Abstract
No studies have addressed the spatial complexity of Anopheles arabiensis populations in Zambia or the effects of drought on the genetic structure of this species. We genotyped approximately 420 An. arabiensis at 12 microsatellite loci representing 18 collections from the Southern Province of Zambia. Collections spanned three transmission seasons and covered a wet year–drought year–wet year cycle. Anopheles arabiensis within the 2,000 km2 of the Macha study region were panmictic, with high gene flow between Macha and Namwala, Zambia, which are 80 km apart. There was little evidence for genetic structuring among years, with no significant shifts in allele frequency distributions or observed heterozygosity, and no evidence for a genetic bottleneck despite a drastic reduction in mosquito numbers during the drought year. Anopheles arabiensis in southern Zambia has a large deme size, and the regional genetic structure of this species was little affected by an extended drought period.
INTRODUCTION
Anopheles gambiae s.s. Giles and An. arabiensis Patton are two of the most widespread and important malaria vectors in Africa.1,2 The need to understand the population structure of these vectors has generated increasing interest towards the interpretation of ecologic and biologic events in a genetic context. For example, data generated by population genetic studies on An. gambiae s.s. or An. arabiensis can be directly applied towards monitoring and predicting the spread of genetic elements conferring insecticide resistance3–5 or the spread of transgenes potentially introduced as control strategies for vector populations.6,7 Genetic studies on An. gambiae s.s. in west Africa have uncovered extraordinary intraspecific genetic complexity; this species represents not one homogenous gene pool but is comprised of five discrete chromosomal forms.8,9 In addition to cytogenetics, microsatellite markers developed for An. gambiae s.s.10,11 have been integral to studies aimed at understanding the genetic structure and patterns of gene exchange among geographically and seasonally diverse mosquito populations.8,12–15 More recently, microsatellites identified in An. gambiae have been applied with varying degrees of success in studies targeting An. arabiensis.15–22 Although relatively less studied, An. arabiensis serves as a major, and sometimes the primary, vector of Plasmodium falciparum throughout much of sub-Saharan Africa.23–25
Much less structure is evident among and within An. arabiensis populations compared with its sister taxon, An. gambiae s.s. However, there is evidence of genetic differentiation within this species according to habitat cline, topography, and geographic distance across Africa.17–22 In general, deme sizes of An. arabiensis appear to be large, with little genetic differentiation among mosquito collections separated by less than 200-250 km.15–17,21 Given the limited flight range observed in An. arabiensis,26,27 studies have attributed the findings of high gene flow between widely separated mosquito collections to large effective population sizes and/or recent range expansion rather than extensive contemporary gene flow or mass migra tion.15–17 In comparison, Donnelly and Townson17 found extensive differentiation among collections of An. arabiensis separated by > 200 km along a north-south transect in east Africa. Onyabe and Conn22 also reported significant population structuring along a cline of ecologic zones in Nigeria. These studies demonstrate that geographic distance as well as changes in habitat contribute to the differentiation observed among An. arabiensis from different locations. These findings of genetic differentiation across Africa are consistent with observations of differential blood host preference and resting behavior in An. arabiensis from east Africa, west Africa, and Madagascar.25,28–30 In some instances, landforms such mountainous terrain and islands have been noted to limit genetic exchange between An. arabiensis populations from geographically similar locations.17,18,20
Apart from physical distance and topography influencing population structure, climate can exert a significant selective pressure on the genetic make-up of a mosquito population. Although microsatellites are characteristically neutral to selection, chromosome inversions present in the An. gambiae complex such as 2Rbc and 2La were more commonly observed in mosquitoes inhabiting arid versus wet climates in Nigeria.31 Similarly in Mali, the Mopti chromosomal form of An. gambiae s.s. was more abundant during the dry season and early rainy season whereas the Bamako and Savanna forms predominated during the late rainy season.32 Inversion 2Rbc varied in frequency from nearly 90% during the dry season to 30% in the wet season.32 Thus, shifts in microsatellite allelic diversity and frequency may be evident at loci located within chromosomal inversions that are subject to selection.
The Southern Province of Zambia experiences hyperendemic transmission of P. falciparum by both An. arabiensis and An. funestus s.s. Giles in the apparent absence of An. gambiae s.s.33 To date, no studies have examined the genetic structure of An. arabiensis populations within Zambia, and until recently, no studies had been published on the entomo-logic parameters of malaria transmission in Zambia in more than 25 years.25 Characterizing the population structure of mosquitoes in this region would help guide future malaria research and control efforts.
In conjunction with studies on seasonal malaria transmission dynamics in this region, our specific aims were to 1) evaluate additional microsatellite markers for use in An. arabiensis, 2) characterize the genetic structure of An. arabiensis collected throughout the 2000-km2 Macha region in the Southern Province of Zambia to determine any barriers to gene flow, and 3) examine the data for evidence of genetic drift across a wet year–drought year–wet year cycle. We hypothesized that An. arabiensis in this study area would represent one panmictic population given no obvious topo-graphic barriers that might restrict gene flow. However, highly focal transmission intensity recorded among closely situated villages (5 km)25 suggested minimal movement of mosquitoes between villages in this region. Considering temporal fluctuations in the mosquito population density, we also sought to determine whether drought conditions extending throughout the 2004–2005 rainy season resulted in a genetic bottleneck in An. arabiensis.
