The complex, three-dimensional structure of the normal ear makes auricular reconstruction a challenging undertaking. A congenitally small and/or malformed external ear is a condition associated with psychological morbidity and one in which surgical reconstruction provides significant relief. Although the autologous rib cartilage graft technique has gained wide acceptance since its introduction more than 50 years ago, other techniques have been devised and developed, in addition to classification systems to guide diagnosis and research. Contributors to this article, who are leaders in the field, comment on the advantages and disadvantages of each technique and describe their own methods.
Keywords: Auricular reconstruction, Autologous reconstruction, Ear, Microtia, Medpor implant, Prosthetic reconstruction
Abstract
Several surgical techniques have been described for auricular reconstruction. Autologous reconstruction using costal cartilage is the most widely accepted technique of microtia repair. However, other techniques have certain indications and should be discussed with patients and families when planning for an auricular reconstruction. In the present review, the authors discuss the main surgical techniques for auricular reconstruction including autologous costal cartilage graft, Medpor (Stryker, USA) implant and prosthetic reconstruction. To further elaborate on the advantages and disadvantages of each technique, the authors invited leaders in this field, Dr Nagata, Dr Park, Dr Reinisch and Dr Wilkes, to comment on their own technique and provide examples of their methods.
Abstract
Plusieurs techniques chirurgicales de reconstruction auriculaire ont déjà été décrites. La reconstruction autologue à l’aide de cartilage costal est la technique la plus acceptée pour la réparation des microties. Cependant, d’autres techniques sont parfois indiquées et devraient être proposées aux patients et à leur famille au moment de planifier une reconstruction auriculaire. Dans la présente analyse, les auteurs traitent des principales techniques chirurgicales de reconstruction auriculaire, y compris la greffe de cartilage costal autologue, l’implant Medpor (Stryker, États-Unis) et la reconstruction prosthétique. Pour traiter des avantages et inconvénients de chaque technique, les auteurs ont invité les docteurs Nagata, Park, Reinish et Wilkes, chefs de file dans ce domaine, à commenter leur propre technique et à donner des exemples de leurs méthodes.
Microtia describes a congenitally small and malformed external ear. The deformtiy occurs with a frequency of one in every 7000 to 8000 live births (1). The prevalence is significantly higher in Hispanics, Asians, Native Americans and Andeans. Males are affected more than females, and most cases of microtia are unilateral with a higher rate of occurrence on the right side. Environmental and genetic factors are important in the etiology of microtia (2). Microtia can cause psychological morbidity and surgical repair results in significant relief (3).
The normal external ear is a complex three-dimensional structure and, as such, reconstruction of the ear is a demanding undertaking. A new era in ear reconstruction began in 1959 when Tanzer (4) introduced his multistage autologous rib cartilage technique. Since then, modifications have been made to the original technique of Tanzer, mainly by Brent (5) and then Nagata (6), to improve esthetic results and decrease complication rates. The autologous rib cartilage graft has gained wide acceptance by surgeons; however, some other methods have been devised for auricular reconstruction including prosthetic and implant reconstruction, among others.
Classification systems have been developed to facilitate diagnosis, surgical repair and research studies of microtia. Hermann Marx described the first classification system in 1926, which was later modified by Meurman. Marx/Meurman classified microtia into four degrees based on the vestigial remnant. In grade I microtia, all of the structures are present but with variable degrees of hypoplasia of the auricle, with cupping and variable external auditory stenosis. In grade II, variable hypoplasia of the concha is often accompanied by the absence of the external auditory canal. Grade III is the classic ‘peanut ear’, in which the auricle is absent and the lobule has an abnormal shape and position. Grade IV, known as anotia, is the most severe from of microtia, which is characterized by the complete absence of external ear (7,8).
Nagata (6) defined five types of microtia based on the surgical technique for each deformity. In the anotia type, the external ear is completely absent. Lobule type includes a variably shaped remnant cartilaginous anlage and a vertically oriented lobule, with no acoustic meatus, concha or tragus. Large conchal type is characterized by the presence of lobule, concha (with or without acoustic meatus), tragus, and intertragal notch but with varying degrees of deformity of the upper pole of the auricle. The small conchal type is similar to the lobule type but with a small indentation in the region of the conchal bowl. Atypical type includes all of the deformities that do not fit in the other categories (7).
