Abstract
Objectives
We sought to determine whether the association between cricopharyngeus muscle activity and upper esophageal sphincter pressure may change in a task-dependent fashion. We hypothesized that more automated tasks related to swallow or airway protection would yield a stronger association than would more volitional tasks related to tidal breathing or voice production.
Methods
Six healthy adult subjects underwent simultaneous intramuscular electromyography of the cricopharyngeus muscle and high-resolution manometry of the upper esophageal sphincter. Correlation coefficients were calculated to characterize the association between the time-linked series.
Results
Cricopharyngeus muscle activity was most strongly associated with upper esophageal sphincter pressure during swallow and effortful exhalation tasks (r = 0.77 and 0.79, respectively; P < .01). The association was also less variable during swallow and effortful exhalation.
Conclusions
These findings suggest a greater coupling for the more automatic tasks, and may suggest less coupling and more flexibility for the more volitional, voice-related tasks. These findings support the important role of central patterning for respiratory- and swallow-related tasks.
Keywords: cricopharyngeus, deglutition, electromyography, high-resolution manometry, upper esophageal sphincter
Introduction
The upper esophageal sphincter (UES) is a high-pressure zone located at the junction between the hypopharynx and the upper esophagus. This high-pressure zone primarily comprises the cricopharyngeus (CP) muscle, with possible contributions from the thyropharyngeus portion of the inferior pharyngeal constrictor and the rostral esophageal musculature. At rest, the UES is closed because of CP contraction and passive pressure imposed by surrounding pharyngeal and laryngeal structures.1–3 During a swallow, the CP muscle relaxes to allow the UES to open and the bolus to pass into the esophagus. This CP muscle quiescence and drop in UES pressure is followed by a burst in muscle activity and by pressure to levels higher than baseline and then a gradual return to resting activity and pressure.1,4–6 The anterosuperior movement of the hyolaryngeal complex through contraction of the suprahyoid muscles1,7,8 and pressure imposed by the bolus8–10 also serve to open the UES during swallowing. Given its anatomic location near the upper airway, at or above the thoracic inlet, the CP muscle may also serve an important non-swallow role to regulate UES closure—especially for tasks that may require increased thoracic and pharyngeal pressure to guard against air movement into the esophagus and fluid movement in the pharynx. Increased UES pressure and CP muscle electromyographic (EMG) activity have been found during inhalation and exhalation2,5,11–13 and during phonation.14,15 The UES pressure and CP activity may also change with posture,2,16,17 state of arousal,2 and distention of the esophagus.18,19 Thus, the UES may play an important role in non-swallow activity.
Because the UES is defined as more than the CP muscle, different aspects of UES dynamics have been examined by a variety of means, including 1) intramuscular EMG testing to measure CP muscle activity; 2) manometry, to measure contact pressure patterns within the UES; and 3) videofluoroscopy, scintigraphy, endoscopy, magnetic resonance imaging, and ultrasonography, to visualize UES patency, pharyngeal and esophageal movements, or other events that contribute to UES opening.1,2,6–8,20,21
Prior manometric studies used older catheters that included only 2 to 6 unidirectional sensors. Technical limitations of this type of catheter preclude a thorough analysis of the asymmetric,1,8,22 highly mobile6,10,23 UES. To overcome these limitations, the current study used high-resolution manometry (HRM) with 36 pressure sensors spaced 1 cm apart, which acquire circumferential pressure data with a resolution that can capture the rapid pressure changes that occur at the level of the UES.10,24 Although 4 to 6 sensors would span the entire UES, the UES is highly mobile during swallowing and could move beyond the range of the catheter. Using a catheter with 36 sensors ensures that pressure can still be measured, even when the UES moves during the swallow. It is clear that the laryngopharynx is a complex organ system involved in nearly every activity of living. The larynx has been extensively studied to elucidate the interaction of tasks that require glottic movement, such as respiration and swallowing.25 Recent work using pattern-recognition programming has suggested that identifiable pressure changes at the level of the UES may be highly valuable in classifying normal versus abnormal swallow pressure patterns.26 The manner in which UES pressure is modulated is not fully understood, and the contributions of the CP muscle to UES pressure modulation during swallow and non-swallow tasks remain unknown.
The aim of the present study was to evaluate the association between UES pressure and CP muscle activity during swallow and non-swallow tasks. We hypothesized that UES pressure and CP activity would be positively associated for each task, and that the strength of the relationship would be task-dependent.
Materials and Methods
Equipment
The UES pressure was recorded with a solid-state high-resolution manometer (ManoScan 360; Sierra Scientific Instruments, Los Angeles, California). The manometric catheter has an outer diameter of 2.75 mm and 36 circumferential pressure sensors, each spanning 2.5 mm, spaced 1 cm apart. Each sensor receives input from 12 circumferential sectors; the data are then averaged and a mean pressure is recorded as the pressure detected by that sensor. The system is calibrated to record pressure between –20 and 600 mm Hg, with a fidelity of 2 mm Hg. Data were recorded at a sampling rate of 50 Hz (ManoScan Data Acquisition; Sierra Scientific Instruments). The catheter was calibrated before use with each participant according to manufacturer specifications.
