Incidence data regarding late hematoma following breast augmentation do not exist, nor has its etiology been elucidated. Hematomas have been reported to develop months to decades after augmentation with various types of implants, even in the absence of trauma. This study reviewed the occurrence of late hematoma in five patients who received smooth, round silicone gel implants in a single-surgeon practice over a 30-year period. All patients presented with progressive enlargement of the involved breast, which appeared to result from multiple and recurrent bouts of bleeding rather than from an isolated event. Histological analysis of capsules provided insight into the pathogenesis of this rare phenomenon.
Keywords: Breast implants, Complications, Late hematoma
Abstract
BACKGROUND:
Late hematoma after breast augmentation is a rare phenomenon with unproven etiology.
METHODS:
Five patients presented with a late unilateral hematoma after receiving bilateral, submuscular, smooth, round, silicone gel implants for cosmetic breast augmentation. All patients underwent explantation and capsulectomy. Extensive clinical and histological analysis of all capsules was performed.
RESULTS:
All patients presented with progressive unilateral breast enlargement with at least a doubling of their breast size. Breast enlargement developed nine, 12, 14, 22 and 38 years, respectively, after initial implant insertion and in the absence of any known trauma. Patients presented for treatment one, three, four, nine and 12 months, respectively, after initially noticing breast enlargement. This enabled a sequential analysis of the events occurring on the surface of the capsule and within the structure of the capsule. Macroscopic and microscopic analysis of all capsules demonstrated multiple areas of recent and older hemorrhage, both within the structure of capsules and on the surface of the capsule. Continuing recurrent bouts of acute hemorrhage were observed even nine and 12 months after initial hematoma development. The position of medium-size vessels within the capsules corresponded to the sites of recurrent bleeding within capsules.
CONCLUSIONS:
Late hematomas presented from nine to 38 years after submuscular breast augmentation with a doubling of breast size. Late hematomas appeared to result from multiple recurrent bouts of bleeding within the structure of the capsules. This leads to recurrent bleeding on the surface of the capsules. Breast enlargement develops progressively over time rather than acutely. Continuing acute hemorrhage persisted up to one year after initial hematoma development. This progression suggests a process analogous to a chronic expanding hematoma that has been described in other areas of the body. Failure to remove a capsule could result in long-term continuing bleeding from vessels within the capsule.
Abstract
HISTORIQUE :
L’apparition d’un hématome tardif après une augmentation mammaire est un phénomène rare dont l’étiologie n’est pas démontrée.
MÉTHODOLOGIE :
Cinq patientes ont consulté à cause d’un hématome unilatéral tardif consécutif à l’installation d’implants mammaires bilatéraux en gel de silicone à surface lisse et de forme ronde en position rétromusculaire en vue d’une augmentation mammaire esthétique. Toutes les patientes ont subi une explantation et une capsulectomie. Les chercheurs ont effectué une analyse clinique et histologique approfondie de toutes les capsules.
RÉSULTATS :
Toutes les patients présentaient une hypertrophie mammaire unilatérale progressive ayant au moins doublé la taille de leur sein. Cette hypertrophie s’est manifesté neuf, 12, 14, 22 et 38 ans, respectivement, après l’insertion initiale des implants, en l’absence de tout traumatisme connu. Les patientes ont consulté pour être traitées un, trois, quatre, neuf, et 12 mois, respectivement, après avoir remarqué l’hypertrophie. Il a donc été possible de procéder à une analyse séquentielle des événéments se produisant à la surface et dans la structure de la capsule. L’analyse macroscopique et microscopique de toutes les capsules a démontré de nombreuses zones d’hémorragies récentes et anciennes, à la fois dans la structure et à la surface des capsules. Des accès récurrents d’hémorragie aiguë ont même continué de se produire neuf et 12 mois après l’apparition de l’hématome. La position de vaisseaux de taille moyenne dans les capsules correspondait aux foyers de saignements récurrents s’y produisant.