In the present article, we review the main surgical techniques for total auricular reconstruction. The significance of the review is the contribution of leaders in this field. They were each invited to contribute example cases and discuss the advantages and disadvantages of their own techniques.
AUTOLOGOUS COSTAL CARTILAGE GRAFT
Despite the advances in other methods, autologous costal cartilage graft remains the mainstream of ear reconstruction surgery. The acceptable aesthetic results and durability of the cartilaginous framework in long-term follow-up have contributed to the success of this method (9). The Tanzer (4), Walton and Beahm (9) and Brent (10) methods have provided the basis for current autologous reconstruction techniques. Historically, it has required three or four stages to create an ear. Current techniques have evolved to reduce the number of required stages.
Nagata technique
The Nagata technique for auricular reconstruction encompasses two stages. The first is performed no earlier than 10 years of age and after the chest circumference at the level of the xyphoid has grown to at least 60 cm. In the first stage, the lobule is split and transposed, and a three-dimensional (3D) costal cartilage framework is constructed and inserted in a subcutaneous pocket. At the second stage, the ear is projected with a second costal cartilage block, and the retroauricular sulcus is created and is covered with a fascial flap and skin graft.
First stage:
Nagata harvests the ipsilateral sixth to ninth costal cartilages to fabricate the framework. The sixth and seventh costal cartilages are used to construct the base frame. The ninth costal cartilage forms antihelix, superior and inferior crus. The helical rimhelix unit and crus helicis are constructed using the eighth costal cartilage. Conchal bowl elements are created from rib cartilage remnants. Fine-gauge wire sutures are used to assemble the constructed units and create the one-piece, 3D framework (11).
To avoid the complications associated with costal cartilage harvest for total auricular reconstruction, Kawanabe and Nagata (12) preserve the perichondrium at the donor site. After the fabrication of the 3D framework, the remaining cartilages are cut into 2 mm to 3 mm blocks and returned to the perichondrial pockets. In 273 cases, with a follow-up period ranging from six to 43 months, they reported one pneumothorax, one case of methicillin-resistant infection and no chest wall deformities (12). They have also confirmed the regeneration of the cartilage at the donor site. In four representative cases, regenerated cartilage was sampled during the second stage. The regenerated cartilage demonstrated gross and histological characteristics of normal cartilage. Hence, the regenerated cartilage can be used for secondary auricular reconstruction and bilateral cases, in which the availability of cartilage donor may be limited (13).
To cover his 3D cartilage framework, Nagata creates four skin flaps. The lobule is split to form an anterior and a posterior skin flap. The posterior lobule flap remains attached to the mastoid skin flap, which is thus increased in surface area. An anteriorly based tragal flap is used to surface the external surface of the tragus. Skin incisions defining the margins of the mastoid and posterior lobule flaps form a ‘lazy W’. The middle adjacent limbs of the ‘W’ will meet to form an inverted cone and the depth of the intertragal notch. The W-flap and anterior lobule flap will reciprocally transpose in a z-plasty fashion. Vascularity of the W flap is increased by maintaining a subcutaneous pedicle in the floor of the conchal bowl. The above described incisions provide access for excision of the rudimentary auricular cartilage and for creation of the subcutaneous pocket. The 3D framework is introduced into the subcutaneous pocket around the subcutaneous pedicle. The skin flaps are secured over the 3D framework with sutured bolsters, which are left in the place for two weeks (11).
Second stage:
At least six months after the first operation, the patient undergoes the second stage of surgery, which involves the elevation of reconstructed ear. An incision, made approximately 1 cm posterior to the helix, is used to elevate the framework. A crescent-shape wedge of autologous rib cartilage harvested from the fifth rib is placed under the ear to prevent repositioning of the framework. A temporoparietal fascia flap is raised and tunnelled subcutaneously toward the posterior aspect of the construct to provide coverage for the posterior surface of ear and cartilage graft and mastoid surface. The retroauricular skin is advanced anteriorly and a split-thickness skin graft is harvested from parietal-occipital scalp to cover the exposed flap (Figure 1).
Figure 1).