The CP-EMG signals were recorded with a 50-µm-diameter, bipolar hook-wire intramuscular electrode (MicroProbes, Gaithersburg, Maryland) and a surface ground electrode (A10058-SRT; Vermed, Bellows Falls, Vermont) placed on the forehead. The EMG signals were amplified, bandpass-filtered from 100 Hz to 6 kHz (model 15LT; Grass Technologies, Warwick, Rhode Island), and digitized at 20 kHz (LabChart version 6.1.3; ADInstruments, Colorado Springs, Colorado).27,28 The UES pressure and CP-EMG data were time-linked by means of a transistor-to-transistor logic signal.
Participants
Our study included 6 participants (4 male), 21 to 25 years old, without a history of swallowing, respiratory, or neurologic deficits. Each participant provided informed consent, and the protocol was approved by the Institutional Review Board of the University of Wisconsin–Madison. The participants were instructed not to eat for 4 hours and not to drink for 2 hours before testing to avoid any potential confounding effect of satiety.
Procedures
Topical 2% viscous lidocaine hydrochloride was applied to the nasal passages and to the manometric catheter as a topical anesthetic and lubricant to ease passage of the catheter through the nasal cavity and pharynx. Before CP electrode insertion, 1 mL of 1% lidocaine hydrochloride with epinephrine (1:100,000) was subcutaneously injected into the neck through a 30-gauge needle. The intramuscular electrode was then inserted with a 27-gauge needle. The characteristic CP muscle pattern of quiescence during a swallow followed by a burst of activity after the swallow was consistent with accurate placement.5 Bilateral surface EMG electrodes were placed in the submental region between the mandible and the hyoid bone, each at 1 cm from midline. Once the catheter and electrodes were inserted, the participants rested for approximately 5 minutes to adjust to the catheter and electrodes before performing the experimental tasks.
The following tasks were performed 5 times each: 1) restful tidal breathing; 2) comfortable phonation; 3) loud phonation; 4) passive exhalation from maximum lung volume; 5) forceful exhalation from maximum lung volume; and 6) swallowing. Each task was performed while the participant sat comfortably in an examination chair while looking straight ahead with the chin in a neutral position. For restful tidal breathing, the participants sat quietly without speaking or swallowing. For comfortable phonation, they took a breath and sustained “ah” at a constant, comfortable pitch and sound pressure level for 10 seconds. For loud phonation, they took a breath and sustained “ah” at a constant, comfortable pitch at 10 dB greater than comfortable loudness. Pitch was monitored by a trained singer and voice specialist (M.J.H.). The sound pressure level was monitored with a sound level meter (Quest Technologies, Oconomowoc, Wisconsin) with the microphone held 5 cm from the corner of the mouth. For passive exhalation from maximal lung volume (hereinafter referred to as passive exhalation), the participants inhaled as fully as possible, then passively exhaled at a constant pressure for 10 seconds. For forceful exhalation from maximal lung volume (hereinafter referred to as forceful exhalation), the participants inhaled as fully as possible, then exhaled as forcefully as possible for 10 seconds. Air pressure in the mouth was measured during exhalation with a pressure sensor (Fluke, Everett, Washington) coupled to a snorkel-style mouthpiece by a semi-occluded tube fitted with a 2-mm-diameter leak. Both nostrils were occluded during the exhalation tasks with a standard nose clip. For the swallow task, 5 mL of room-temperature water was delivered to the mouth via a 10-mL syringe. The participants held the bolus in the mouth until cued to swallow. Task order was randomized by task type. Only satisfactory trials were included in the analysis. Examples of unsatisfactory trials are instances in which the participant stopped the task prematurely, started the task before being cued to do so, or increased the pitch of the sustained “ah” as he or she increased loudness. One subject completed only the swallowing activity portion of the entire research protocol. Thus, 30 trials (5 for each of 6 tasks) were analyzed for each of the other 5 participants.
Data Analysis
The UES pressure and CP-EMG data were analyzed with a customized Matlab program (MathWorks, Natick, Massachusetts). The CP-EMG signals were rectified, low-pass-filtered, then resampled to 50 Hz to match the sampling rate of the HRM signals. The segment of time-series data for UES pressure and the CP muscle voltage were time-aligned for each trial.