MATERIALS AND METHODS
Study area
The Johns Hopkins Malaria Research Institute's field station in Macha, Zambia is located in the Southern Province at an elevation approximately 1,000 meters above sea level. The habitat is characterized as miombo woodland. Average annual rainfall varies greatly in Zambia, but averages 600–1,000 mm (24–40 inches) in the southern parts of the country.2 There is one rainy season each year that lasts from approximately November to April, followed by cool dry (April–August), and hot dry (August–November) seasons. During 2004–2005, mosquito activity was substantially reduced because of an extended regional drought.25 Total rainfall in Macha between November 2004 and May 2005 was 457mm (18 inches) and concentrated at the beginning of the season; total rainfall between November 2005 and May 2006 was 915 mm (36 inches). The dry conditions during the 2004–2005 season coupled with temperatures averaging more than 25°C between December 2004 and March 2005 resulted in 40–90% loss of the maize crop and scarce water availability in the Southern Province during 2004–2005.34 Namwala, Zambia is located approximately 80 km northwest of Macha on the Kafue River delta and was not as severely affected by the drought.
Mosquito collection and handling
To aid in the random selection of collection sites, the greater Macha region (2,000 km2) was divided into 5 km × 5 km grids (Figure 1). Anopheles arabiensis mosquitoes were collected by pyrethrum spray catch30 from 15–20 representative houses in each of 20 randomly-selected grids. Spray catches were executed with locally purchased DOOM Super® (Adcock Ingram Ltd., Bryanston, South Africa) combination synthetic pyrethroid (D-phenothrin, 0.92g/kg; prallethrin, 0.4 g/kg; and imiprothrin, 0.25 g/kg). Two or three village areas were sampled per day over a period of two weeks during early (November 2004 and 2005), middle (January 2005 and 2006), and late (March 2005 and 2006) rainy season. Additionally, preliminary collections were obtained by spray catch from 10 village areas during the 2003–2004 rainy season. All spray catches were performed between 7:00 AM and 10:00 AM. The houses selected varied throughout the course of the season. Mosquitoes in Namwala were collected in CDC light traps35 in February 2006 as part of a preliminary sampling effort for an independent project. Immediately after collection, specimens were killed by freezing, morphologically identified,1,36 and packed individually in tubes containing silica gel desiccant (J. T. Baker, Phillipsburg, NJ) and cotton and stored at room temperature. Mosquito samples included in genetic analysis are listed in Table 1.
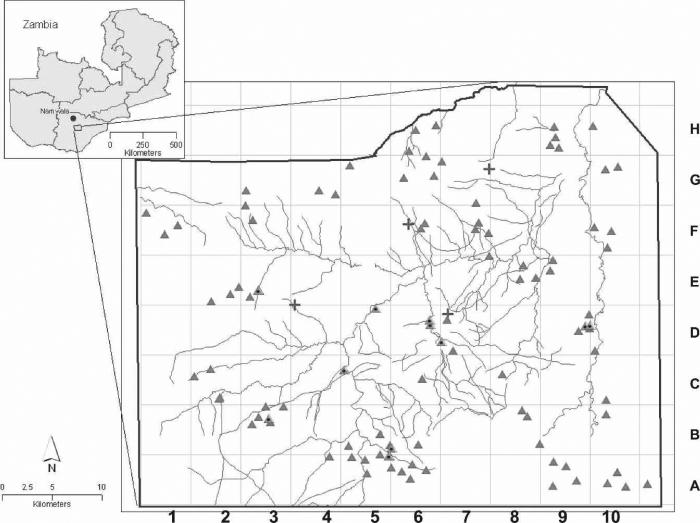
FIGURE 1.
Map of Anopheles arabiensis collection sites in Macha, Zambia. + represent rural health centers and triangles depict mapped households comprising the village areas in grids randomly selected for study. Mapped anopheline breeding sites are shown as points enclosed inside triangles, and rivers are depicted as lines. Each of these village areas was sampled during regional pyrethrum spray collections in 2004–2005 and 2005–2006 and corresponds to samples listed in Table 1. The location of Namwala, Zambia is represented as a dot to the northwest of Macha on inset map of Zambia.
Table 1.
Anopheles arabiensis mosquito collections used in genetic analysis from 2003–2004 (wet), 2004–2005 (drought), and 2005–2006 (wet) transmission seasons*
| Year | Collection grid | GPS location | No. of mosquitoes tested |
|---|---|---|---|
| 2003–2004 | A09 | S –16.53310, E 26.90134 | 9 |
| C10 | S –16.47858, E 26.94370 | 19 | |
| D10 | S –16.39383, E 26.92084 | 2 | |
| E02 | S –16.36836, E 26.58828 | 38 | |
| E06 | S –16.36319, E 26.76670 | 6 | |
| 2004–2005 | C07 | S –16.42302, E 26.79061 | 7 |
| E06 | S –16.36319, E 26.76670 | 15† | |
| E06 | S –16.36319, E 26.76670 | 25 | |
| 2005–2006 | A06 | S –16.52354, E 26.76617 | 9 |
| A10 | S –16.53292, E 26.95307 | 16 | |
| B05 | S –16.50541, E 26.76616 | 26 | |
| C10 | S –16.47858, E 26.94370 | 31 | |
| D10 | S –16.39383, E 26.92084 | 22 | |
| E02 | S –16.36836, E 26.58828 | 12 | |
| E06 | S –16.36319, E 26.76670 | 10 | |
| E09 | S –16.34218, E 26.88820 | 50 | |
| G03 | S –16.39430, E 26.79049 | 53 | |
| H09 | S –16.20747, E 26.89604 | 12 | |
| Namwala | S –15.75879, E 26.43274 | 48 |
Collection grid column refers to reference grids depicted in Figure 1. GPS = global positioning system.
