Abstract
The transcription factor Pax6 plays a pivotal role in eye development, as eye morphogenesis is arrested at a primitive optic vesicle stage in homozygous Pax6 mutant mouse embryos. The arrested optic vesicle development has led to the assumption that cellular differentiation programs are unable to initiate. Contrary to this, we found that neurogenesis in Pax6 mutant optic vesicles was not arrested, but instead accelerated as numerous neurons differentiated precociously, more than a day earlier than normal. To identify potential mechanisms for Pax6 repression of neuron differentiation, we examined retinal proliferation and differentiation. Mutant optic vesicles had reduced proliferation, coupled with precocious activation of the proneural gene, Mash1. Ectopic expression of Mash1 was sufficient to induce precocious neuron differentiation. Subsequently, precocious neurons adopted a generic rather than a specific retinal neuron fate. Thus, Pax6 regulates the timing of retinal neurogenesis and couples it with specific neuron differentiation programs.
Keywords: Retina, Mouse embryo, Mutant, Sey, Small eye, Aniridia, Transcription factor, Neurogenesis, Neuron differentiation, Proneural, bHLH, Mash1, Brn3b, Isl1, Timing
Introduction
The development of the vertebrate retina requires the differentiation and assembly of a network of several distinct types of neurons and glia. This process is precisely regulated to produce differentiated cells at specific times, and in appropriate types and numbers. For example, the first differentiating retinal cells in all vertebrates are retinal ganglion cells (RGCs), followed by differentiation of other cell types in a stereotyped sequence (Rapaport et al., 2004; Young, 1985). This differentiation pattern suggests that the timing of terminal division is somehow integrated with the process of cell-type determination, though the mechanism for integrating timing and determination is unclear. All retinal cell types derive from a common retinal progenitor cell (RPC) population, implying both that lineage restrictions are relatively unimportant (Holt et al., 1988; Turner et al., 1990; Wetts and Fraser, 1988), and that the differentiation potential of progenitor cells shifts as development proceeds. For temporal regulation, the switch from dividing progenitor to terminal division is controlled by general cell-cycle regulators (Ohnuma and Harris, 2003; Ohnuma et al., 2002). In addition, at least one bHLH transcription factor, Hes1, is a repressor of CNS neuron differentiation, in that Hes1 mutant mouse embryos form neurons earlier than wildtype (Ishibashi et al., 1995; Tomita et al., 1996a). Genetic mechanisms for cell type determination are more extensively defined, with combinations of specific proneural bHLH and homeodomain transcription factors driving differentiation programs for specific cell types (Cepko, 1999; Kageyama and Nakanishi, 1997; Vetter and Brown, 2001). The specific mechanisms in the retina that regulate the timing of differentiation, and couple differentiation to determination programs, however, remain largely undefined.
The Pax6 gene is a key regulator of multiple aspects of eye development and encodes a transcription factor with both paired and homeodomain DNA binding motifs (Walther and Gruss, 1991). Mutations in Drosophila, mouse, rat, and human Pax6 demonstrate its evolutionarily conserved requirement for eye development (Glaser et al., 1992; Hill et al., 1991; Quiring et al., 1994). Furthermore, Pax6 has the potent ability to activate de novo eye development, since ectopic Pax6 induces ectopic eyes with complete networks of differentiated retinal cell types (Chow et al., 1999; Halder et al., 1995).
Genetic analysis of Pax6 function in mice has begun to define specific mechanisms for Pax6 action in eye development. Pax6 is expressed very early, in the evaginating optic vesicle and subsequently the entire retinal progenitor population preceding differentiation (Walther and Gruss, 1991). Despite its early expression in the optic vesicle, Pax6 is not required for optic vesicle outgrowth or identity, as optic vesicles form in homozygous Pax6 mutants (Hill et al., 1991; Hogan et al., 1986), and furthermore express several transcription factors associated with optic-vesicle identity (Otx2, Lhx2, Rx, Six3, Pax6 itself, and others; Bernier et al., 2001; Jean et al., 1999; Zhang et al., 2000). However, subsequent development of the optic vesicle in Pax6 mutants is highly abnormal in several respects, with failure to form optic cup, neural retina, pigmented epithelium, or optic stalk, and the associated epithelial lens placode fails to invaginate to form the lens (Grindley et al., 1995; Hogan et al., 1986). These developmental and morphological abnormalities have been interpreted as an arrest at an early, presumably pre-neurogenic, stage of optic development. Thus, Pax6 is necessary for eye morphogenesis, and uncommitted optic vesicle cell progression to competent retinal progenitor cells.
Pax6 expression is maintained throughout retinal development, including early expression in all retinal progenitor cells, continued expression in the proliferative margin of the retina, and expression in three types of retinal neuron: RGC, amacrine, and horizontal (Hitchcock et al., 1996; Puschel et al., 1992; Walther and Gruss, 1991). To investigate later functions of Pax6, a recent study performed Cre-lox mediated deletion of Pax6 in retinal progenitor cells after optic cup formation (Marquardt et al., 2001). Here, Pax6 was removed specifically from peripheral neural retina, causing reduced rates of proliferation, up-regulation of the proneural factor NeuroD, and differentiation of amacrine cells at the expense of other retinal cell types. Therefore, one function of Pax6 is to maintain the full potency of RPCs to generate all retinal cell types, with amacrine cells as a Pax6-independent default fate. Thus, Pax6 has both an early role in morphogenesis, and a distinct later function in retinal neurogenesis.
The rudimentary morphology of the arrested optic vesicle in Pax6 mutants has led to the assumption that neurogenesis is either arrested or fails to initiate. However, we discovered that neurons differentiate in Pax6 mutant optic vesicles, and investigated mechanisms of differentiation and specification. These results identify previously undiscovered functions for Pax6 in controlling both the timing of retinal neurogenesis, and the determination of specific neuron types.
Materials and methods
Mouse embryos
Wildtype mouse embryos were obtained from timed matings of FVB/N mice. Noon of the day of a vaginal plug was designated E0.5. Embryos were fixed in 4% paraformaldehyde/0.1 M phosphate buffer at 4°C: for βIII-tubulin antibody labeling or in situ hybridization, fixation was for 1–4 weeks, while for Mash1 and cell-type specific antibodies, fixation was for only 1 h at 4°C.
In all mutant experiments, the SeyNeu1 null allele of Pax6 was used in an FVB/N background. Mutant embryos were obtained using crosses between Pax6+/−males and females. To distinguish between Pax6+/−and +/+ embryos, the genotype of each embryo was determined using PCR as described (Grindley et al., 1995).
In some experiments, Math5lacZki/+ mice (insertion of the lacZ marker gene into the Math5 locus) were crossed with Pax6+/−mice. Mice carrying one copy of each mutation were intercrossed, embryos from E8.5–E10.5 collected, and Math5-LacZ expression detected by X-gal staining. Yolk sac tissue was used to genotype for the presence of each allele by PCR.