Great care was taken to identify the HRM sensor that best represented the task-related UES pressure. The UES is a 3-dimensional structure that may move as an individual breathes, phonates, or swallows. For example, the UES may ascend up to 3 cm6,10 during a swallow, and the UES typically descends during voice onset. The HRM sensors used in the present study are spaced 1 cm apart. Thus, 3 to 4 sensors may be positioned within the UES. As the UES rises during a swallow, the specific sensors positioned within the UES may change. Therefore, the UES manometric signal was defined by 5 sensors. The first sensor was the most caudal sensor to register tonic UES resting pressure. The remaining 4 sensors were immediately rostral from the first. During the phonation and exhalation tasks, the band of high pressure at the UES typically shifted caudally at the start of the task, likely because of a change in laryngeal posturing coupled with catheter movement related to velopharyngeal port closure.6 In these tasks, the 5 sensors entered into the correlation were shifted to accommodate the change in UES pressure registration. Each of these 5 sensors was carefully examined for each trial. To best represent the association between CP muscle activity and UES pressure for each task, the manometric sensor with the highest correlation coefficient was selected.
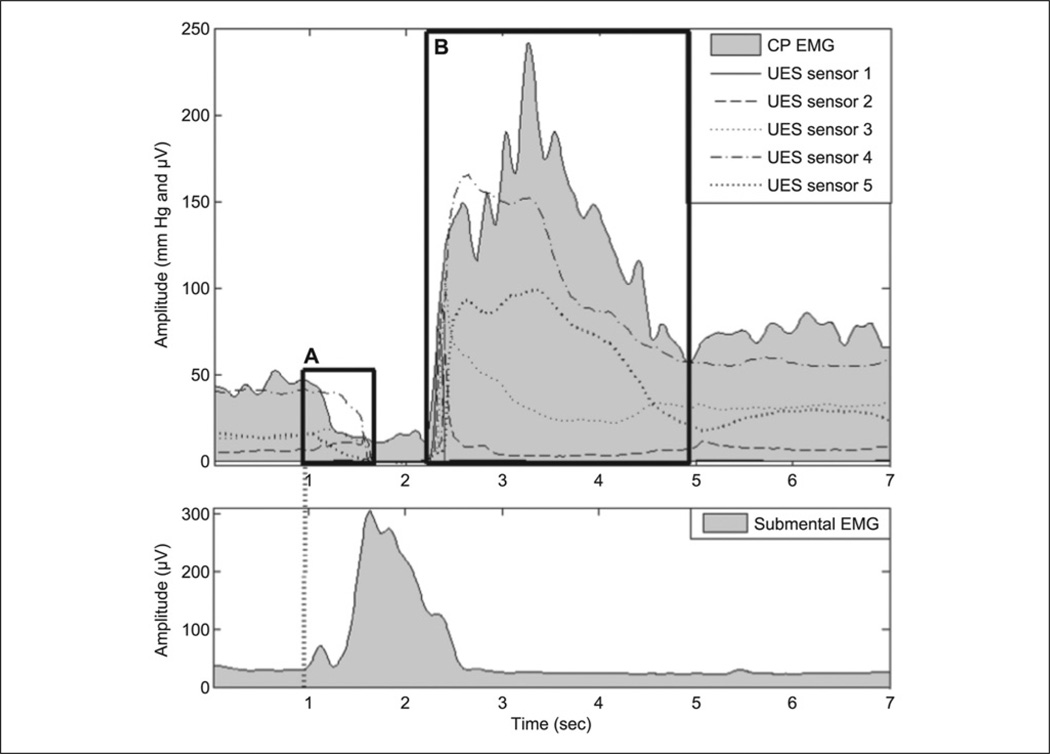
For each restful tidal breathing, phonation, and expiration trial, the entire 10-second data segment was analyzed. For swallow trials, we used two different approaches. Similar to the phonation and expiration trials, the entire 10-second data segment was analyzed. In addition, we analyzed a pre–UES-nadir segment and a post–UES-nadir segment (Figure 1). The pre–UES-nadir segment began with the onset of submental surface EMG activity associated with the swallow and ended when the last manometric signal reached a low-pressure nadir (typically observed when the UES began to open). The post–UES-nadir segment began when the first manometric signal started to rise from the low-pressure nadir (typically observed when the UES began to close) and ended when the postswallow CP-EMG signal reached a plateau. The pre–UES-nadir and post– UES-nadir segments lasted an average of 1.69 and 2.41 seconds, respectively. Because the CP muscle is relatively quiet during the swallow,1,2 pressure changes within the UES related to intrabolus pressure during the swallow may confound the correlations. In addition, pressure events related to the pharyngeal swallow last less than 2 seconds.29 Therefore, the preswallow and postswallow data segments may more accurately represent the swallow-related association between the CP muscle and the UES.
Figure 1.
Upper plot is composite of cricopharyngeal electromyographic (CP-EMG; shaded in gray) and upper esophageal sphincter (UES) sensors from high-resolution manometry (HRM; solid and dashed black lines). Lower plot shows submental surface electromyography. (A) Pre–UES-nadir segment. Left boundary starts at onset of submental EMG activity, and right boundary ends when last UES HRM sensor reaches nadir. (B) Post–UES-nadir segment. Left boundary starts when first UES HRM sensor rises from nadir, and right boundary ends when CP-EMG activity reaches plateau.
