulation via IL-2 and antagonism by Bim during homeostatic perturbation. This role of the Mcl-1 and Bim axis in driving the return of Treg cells to homeostatic levels represents a potential intervention point for therapeutic manipulation.
Online Methods
Mice
Bak−/− (ref. 18), Baxfl/fl (ref. 47), Bcl-x(Bcl2l1)fl/fl (ref. 25), Bim(Bcl2l11)fl/fl (ref. X), CD11c-Cre-Tg (ref. 48), CD127-Cre-Tg (ref. 27), Foxp3GFP (ref. 49), Foxp3YFPCre (ref. 24), Foxp3Thy1.1 (ref. 50), IL-2.BAC-Thy1.1 mice (manuscript in preparation, RJ Luther and CT Weaver), Mcl1fl/fl26, Rag2−/− (ref. 51), Rag2GFP-Tg (ref. 52), Rosa26-flstopfl-YFP (ref. 53), Rosa26-flstopfl-DTR (ref. 54) and Rosa26Cre-ERT2 (ref. 55) mice were all generated on, or backcrossed to, the C57BL/6 background. Foxp3DTR mice56 were backcrossed to the C57BL/6.Ly5.1 background. Experimental mice were housed under specific pathogen–free conditions. Disease development was monitored by frequent observation and post-mortem analysis. Cohorts of mice for the survival test were removed from the study at death or when veterinary advice indicated likely death within 48 h. All experiments were approved by the University of Leuven animal ethics committee or the WEHI animal ethics committee. Histological examination was performed by Histology Consultation Services and pathology reports were generated by BioGenetics.
Design of animal experiments
All endpoint experiments used 3 or more mice of the specified genotypes and age-range to enable statistical comparisons. Mice were assigned to groups based on a semi-randomization process that included a post-randomization reassignment to ensure equal age-sex distribution within groups. Investigators performing experiments were only aware of mouse ID numbers, not genotype. Genotypes were revealed only upon analysis.
Foxp3DTR/Thy1.1 heterozygous females, 6–8 weeks of age, were injected intraperitoneally with a dose of 50 μg/kg of DT (Sigma-Aldrich) diluted in saline on days 0 and 1 (for CD11c depletion, on days 0, 1, 2 and 3) of the experiment. A daily dose of 50 μg of IL-2–neutralizing antibody (S4B6) or IgG2a isotype-matched control antibody (eBR2a, eBioscience) was administered intraperitoneally starting on the day of the first DT injection. CTLA4-Ig (abatacept, Bristol-Myers Squibb) was injected intraperitoneally (25 mg/kg) on days 0, 2 and 4.
Cd127Cre Mcl1wt/fl-huCD4 female mice, 6–8 weeks of age, were injected intraperitoneally with IL-2 complex at a dose of 16.5 μg/mouse per day. IL-2 complex was generated by mixing mouse recombinant IL-2 (eBioscience) with anti-mouse IL-2 antibody (JES6-1A12, eBioscience) at a 1:10 ratio, and was injected intraperitoneally at a dose of 16.5 μg/mouse per day, as per the published protocol57.
Bone-marrow chimeric experiments were performed using recipient C57BL/6 Rag2−/− female mice sub-lethally irradiated with 9.5Gy at 8–12 weeks of age and reconstituted within 24 h using an intravenous injection with a total of 2 × 106 hematopoietic cells from bone marrow donors. Chimeric mice were analyzed at or after 6 weeks after reconstitution. For inducible deletion of Bcl-x or Mcl1, chimeric mice were given two doses of 200 mg/kg of tamoxifen (Sigma, T5648) via oral gavage on days 0 and 1.
BrdU exposure was initiated in C57BL/6 male mice, 6–8 weeks of age, via an intraperitoneal injection of 100 μg/100 μl BrdU (Sigma). A subset of mice were additionally given continuous exposure to 8 mg/ml BrdU in the drinking water, changed daily.