Immunofluorescence and in situ hybridization
Fixed embryos were infiltrated with sucrose and gelatin, embedded in 15% sucrose/7.5% gelatin/0.1 M phosphate, and frozen for cryosectioning on a nasal-temporal plane. For antibody labeling, the slides were incubated 1 h in blocking solution (4% milk TST) consisting of 4% powdered milk dissolved in 10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20 (Sundin and Eichele, 1990), then incubated overnight with antibodies diluted in 4% milk TST. After several washes in TST, the slides were incubated 2 h with biotinylated secondary antibodies (Vector), followed by Cy2- or Cy3-conjugated streptavidin (Vector). For counterstaining, a 1-min wash in a DAPI solution was followed by coverslipping and fluorescence microscopy.
Primary antibodies used were rabbit neuronal class βIII-tubulin (1:3000, Covance), goat anti-Doublecortin (1:2000, Santa Cruz), goat anti-Brn3b (1:100, Santa Cruz), mouse anti-Brn3a (1:1000; E. Turner, UCSD; Fedtsova and Turner, 1995), mouse anti-Syntaxin (1:1000, Sigma), mouse anti-NF-160 (1:500, Sigma), mouse anti-Isl1 (1:20, Developmental Studies Hybridoma Bank, Univ Iowa), rabbit anti-Dlx (1:40; Panganiban et al., 1995), mouse anti-VC1.1 (1:400, Sigma), and mouse Mash1 (1:100, BD PharMingen). Secondary antibodies were Cy2- or Cy3-conjugated goat anti-rabbit IgG and donkey anti-mouse IgG (1:200, Jackson ImmunoResearch Lab).
In situ hybridization was carried out using Math5, Mash1, Ngn2 or NeuroD cDNAs as templates for digoxygenin antisense riboprobes, as described in Brown et al. (1998). Embryos were embedded in gelatin/sucrose/PBS, cryosectioned at 10 µm and imaged using a Leica compound microscope, equipped with an Orca camera and Openlab 3 and Adobe Photoshop 6.0 software.
To estimate the number of neurons in optic vesicles, 10 µm sections were labeled with βIII-tubulin antibody. Positively labeled cell bodies were summed from complete series of sections through both eyes. Only strongly labeled cells were included in the total neuron count, constituting a conservative threshold, and double-counting of bisected cells was minimized by comparing cell profiles and nuclear labels from adjacent sections.
Proliferative labeling of retinal progenitors
BrdU (bromodeoxyuridine) injections on E10.5 and antibody labeling were made to identify retinal progenitors in S-phase. A BrdU solution (Sigma) was injected into the peritoneal cavity of pregnant female mice at 0.5 ml of 6 mg/ ml solution per 30 g body weight, i.e., 0.1 mg/g (Mastick and Andrews, 2001). Because of the short clearance time of BrdU, the injection was repeated 20 min later to increase labeling intensity. At 1 h after the first injection, embryos were collected, fixed, and processed for cryosectioning and BrdU antibody labeling. Three fields from the distal optic vesicle/cup were examined for each of three embryos for each of the genotypes (+/+, +/−, −/−), and the percentage of BrdU+ cells calculated by dividing by the total number of DAPI+ nuclei.
Chick embryo electroporation
To mis-express Mash1 in the early optic vesicle, a Mash1 expression plasmid was electroporated into chick embryos, using standard techniques (Muramatsu et al., 1997; Swartz et al., 2001). A Mash1 expression plasmid (Nakada et al., 2004) (2 mg/ml in PBS) was mixed with a pCA-GFP plasmid (1 mg/ml), with Fast Green dye added. For control electroporations, GFP plasmid alone was used. DNA was injected into the lumen of one optic vesicle at stage HH 10–12 (Hamburger and Hamilton, 1992), followed by electroporation with 5 pulses for 50 ms of 15 V, using a BTX Electroporator (San Diego, CA). The embryos were allowed to develop for 20–24 h (stages 15–17), then collected and fixed. Embryos with strong GFP expression were selected for embedding, sectioning, and βIII-tubulin antibody labeling. Ectopic Mash1 expression in GFP+ cells was verified with Mash1 antibody (Pharmingen). About 37% of electroporated cells co-expressed GFP and Mash1 (of 111 GFP+ cells examined, 41 were Mash1+). Of 10 embryos with GFP+ eyes, 8 had strong GFP labeling and all 8 had precocious βIII-tubulin expression in a subset of the GFP+ cells. About 28% of electroporated cells co-expressed GFP and βIII-tubulin (of 165 GFP+ cells examined, 46 were βIII-tubulin+).
Results
The Pax6 mutant optic vesicle retains a primitive morphology
As previously described (Hogan et al., 1986), morphological development of the optic vesicle is severely disrupted in Pax6 mutants (Fig. 1). In the E10.5 wildtype eye, roughly a half-day before differentiating neurons first appear, eye morphogenesis is largely complete, with the optic vesicle rearranged into a distinct cup-shaped neural retina and pigmented epithelium, lens primordium, and optic stalk. In the absence of Pax6, none of these structures are apparent. A simple morphology is retained, with the distal optic vesicle consisting of a thin epithelial sheet containing relatively few cells. Thus, Pax6 is required for major morphogenetic events in the primitive optic vesicle, and presumably a blockade of any subsequent cellular differentiation.
Fig. 1.
Pax6 mutant eye structures arrest at a primitive optic vesicle stage. Sections through wildtype and Pax6−/− optic vesicles at E10.5, labeled with the nuclear stain DAPI to reveal tissue organization. (A) Wildtype embryos have a highly organized optic cup structure, including optic stalk (os), retinal pigmented epithelium (rpe), cup-shaped neural retina (nr), and early cellular lens (le). (B) Pax6−/− embryos have a primitive optic vesicle (op ves) evaginating from the forebrain. Undifferentiated characteristics of the optic vesicle include a wide-open optic stalk that fails to constrict, failure in optic cup morphogenesis, lack of a thick neural retina, and lack of a lens. Scale bar: 100 µm
The Pax6 mutant optic vesicle contains differentiating neurons
A molecular marker for very early stages in neuron differentiation is the neuron-specific βIII-tubulin protein (βIII-tub, TuJ1 antibody; Easter et al., 1993). In the retina, βIII-tub expression can be detected within 4 h after terminal division of neuronal progenitors (Milton, Wong, and Mastick, unpublished). In previous studies, we examined patterns of neurogenesis in the forebrain of wildtype and Pax6 mutant embryos (Mastick and Andrews, 2001; Mastick and Easter, 1996; Mastick et al., 1997). During the course of these studies, we noticed βIII-tub+ cells in Pax6 mutant optic vesicles. The finding of cells expressing a neuron differentiation marker was unexpected and prompted further investigation.