CONCLUSIONS :
Les hématomes tardifs se sont manifestés de neuf à 38 ans après une augmentation mammaire en position rétromusculaire et ont doublé la taille du sein. Ils semblaient découler de multiples accès récurrents de saignements dans la structure des capsules. L’hypertrophie mammaire s’est produite progressivement plutôt que de manière subite. Les hémorragies aiguës ont persisté jusqu’à un an après l’apparition initiale de l’hématome. Cette progression laisse supposer un processus analogue à l’expansion chronique des hématomes décrite dans d’autres parties du corps. Si on n’extrait pas la capsule, les saignements risquent de s’y poursuivre à long terme.
Late hematoma after breast implants is a rare phenomenon. Most studies in the literature are in the form of individual case reports (1–7); no data regarding the actual incidence of this phenomenon exists. It has been reported with smooth and textured silicone gel implants, saline implants and polyurethane-covered silicone gel implants. It has occurred after breast augmentation and breast reconstruction, and usually develops two to 10 years after implant insertion and in the absence of any known trauma. The exact etiology of hematoma development has not been elucidated.
The present study reports on five patients who presented with a late unilateral hematoma after receiving bilateral, submuscular, silicone gel implants for cosmetic breast augmentation. Careful analysis of the capsule histology in these patients provides further insight to the etiology and pathogenesis of this phenomenon.
METHODS
A review of a single-surgeon practice over a 30-year period demonstrated the occurrence of a late hematoma in five patients. Four patients had their initial augmentation performed by the senior author, and the other received her implants in another city. All capsules were carefully evaluated by a designated soft-tissue pathologist. Careful histological examination of the capsules provided an analysis of the sequential events occurring on the surface of the capsules and within their structure.
RESULTS
All patients had received smooth, round, silicone gel implants that were inserted in the submuscular plane. Patients 1 and 3 to 5 had received Surgitek (Bristol-Myers, USA) silicone gel implants (Table 1). Patient 2 had received Dow Corning (Dow Corning, USA) implants. Implant sizes ranged from 180 mL to 275 mL. In cases 1, 3 and 4, the implants were intact, and ruptured in patients 2 and 5. All five patients presented with a progressive unilateral breast enlargement with at least a doubling of their breast size (Figure 1). There was no known history of trauma, anticoagulation or steroid use. All five patients presented with a Baker IV contracture in the involved breast (8). This firmness was likely due to the expanding hematoma volume, rather than to an isolated capsular contracture. Patients initially developed their unilateral hematomas nine, 12, 14, 22 and 38 years after receiving implants (Table 1). Patients presented one, three, three, four and 12 months after they initially recognized breast enlargement. Careful histological examination of the capsules provided an analysis of the sequential events occurring on the surface of the capsules and within their structure.
TABLE 1.
Late hematomas after breast augmentation with smooth round silicone gel implants
| Patient | Implant (size) | Onset, years | Duration, months |
|---|---|---|---|
| 1 | Surgitek* (220 mL, intact) | 9 | 1 |
| 2 | Dow Corning (275 mL, ruptured) | 38 | 3 |
| 3 | Surgitek (180 mL, intact) | 14 | 4 |
| 4 | Surgitek (220 mL, intact) | 12 | 12 |
| 5 | Surgitek (270 mL, ruptured) | 22 | 3 |
Bristol-Myers, USA
Figure 1).
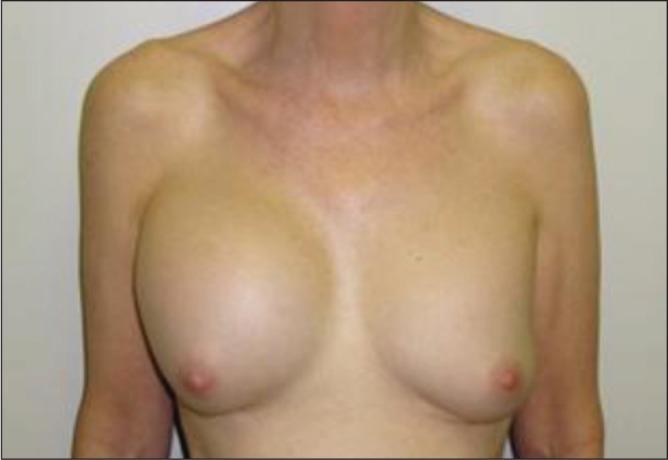
Patient 2. The patient is shown three months after the onset of progressive right breast enlargement. Her original surgery was performed 38 years previously. The size of her right breast had progressively more than doubled over the preceding three months. All five patients in the present study presented with a similar major enlargement of their breast size
At exploration, all implant pockets demonstrated a sudden gush of watery, chocolate-brown fluid under pressure, suggesting a liquefied hematoma. All patients also had evidence of a semisolid blood clot. Clinically, this had the appearance of granulation tissue on the surface of the capsules. All pockets were irrigated with bacitracin saline solution and a capsulectomy was performed. Capsule specimens were sent for aerobic and anaerobic bacterial and fungal cultures. A designated pathologist carefully evaluated all capsules.