Flow cytometry
Leukocytes from peripheral blood, thymus, spleen or lymph nodes were analyzed using the following antibodies: anti-BrdU-APC (1/50) (B44, BD), anti-CD25-PECy7(1/300) (PC61.5), anti-CD25-PE (1/300) (BD), anti-CD4-APC-H7 (1/250) (GK1.5, BD), anti-CD4-PerCP (1/200) (GK1.5, BioLegend), anti-huCD4-PE (1/50) (OKT4, BD), anti-CD4-PE (1/200) (GK1.5), anti-CD4-FITC (1/200) (GK1.5), anti-huCD4-PE-Cy7 (1/25) (RPA-T4), anti-TCR-beta-PE-Cy7 (1/100) (H57.59.1, BioLegend), anti-CD44-PerCP-Cy5.5 (1/500) (IM7), CD62L-PE-Cy7 (1/500) (MEL-14), anti-CD8-PerCP-Cy5.5 (1/300) (53-6.7), anti-CD8-APC-eFluor780 (1/300) (53-6.7), anti-CD8-Qdot-655(1/300), anti-Foxp3-APC (1/100) (FJK-16s), anti-Foxp3-FITC (1/100) (FJK-16s), anti-GFP–Alexa Fluor 488 (1/200) (polyclonal, Invitrogen), anti-Ki67-PE (1/50) (B56, BD), anti-Ki67-FITC (1/50) (B56, BD) and anti-Thy1.1-PerCP-Cy5.5 (1/250) (HIS51), anti-IFN-γ-PerCP-Cy5.5 (1/350) (XMG1.2), anti-IL-17A-APC (1/100) (17B7), anti-IL-4-PECy7 (1/100) (BVD6-24G2) (all eBioscience unless indicated otherwise). Intracellular staining for Ki67 and Foxp3 was performed after fixation and permeabilization using the reagents from the eBiosciences Foxp3 staining kit. Intracellular staining for BrdU was performed after Foxp3 staining, using the BrdU staining kit (BD). For intracellular cytokine staining, cells were stimulated for 4 h in complete RPMI in presence of Phorbol myristate acetate (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and monensin (1/1,000; BD), reagents from the BD cytofix/cytoperm kit were used. Apoptosis was assessed using the Abcam active Caspase-3 FITC Staining Kit.
Biochemical analyses
Anti-dsDNA titers in individual plasma samples were determined by enzyme-linked immunosorbent assay (ELISA). IgE levels were measured with a mouse IgE Ready-SET-Go! ELISA assay (eBioscience). IL-2 concentrations were determined with a mouse IL-2 High Sensitivity ELISA (eBioscience). For in vitro stimulation of Treg cells with IL-2, pooled splenic and lymph node cells from Foxp3Cre mice were labeled with anti-CD4 microbeads and the CD4+ T cells enriched on an AutoMACS separator (Miltenyi Biotec). Enriched CD4+ cells were stained with anti-CD4-PerCP-Cy5.5 and anti-CD25-PE and YFP+ Treg cells or YFP− conventional T cells were purified by cell sorting on a MoFlo FACS machine (Cytomation). Treg cells were plated at 105 cells per well in Complete medium with 200 U/well IL-2 (Peprotech). The pan-caspase inhibitor QVD-OPH was added where indicated to prevent Treg apoptosis in the absence of IL-2. After 24 h of culture, Treg cells were recovered in lysis buffer and lysates were separated by SDS-PAGE, then transferred to PVDF membrane for probing with rabbit anti-Mcl-1 (Rockland Immunochemicals), HRPO conjugated anti-rabbit immunoglobulin (Southern Biotech) and development with ECL reagents (GE Healthcare).
Statistics and bioinformatics
Statistical analysis was performed on all data point, excluding only technical failures. No data-points were excluded on the basis of being outliers. All data points shown and used for analysis are biological replicates. Differences in animal survival rates were analyzed using a log rank test (Prism). All other statistical analyses were performed through an ANOVA with Tukey’s post-test, followed by individual unpaired two-tailed t-test comparisons between two groups, with P < 0.05 used as the threshold for statistical significance. Tests were picked in advance of data generation based on experimental design, rather than post-hoc data analysis of data distribution. Data are presented as mean ± s.d., with individual data points overlaid where appropriate.
Prediction of caspase cleavage sites was performed using CASVM58, using the P14-P10′ scanning window size and allowing aspartic acid and glutamic acid at P1.