To characterize βIII-tub expression in the Pax6−/− optic vesicle, sections of E10.5 optic vesicles were labeled with βIII-tub antibody (Fig. 2). The βIII-tub+ cells had three distinct cell morphologies, similar to the initial stages of neuron differentiation observed elsewhere in the developing CNS (Easter et al., 1993): an elongated columnar shape typical of newly born neurons, rounded cells at the outer (i.e., pial, vitreal) surface of the epithelium, and a population with distinct neurite projections, likely the initial stages of axon formation (Fig. 2).
Fig. 2.
The Pax6 mutant optic vesicle contains βIII-tubulin-expressing neurons. The panels show high magnification views of sections of Pax6−/− optic vesicles from E10.5 embryos, labeled with an antibody against neuron-specific class III β-tubulin. (A) Some βIII-tub+ cells have an elongated morphology typical of neurons in the earliest stage of differentiation, with processes spanning the thickness of the wall of the optic vesicle. (B) Numerous βIII-tub+ cells have rounded cell bodies at the outer (pial) surface. (C) Other βIII-tub+ cells show neurite projections along the pial surface (arrowhead). Scale bar: 20 µm.
Within the Pax6−/−optic vesicle, neurons were scattered throughout the most distal (lateral) part of the optic vesicle, the expected location of the neural retina. A non-neuronal region, presumably RPE and optic stalk, separated the optic neurons from diencephalon (Fig. 3F). βIII-tub+ cells first appeared in the dorsal optic vesicle of Pax6 mutants, a dorsal shift relative to wildtype, consistent with the ventralization of the mutant optic vesicle recently reported (Baumer et al., 2002, 2003).
Fig. 3.
Neurogenesis is precocious and extensive in Pax6−/− optic vesicles. Sections of optic vesicles were labeled with βIII tubulin antibody from wildtype (Pax6+/+) and Pax6−/− embryos at various developmental stages. (A–D) Timing of neuron differentiation in Pax6+/+ optic vesicles. No neurons were observed from embryos ranging from 27 somites (E9.5) to 38 somites (E1 1.0). After the first appearance at somites 39–40 in the central optic cup (C, arrowhead), numbers rapidly increased at later stages (D, 46 somites, E11.5). (E–H) Timing of neuron differentiation in Pax6−/− optic vesicles. Neurons first appeared at somites 26–27 (E, arrowhead), a day earlier than in wildtype. The number of neurons in the distal optic vesicle (F, demarcated by lines) increased by 36 somites. (I) Time course of optic neuron differentiation in wildtype and mutant embryos. The total number of βIII-tub+ cells was determined by summing the number of βIII-tub+ cell profiles from a complete set of optic vesicle sections (both eyes) from each embryo examined (wildtype, filled triangles, n = 17 embryos; Pax6−/− , hollow triangles, n = 21; Pax6+/−, gray triangles, n = 10). Neuron differentiation in wildtype optic vesicles began at 39–40 somites, and rapidly increased in number after E1 1.5 (approximately 48 somites; data from older stages not shown). Neurogenesis within the Pax6−/− optic vesicle was first observed at 26 somites, then rapidly increased to a peak greater than 200 by the mid-30 somite stages. The number of neurons gradually declined over the next day, and virtually disappeared by 62 somites (see Fig. 7). In Pax6+/− embryos, neurogenesis did not begin until 46 somites. Scale bar: 100 µm.
Neurogenesis within Pax6−/− optic vesicles is precocious
To define the time of first appearance of βIII-tub+ neurons, sections through wildtype and Pax6−/−optic vesicles from E9.5–11.5 were labeled with βIII-tub antibody, and the total number of neurons per embryo counted (i.e., neurons counted in a complete set of eye sections in each embryo). The first appearance of βIII-tub+ cells in wildtype mouse retina had not been previously reported, so this became our first objective (Figs. 3A–D). No neurons were observed from E9.5 (27 somites) through E10.5 (34–38 somites) (Fig. 3I). Only at the 39 or 40 somite stage (E11.0) did one or a few neurons appear. From this initial differentiation, the number of neurons exponentially rose, so that by 50 somites, the neuron population exceeded 700 neurons (data not shown).
The time course of neurogenesis in Pax6−/− embryos differed greatly from wildtype embryos (Figs. 3E – H). Neurogenesis in Pax6−/− optic vesicles occurred precociously (prematurely early), first appearing at 26–27 somites (Fig. 3E). During the next half-day, the neuron population increased, reaching a peak of about 250 cells at 36 somites (E10.5). A gradual decrease in the neuron population was apparent over the next day (analysis of older stages is described below). Thus, in the Pax6−/− optic vesicle, there is a burst of neurogenesis, beginning more than a day earlier than in wildtype. Together, these findings demonstrate that Pax6−/− retinal progenitor cells are capable of differentiating as neurons, and that Pax6 has a key role in setting the timing of retinal neurogenesis.
To date, no early cellular defects have been identified in Pax6+/− optic vesicles. However, Pax6 has a strong dose-dependent effect, shown by a reduced adult eye size in heterozygotes (Grindley et al., 1995; Hill et al., 1991; Hogan et al., 1986). Therefore, we examined neuron differentiation in heterozygous embryos, whose embryonic eye morphology appeared normal. One might predict that Pax6+/− embryos would have either wildtype timing of neuron differentiation, or an intermediate phenotype such as slightly precocious neuron differentiation. Unexpectedly, Pax6+/−embryos had a delay in neuron differentiation relative to wildtype of about one-half day (Fig. 3I). Neurons did not appear until 46 somites, though neuron numbers in Pax6+/− embryos then rapidly increased to near wildtype by 50 somites.
Retinal proliferation is reduced in Pax6 mutants
The precocious neurogenesis in Pax6−/− embryos implies that a wildtype function of Pax6 is to suppress neuron differentiation until the 39–40 somite stage. Factors that accelerate proliferation inhibit differentiation, and likewise the inhibition of proliferation tends to promote differentiation (Ohnuma and Harris, 2003). Since Pax6 is known to regulate proliferation in the developing cerebral cortex (Estivill-Torrus et al., 2002; Gotz et al., 1998; Warren et al., 1999), and proliferation in E12.5–18.5 retina (Marquardt et al., 2001), we examined proliferation in the early optic vesicle. At E10.5, embryos were pulse-labeled in utero by bromodeoxyuridine (BrdU) injections, with collection and fixation after 1 h. BrdU is a thymidine analog that is incorporated by progenitor cells in S-phase. In wildtype, a BrdU pulse labels a thick layer of nuclei in the neural retina (Figs. 4A, D). In contrast, in Pax6 mutant optic vesicles, BrdU+ cells were sparser within the thin neuroepithelium (Fig. 4C), though overall nuclei appeared more tightly packed (Fig. 4F). The percentage of BrdU+ nuclei was determined for each genotype (Fig. 4G), and was much lower in Pax6−/− embryos than in wildtype (P ≪ 0.01, t test). This reduction in proliferation was similar to that seen in E12.5 retina after conditional inactivation of Pax6 (Marquardt et al., 2001), suggesting that Pax6 has both an early and an ongoing function in regulating progenitor proliferation. We also examined heterozygotes, finding that Pax6+/− embryos had an intermediate percentage of BrdU+ nuclei, but also significantly lower than wildtype (P < 0.01). Double-labeling of Pax6 mutant sections with both BrdU and βIII-tubulin antibodies showed that the βIII-tub+ cells did not incorporate BrdU (data not shown), providing additional evidence that these cells are differentiating. Thus, in the absence of Pax6, proliferation of retinal progenitors is diminished. This reduction in proliferation coincides with both the failure to undergo optic cup morphogenesis and the precocious appearance of differentiating neurons.