Macroscopic examination of the external surface of all capsules demonstrated multiple patchy areas of recent (bright red) and older (dark red) hemorrhage (Figure 2). This suggested that bleeding on the surface of the capsule was recurrent in nature, rather than occurring as an isolated event.
Figure 2).
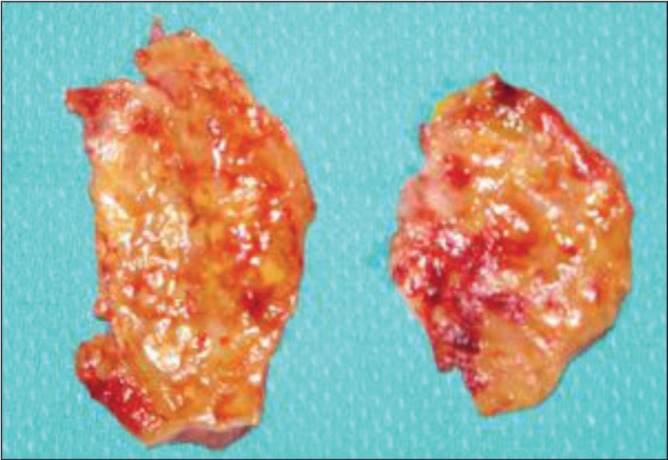
Patient 2. The hematoma had been present for three months. Macroscopic appearance of the capsule showed multiple patchy areas of recent (bright red) and older hemorrhage (dark red). This suggested that bleeding was recurrent in nature, rather than as an isolated event
Histological analysis of the capsules demonstrated a progression of findings over time. In patient 1, the hematoma had been present for only one month. Histopathology, using WHO stain (hematoxylin, phloxin, saffron and alcian green), showed that the surface of the capsule was covered by bright red areas of recent bleeding and paler areas of organizing hematoma (Figure 3). Within the capsule, there were several large engorged vessels. One of these vessels may have undergone erosion and bleeding to cause the hematoma. Within other areas of the capsule, numerous areas of hemorrhage were present, indicating multiple episodes of recurrent bleeding (Figure 4).
Figure 3).
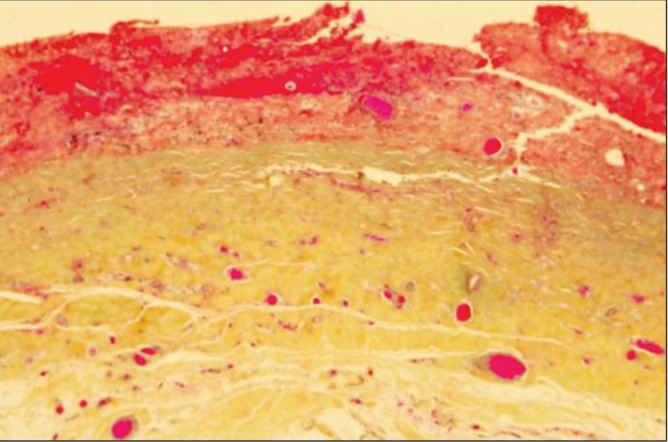
Patient 1. The hematoma had been present for one month. WHO stain of the capsule showed bright red areas on the surface of the capsule representing recent hematoma. The paler areas represent older organizing hematoma. Within the capsule, there are many large engorged vessels. One of these vessels may have undergone erosion and bleeding, causing the hematoma (original magnification ×12.5)
Figure 4).