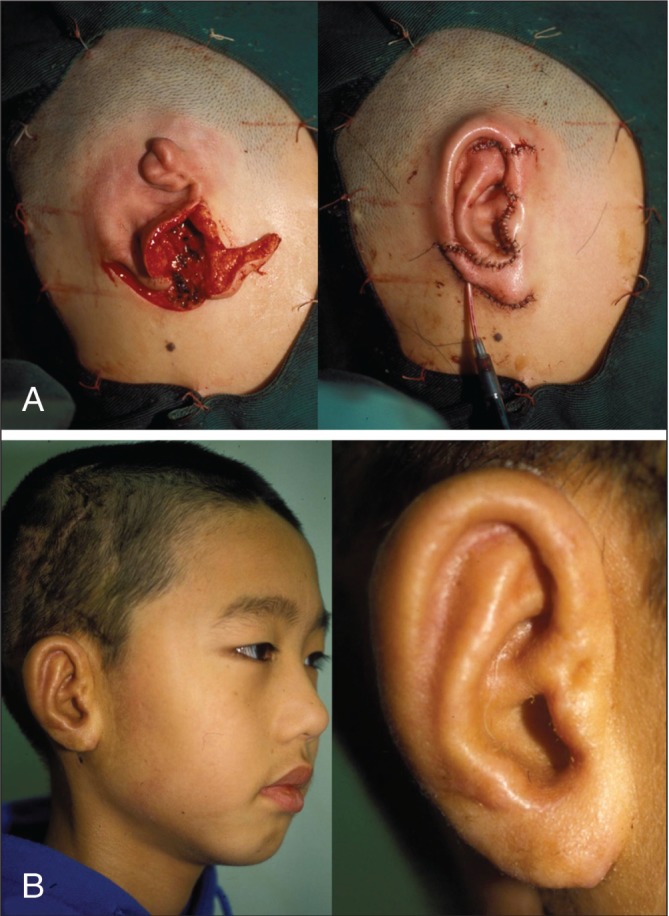
Nagata technique. A Intraoperative view. B Postoperative view (Courtesy of Dr Satoru Nagata, Akiba Hospital, Urawa, Saitama, Japan)
Chen et al (14) have developed a modification to Nagata’s second stage. They create continuous skin coverage for the ear by designing a leaf-like flap composed of an ultradelicate, split-thickness scalp skin graft in continuity with the full-thickness skin of the anterior surface of reconstructed auricle.
Tissue expansion and autologous costal cartilage graft (Park method)
Park (15) described the expanded two-flap method for total auricular reconstruction. Three stages are included in this method. The first involves the insertion of a tissue expander in a pocket created under the fascial layer in the mastoid area. Gradual inflation of expander by saline infusion begins three weeks after the insertion and continues for five months to reach a final volume of approximately 80 mL to 90 mL. Both overlying fascial layer and skin are expanded.
In the second stage, contralateral rib cartilages are harvested to fabricate the framework. The tissue expander is explanted and the expanded fascial layer and skin flap are separated to provide a space for placement of the framework. Skin is undermined anterior to the vestigial cartilage to accommodate the tragal element of the framework. A medium-size hole is created in the fascial flap to place the crus helicis. The upper part of the base frame is placed between the skin and fascial flaps and the lower portion is inserted in the earlobe envelope. The anterior surface of the construct is covered by the skin flap. The fascial flap drapes the posterior portion. A skin graft is harvested from the groin or scalp to provide coverage for the exposed fascial flap.
The third stage consists of various skin incisions over the anterior part of the framework to shape the tragus, crus helicis, conchal floor, intertragic notch and a hollow mimicking the external auditory meatus (Figure 2) (15).
Figure 2).