Supplementary Material
Acknowledgments
We thank P. Fink (University of Washington, Seattle, USA) for providing Rag2–GFP-Tg mice backcrossed to the B6 background, L. Hennighausen (National Institutes of Health, Bethesda, USA) for providing Bcl2l1flox mice, A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, USA) for providing Foxp3GFP, Foxp3Cre, Foxp3Thy1.1 and Foxp3DTR mice, G. Kelly and S. Grabow (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for CreERT2Mcl1flox and CreERT2Bcl2l1flox mice and Bcl-2−/− fetal liver samples, F. Kupresanin, G. Siciliano, G.-F. Dabrowski, K. Humphreys and E. Lanera (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for technical assistance, S. Korsmeyer (Harvard Medical School, Boston, USA) for BaxfloxBak−/− mice, and A. Kallies (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for critical feedback on the manuscript. This work was supported by grants from the VIB, Marie Curie (TREG to A.L.), European Research Council (IMMUNO to A.L.), Interuniversity Attraction Poles (VII/39 to A.L and P.M.), QSIS (to A.A.F.) and the Australian National Health & Medical Research Council (CDF-1 #637353 to D.H.D.G.). W.P. is funded by Agentschap voor Innovatie door Wetenschap en Technologie. B.C., S.M.S. and S.H.-B. are funded by the Fonds Wetenschappelijk Onderzoek. This work was made possible through Victorian State Government Operational Infrastructure Support and the Australian Government National Health & Medical Research Council Independent Research Institutes Infrastructure Support Scheme.
Footnotes
Author Contributions
W.P., B.C., A.P., S.M.S., J.B., M.H., S.S., D.F., S.H.-B., L.L. and J.D. performed the experiments. D.H., A.S., P.B., R.J.L. and C.T.W. provided key reagents. S.M.S., A.A.F., P.M., J.D., D.H.D.G. and A.L. designed the study. W.P., D.H.D.G. and A.L. wrote the manuscript. All authors read and approved the manuscript.
The authors declare no competing financial interests.
References
- 1.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, et al. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 4.Chougnet CA, et al. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siggs OM, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian L, et al. Foxp3+ regulatory T cells exert asymmetric control over murine helper responses by inducing Th2 cell apoptosis. Blood. 2011;118:1845–1853. doi: 10.1182/blood-2011-04-346056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyara M, et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lages CS, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynor J, et al. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24:482–487. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu J, et al. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 11.Baba J, et al. Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood. 2012;120:2417–2427. doi: 10.1182/blood-2012-02-411124. [DOI] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 13.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 14.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, et al. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell checkpoint: both IL-2 knockout and Fas mutation prolong lifespan of Scurfy mice but by different mechanisms. J Immunol. 2007;179:8035–8041. doi: 10.4049/jimmunol.179.12.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda T, et al. Dual effects of TRAIL in suppression of autoimmunity: the inhibition of Th1 cells and the promotion of regulatory T cells. J Immunol. 2010;185:5259–5267. doi: 10.4049/jimmunol.0902797. [DOI] [PubMed] [Google Scholar]
- 17.Zhan Y, et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187:1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. Preferential control of induced regulatory T cell homeostasis via a Bim/Bcl-2 axis. Cell Death Dis. 2012;3:e270. doi: 10.1038/cddis.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, et al. Selective impairment of CD4+CD25+Foxp3+ regulatory T cells by paclitaxel is explained by Bcl-2/Bax mediated apoptosis. Int Immunopharmacol. 2011;11:212–219. doi: 10.1016/j.intimp.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Tischner D, et al. Defective cell death signalling along the Bcl-2 regulated apoptosis pathway compromises Treg cell development and limits their functionality in mice. J Autoimmun. 2012;38:59–69. doi: 10.1016/j.jaut.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motoyama N, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 23.Ma A, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubtsov YP, et al. IL-10 produced by regulatory T cells contributes to their suppressor function by limiting inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Wagner KU, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 26.Vikstrom I, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330:1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Dzhagalov I, et al. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 30.Strasser A, et al. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 32.Gray DH, et al. The BH3-only proteins Bim and Puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity. 2012;37:451–462. doi: 10.1016/j.immuni.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyman O, et al. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 34.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Tai X, et al. Foxp3 is pro-apoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013 doi: 10.1016/j.immuni.2013.02.022. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker LS. CD4+ CD25+ Treg: divide and rule? Immunology. 2004;111:129–137. doi: 10.1111/j.0019-2805.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeill A, et al. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol. 2007;65:63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 39.Suffner J, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 40.Guy CS, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol. 2013;14:262–270. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 42.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dummer W, et al. Autologous regulation of naive T cell homeostasis within the T cell compartment. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 44.Gorelik L, et al. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 46.Liston A, et al. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi O, et al. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caton ML, et al. Notch-RBP-J signaling controls the homeostasis of CD8-dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Liston A, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci USA. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 52.Yu W, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 53.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 55.Seibler J, et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003;31:e12. doi: 10.1093/nar/gng012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JM, et al. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 57.Létourneau S, et al. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci USA. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wee LJ, et al. CASVM: web server for SVM-based prediction of caspase substrates cleavage sites. Bioinformatics. 2007;23:3241–3243. doi: 10.1093/bioinformatics/btm334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.