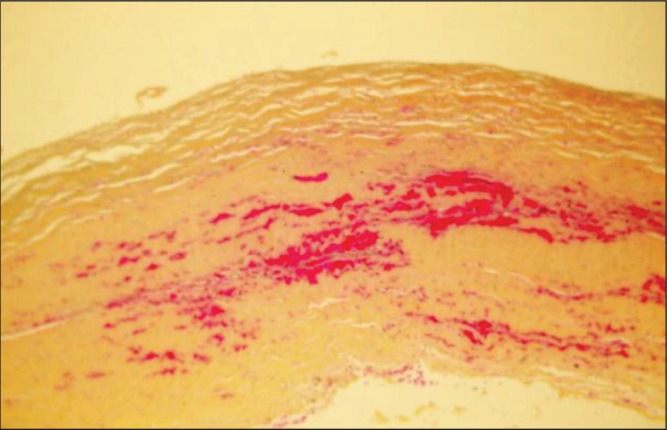
Patient 1. WHO stain of other areas of the capsule shown in Figure 3. There are numerous areas of hemorrhage within the capsule indicating multiple episodes of recurrent bleeding (original magnification ×50)
In patient 2, the hematoma had been present for three months. Hematoxylin and eosin stain of the capsule showed multiple areas of recurrent acute hemorrhage (Figure 5). In patient 3, the hematoma had been present for four months. WHO stain of the surface of the capsule showed hematoma at different stages of organization (Figure 6). The bright red areas on the surface of the capsule indicate areas of recent recurrent bleeding. The darker areas indicate areas of older bleeding. With the capsule, there were numerous bright red areas showing multiple areas of recent recurrent bleeding.
Figure 5).
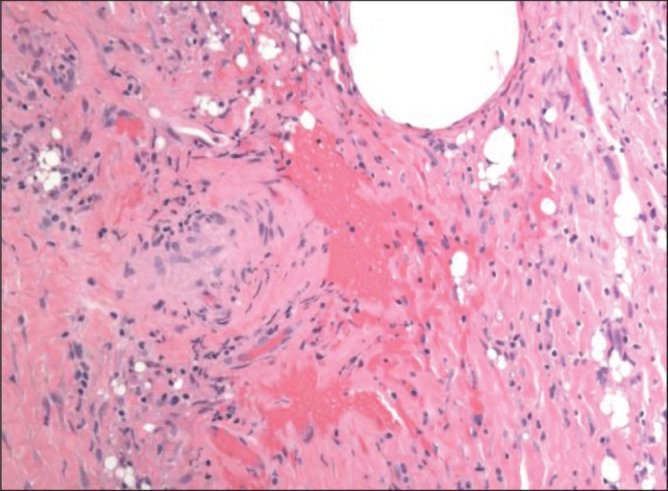
Patient 2. The hematoma had been present for three months. Hematoxylin and eosin stain of the capsule showing multiple areas of recent acute hemorrhage (original magnification ×200)
Figure 6).
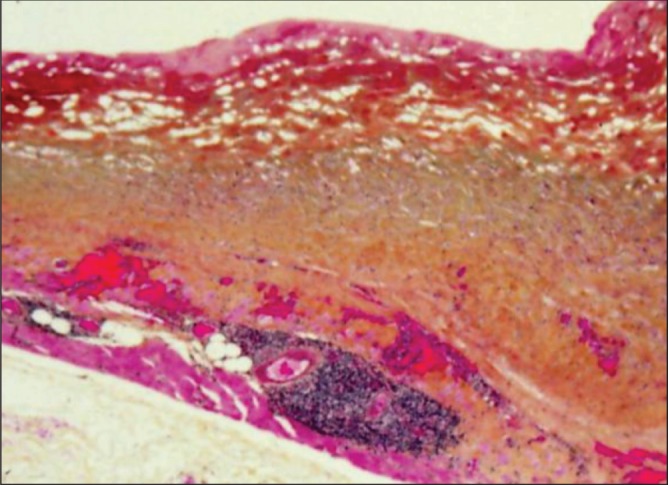
Patient 3. The hematoma had been present for four months. WHO stain showing organizing early (bight red) and late (dark red) hematoma on the surface of the capsule. Within the capsule, there were multiple areas of recurrent bleeding (with aggregates of lymphocytes) (original magnification ×12.5)
In patient 4, the hematoma had been present for one year. The capsule was 5 mm to 6 mm thick compared with 1 mm to 2 mm in the contralateral breast. Clinically, the surface of the capsule was covered with a layer of granulation tissue. Within the capsule, there were extensive areas of chronic inflammation surrounding numerous areas of old hemorrhage (with hemosiderin pigment), with areas of more recent organizing hematoma (Figure 7). These findings extended through the inner one-half of the thickness of the capsule.