To examine the association between UES pressure and CP muscle activity, we calculated a Pearson product-moment correlation coefficient for each trial. For each task, the mean correlation was calculated for each subject. These correlations served as the dependent variable used in the analysis of variance that followed. The correlation coefficient can be thought of as a random variable that may be influenced by other factors. Evaluating factors that may have an impact on the correlation coefficient can be done by analysis of variance. Thus, task differences were analyzed by repeated-measures analysis of variance with pairwise comparisons using Fisher’s protected least significant difference tests. Post hoc task grouping was performed with Fisher’s protected least significant difference tests. The criteria for grouping consisted of nonsignificant differences for all tasks within the same group and significant differences for all tasks in separate groups. Group differences were calculated with Student’s t-test. A significance (α) level of 0.05 was used for the t-test, as well as for the analysis of variance.
Results
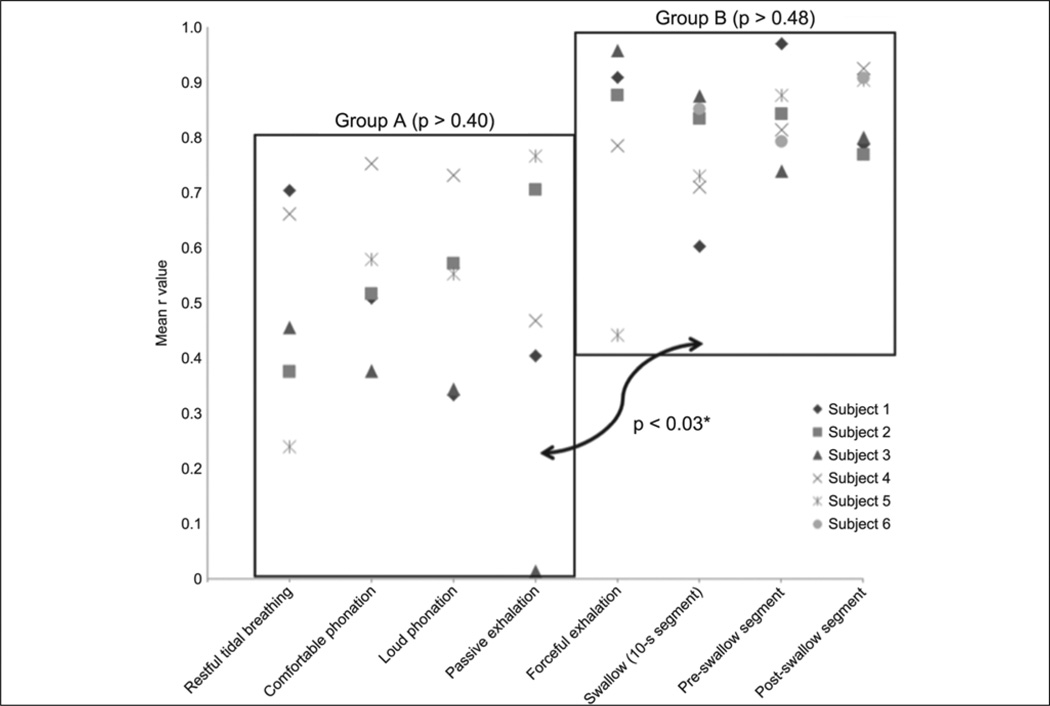
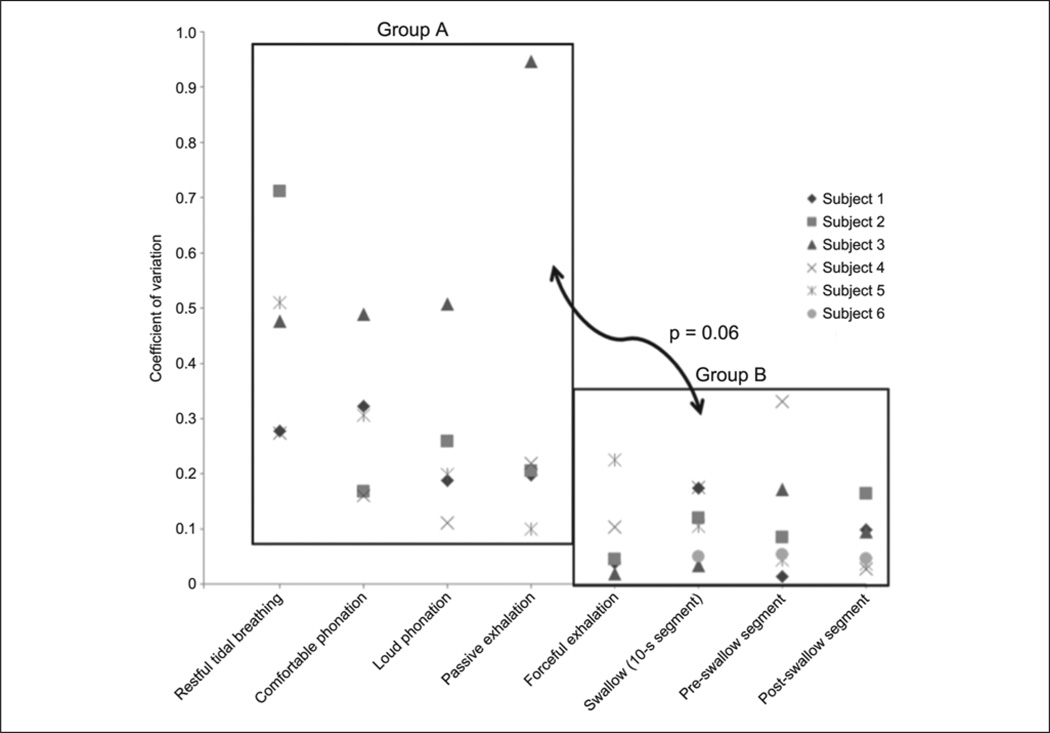