One collection of An. quadriannulatus.
DNA preparation and polymerase chain reaction (PCR)
DNA was extracted from mosquitoes by a modified salt procedure as described.37 Prior to homogenization, dry field specimens were rehydrated at room temperature in 20 μL of double-distilled H20 for 20 minutes. The relative quality of all DNA extractions was checked by PCR amplification of a fragment of the mitochondrial NADH dehydrogenase subunit 4 (ND4) using arthropod-specific primers.38 The identity of all specimens morphologically identified as An. gambiae s.l. was molecularly confirmed by PCR.39 All DNA amplifications were completed on a MJ Research® PTC-200 thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA) and visualized on 2% agarose gels stained with ethidium bromide. All gels were run with a GeneRuler 100-basepair DNA ladder (Fermentas Life Science, Hanover, MD).
Microsatellite amplification
Twenty microsatellite loci originally identified in An. gambiae s.s. were evaluated, and 12 were ultimately optimized for use with An. arabiensis (Table 2). Loci were used if they amplified successfully from all samples and were polymorphic (contained greater than one allele). Selected loci spanned chromosomes 2 and 3 and included microsatellites presumed to be located both within and outside inversions. Four hundred twenty-two specimens representing 19 collections were genotyped at all 12 micro-satellite loci. All PCRs were performed in 20-μL reaction volumes containing 10 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 1.0 mM dNTPs, 1.0 units of Taq polymerase, and 25 pmol each of forward and reverse primer. An initial denaturation of 2 minutes at 95°C was followed by 29 cycles of 94°C for 20 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The final 72°C extension step was 1 hour. The forward primer in each reaction was labeled with a fluorescent marker (FAM, TET, or HEX) compatible with ABI PRISM (Perkin-Elmer, Norwalk, CT) capillary electrophoresis. Single locus PCR products were mixed for multiplexed analysis in the following combinations: 119/750/59, 788/249/ 143, 93/128/746, and 95/79/811.
Table 2.
Microsatellite loci described by Zheng and others10 selected for population genetic analysis of Anopheles arabiensis in Zambia*
| Locus | Chromosome | Repeat motif10 | Location relative to inversion | Total no. of alleles | Observed allele size range |
|---|---|---|---|---|---|
| AG2H143 | 2L | (TC) 9 | Inside fixed 2La20 | 10 | 151–167 |
| AG2H95 | 2R | (GT) 5 + 2 + 2 | Outside | 8 | 100–118 |
| AG2H788 | 2R | (GT) 8 | Inside polymorphic 2Ra | 5 | 77–87 |
| AG2H79 | 2R | (GT) 20 | Inside polymorphic 2Rb23 | 5 | 167–175 |
| AG3H59 | 3R | (GT) 9 | Outside | 9 | 112–136 |
| AG3H119 | 3R | (GT) 6 | Inside polymorphic 3Ra | 6 | 174–186 |
| AG3H128 | 3R | (GT) 21 | Outside | 21 | 76–128 |
| AG3H750 | 3R | (GT) 8 | Outside | 6 | 80–92 |
| AG3H811 | 3R | (TG) 9 | Outside | 34 | 122–190 |
| AG3H249 | 3R | (GT) 15 | Outside | 21 | 106–158 |
| AG3H746 | 3R | (GT) 14 | Outside | 32 | 83–157 |
| AG3H93 | 3R | (gt) 4 + 7 | Outside | 25 | 122–174 |
Statistical analysis
Multiplexed PCR products were evaluated on an ABIPrism 3100-Avant Genetic Analyzer (Applied Biosystems, Foster City, CA) and data analyzed using GeneScan and GenoTyper Fragment Analysis software packages to derive microsatellite genotypes and allele sizes (Applied Biosystems). Arlequin version 2.040 was used to calculate observed (Ho) and expected heterozygosity (He), allele frequencies, test for compliance to Hardy-Weinberg equilibrium (HWE), and estimate F ST 41 and N m 42 values. Departures from HWE at each locus were evaluated using the Markov chain algorithm of Guo and Thompson43 with 100,000 steps in the Markhov chain and 1,000 dememorization steps. The proportion of observed heterozygote deficiencies (D), the frequencies of null alleles explaining those deficiencies (r), and the expected number of individuals homozygous for a null allele for each collection (F) were calculated according to the methods of Chakraborty and others.44 Linkage equilibrium for all pairs of loci was evaluated by exact test.45
To avoid artifacts caused by the Wahlund effect,46 collections were partitioned by both year and grid location, as listed in Table 1. The initial 12 loci were reduced to a set of nine, for which at least 15 of 19 of the collections were in compliance with HWE after Bonferroni correction. Collections were evaluated for genetic structuring across all nine loci, or for loci only on chromosome 2 or 3, or for loci located either inside or outside of inversions. Anopheles arabiensis from Namwala, Zambia were included as a geographic outgroup, and An. quadriannulatus (Theobald) served as a sympatric, taxanomic outgroup because An. gambiae s.s. Giles is not present in this area.25 Structural analysis of molecular variance (AMOVA)41 was performed on Macha An. arabiensis collections grouped by year for each scenario (all loci, by chromosome, by inversion) to test the hypothesis that there is no difference in genotype frequency among collections between wet and drought years.