Figure 7).
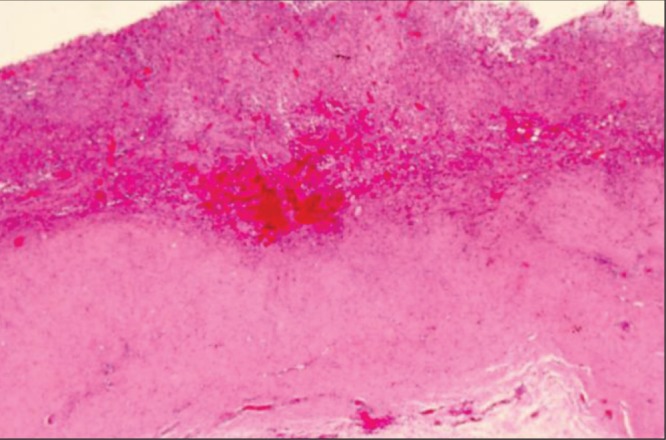
Patient 4. The hematoma had been present for one year. The capsule was 6 mm to 7 mm thick, compared with 1 mm to 2 mm on the contralateral side. Hematoxylin and eosin stain of the capsule showing extensive areas of chronic inflammation surrounding areas of organizing hematoma extending through the inner one-half of the thickness of the capsule (original magnification ×12.5)
Aerobic and anaerobic bacterial and fungal cultures or the capsules were negative except for patient 3, whose culture showed a coagulase-negative Staphylococcus species. This was not treated with antibiotics because it was likely an insignificant colonizing organism. All five patients in the present study requested removal of both of their implants without subsequent implant insertion. Patients 1, 3, 4 and 5 were followed-up for three years; patient 2 was followed-up for one year. All patients remained symptom free over these time periods.
DISCUSSION
In 1979, Georgiade et al (1) first reported on a late hematoma developing around a breast implant. It developed 2.5 years after breast augmentation with a saline-filled prosthesis containing 40 mg of triamcinolone acetonide. At exploration, a medium-size capsular artery was found to be eroded and actively bleeding. The authors suggested that the large dose of corticosteroid was responsible for this erosion.
Over the years, other authors have sporadically reported on late hematomas that had developed around various types of breast implants. In 2004, Brickman et al (2) described a case of late hematoma that appeared nine years after breast augmentation with polyurethane-covered silicone gel implants. These investigators suggested that these hematomas were caused by the intense, highly vascular inflammatory response that was induced by the polyurethane coating. In 2005, Veiga et al (3) described a late hematoma that developed one year after insertion of subglandular, textured, silicone gel implants. They suggested that friction between the rough surface of the textured implant and the fibrous capsule may have contributed to erosion of a small vessel. In 2007, Peters and Fornasier (4) described three patients who developed hematomas nine, 12 and 14 years, respectively, after receiving submuscular smooth silicone gel implants. In 2011, Ibrahim and Atiyeh (5) described a late capsular hematoma that had developed in a patient with a bleeding disorder.
In 2013, Grippaudo et al (6) described a patient who developed a hematoma two years after breast reconstruction with Biocell textured implants (Naturelle; Allergan Inc, USA) (6). This case was unique because a double capsule was present, with a hematoma between the two layers of the capsule. These authors suggested that mechanical friction and subsequent “implant degloving” between the textured implant and the inner capsule could result in bleeding.
Analysis of the five cases in the current study additional light on the etiology and pathogenesis of late hematomas after breast augmentation. These hematomas all developed many years after implant insertion (nine, 12, 14, 22 and 38 years). All patients presented with a progressive enlargement of their involved breast. The development of hematomas appeared to result from multiple and recurrent bouts of bleeding rather than from an isolated event. The macroscopic appearance of the surface of the capsules showed multiple areas of recent and old hemorrhage (Figure 2). The microscopic appearance of the capsules also showed multiple areas of recurrent bleeding both within the structure of the capsules and on the surface of the capsules (Figures 3 to 7). Medium-size arteries were demonstrated within the structure of the capsules (Figure 3) and the position of these vessels corresponded to the sites of recurrent bleeding within the capsules. Goyal and Mansel (7) suggested that the chronic bleeding in these patients was related to microfractures in the periprosthetic capsule. The rigidity of the capsule could prevent retraction of a damaged vessel, allowing additional bleeding episodes. This hypothesis may help to explain the recurrent episodes of bleeding that occurred in all patients in the current study.