A sample of HRM and EMG tracings from 1 subject can be found in Figure 2. Summary statistics for each task are displayed in Table 1. The mean correlation coefficient for each of the 6 subjects was used to calculate the group means and standard deviations displayed in Table 2. Moderate correlations were observed for tasks in group A (restful tidal breathing, comfortable phonation, loud phonation, and passive exhalation). Strong correlations were observed for tasks in group B (forceful exhalation and swallow tasks). There was a significant effect of task on the degree of CP-EMG and HRM UES pressure correlation [F(7,35) = 5.47; P < .001]. Correlations were significantly higher for the tasks in group B than for those in group A (P < .03). Within each task group, the differences were nonsignificant (P = .40 to .95). Coefficients of variation for tasks in each group were collapsed by subject. The mean coefficients of variation were higher for the tasks in group A than for those in group B, although the differences did not reach statistical significance (t(5) = 2.52; P = .06). Distributions of mean correlation coefficients and coefficients of variation of correlation coefficients can be found in Figures 3 and 4, respectively.
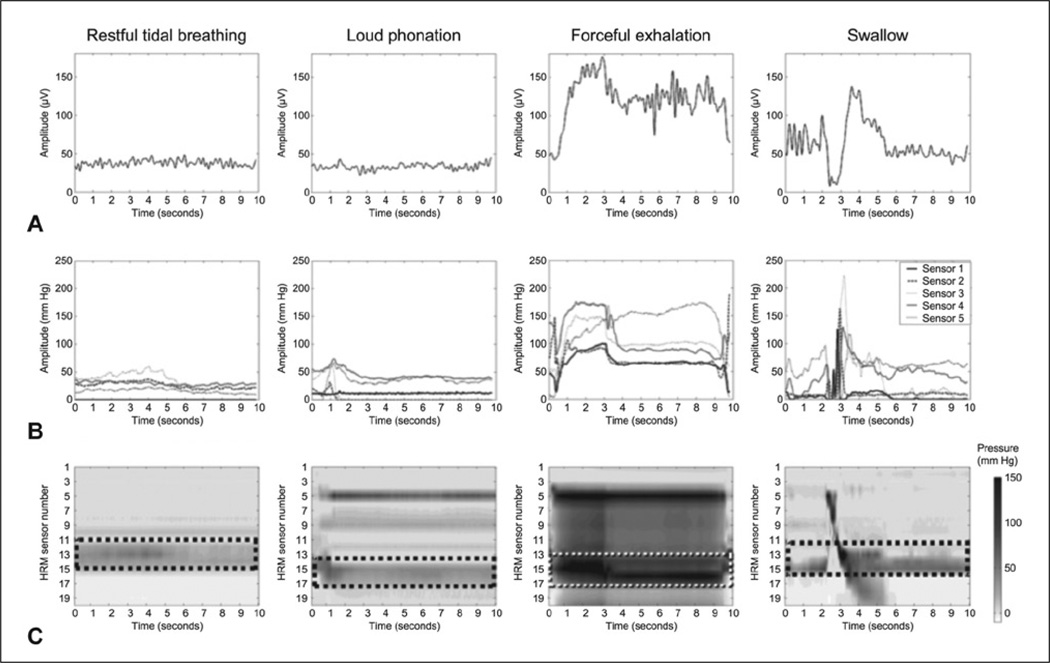
Figure 2.
(A) Rectified CP-EMG plots. (B) HRM line plots of 5 sensors (superior to inferior) located in UES. (C) HRM spatiotemporal plots, with data from 5 UES sensors displayed within dashed rectangle. In these plots, darker-gray and black tones correspond to higher pressures.
Table 1.