Analyses were performed with all individuals listed in Table 1, as well as a random sample of 32 mosquitoes (from 2 or 3 randomly selected collections) from each year to reduce sampling bias. Allele frequency distributions for loci located outside inversions, inside fixed inversions and inside polymorphic inversions and for Macha An. arabiensis were analyzed by the Mann-Whitney U test to test the hypothesis that allele frequency distributions at each locus were the same between collections from 2004 through 2005, 2005 through 2006, and 2004 through 2006. Observed heterozygosity for Macha An. arabiensis was similarly analyzed by the Mann-Whitney U test to test the hypothesis that heterozygosity was the same between collections from 2004 through 2005, 2005 through 2006, and 2004 through 2006. To evaluate temporal fluctuations in gene flow, Macha collections (excluding An. quadriannulatus) from the same year were pooled after confirmation of infinite gene flow among collections on a spatial scale.
Pooled collections within each year were analyzed as above, as well as evaluated for evidence of a genetic bottleneck using the software Bottleneck.47 Bottleneck compares the observed heterozygosity against the expected equilibrium heterozygosity at each locus based on the number of alleles and sample size to determine if there is a gene diversity excess or deficit.47 Populations that have recently undergone a genetic bottleneck should display excess heterozygosity as compared with what would be expected for populations under mutation-drift equilibrium. The same set of 9 loci ultimately used in the genetic analysis were evaluated for excess heterozygosity by the sign test and the Wilcoxon sign-rank test using 1,000 permutations.47 Calculations were performed under the Infinite Alleles Model (IAM), Stepwise Mutation Model (SMM), and Two-Phased Model of Mutation (TPM) with 85% of SMM in TPM.
RESULTS
Hardy-Weinberg equilibrium and linkage disequilibrium
Approximately 420 An. arabiensis from Macha and Namwala, Zambia, and 15 An. quadriannulatus from Macha were geno-typed at 12 microsatellite loci (Tables and 2). Microsatellite loci that were tested but either did not amplify well or gave ambiguous results were AG2H802, AG3H817, AGXH412, AG3H83, AG2H26, AG3H311, AG3H544, and AG2H637. Of the 228 tests (12 loci × 19 populations) for conformance to HWE at the locus level within populations, 17 tests (7.46%) showed significant deviation from HWE after Bonferroni correction for multiple comparisons. These deviations were predominantly due to loci AG3H746 and AG2H788. The expected frequency of null alleles for these two loci ranged from 0.13 to 1.00 for AG2H788 and from 0.01 to 0.32 for AG2H746. Therefore, these two loci were excluded from all further analyses. AG2H79 was either significant or borderline significant in seven populations, and was also excluded. In exact tests for linkage disequilibrium, only 8 (1.16%) of 684 comparisons were significant after Bonferroni correction.
Observed proportional heterozygote deficiencies, the expected frequency of null alleles, and the estimated number of mosquitoes homozygous for a null allele in each collection were all very low for the nine analyzed loci (Supplementary Table 1). The expected frequency of null alleles was generally less for these microsatellite loci in An. arabiensis than for other loci previously examined in An. arabiensis.21,22 These parameters were also similar between An. arabiensis and An. quadriannulatus in this study. A complete table of observed and expected heterozygosity and null allele data for the nine loci in all 19 populations can be found in the supplementary data (Supplementary Table 1).
Spatial analysis of genetic differentiation
As expected, An. arabiensis populations sampled throughout the Macha region and between Macha and Namwala were panmictic (Table 3). There was infinite gene flow among the 18 collections across all nine analyzed loci (Table 3). There was modest structuring among collections when loci on chromosome 2 or inside inversions were analyzed as compared with loci on chromosome 3 or outside inversions. However, gene flow remained high and these observations were not significant. Two An. arabiensis collections were consistently identified as having reduced gene flow relative to all others. These two collections, E02_2006 and E06_2005, accounted for the low Nm values estimated for these population comparisons. For the analyses of chromosome 2 and inside inversions, collection E02_2006 had reduced gene flow, and for chromosome 3 and outside inversions, collection E06_2005 had reduced gene flow relative to the other collections. It is unclear whether there is a biologic explanation for these unexpected observations, or if they represent technical anomalies.
Table 3.
Spatial and temporal comparison of estimated FST and Nm values for all collections across all nine loci by inversion and by chromosome*
| All nine loci |
Outside inversions |
Inside inversions |
Chromosome 2 |
Chromosome 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | FST | Nm | FST | Nm | FST | Nm | FST | Nm | FST | Nm |
| Within Macha arab | 0.00000 | Inf | –0.0008–0.000 | 31838–inf | –0.04926–0.11622 | 3.8–inf | –0.0697–0.1485 | 2.9–inf | –0.0004–0.0003 | 2198–inf |
| Macha–Namwala arab | 0.00000 | Inf | –0.0002–0.000 | 22287–inf | –0.04018–0.00487 | 10–inf | –0.0281–0.0725 | 6.4–inf | –0.0002–0.0002 | 2287–inf |
| Macha arab–quad | 0.00000 | Inf | 0.00000 | 2235–inf | 0.00574–0.0503 | 4.2–86.6 | 0.0512–0.2231 | 1.7–9.3 | 0.000–0.00022 | 2235–inf |
| Macha 2003/4–2004/5 | 0.00000 | Inf | 0.00011 | 4403 | –0.00035 | Inf | 0.01084 | 45.6 | 0.00023 | 2204 |
| Macha 2004/5–2005/6 | 0.00000 | Inf | 0.00018 | 2772 | 0.00374 | 133.2 | 0.00116 | 430 | 0.00017 | 8388 |
| Macha 2003/4–2005/6 | –0.00001 | Inf | 0.00005 | 10866 | –0.00121 | Inf | 0.01214 | 40.7 | 0.00006 | 2926 |
Only the FST for Anopheles arablensls (arab) and An. quadriannulatus (quad) on chromosome 2 were significant after Bonferroni correction (P < 0.0003). These values are in bold. Inf = infinity.