Fig. 4.
Proliferation of retinal progenitors is reduced in Pax6 mutants. Litters of embryos were labeled by in utero BrdU injection on E10.5. To identify optic vesicle cells in S-phase, embryos were collected and fixed 1 h after the BrdU pulse. Sections from 33–35 somite embryos were labeled with BrdU antibodies (panels A–C, yellow) and, on the same section, the nuclear dye DAPI (panels D–F, blue). All panels are at the same magnification. (G) Quantification of BrdU+ nuclei, expressed as a percentage of the total number of nuclei (mean ± SEM). Homozygous mutant embryos had much lower percentages of BrdU+ nuclei than wildtype (P ≪ 0.01, t test), and heterozygotes had intermediate values. Scale bar in A: 50 µm.
The proneural gene Mash1 is up-regulated in Pax6 mutants, and can induce early neuronal differentiation
Neuron differentiation is driven by specific transcription factors, notably the bHLH factors that are potent inducers of specific types of retinal neurons (Cepko, 1999; Kageyama and Nakanishi, 1997; Vetter and Brown, 2001). For example, Math5 is the earliest bHLH factor expressed in wildtype, and is necessary for the earliest neurons, the RGCs (Brown et al., 1998, 2001; Kanekar et al., 1997; Liu et al., 2001; Wang et al., 2001). One obvious mechanism for precocious differentiation of neurons in Pax6 mutants would be the precocious activation of one or more proneural bHLH factors. Therefore, we examined the mRNA expression of four retinal bHLH factors (Math5, Ngn2, NeuroD, and Mash1) in Pax6 mutants up to and during the period of precocious neurogenesis, E8.5–E10.5. These genes were chosen based upon their loss- and gain-of-function phenotypes in the retina, and because their regulation by Pax6 has been reported at later stages of retinal development (Marquardt et al., 2001).
Of the four bHLH factors tested, several were not expressed in early mutant optic vesicles. We did not detect Math5, Ngn2, or NeuroD mRNA in wildtype, Pax6+/−or Pax6−/− optic vesicles from E8.5 to E10.5 (data not shown; n = 3 litters for each probe at each age). The lack of Math5 expression was further verified by assaying lacZ expression in embryos heterozygous for a Math5-lacZ knock-in allele (Brown et al., 2001) and containing varying dosages of the Pax6 mutation (data not shown). No βgal expression was detected at any age (n = 2 independent litters per age). Thus, differentiation of neurons in the Pax6 mutant optic vesicle occurs independently of Math5 expression.
Only Mash1 showed altered expression in Pax6 mutants. Normally, Mash1 is expressed briefly in the wildtype optic vesicle at E9.5, at low levels, and in a few cells (Figs. 5A, C) (Guillemot and Joyner, 1993). This early optic expression of Mash1 was transient, disappearing by E10.5, and not reactivated until a much later secondary phase of expression at E14.5 (Brown et al., 1998; Jasoni and Reh, 1996). In contrast, Pax6−/− optic vesicles contained more cells that expressed both Mash1 mRNA and protein throughout the period of precocious neurogenesis (Figs. 5B, D, F, H). Mash1 mRNA appeared as early as 20 somites in Pax6 mutants (data not shown). Mash1+ cells did not co-express βIII-tubulin by double antibody labeling of Pax6 mutant optic vesicles during the period of precocious neurogenesis, specifically 32–34 somites; examination of βIII-tubulin+ cells at high magnification showed that none had Mash1 expression (0 Mash1+ of 64 βIII-tub+; data not shown). This observation is consistent with previous findings that Mash1 expression is restricted to progenitors, and down-regulated at or prior to terminal division (Andrews et al., 2003; Torii et al., 1999; Yun et al., 2002). At E8.5, we also observed ectopic Mash1 expression in the dorsal forebrain of Pax6 heterozygous (not shown) and homozygous mutant embryos (Figs. 5I, J), well before any optic vesicle expression. The requirement of Pax6 to suppress Mash1 in a dosage-dependent manner suggests that this genetic relationship might be widespread throughout the forming CNS and contrasts with the requirement for Pax6 to activate Mash1 in the later retina (Marquardt et al., 2001).
Fig. 5.
The proneural bHLH factor Mash1 is up-regulated in Pax6 mutants. (A–D) Sections through optic vesicles of E9.5 wildtype (A, C) and Pax6−/− (B, D) embryos, labeled with Mash1 antibody (red in A, B) or by Mash1 in situ hybridization (purple in C, D). In E9.5 wildtype optic vesicles, a few Mash1+ cells were observed, as previously reported (Guillemot 1995). Mash1+ cells were more numerous in Pax6−/− optic vesicles, assayed either for mRNA and protein. Arrowheads point to examples of Mash1+ cells. (E–H) Mash1 labeling of sections through E10.5 wildtype (E, G) and Pax6−/− (F, H) embryos. Mash1 expression was absent in wildtype, but present in mutant optic vesicles. (I, J) Mash1 is up-regulated in the forebrain of E8.5 Pax6−/− embryos, as assayed by whole mount in situ hybridization. No Mash1 expression was observed in the anterior neural plate of wildtype embryos. (I) Scale bar in A: 100 µm for A, B, E, F, I, J; 50 µm for C, D, G, H.