Summary Data for Each Task.a
| Task | Maximum UES Pressure, mm Hg |
Maximum CP Amplitude, uV |
Sound Pressure Level, dB |
Air Pressure, cm H2O |
|---|---|---|---|---|
| Restful tidal breathing (n = 30) | 175 ± 116 | 153.45 ± 61.41 | ||
| Comfortable phonation (n = 30) | 321 ± 266 | 146.51 ± 122.29 | 83.8 ± 3.63 (range, 80–88) | |
| Loud phonation (n = 30) | 304 ± 221 | 200.38 ± 222.80 | 93.8 ± 3.63 (range, 90–98) | |
| Passive exhalation (n = 30) | 164 ± 117 | 66.10 ± 20.82 | 10.76 ± 5.66 (range, 6.5–14.5) | |
| Forceful exhalation (n = 30) | 701 ± 451 | 393.29 ± 230.85 | 105.76 ± 28.69 (range, 64–182) | |
| Swallow (10-second segment; n = 35) | 703 ± 408 | 420.51 ± 237.32 | ||
| Pre–UES-nadir segment (n = 35) | 320 ± 250 | 116.37 ± 61.20 | ||
| Post–UES-nadir segment (n = 35) | 630 ± 375 | 413.23 ± 242.36 |
Data are mean ± SD. Maximum values for upper esophageal sphincter (UES) pressure and cricopharyngeal (CP) amplitude were normalized to median during restful tidal breathing task, and reflect percentages of median.
Table 2.
Correlation Coefficients (UES Manometry Versus CP Electromyography) for Each Task.a
| Task | Group Mean | Group Mean Dispersion |
|---|---|---|
| Group A | ||
| Restful tidal breathing | 0.49 ± 0.19 | 0.20 ± 0.05 |
| Comfortable phonation | 0.55 ± 0.14 | 0.15 ± 0.04 |
| Loud phonation | 0.51 ± 0.17 | 0.16 ± 0.10 |
| Passive exhalation | 0.47 ± 0.30 | 0.14 ± 0.09 |
| Group B | ||
| Forceful exhalation | 0.79 ± 0.21 | 0.08 ± 0.09 |
| Swallow (10-second segment) | 0.77 ± 0.10 | 0.08 ± 0.04 |
| Pre–UES-nadir segment | 0.84 ± 0.07 | 0.09 ± 0.09 |
| Post–UES-nadir segment | 0.85 ± 0.07 | 0.06 ± 0.04 |
Mean correlation coefficient for each of 6 subjects was used to calcu-late group mean and SD. Significant differences were observed only between tasks in group A and tasks in group B (P < .03). Group mean dispersion is representative of average SD among subjects.
Figure 3.
Distribution of mean correlation coefficients (r) of UES HRM and CP-EMG data across tasks. Pairwise comparisons revealed no significant difference within group A or group B (P > .3 and P > .4, respectively). Correlations from tasks in group B, however, were significantly stronger than those in group A (P < .03).
Figure 4.
Distribution of coefficients of variation of correlation coefficients (r) of UES HRM and CP-EMG data across tasks. Difference in coefficients of variation between group A and group B approached significance (P = .06).
Discussion
This was the first study to examine the association between UES pressure and CP-EMG activity during swallow and nonswallow tasks by using modern manometric technology. This new technology, combined with novel analysis techniques, has allowed for a more precise distinction between CP muscle and UES activity across tasks than has previously been reported. Moderate positive correlations were found between UES HRM and CP-EMG results during quiet resting, sustained phonation, and passive exhalation, and strong positive correlations were found during forceful exhalation and swallowing. These results suggest that the CP muscle is active in producing UES pressure in all of the above tasks, and that it may contribute more to pressure changes during forceful exhalation and swallowing.
Differences in correlations seen between tasks may reflect different physiological mechanisms contributing to pressure changes at the UES. The swallowing task was brief, and thus may have required motor planning different from that of the other, sustained tasks.30 The pressure profile in the UES during quiet resting, sustained phonation, and passive exhalation may have a component of passive tissue elasticity or laryngeal and pharyngeal posturing,1,3,31 forces not measured in this study. The increased CP muscle activity and correlative UES pressure seen during the forceful exhalation task may be due to higher recruitment of motor units as a protective mechanism against pharyngoesophageal reflux, similar to that found during esophageal distention.18,19 In the case of forceful exhalation, however, the stimulus to trigger the protective mechanism would be an increase in intrathoracic pressure, as opposed to esophageal distention.
In the exhalation tasks, the resistance of the semioccluded tube appeared to equalize the pressures in the thoracic, pharyngeal, and oral cavities.32,33 This equalization of pressure can be observed on HRM between the nasopharynx and the UES (as seen in Figure 2C, third column). Nasopharyngeal and UES contact pressures were differentiated from the air pressure artifacts by the higher pressure amplitudes registered. Nonetheless, the similar modulations of pressure suggest an interaction between the degree of air pressure resistance and the UES pressure generated.