As expected, FST values were significant (P < 0.0003) between Macha An. arabiensis and An. quadriannulatus on chromosome 2 (Table 3). Gene flow between An. quadriannulatus and An. arabiensis was reduced to 1.7–9.3 migrants per generation when considering only loci on chromosome 2 or to 4.2–86.6 migrants per generation for loci inside inversions. Anopheles quadriannulatus contained 14 unique alleles in seven loci compared with more than 400 specimens of An. arabiensis. These were AG2H95: alleles 108 and 128; AG3H119: 194 and 216; AG3H128: 96 and 82; AG2H143: 149 and 147; AG3H750: 76 and 78; AG2H788: 67, 71, and 75; and AG3H746: 83.
Temporal analysis of genetic differentiation
Examinations for population differentiation across wet and dry transmission seasons used three strategies. First, because there was infinite gene flow among collections throughout the Macha region on a spatial scale, collections from the same year were pooled to analyze genetic structure and gene flow among Macha An. arabiensis from year to year. There were modest fluctuations in gene flow observed from year to year when data were analyzed by chromosome or inversion status, although Nm values were still high (Table 3). Second, an AMOVA was performed on all as well as a random set of 32 mosquitoes from each year. Data were structured by both collection grid and year. All of the observed variation was within groups (years); there was no significant structuring evident among An. arabiensis collections from year to year. Mann-Whitney U tests on allele frequency distributions showed no significant shifts in allele frequencies between wet and drought years for loci located outside inversions, inside fixed inversions, or inside polymorphic inversions (P > 0.05). Observed heterozygosity also did not fluctuate significantly between wet and drought years (P > 0.05).
Finally, pooled collections from each year were analyzed for evidence of a genetic bottleneck at each locus and across all loci. Despite some isolated locus-specific events of excess heterozygosity, there was no significant evidence of a genetic bottleneck in An. arabiensis collections from any of the three years under any of the mutation models. None of the years contained significant excess heterozygosity by the sign test for any of the mutation models (P > 0.05). Heterozygosity for each of the three years conformed to an L-shaped distribution typical of populations in mutation-drift equilibrium.
DISCUSSION
We examined the spatial and temporal genetic structuring of An. arabiensis in Macha, Zambia. Of the 20 loci initially selected, we obtained consistent heterologous amplification of alleles that were easily scored in 12 of these loci. Unlike Donnelly and others,16 we had good success with AG3H93 and AH3H249. These observations suggest that the utility of some loci in An. arabiensis may vary from region to region due to intrinsic variations in priming site sequence among different mosquito populations. Such sequence divergences are expected when locus-specific primers designed for one species are applied to a closely-related species.17,48
As expected, significant genetic differentiation was evident between An. arabiensis and An. quadriannulatus on chromo-some 2. However, estimates of gene flow among our collections of An. arabiensis and An. quadriannulatus were high compared with previously reported gene flow estimates between sibling species in the An. gambiae complex.8,14,22 Infinite gene flow between An. arabiensis and An. quadriannulatus is biologically implausible because these species are considered to be largely reproductively isolated taxa. Hybrid specimens of An. arabiensis × An. quadriannulatus in nature have been documented from Zimbabwe, although at a frequency < 0.1% of the total sample.49 High estimates of gene flow between An. arabiensis and An. quadriannulatus are not necessarily the result of contemporary introgression, but may reflect either shared ancestral alleles or convergence back to shared alleles due to limitations in the allele model. Although gene flow between these two species was unexpectedly high, there were several unique alleles identified in An. quadriannulatus in seven of the 12 loci, which is indicative of reproductive isolation and speciation. Speciation of members of the An. gambiae complex is thought to be relatively recent, as demonstrated by shared ancestral ND5 haplotypes between An. gambiae s.s. and An. arabiensis.50 Large numbers of shared mitochondrial haplotypes have also been documented between An. gambiae s.s. and An. bwambe.51 Still, we observed only slightly less amplification success of alleles in An. quadriannulatus relative to An. arabiensis across the set of nine loci, which extended the usefulness of these loci into this previously unexplored taxon. Furthermore, there was a relatively low expected frequency of null alleles for each of these two species.
Despite physical distance and very different habitat types, there was infinite gene flow among An. arabiensis populations within the Macha region and between Macha and Namwala, which predicted a deme size that covers an area greater than 2,000 km2, with a radius greater than 80 km. These estimates are consistent with those previously reported for An. arabiensis, in which population differentiation using microsatellites was not detected at distances less than 200–250 km15–17,21 where no topographic barrier was present. Limited structuring detected for loci analyzed on chromosome 2 and within inversions is consistent with most inversions being present on chromosome 2, thus limiting genetic exchange through recombination.8,19 Lanzaro and others8 reported greatly reduced gene flow among chromosomal forms of An. gambiae s.s. on chromosome 2 (Nm = ~3–4) compared with chromo-some 3 (Nm = ~20–infinity). Temu and Yan19 also observed greater structuring when loci were analyzed relative to inversion. Microsatellite loci within polymorphic inversions showed 4–7 times higher genetic differentiation by comparison of FST than markers within fixed inversions or outside of inversions. Therefore, the careful selection of particular microsatellite loci appears to be a critical determinant in the degree of population sub-structure ultimately showed by genetic analysis.