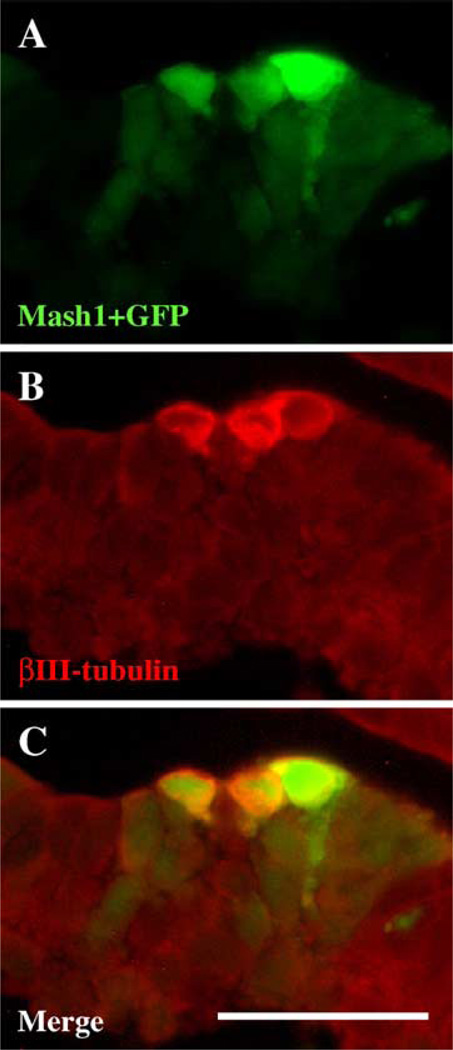
To directly test if Mash1 is sufficient to induce precocious neuron differentiation, Mash1 was misexpressed by electroporation into the optic vesicle of early (stages 10–12) chick embryos, along with a GFP marker plasmid to identify transfected cells. The embryos were analyzed after a day of development, i.e., stages 15–17, prior to any endogenous neuron differentiation (Waid and McLoon, 1995). GFP was visible at varying levels in patches of cells in the optic cup (Fig. 6A). These GFP+ patches contained βIII-tubulin+ cells (Fig. 6B; Fig. 8 of 8 electroporated embryos). In contrast, βIII-tubulin expression was not seen in neighboring regions that were untransfected. As a further control, electroporation of GFP alone did not induce any βIII-tubulin expression (n = 3 embryos; data not shown). Ectopic Mash1 expression, verified by Mash1 antibody labeling, was limited to a subset of the GFP+ cells (37%; of 111 GFP+ cells examined, 41 were Mash1+). Within patches of transfected cells, βIII-tubulin expression was limited to a subset of the brightest GFP cells (28%, 46 of 165 cells examined), a percentage slightly lower than for Mash1 expression, perhaps because some cells were either not responsive to ectopic Mash1, or contained only subthreshold levels of Mash1. Overall, we conclude that ectopic Mash1 expression is sufficient to induce precocious neuron differentiation in very early retinal progenitor cells, consistent with its up-regulation causing precocious neuron formation in Pax6 mutants.
Fig. 6.
Ectopic Mash1 expression induces neuron differentiation. A Mash-1 expression vector was co-electroporated with a GFP marker plasmid into early optic vesicles of chick embryos. Embryos were collected after 20–24 h of development (prior to any endogenous retinal neuron differentiation), and sections labeled with βIII-tub antibody. (A) A cluster of GFP+ cells (green) following electroporation. (B) The same section labeled with βIII-tub antibody labeling (red). (C) Merge of GFP and βIII-tub images, showing that the brightest GFP+ cells were βIII-tub+. Scale bar in C, 50 µm.
Fig. 8.
Fate of precocious neurons in Pax6 mutants: abnormal morphology of the Pax6 mutant optic remnant and loss of precocious neurons. Sections through Pax6 mutant at E13 (60 somite stage) labeled with the nuclear marker DAPI and βIII-tubulin antibody. (A) Low magnification view of a DAPI-labeled section, showing the small remnant of the optic vesicle (opt), buried in mesenchymal tissue of the head, and connected to the third ventricle (3rdV) by an optic stalk (os). (B) A higher magnification view of the same section, showing a lack of recognizable optic cup or other eye structures. (C) βIII-tubulin antibody labeling of the same section. The precocious neurons have largely disappeared, with only a single neuron (arrowhead) labeled in the sections of the entire optic remnant. Scale bar in C: 200 µm for A; 100 µm for B; 50 µm for C.
Pax6 is required for specification of early retinal neurons
Since the timing of differentiation was advanced in Pax6 mutants, an important issue was what neuron type(s) were generated. A first prediction was that if neuron differentiation followed a wildtype sequence, then the precocious neurons would differentiate as RGCs. Alternatively, the conditional inactivation of Pax6 at E10.5 leads exclusively to amacrine differentiation (Marquardt et al., 2001). A third possibility is suggested by the up-regulation of Mash1, which can induce bipolar neuron differentiation, at least when ectopically expressed in wildtype (i.e., Pax6+) retinal progenitors (Tomita et al., 1996b). Thus, a bipolar neuron identity could also be predicted, even though most bipolar cells are normally born postnatally (Rapaport et al., 2004).
To distinguish among these possibilities, sections through Pax6 mutant optic vesicles were labeled with a battery of retinal neuron markers (Fig. 7) and included colabeling with βIII-tubulin. Mutant embryos were examined both at E10.5 and E11.5, to allow precocious neurons to advance through differentiation.
Fig. 7.
Precocious neurons express generic neuronal markers, but lack definitive markers for RGC or amacrine neurons. (A-H) Sections through E10.5 Pax6−/−optic vesicles labeled with βIII-tubulin antibody (green) compared with other neuronal antibody markers (red). Filled arrowheads indicate double labeled cells; empty arrowheads indicate cells that are βIII-tub+ only. (I) Cell type specificity of antibody markers (after Marquardt et al., 2001; and observations of wildtype embryos, data not shown). Symbols for labeling in Pax6−/− optic vesicles: “+”, expression in precocious neurons; “+/−”, expression in some but not all βIII-tub+ cells; “−”, no co-expression with βIII-tub. Scale bar in A applies to all panels, 20 µm.
We tested several markers that are specifically expressed in differentiating RGCs. Doublecortin. All of the βIII-tub+ cells expressed doublecortin, a cytoplasmic protein expressed in differentiating RGCs (Rachel et al., 2002), but also very early in the differentiation of precocious neurons (Fig. 7A). Cells that expressed only doublecortin were abundant in the mutant optic vesicle (Fig. 7A). NF-160. NF-160 is a neurofilament protein that is expressed in RGCs, and later horizontals (Nixon et al., 1989). NF-160 expression marked a subset of the precocious neurons (Fig. 7B).