Three limitations should be noted. First, a modest-size sample consisting only of normal, young subjects was included. Although this is an important first step in investigating the relationship between muscle activity and pressure generation in a variety of tasks, it would be interesting to determine how these relationships change in disease states. Second, as with all studies using intramuscular EMG testing, there was some difficulty in placing electrodes properly in the muscle of interest. Placing electrodes in the CP muscle provides a unique challenge, in that direct visualization would require general anesthesia, as was used in earlier studies.1–3,11,13,20 This may explain, at least in part, why we observed a lower average correlation coefficient between CP muscle activity and UES pressure than did Lang et al,2 who found a correlation of 0.87 (P < .01). This difference may be due to the difference in models (human versus dog) and thus in electrode and sensor placement methods. Future human studies would benefit from guided approaches, including ultrasound or videofluoroscopy, to more accurately place EMG electrodes in the CP muscle and manometric sensors in the UES. Third, movement of the UES was not directly measurable in this experimental setup. This limitation was corrected for in the non-swallow tasks by adjustment of the HRM sensors included in the analysis. Further studies using some visualization technique to track UES movement during swallowing would be advantageous. Improving the reliability of electrode placement for future studies combining HRM with EMG testing is desirable to ensure the validity of the EMG signal and to increase the number of subjects included in these types of studies.
The findings of this study confirm that the contributions of the CP muscle to UES pressure may be task-dependent. Although each task in our study likely includes some level of voluntary control,34,35 it seems reasonable to suggest that tasks of a more voluntary nature (eg, voice, passive exhalation) may be more cortically influenced, and as relatively more complex tasks, they may exhibit more variability and more independence between the UES and the striated CP muscle. Thus, it is interesting that the manometric-EMG associations were more modest—and the associations were most variable—for these more voluntary tasks. In contrast, the ingestive and protective nature of swallow and forceful exhalation—required for cough and airway protection— may require less voluntary control or cortical influence, and thus may yield a more specific, less variable, and less complex pattern. Interestingly, the manometric-EMG associations were strongest—and the associations were least variable—for these tasks. A brief comment on the difference between the two exhalation tasks (forceful versus passive) may be helpful. Each task required subjects to demonstrate volitional control. However, the pressures generated for forceful exhalation (between 80 and 120 cm H2O) are more typical of airway protection (eg, cough), whereas the pressures generated during “passive” exhalation (between 5 and 15 cm H2O) are within the range observed during voice and speech. Our results provide an interesting example of how the UES and CP muscle may coordinate differently depending on the task requirements, and may represent an example of task-dependent tuning of central pattern generators. It could also be possible that the correlations between UES and CP muscle signals were simply dependent on the magnitude of the signals generated. Therefore, our results highlight how the complexity, force requirements, and voluntary nature of a task may influence the role of the CP muscle in regulating UES pressure. Our initial findings suggest that these are important avenues to explore in future studies.
Conclusions
These findings are of both scientific and clinical interest. From a scientific standpoint, combined HRM-EMG testing may be a valuable tool as we learn more not only about the complex pressure patterns of the pharynx, but also about how they are generated. Additionally, strong correlations with swallowing may signify that HRM alone, rather than combined HRM-EMG testing, may be adequate in future examinations of CP muscle function during the swallow. Clinically, it may be beneficial to perform a more comprehensive examination of patients with CP muscle dysfunction, a fairly common cause of dysphagia. Although evaluation of this disorder is understandably focused on dysphagia, asking patients about respiratory or phonatory difficulties appears warranted on the basis of the contributions of the CP muscle to these tasks. This is an important step amid the increase in popularity and clinical acceptability of pharyngeal HRM and its use for decision-making in cases of dysphagia.