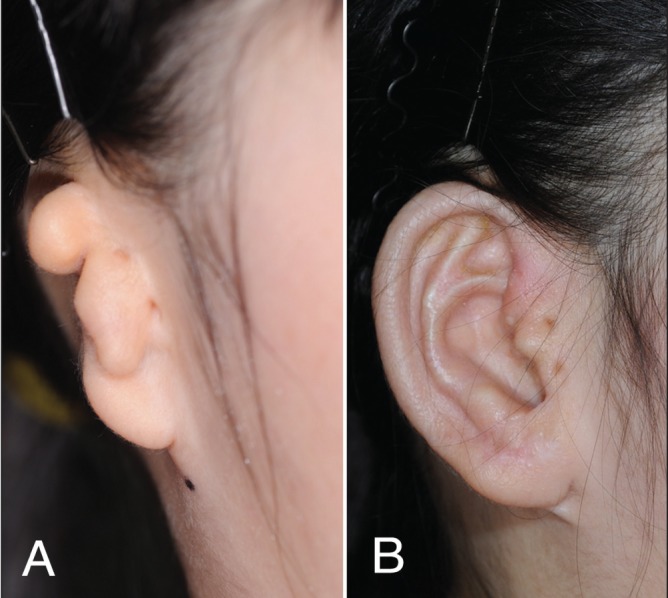
Park technique. A Preoperative view. B Postoperative view (Courtesy of Dr Chul Park, Korea University Anam Hospital, Seoul, South Korea)
IMPLANT RECONSTRUCTION
Medpor (Stryker, USA) is a synthetic biocompatible porous polyethylene implant. Reinisch (16) has pioneered its use as an alternative to conventional autologous rib cartilage graft for ear reconstruction. Through the past several decades, he has made several modifications to both the implant and his surgical technique to decrease the complications of this method.
Reinisch and Lewin (16) use a temporoparietal fascial flap (TPF) to wrap the implant. To cover the entire implant, the TPF should measure at least 11 cm wide and 12 cm in vertical height from the mid-concha. The initial Y-shaped incision of scalp has been replaced over time by a zigzag and then a horizontal incision, which is associated with less visible scar. He now uses a lighted retractor to elevate the flap from the auricular incisions to avoid a scalp scar. One drain is placed under the implant and another drain is placed beneath the elevated scalp. The lateral surface of the implant is covered by an anteriorly based skin flap consisting of the skin of microtic ear and of the mastoid area. In most cases, in addition to the skin flap, a full-thickness skin graft from the postauricular surface of the normal side ear is needed to complete the coverage of the entire lateral surface of the ear. To cover the postauricular surface of the reconstructed ear, a full-thickness skin graft is harvested from the lower abdomen, inner upper arm or supraclavicular neck. Because the split-thickness skin graft from the scalp can cause some postauricular contraction and small inclusion cysts, it is limited to patients who require a second-stage surgery, when the contraction can be corrected with the sulcus release and full-thickness skin graft. Silicon mold and a hard ear cup are used during the postoperative course to decrease swelling and protect the ear, respectively (Figure 3) (16).
Figure 3).
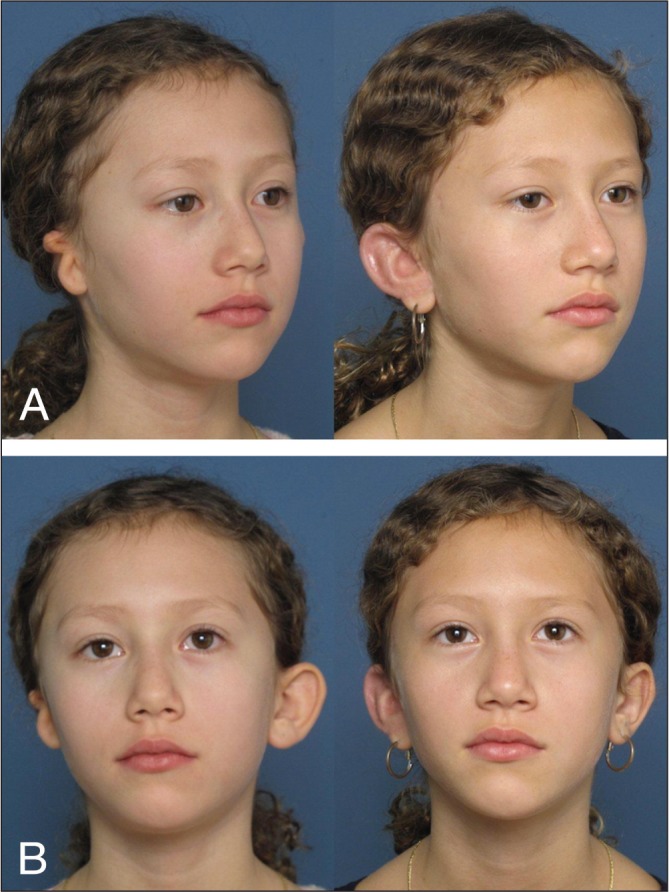
Reinisch technique. A Pre- and postoperative oblique view. B Pre- and postoperative anteroposterior view (Courtesy of Dr John Reinisch, Cedars-Sinai Medical Center, Beverly Hills, California, USA)
PROSTHETIC RECONSTRUCTION
The ear prosthesis or epithesis is an alternative to plastic surgery. There are several methods for retention of prosthesis. However, the osseointegration described first by Brånemark (17) during the 1950s has become the most reliable and durable method for fixation of the prosthesis. Using titanium implants to integrate facial or cranial prostheses into living bone has been proven to be safe and is associated with predictable esthetic results (17).