Next, four transcription factors with early expression in wildtype were tested for expression in Pax6 mutants. These factors have the key feature of being activated just before, or within hours of, the terminal division of the first differentiating neurons (Milton, Wong, and Mastick, in prep). Dlx. The Dlx family of homeodomain transcription factors include Dlx1 and 2, normally expressed very early (E11.0) in a subset of progenitors and later in RGC, horizontal, amacrine, and bipolar neurons (de Melo et al., 2003). A pan-DLX antibody was used that recognizes both Dlx1 and 2. The E10.5 mutant optic vesicle contained Dlx+ βIII-tub+ neurons, but singly positive cells were also seen for both markers (Fig. 7C), consistent with the wildtype pattern of a subset of Dlx+ progenitors (βIII-tub–) and postmitotic neurons (βIII-tub). Islet1. Islet1 is a homeodomain protein associated with several specific populations of early differentiating neurons in the CNS (Ericson et al., 1995), including postmitotic retinal neurons (RGC, amacrine, bipolar) (Galli-Resta et al., 1997). In Pax6 mutants, Islet1 expression was present, expressed at high levels but only in a subset of the βIII-tub+ neurons (Fig. 7D). Brn3a, Brn3b. Brn3a and 3b are homeodomain proteins specifically expressed in early differentiating RGC neurons, and are required for differentiation of this cell type (Gan et al., 1996; Liu et al., 2000; Trieu et al., 2003; Xiang et al., 1993). Neither Brn3 protein was expressed in Pax6 mutants (Figs. 7E, F).
Syntaxin, VC1.1. We tested two amacrine markers, syntaxin and VC1.1 (Alexiades and Cepko, 1997; Arimatsu et al., 1987; Barnstable et al., 1985). Neither syntaxin nor VC1.1 were expressed by the precocious neurons (Figs. 7G, H), suggesting that the precocious neurons did not have an amacrine identity. This result contrasts with the abundant and exclusive differentiation of syntaxin+ amacrine cells reported in retinas conditionally mutant for Pax6 (Marquardt et al., 2001).
PKC. Finally, based on the prediction that precocious Mash1 expression might activate bipolar differentiation, we tested the bipolar marker PKC, and found no labeling in the mutant optic vesicle (data not shown).
In summary, the precocious neurons were positive for several retinal neuron markers (doublecortin, NF-160, Dlx, and Islet1). However, the precocious neurons did not express key RGC, amacrine, or bipolar markers. Taken together, the analysis highlights that optic vesicle cells have the ability to become neurons by E9.5 but in the absence of Pax6 are unable to adopt specific retinal fates, despite the overall retinal identity of the progenitor population. A caveat to this conclusion is that some of these cell type markers may require longer times of differentiation for activation.
Precocious neurons do not persist
To follow the fate of the precocious neurons, embryos at an older stage (E13) were sectioned and labeled with βIII-tubulin antibody (Fig. 8). Remnant optic structures could be identified as lateral protrusions from the rostral forebrain (Fig. 8A). The optic remnants had a highly abnormal morphology, with no recognizable eye structures (Fig. 8B). Strikingly, βIII-tubulin labeling was very sparse, with only one neuron observed in the E13 Pax6−/− embryos examined (Fig. 8C). Thus, the large population of precocious neurons born between E9.5–E10.5 does not survive past E13, consistent with the gradual decline from E10.5 to E11.5 (Fig. 3I).
Discussion
The transcription factor Pax6 is central to current models of eye development. Pax6 expression is sufficient to induce ectopic eyes, albeit in a tissue- and stage-specific manner (Chow et al., 1999; Halder et al., 1995). Pax6 mutant mice have identified multiple roles for Pax6, including optic cup, RPE, and lens morphogenesis (Grindley et al., 1995; Hogan et al., 1986), dorsal–ventral patterning in the optic cup and stalk (Baumer et al., 2002, 2003), and retinal cell type specification (Marquardt et al., 2001). Here we have significantly extended Pax6 function, showing that Pax6 regulates the timing of neuron differentiation and has an additional early role in neuron specification. Together, these results have important implications for understanding and integrating the multifaceted functions of Pax6 in eye development.
Pax6 controls the timing of neurogenesis in the retina
Our main finding is that Pax6 genetically suppresses retinal neurogenesis, since numerous neurons differentiate early in the optic vesicle of Pax6 mutant mouse embryos. The primary line of evidence is the expression of neuronspecific βIII-tubulin, as well as several other neuronal proteins. These βIII-tub+ cells have morphologies typical of early retinal neurons (Fig. 2), and are post-mitotic. Thus, in the absence of Pax6, the optic vesicle generates competent neuronal progenitors. Moreover, neuron differentiation initiates a day earlier in Pax6 mutant optic vesicles than in wildtype (Fig. 3). Evidently, Pax6 has a negative regulatory influence on the differentiation of the initial progenitors. The implication is that the absence of Pax6 releases promoter(s) of neuron differentiation, or inhibitors of proliferation. Pax6 has prominent expression in the optic vesicle and so likely acts cell-autonomously in retinal progenitor cells to inhibit neuron differentiation. Alternatively, Pax6 is also expressed in the lens, so a non-autonomous effect of Pax6-dependent signals from the lens might also effect differentiation of retinal progenitor cells.
How does Pax6 repress neurogenesis in the retina? First, the expression of the proneural bHLH transcription factor, Mash1, is up-regulated in Pax6 mutant optic vesicles and dorsal forebrain (Fig. 5), and ectopic Mash1 is sufficient to drive precocious neuron differentiation (Fig. 6). The prolonged early Mash1 expression in Pax6 mutant optic vesicles could result either from abnormal retention or extra activation of Mash1. It is unknown whether Pax6 regulation of Mash1 is direct or indirect, but it should be noted that Mash1 expression can be both up- and down-regulated in Pax6 mutants, depending on the brain region (Yun et al., 2001). Interestingly, Pax6 regulates another proneural bHLH gene, Ngn2, by directly activating it (Scardigli et al., 2003). In the early optic vesicle, we found that Pax6 affected Mash1 but not Ngn2, and involved repression rather than activation. The complexity of the genetic interactions raises the possibility that Pax6 controls both positive and negative rates of neurogenesis via the expression of bHLH genes.
A second, perhaps related, mechanism for Pax6 control of the timing of retinal neurogenesis is implicated by a decrease in proliferation in Pax6 mutants. A lower proliferation rate could sensitize mutant RPCs to prodifferentiation factors, either extrinsic or intrinsic (such as Mash1), resulting in precocious neuron differentiation instead of proliferation. As a direct analogy, cell cycle inhibitory proteins can potentiate the differentiation of retinal neurons (Ohnuma et al., 2002). Proliferation rates are also altered in the thalamus of Pax6 mutants (Warren and Price, 1997). Similarly, in the early cortex, Pax6 mutant progenitors progress more quickly to activate expression of neuronal markers (Estivill-Torrus et al., 2002). Thus, regulation of proliferation, along with setting the timing of neurogenesis, appears to be a general function of Pax6 in the CNS.
Unexpectedly, Pax6 heterozygous embryos have a delay in neurogenesis relative to wildtype (Fig. 3I). This delay was less dramatic than the accelerated differentiation schedule in homozygous mutants. This type of heterozygotic genetic effect is unique, to our knowledge, but may identify a complex regulatory mechanism potentially present in other developmental pathways. Future experiments to determine the cellular and genetic basis for the heterozygous delay in neurogenesis may provide insight into the human Pax6 heterozygous condition of Aniridia.