Although spatial analysis did not demonstrate any significant barriers to gene flow among regional collections of An. arabiensis, extreme drought conditions during the study period presented the opportunity to analyze the data for evidence of genetic drift events. When data were examined for evidence of a genetic bottleneck, there was no convincing pattern of excess heterozygosity in any of the years. Structure analysis also did not show any significant differentiation in An. arabiensis collections between years. Certain polymorphic chromosomal inversions are known to be influenced by arid climate.31,32 An alternative arrangement to 2Rbc in Sudan populations of An. arabiensis,52 2Rb is common in An. arabiensis populations inhabiting arid environments, and varies in frequency between wet and dry seasons.31,32 Inversion 2Rb has also been associated with indoor biting and resting behaviors in An. arabiensis,31,53 an observation consistent with the highly anthropophilic nature of An. arabiensis at our study site.25 Because locus AG2H79 is located within inversion 2Rb, we questioned whether there would be a shift in allele frequencies at AG2H79 as a result of climate induced selection pressures on inversion 2Rb during extended drought conditions. However, no significant shifts in allele frequency were observed.
In conclusion, relatively high estimates of gene flow, constant observed heterozygosity among years, no significant shifts in allele frequency distributions, and a large deme size collectively argue that the 2004–2005 drought in southern Zambia had little overall impact on the genetic diversity of An. arabiensis in the Southern Province. Therefore, heterogeneous transmission intensity among closely-situated villages during this time period was not due to genetically partitioned mosquito populations.25 Our results are in accord with those of Simard and others15 who demonstrated a large deme size, relatively high effective population size, and constant mean observed heterozygosity in populations of An. arabiensis in Senegal, west Africa, between rainy and dry seasons over three consecutive years. Furthermore, additional estimates of large effective population sizes of An. arabiensis during the dry season in Mali, Nigeria, and Burkina Faso support the hypothesis that An. arabiensis populations are maintained continuously throughout the year with seasonal reductions rather than severe bottlenecks or extinctions.54 Therefore, neither predictable annual dry seasons nor unexpected extended drought periods appear to have an appreciable effect on the genetic diversity of this arid-adapted mosquito.
There are several possible explanations for the lack of a genetic bottleneck in spite of extreme and extended drought conditions. First, too few generations may have been completed due to mosquitoes entering estivation or reproducing infrequently to see any temporal effects of genetic drift. Second, the effective population size may be so large that there is no evident effect of the drought on the overall genetic constitution of An. arabiensis over a large spatial scale.15,54 Third, because of drought conditions and the extensive elimination of surface water, mosquitoes may have migrated farther in search of breeding sites.55 In this case, the high Nm values we observed may be in part due to actual mosquito movement across the Macha catchment region. In agreement with those results from west Africa, An. arabiensis in Macha, Zambia most likely persisted throughout the extended drought in a diffused deme, in which mosquitoes survived the dry conditions in small numbers below a sampling threshold in individual villages, but were still part of a large overall population.15,56 It is tempting to speculate on extended drought conditions wiping out mosquito populations given the reduction in malaria transmission. However in this case, extended drought conditions appeared to have little observable impact on the regional genetic diversity of An. arabiensis and malaria transmission in the following season despite greatly reduced mosquito numbers.
Supplementary Material
Acknowledgments
We thank Harry Hamapumbu for organizing and managing the field team, and Petros Moono, Patricia Muleya, Pamela Sinywimaanzi, Fidelis Chanda, Lusyomo Chikobolo, Collence Munsanje, Rodwell Moono, Peter Simakwati, Guide Hansumo, Scene Mudenda, Betham Dubeka, Frederick Mwiinga, Buster Musanje, Maron Mulota, and Kalizya Sinyangwe for collecting mosquitoes.
Financial support. This study was supported in part by funding to Douglas E. Norris from the Johns Hopkins Malaria Research Institute, a Johns Hopkins Malaria Research Institute pre-doctoral fellowship award to Rebekah J. Kent, a Johns Hopkins School of Public of Medicine Global Field Experience Fund award to Rebekah J. Kent, a Frederik B. Bang award to Rebekah J. Kent, and a National Institute of Environmental Health Sciences training award (T32ES07141) to Rebekah J. Kent.
Footnotes
Note: Supplementary Table 1, Observed and Expected Heterozygosity for Anopheles arabiensis Collections in Macha, Zambia, appears online at www.ajtmh.org.