Erosion of a medium-size vessel within the capsule could result from various causes, including normal wear and tear from friction of the implant against the capsule; capsular contracture; the force of capsular contracture against a ‘knuckle’ of an otherwise intact implant (such as in patients 1, 3 and 4); friction between the capsule and the rough surface of a textured implant; an intense, highly vascular, inflammatory response, particularly with polyurethane-coated implants; and trauma, which would increase existing friction between the implant and the capsule.
The hematomas in these five cases appeared to be examples of ‘chronic expanding hematomas’. This process was originally described by Reid et al (9). It is well recognized in the neurosurgical literature. More recently, it has also been demonstrated in four patients 30 years after thoracic surgery and in other patients involved other areas of their musculoskeletal system (10). The self-perpetuating nature of the condition appears to result from irritant effects of blood and its breakdown products, which cause repeated exudation and bleeding from capillaries in the granulation tissue.
All of the patients in the present study underwent a capsulectomy with a careful histological assessment of their capsules. This is important to rule out any other pathological findings in these patients. The differential diagnosis of a late progressive enlargement after breast augmentation is extensive. Some of these diagnoses are relatively implant specific. The list includes the following:
Late seroma: In these patients, there is no actual bleeding. Breast enlargement is due to seroma formation. This condition is seen primarily with aggressively textured Biocell implants (11,12). Hall-Findlay (11) has suggested that the initial adherence of the capsule to the textured implant could become separated. Subsequent shear forces between the two rough surfaces could create a seroma. This finding has also been observed with polyurethane implants. There can be a small seroma with a Siltex (Mentor, USA) textured implant, but it is not seen with smooth-walled implants.
Late infection: This is very rare and cases are largely anecdotal (4). Early infections are usually caused by endogenous bacteria from the skin or from the milk ducts. In contrast, the pathogenesis of a late infection is believed to involve a bacteremia with seeding of the implant or capsule from a distant site (13,14). A bacteremia has been postulated to occur in association with dental procedures, upper respiratory tract infections and urinary tract infections. The incidence of a transient bacteremia ranges from 5% for colonoscopy to 88% for periodontal surgery. This bacteremia could result in a periprosthetic infection.
Malignancy: Breast cancer is a common disease. One in eight women will ultimately be diagnosed with primary breast cancer. Fewer than 0.5% of these cancers are non-Hodgkins lymphoma. Recently, 42 cases of non-Hodgkin’s lymphoma of the breast have been reported in association with breast implants (15). Most of these have been T cell in origin; 35 were anaplastic large-cell lymphoma. More recently, a subtype of anaplastic large-cell lymphoma has been described in which malignant cells are confined to a seroma within the capsular pocket.
Autoinflation: This is a rare phenomenon that occurs with saline-filled implants and double-lumen implants with saline in the outer lumen. Most studies are anecdotal. In 1997, Robinson and Benos (16) described five patients who developed unilateral inflation four to nine years after receiving saline implants. In 2006, Peters (17) described three patients who developed a 50% to 88% unilateral autoinflation four, 10 and 23 years after receiving saline-filled implants. The two most likely mechanisms for autoinflation are an initial hypertonic filling solution or alterations in the valve mechanism, which could allow certain molecules to enter the implant. This could create an osmotic gradient allowing water to enter by diffusion. The finding of elevated levels of uric acid and glucose in some patients would be consistent with this mechanism.