An early role for Pax6 in specification of retinal neuron fate
The precocious neurogenesis in Pax6 mutants offers a test of the role of timing in determining the differentiation potential of retinal progenitors. Several general and retinal neuron type-specific markers were expressed in precocious neurons, supporting the identification of these βIII-tub+ cells as neurons (Fig. 7). However, these cells lacked any of several markers for more specific neuronal cell types. The precocious neurons did express a subset of RGC markers, including doublecortin, NF160, Dlx, and Islet1, and this combination is seen only in RGC cells in the mature retina. However, none of these are definitive for RGC differentiation, and the key determinant Brn3b was not expressed, correlating with the lack of its transcriptional activator Math5.
These results demonstrate that, in the absence of Pax6, retinal progenitor cells are competent to differentiate, actually significantly earlier than in wildtype, yet fail to activate definitive retinal cell differentiation programs, at least for the cell types tested. It is possible that the partial set of RGC markers signifies aborted RGC specification. This would imply that, in the absence of the Math5/Brn3b determination factors, an alternative pathway can lead to neuron differentiation, but results in incomplete or errant cell type determination. Interestingly, each of the individual markers expressed by the Pax6−/− precocious neurons is normally expressed by a variety of non-retinal neurons. Potentially, these Pax6 mutant progenitors are exhibiting a shift in regional identity to forebrain or elsewhere. However, the retention of retinal identity in Pax6 mutant optic vesicles is well documented (Bernier et al., 2001; Jean et al., 1999; Zhang et al., 2000). The implication is that Pax6 acts to couple general neuron-inducing signals to specific retinal neuron differentiation programs.
Pax6 functions shift as retinal development proceeds
Retinal progenitor cells express Pax6, both early in optic vesicle formation and later in proliferative regions of the neural retina. This continuity of Pax6 expression, however, conceals a dramatic shift in Pax6 function at different stages of development. This shift in Pax6 function is clear when our results are compared with those of Marquardt et al. (2001). In their study, a conditional inactivation strategy used a Cre-lox allele to remove Pax6 function from RPCs in peripheral retina beginning about E10.5. Such embryos therefore retain Pax6 expression throughout the optic vesicle during early development, and lose Pax6 only in the peripheral retina. This conditional inactivation causes exclusive differentiation of amacrine neurons, at the expense of all other retinal cell types. The basis for this appears to be the activation of the proneural factor NeuroD that channels cell differentiation to an amacrine or rod fate (Morrow et al., 1999). In contrast, our study of completely mutant optic vesicles identified differentiating neurons but a clear lack of amacrine cell differentiation, and expression of the proneural factor Mash1 instead of NeuroD. One possible explanation for the failure of amacrine markers is the requirement for co-expression of NeuroD with either Pax6 or Six3, to promote amacrine cell genesis (Inoue et al., 2002). Our work argues towards a Pax6-dependent step in amacrine cell determination by pointing out that in the Pax6floxΔ RPCs, Six3 expression promotes amacrine fates in combination with NeuroD.
The most likely path to reconciling our results with those of Marquardt et al. (2001) lies in considering the possibility that Pax6 function changes as development proceeds. The main differences in experimental strategy are differences in developmental timing (null Pax6 allele vs. conditional deletion around E10.5) and location in retina (an entirely Pax6−/− optic vesicle vs. Pax6floxΔperipheral progenitors surrounding a Pax6+ central retina). The difference in location may be important, though both the central and distal retina progenitor populations normally express Pax6. Related to this is the proportion of optic cup cells that are mutant. For instance, in the Pax6floxΔexperiment, the number of cells in the distal optic cup that lacked Pax6 function was small enough that partial non-autonomous rescue might occur. In partial support of this idea, Pax6floxΔ/floxΔ patches appear to proliferate (though slower) and differentiate, but only adopt an amacrine fate. This suggests that the loss of Pax6 can be partially overcome.
Another potential difference between this study and that of Marquardt et al. were the different Pax6 alleles used. In this study, we used the Sey1Neu allele, a spontaneous point mutation causing a splicing defect and predicted premature truncation in the carboxy terminal third of the protein (Hill et al., 1991). Several lines of evidence indicate that this allele is null, including heterozygous eye phenotypes identical to whole scale deletions of Pax6 (Hill et al., 1991), phenotypes identical to seven other truncating point mutations (Favor et al., 2001), and null activity in cell transfection assays (Glaser et al., 1994). Nonetheless, it remains possible that, in Sey1Neu mutants, some partial genetic activity may remain to influence RPC differentiation. In contrast, the Cre-lox approach of Marquardt et al. resulted in a prolonged and full dose of Pax6 in RPCs, only gradually lost following a sequence of α-Cre expression, subsequent Cre activity, lox-mediated deletion, and eventual decay of Pax6 mRNA and protein. Additionally, the central retina and lens retained Pax6 expression, while the efficiency of excision in peripheral retina is not 100%, raising the potential for signals from Pax6-expressing tissues to influence neuron differentiation in Pax6floxΔ mutant cells. Further experiments will be required to reconcile how these alleles result in phenotype differences, though both approaches lead to the conclusion that Pax6 mutant RPCs are competent for neuron differentiation.
During normal retinal development, progenitors produce a sequence of cell types, suggesting shifting regulatory contexts, that is, the varying presence of factors that change the potential of progenitor cells. In this light, the role of Pax6 may allow (or drive) progenitor progress through competence states. The neuronal subtype adopted in the absence of Pax6 would therefore depend on a progenitor’s competence state at the time of Pax6 removal. For example, at the time when Pax6 function was removed in the Pax6floxΔ experiments, progenitors became shunted to amacrine fates but if Pax6 were selectively removed later, the bdefaultQ might be a later cell type, such as a rod or bipolar neuron. The identity of key factors is currently unknown, but the set of retinal identity genes (Rx, Otx2, Six3, Six6, Lhx2) are good candidates, as they are expressed in the early optic vesicle, appear to be Pax6-independent, and may have activity that modulates or drives neuron specification. Therefore, comparison of gene expression patterns in early and late optic vesicles may allow the identification of key developmentally regulated factors that interact with Pax6 to regulate neuron differentiation and specification.