REFERENCES
- 1.Gillies MT, Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. South African Institute for Medical Research Publication no. 55; Johannesburg: 1987. [Google Scholar]
- 2.Coetzee M, Craig M, Le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–77. doi: 10.1016/s0169-4758(99)01563-x. [DOI] [PubMed] [Google Scholar]
- 3.Chandre F, Manguin S, Brengues C, Yovo JD, Darriet F, Diabate A, Carnevale P, Guillet P. Current distribution of a pyre-throid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999;41:319–322. [PubMed] [Google Scholar]
- 4.Weill M, Chandre F, Brengues C, Manguin S, Akogbeto M, Pasteur N, Guillet P, Raymond M. The kdr mutation occurs in the Mopti form of Anopheles gambiae s.s. through introgression. Insect Mol Biol. 2000;9:451–455. doi: 10.1046/j.1365-2583.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Diabaté A, Baldet T, Chandre F, Dabire KR, Simard F, Ouedraogo JB, Guillet P, Hougard JM. First report of a kdr mutation in Anopheles arabiensis from Burkina Faso, west Africa. J Am Mosq Control Assoc. 2004;20:195–196. [PubMed] [Google Scholar]
- 6.James AA, Beerntsen BT, Capurro Mde L, Coates CJ, Coleman NJ, Jasinskiene N, Krettli AU. Controlling malaria transmission with genetically-engineered, Plasmodium-resistant mosquitoes: milestones in a model system. Parassitologia. 1999;41:461–471. [PubMed] [Google Scholar]
- 7.Tripet F, Dolo G, Lanzaro GC. Multilevel analyses of genetic differentiation in Anopheles gambiae s.s. reveal patterns of gene flow important for malaria-fighting mosquito projects. Genetics. 2005;169:313–324. doi: 10.1534/genetics.104.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzaro GC, Touré YT, Carnahan J, Zheng L, Dolo G, Traoré S, Petrarca V, Vernick KD, Taylor CE. Complexities in the genetic structure of Anopheles gambiae populations in west Africa as revealed by microsatellite DNA analysis. Proc Natl Acad Sci U S A. 1998;95:14260–14265. doi: 10.1073/pnas.95.24.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L, Benedict MQ, Cornel AJ, Collins FH, Kafatos FC. An integrated genetic map of the African human malaria vector mosquito, Anopheles gambiae. Genetics. 1996;143:941–952. doi: 10.1093/genetics/143.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann T, Hawley WA, Kamau L, Fontenille D, Simard F, Collins FH. Genetic differentiation of Anopheles gambiae populations from east and west Africa: comparison of microsatellite and allozyme loci. Heredity. 1996;77:192–200. doi: 10.1038/hdy.1996.124. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann T, Licht M, Elissa N, Maega BTA, Chimumbwa JM, Watsenga FT, Wondji CS, Simard F, Hawley WA. Population structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- 13.Carnahan J, Zheng L, Taylor CE, Toure YT, Norris DE, Dolo G, Diuk-Wasser M, Lanzaro GC. Genetic differentiation of Anopheles gambiae s.s. populations in Mali, west Africa, using microsatellite loci. J Hered. 2002;93:249–253. doi: 10.1093/jhered/93.4.249. [DOI] [PubMed] [Google Scholar]
- 14.Taylor C, Touré YT, Carnahan J, Norris DE, Dolo G, Traoré SF, Edillo FE, Lanzaro GC. Gene flow among populations of the malaria vector Anopheles gambiae, in Mali, west Africa. Genetics. 2001;157:743–750. doi: 10.1093/genetics/157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simard F, Lehmann T, Lemasson J-J, Diatta M, Fontenille D. Persistance of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol Biol. 2000;9:467–479. doi: 10.1046/j.1365-2583.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly MJ, Cuamba N, Charlwood JD, Collins FH, Townson H. Population structure in the malaria vector, Anopheles arabiensis Patton, in east Africa. Heredity. 1999;83:408–417. doi: 10.1038/sj.hdy.6885930. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly MJ, Townson H. Evidence for extensive genetic differentiation among populations of the malaria vector Anopheles arabiensis in eastern Africa. Insect Mol Biol. 2000;9:357–367. doi: 10.1046/j.1365-2583.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Simard F, Fontenille D, Lehmann T, Girod R, Brutus L, Gopaul R, Dournon C, Collins FH. High amounts of genetic differentiation between populations of the malaria vector Anopheles arabiensis from west Africa and eastern outer islands. Am J Trop Med Hyg. 1999;60:1000–1009. doi: 10.4269/ajtmh.1999.60.1000. [DOI] [PubMed] [Google Scholar]
- 19.Temu EA, Yan G. Microsatellite and mitochondrial genetic differentiation of Anopheles arabiensis (Diptera: Culicidae) from western Kenya, the Great Rift Valley, and coastal Kenya. Am J Trop Med Hyg. 2005;73:726–733. [PubMed] [Google Scholar]
- 20.Nyanjom SR, Chen H, Gebre-Michael T, Bekele E, Shililu J, Githure J, Beier JC, Yan G. Population genetic structure of Anopheles arabiensis mosquitoes in Ethiopia and Eritrea. J Hered. 2003;94:457–463. doi: 10.1093/jhered/esg100. [DOI] [PubMed] [Google Scholar]
- 21.Kamau L, Mukabana WR, Hawley WA, Lehmann T, Irungu W, Orago AA, Collins FH. Analysis of genetic variability in Anopheles arabiensis and Anopheles gambiae using microsatellite loci. Insect Mol Biol. 1999;8:287–297. doi: 10.1046/j.1365-2583.1999.820287.x. [DOI] [PubMed] [Google Scholar]
- 22.Onyabe DY, Conn JE. Population genetic structure of the malaria mosquito Anopheles arabiensis across Nigeria suggests range expansion. Mol Ecol. 2001;10:2577–2591. doi: 10.1046/j.0962-1083.2001.01387.x. [DOI] [PubMed] [Google Scholar]
- 23.Fontenille D, Lochouarn L, Diatta M, Sokhna C, Dia I, Diagne N, Lemasson J-J, Ba K, Tall A, Rogier C, Trape J-F. Four years’ entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and An. arabiensis. Trans R Soc Trop Med Hyg. 1997;91:647–652. doi: 10.1016/s0035-9203(97)90506-x. [DOI] [PubMed] [Google Scholar]
- 24.Shililu J, Ghebremeskel T, Mengistu S, Fekadu H, Zerom M, Mbogo C, Githure J, Novak R, Brantly E, Beier JC. High seasonal variation in entomologic inoculation rates in Eritrea, a semi-arid region of unstable malaria in Africa. Am J Trop Med Hyg. 2003;69:607–613. [PubMed] [Google Scholar]
- 25.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis following an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 26.Adams PC. Some observations on the flight of stained anophelines at Nkana, northern Rhodesia. Ann Trop Med Parasitol. 1940;34:35–43. [Google Scholar]
- 27.Thompson MC, Connor SJ, Quinones ML, Jawara M, Todd J, Greenwood BM. Movement of Anopheles gambiae s.l. malaria vectors between villages in The Gambia. Med Vet Entomol. 1995;9:413–419. doi: 10.1111/j.1365-2915.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 28.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 29.Ralisoa Randrianasolo BO, Coluzzi M. Genetical investigations on zoophilic and exophilic Anopheles arabiensis from Antananarivo area (Madagascar). Parassitologia. 1987;29:93–97. [PubMed] [Google Scholar]
- 30.Ameneshewa B, Service MW. Resting habits of Anopheles arabiensis in the Awash river valley of Ethiopia. Ann Trop Med Parasitol. 1996;90:515–521. doi: 10.1080/00034983.1996.11813077. [DOI] [PubMed] [Google Scholar]
- 31.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 32.Touré YT, Petrarca V, Traoré SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. Distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- 33.Larkin GL, Thuma PE. Congenital malaria in a hyperendemic area. Am J Trop Med Hyg. 1991;45:587–592. doi: 10.4269/ajtmh.1991.45.587. [DOI] [PubMed] [Google Scholar]
- 34.FEWS NET. Food Security Update, February 2005. US Agency for International Development. 2005 Mar 14; Available from http://www.fews.net/centers/?f=zm.