CONCLUSIONS
The results of the present study indicate that late unilateral hematomas can occur from nine to 38 years after breast augmentation. Breast enlargement is progressive rather than acute. Histological analyses of the implant capsules provided a sequential analysis of events occurring within the structure of the capsule and on the surface of the capsule. Multiple areas of recent and older hemorrhage occurred both within the structure of the capsules and on their surface. The position of medium-size vessels within the capsules corresponded to the sites of recurrent bleeding within the capsules. Continuing acute bouts of hemorrhage persisted even up to one year after initial hematoma development. The progressive nature of hematoma development in these patients was similar to that of chronic expanding hematomas that have been shown to occur in other areas of the body.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare. All authors participated in the research, and have reviewed and agree with the content of the article.
REFERENCES
- 1.Georgiade NG, Serafin D, Barwick W. Late development of hematoma around a breast implant, necessitating removal. Plast Reconstr Surg. 1979;64:708–10. [PubMed] [Google Scholar]
- 2.Brickman M, Parsa NN, Parsa FD. Late hematoma after breast implantation. Aesthetic Plast Surg. 2004;28:80–2. doi: 10.1007/s00266-004-3120-8. [DOI] [PubMed] [Google Scholar]
- 3.Veiga DF, Filho JV, Schaider CS, et al. Late hematoma after aesthetic breast augmentation with textured silicone prosthesis: A case report. Aesthetic Plast Surg. 2005;29:431–3. doi: 10.1007/s00266-004-0098-1. [DOI] [PubMed] [Google Scholar]
- 4.Peters W, Fornasier V. Late unilateral breast enlargement after insertion of silicone gel implants. Can J Plast Surg. 2007;15:19–28. doi: 10.1177/229255030701500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim AE, Atiyeh BS. Management of delayed capsular hematoma after breast reconstruction. Aesth Plast Surg. 2011;35:923–7. doi: 10.1007/s00266-011-9685-0. [DOI] [PubMed] [Google Scholar]
- 6.Grippaudo FR, Renzi L, Costantino B, et al. Late unilateral hematoma after breast reconstruction with implants: Case report and literature review. Aesthetic Surg. 2013;33:830–4. doi: 10.1177/1090820X13496249. [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Mansel RE. Hematoma as a late complication after breast reconstruction with implant. Br J Plast Surg. 2003;56:189–91. doi: 10.1016/s0007-1226(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 8.Little G, Baker JL. Results of closed compression capsulotomy for treatment of contracted breast implant capsules. Plast Reconstr Surg. 1980;65:30–3. doi: 10.1097/00006534-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Reid JD, Kommareddi S, Lankerani M, et al. Chronic expanding hematomas: A clinicopathologic entity. JAMA. 1980;244:441–2. [PubMed] [Google Scholar]
- 10.Uramoto H, Nakanishi R, Eifuku R, et al. Chronic expanding hematoma in the chest. J Cardiovasc Surg. 2000;41:143–6. [PubMed] [Google Scholar]
- 11.Hall-Findlay EJ. Breast implant complication review: Double capsules and late seromas. Plast Reconstr Surg. 2011;1:56–66. doi: 10.1097/PRS.0b013e3181fad34d. 127. [DOI] [PubMed] [Google Scholar]
- 12.Spear SL, Rottman SJ, Glicksman C, Brown M, Al-Attar A. Late seromas after breast implants: Theory and practice. Plast Reconstr Surg. 2012;130:423–35. doi: 10.1097/PRS.0b013e3182589ea9. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbogen R. Breast implant encapsulation in association with dental work. Plast Reconstr Surg. 1986;78:541. doi: 10.1097/00006534-198610000-00029. (Lett) [DOI] [PubMed] [Google Scholar]
- 14.Rigg BM. Breast encapsulation following a minor distant infection. Plast Reconstr Surg. 1987;79:505. doi: 10.1097/00006534-198703000-00072. (Lett) [DOI] [PubMed] [Google Scholar]
- 15.Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: Five Australian cases. Plast Reconstr Surg. 2012;129:610e–617e. doi: 10.1097/PRS.0b013e3182450aae. [DOI] [PubMed] [Google Scholar]
- 16.Robinson OF, Jr, Benos DJ. Spontanous autoinflation of saline mammary implants. Ann Plast Surg. 1997;39:114–8. doi: 10.1097/00000637-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Peters W. Autoinflation of saline-filled inflatable breast implants. Can J Plast Surg. 2006;14:219–26. doi: 10.1177/229255030601400403. [DOI] [PMC free article] [PubMed] [Google Scholar]