Acknowledgments
GSM would like to acknowledge Celeste Malinoski (U. Michigan, Ann Arbor) for preparing the initial slides that identified precocious neurons in Pax6 mutants. The Dlx antibody was a generous gift from G. Boekhoff-Falk (U. Wisconsin, Madison); Brn3a antibody was a gift from E. Turner (UC, San Diego); the Mash1 expression plasmid was a gift from J. Johnson (UT Southwestern). We thank Chris von Bartheld (U. Nevada, Reno) for advice on cell counting, Gracie Andrews and Amy Altick (U. Nevada, Reno) for advice on antibody labeling, Tien Le for technical support, and Bill Goossens for assistance with imaging. We thank Steve Easter and Kenny Campbell for critical reading of this manuscript, and Tom Glaser for helpful discussions. Supported by NIH grants R01 HD38069 (GSM) and R01 EY13612 (NLB); undergraduate fellowships from the Nevada Biomedical Research Infrastructure Network (CNS), and undergraduate research grants from the University of Nevada VP for Research (GTP and CNS).
References
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. doi: 10.1242/dev.124.6.1119. [DOI] [PubMed] [Google Scholar]
- Andrews GL, Yun K, Rubenstein JL, Mastick GS. Dlx transcription factors regulate differentiation of dopaminergic neurons of the ventral thalamus. Mol. Cell. Neurosci. 2003;23:107–120. doi: 10.1016/s1044-7431(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Naegele JR, Barnstable CJ. Molecular markers of neuronal subpopulations in layers 4, 5, and 6 of cat primary visual cortex. J. Neurosci. 1987;7:1250–1263. doi: 10.1523/JNEUROSCI.07-04-01250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P. Pax6 is required for establishing nasotemporal and dorsal characteristics of the optic vesicle. Development. 2002;129:4535–4545. doi: 10.1242/dev.129.19.4535. [DOI] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Spieler D, Treichel D, Ashery-Padan R, Gruss P. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development. 2003;130:2903–2915. doi: 10.1242/dev.00450. [DOI] [PubMed] [Google Scholar]
- Bernier G, Vukovich W, Neidhardt L, Herrmann BG, Gruss P. Isolation and characterization of a downstream target of Pax6 in the mammalian retinal primordium. Development. 2001;128:3987–3994. doi: 10.1242/dev.128.20.3987. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr. Opin. Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J. Comp. Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J. Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Favor J, Peters H, Hermann T, Schmahl W, Chatterjee B, Neuhauser-Klaus A, Sandulache R. Molecular characterization of Pax6(2Neu) through Pax6(10Neu): an extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus . Genetics. 2001;159:1689–1700. doi: 10.1093/genetics/159.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech. Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J. Neurosci. 1997;17:7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc. Natl. Acad. Sci. U. S .A. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat. Genet. 1992;2:232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Joyner AL. Dynamic expression of the murine Achaete–Scute homologue Mash-1 in the developing nervous system. Mech. Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila . Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Macdonald RE, VanDeRyt JT, Wilson SW. Antibodies against Pax6 immunostain amacrine and ganglion cells and neuronal progenitors, but not rod precursors, in the normal and regenerating retina of the goldfish. J. Neurobiol. 1996;29:399–413. doi: 10.1002/(SICI)1097-4695(199603)29:3<399::AID-NEU10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J. Embryol. Exp. Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Reh TA. Temporal and spatial pattern of MASH-1 expression in the developing rat retina demonstrates progenitor cell heterogeneity. J. Comp. Neurol. 1996;369:319–327. doi: 10.1002/(SICI)1096-9861(19960527)369:2<319::AID-CNE11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/ hypothalamic axis and the ventral optic stalk. Mech. Dev. 1999;84:31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr. Opin. Genet. Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Liu W, Khare SL, Liang X, Peters MA, Liu X, Cepko CL, Xiang M. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Andrews GL. Pax6 regulates the identity of embryonic diencephalic neurons. Mol. Cell. Neurosci. 2001;17:190–207. doi: 10.1006/mcne.2000.0924. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Easter SS., Jr Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev. Biol. 1996;173:79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Davis NM, Andrew GL, Easter SS., Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997;124:1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Mizutani Y, Ohmori Y, Okumura J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem. Biophys. Res. Commun. 1997;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Lewis SE, Dahl D, Marotta CA, Drager UC. Early posttranslational modifications of the three neurofilament subunits in mouse retinal ganglion cells: neuronal sites and time course in relation to subunit polymerization and axonal transport. Brain Res. Mol. Brain Res. 1989;5:93–108. doi: 10.1016/0169-328x(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–2446. doi: 10.1242/dev.129.10.2435. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Sebring A, Nagy L, Carroll S. The development of crustacean limbs and the evolution of arthropods. Science. 1995;270:1363–1366. doi: 10.1126/science.270.5240.1363. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Gruss P, Westerfield M. Sequence and expression pattern of pax-6 are highly conserved between zebrafish and mice. Development. 1992;114:643–651. doi: 10.1242/dev.114.3.643. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA. Spatiotemporal features of early neurono-genesis differ in wild-type and albino mouse retina. J. Neurosci. 2002;22:4249–4263. doi: 10.1523/JNEUROSCI.22-11-04249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 2003;130:3269–3281. doi: 10.1242/dev.00539. [DOI] [PubMed] [Google Scholar]
- Sundin OH, Eichele G. A homeo domain protein reveals the metameric nature of the developing chick hindbrain. Genes Dev. 1990;4:1267–1276. doi: 10.1101/gad.4.8.1267. [DOI] [PubMed] [Google Scholar]
- Swartz M, Eberhart J, Mastick GS, Krull CE. Sparking new frontiers: using in vivo electroporation for genetic manipulations. Dev. Biol. 2001;233:13–21. doi: 10.1006/dbio.2001.0181. [DOI] [PubMed] [Google Scholar]
- Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996a;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Tomita K, Nakanishi S, Guillemot F, Kageyama R. Mash1 promotes neuronal differentiation in the retina. Genes Cells. 1996b;1:765–774. doi: 10.1111/j.1365-2443.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- Trieu M, Ma A, Eng SR, Fedtsova N, Turner EE. Direct autoregulation and gene dosage compensation by POU-domain transcription factor Brn3a. Development. 2003;130:111–121. doi: 10.1242/dev.00194. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Vetter ML, Brown NL. The role of basic helix-loop-helix genes in vertebrate retinogenesis. Semin. Cell Dev. Biol. 2001;12:491–498. doi: 10.1006/scdb.2001.0273. [DOI] [PubMed] [Google Scholar]
- Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren N, Price DJ. Roles of Pax-6 in murine diencephalic development. Development. 1997;124:1573–1582. doi: 10.1242/dev.124.8.1573. [DOI] [PubMed] [Google Scholar]
- Warren N, Caric D, Pratt T, Clausen JA, Asavaritikrai P, Mason JO, Hill RE, Price DJ. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb. Cortex. 1999;9:627–635. doi: 10.1093/cercor/9.6.627. [DOI] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993;11:689–701. doi: 10.1016/0896-6273(93)90079-7. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat. Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, De Angelis MH, Weinmaster G, Rubenstein JL. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 2002;129:5029–5040. doi: 10.1242/dev.129.21.5029. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–142. [PubMed] [Google Scholar]