- 35.Sudia WD, Chamberlain RW. Battery operated light trap, an improved model. Mosq News. 1962;22:126–129. [PubMed] [Google Scholar]
- 36.Gillies MT, DeMeillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) Second edition. South African Institute for Medical Research; Johannesburg: 1968. Publication no. 54. [Google Scholar]
- 37.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome b. Am J Trop Med Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 38.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87:651–701. [Google Scholar]
- 39.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 40.Schneider S, Kueffer J-M, Roessli D, Excoffier L. Arlequin Version 1.1: A Software for Population Genetic Data Analysis. Genetics and Biometry Laboratory, University of Geneva; Geneva: 1997. [Google Scholar]
- 41.Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution Int J Org Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 42.Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462. doi: 10.1093/genetics/139.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- 44.Chakraborty R, De Andrade M, Daiger SP, Budowle B. Apparent heterozygote deficiencies observed in DNA typing data and their implications in forensic applications. Ann Hum Genet. 1992;56:45–57. doi: 10.1111/j.1469-1809.1992.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 45.Slatkin M, Excoffier L. Testing for linkage disequilibrium in genotypic data using the EM algorithm. Heredity. 1996;76:377–383. doi: 10.1038/hdy.1996.55. [DOI] [PubMed] [Google Scholar]
- 46.Hartl DL, Clark AG, editors. Principles of Population Genetics. Second edition. Sinauer Associates; Sunderland, MA: [Google Scholar]
- 47.Cornuet JM, Luikart G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics. 1996;144:2001–2014. doi: 10.1093/genetics/144.4.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes SH, Hogg JT, Buchanan FC, Crawford AM, Allendorf FW. Microsatellite evolution in congenereic mammals: domestic and bighorn sheep. Mol Biol Evol. 1995;12:1106–1113. doi: 10.1093/oxfordjournals.molbev.a040284. [DOI] [PubMed] [Google Scholar]
- 49.White GB. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg. 1974;68:278–301. doi: 10.1016/0035-9203(74)90035-2. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly MJ, Pinto J, Girod R, Besansky NJ, Lehmann T. Revisiting the role of introgression vs shared ancestral polymorphisms as key processes shaping genetic diversity as recently separated sibling species of the Anopheles gambiae complex. Heredity. 2004;92:61–68. doi: 10.1038/sj.hdy.6800377. [DOI] [PubMed] [Google Scholar]
- 51.Thelwell NJ, Huisman RA, Harbach RE, Butlin RK. Evidence for mitochondrial introgression between Anopheles bwambe, and Anopheles gambiae. Insect Mol Biol. 2000;9:203–210. doi: 10.1046/j.1365-2583.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 52.Petrarca V, Nugud AD, Elkarim Ahmed MA, Haridi AM, Di Deco MA, Coluzzi M. Cytogenetics of the Anopheles gambiae complex in Sudan, with special reference to An. arabiensis: relationships with east and west African populations. Med Vet Entomol. 2000;14:149–164. doi: 10.1046/j.1365-2915.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- 53.Mnzava AEP, Rwegoshora RT, Wilkes TJ, Tanner M, Curtis CF. Anopheles arabiensis and An. gambiae chromosomal inversion polymorphism, feeding and resting behaviour in relation to insecticide house-spraying in Tanzania. Med Vet Entomol. 1995;9:316–324. doi: 10.1111/j.1365-2915.1995.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 54.Taylor CE, Toure YT, Coluzzi M, Petrarca V. Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Med Vet Entomol. 1993;7:351–357. doi: 10.1111/j.1365-2915.1993.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 55.Constantini C, Li SG, Della Torre A, Sagnon N'F, Coluzzi M, Taylor CE. Density, survival, and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Med Vet Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 56.Lehmann T, Hawley WA, Grebert H, Collins FH. The effective population size of Anopheles gambiae in Kenya: implications for population structure. Mol Biol Evol. 1998;15:264–276. doi: 10.1093/oxfordjournals.molbev.a025923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.