Acknowledgment
The authors acknowledge Glen Leverson, PhD, biostatistician, Department of Surgery, for his assistance with statistical analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by NIH grant R21 DC011130A from the National Institute on Deafness and Other Communication Disorders. Dr Hammer also receives funding from NIH grants DC010900, RR025012, and RR023268.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology. 1978;74:514–520. [PubMed] [Google Scholar]
- 2.Lang IM, Dantas RO, Cook IJ, Dodds WJ. Videoradiographic, manometric, and electromyographic analysis of canine upper esophageal sphincter. Am J Physiol. 1991;260:G911–G919. doi: 10.1152/ajpgi.1991.260.6.G911. [DOI] [PubMed] [Google Scholar]
- 3.Medda BK, Lang IM, Dodds WJ, et al. Correlation of electrical and contractile activities of the cricopharyngeus muscle in the cat. Am J Physiol. 1997;273:G470–G479. doi: 10.1152/ajpgi.1997.273.2.G470. [DOI] [PubMed] [Google Scholar]
- 4.van Overbeek JJM, Wit HP, Paping RHL, Segenhout HM. Simultaneous manometry and electromyography in the pharyngoesophageal segment. Laryngoscope. 1985;95:582–584. doi: 10.1288/00005537-198505000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Isberg A, Nilsson ME, Schiratzki H. Movement of the upper esophageal sphincter and a manometric device during deglutition: a cineradiographic investigation. Acta Radiol Diagn (Stockh) 1985;26:381–388. doi: 10.1177/028418518502600404. [DOI] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 8.Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 9.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006;291:G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 11.Jacob P, Kahrilas PJ, Herzon G, McLaughlin B. Determinants of upper esophageal sphincter pressure in dogs. Am J Physiol. 1990;259:G245–G251. doi: 10.1152/ajpgi.1990.259.2.G245. [DOI] [PubMed] [Google Scholar]
- 12.Medda B, Lang I, Dodds W. Control of UES tone by lung inflation reflexes. Gastroenterology. 1990;99:1219A. [Google Scholar]
- 13.Medda B, Lang IM, Layman RD, Hogan WJ, Dodds WJ, Shaker R. Quantification of the relationship between electrical and contractile activities of the upper esophageal sphincter (UES) of the cat. Gastroenterology. 1993;104:A551. [Google Scholar]
- 14.Martin F, Klingholz F. The role of the cricopharyngeal muscle in phonation [in German] Laryngol Rhinol Otol (Stuttg) 1983;62:223–225. [PubMed] [Google Scholar]
- 15.Perera L, Kern M, Hofmann C, et al. Manometric evidence for a phonation-induced UES contractile reflex. Am J Physiol Gastrointest Liver Physiol. 2008;294:G885–G891. doi: 10.1152/ajpgi.00470.2007. [DOI] [PubMed] [Google Scholar]
- 16.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119:369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takasaki K, Umeki H, Kumagami H, Takahashi H. Influence of head rotation on upper esophageal sphincter pressure evaluated by high-resolution manometry system. Otolaryngol Head Neck Surg. 2010;142:214–217. doi: 10.1016/j.otohns.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Babaei A, Dua K, Naini SR, et al. Response of the upper esophageal sphincter to esophageal distension is affected by posture, velocity, volume, and composition of the infusate. Gastroenterology. 2012;142:734–743. doi: 10.1053/j.gastro.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokashiki R, Funato N, Suzuki M. Globus sensation and increased upper esophageal sphincter pressure with distal esophageal acid perfusion. Eur Arch Otorhinolaryngol. 2010;267:737–741. doi: 10.1007/s00405-009-1134-1. [DOI] [PubMed] [Google Scholar]
- 20.Isberg A, Nilsson ME, Schiratzki H. The upper esophageal sphincter during normal deglutition. A simultaneous cineradiographic and manometric investigation. Acta Radiol Diagn (Stockh) 1985;26:563–568. doi: 10.1177/028418518502600511. [DOI] [PubMed] [Google Scholar]
- 21.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of video-fluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4:8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 22.Welch RW, Luckmann K, Ricks PM, Drake ST, Gates GA. Manometry of the normal upper esophageal sphincter and its alterations in laryngectomy. J Clin Invest. 1979;63:1036–1041. doi: 10.1172/JCI109372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahrilas PJ. Upper esophageal sphincter function during antegrade and retrograde transit. Am J Med. 1997;103:56S–60S. doi: 10.1016/s0002-9343(97)00324-0. [DOI] [PubMed] [Google Scholar]
- 24.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 25.McCulloch TM, Van Daele D, Ciucci MR. Otolaryngology head and neck surgery: an integrative view of the larynx. Head Neck. 2011;33(suppl 1):S46–S53. doi: 10.1002/hed.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res. 2012;55:892–902. doi: 10.1044/1092-4388(2011/11-0088). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ertekin C, Pehlivan M, Aydoğdu I, et al. An electrophysiological investigation of deglutition in man. Muscle Nerve. 1995;18:1177–1186. doi: 10.1002/mus.880181014. [DOI] [PubMed] [Google Scholar]
- 28.Ertekin C, Aydogdu I. Electromyography of human cricopharyngeal muscle of the upper esophageal sphincter. Muscle Nerve. 2002;26:729–739. doi: 10.1002/mus.10267. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope. 2010;120:2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatsopoulos N, Joshi J, O’Leary JG. Decoding continuous and discrete motor behaviors using motor and premotor cortical ensembles. J Neurophysiol. 2004;92:1165–1174. doi: 10.1152/jn.01245.2003. [DOI] [PubMed] [Google Scholar]
- 31.Iwarsson J, Sundberg J. Effects of lung volume on vertical larynx position during phonation. J Voice. 1998;12:159–165. doi: 10.1016/s0892-1997(98)80035-0. [DOI] [PubMed] [Google Scholar]
- 32.Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201:401–409. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netsell R, Hixon TJ. A noninvasive method for clinically estimating subglottal air pressure. J Speech Hear Disord. 1978;43:326–330. doi: 10.1044/jshd.4303.326. [DOI] [PubMed] [Google Scholar]
- 34.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 35.Miller AJ. The Neuroscientific Principles of Swallowing and Dysphagia. San Diego, CA: Singular Publishing Group; 1999. [Google Scholar]