Tjellström (18) described the osseointegrated implant procedure as a two-stage technique. The first step involves the insertion of screw-shaped titanium implants through an incision behind the external ear meatus into the temporal bone. The osseointegration of implants is expected to occur three to four months after the insertion. In the second stage, the implants are uncovered and the abutments are attached to the implants. Two to three weeks later, the prosthesis can be fitted to the implant (Figure 4) (18).
Figure 4).
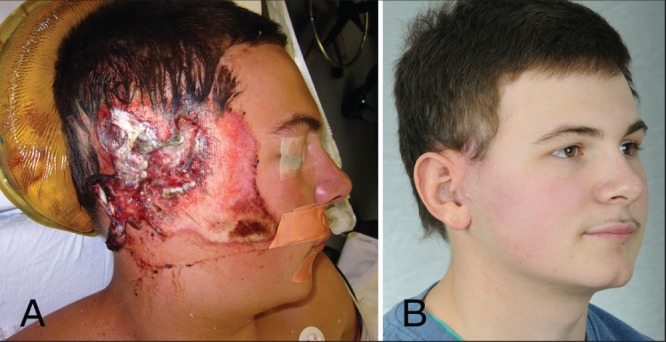
Wilkes technique. A Intraoperative view. B Postoperative oblique view (Courtesy of Dr Gordon Wilkes, University of Alberta, Edmonton, Alberta)
Granström et al (19) reported their experience of osseointegrated implant in 100 pediatric patients. The implant failures were 5.8% of 170 inserted fixtures. Adverse skin reactions appeared in 9.1% of patients over a 21-year follow-up period. Revision surgery was undertaken in 22% of patients because of appositional growth of the temporal bone.
In another study, Korus et al (20) assessed the long-term outcomes of osseointegrated ear reconstruction procedures performed on 69 pediatric and adult patients. In this series, trauma was identified as the most common indication for osseointegrated ear reconstruction, followed by congenital and oncological reasons. The results of that study showed that patients were generally satisfied with the osseointegrated implant.
DISCUSSION
Ear reconstruction is one of the most challenging procedures encountered by plastic surgeons. Many different methods and techniques have been devised. In an effort to provide a comprehensive review, we have described the main reconstruction options. Additionally, we have asked experts in the field, whose techniques are presented in the present review, to discuss the pros and cons of their own techniques (Table 1), and also to demonstrate their surgical outcomes with images.
TABLE 1.
Advantages and disadvantages of different techniques of microtia repair presented by the contributing authors
| Microtia repair technique; contributor | Advantages | Disadvantages |
|---|---|---|
| Autologous costal cartilage graft; Nagata |
|
|
| Subfascial tissue expansion and expanded two-flap method; Park |
|
|
| Implant reconstruction (Medpor, Stryker, USA); Reinisch |
|
|
| Prosthetic reconstruction; Wilkes |
|
|
The Nagata technique enables reconstruction of every detail of the ear and also symmetrical projection of the auricle. With this technique, it is possible to reconstruct the auricle in cases of anotia, low hairline and also for secondary cases. In addition, Nagata’s method of cartilage harvest has eliminated postoperative chest wall deformity. Dr Nagata believes that with the maximal preservation of blood supply in his technique, the risk of resorption of the grafted cartilage is minimal.
Dr Nagata has noted a long learning curve as one disadvantage of his technique. He also believes that the surgeon must have great artistic talent; hence, his technique using autologous rib cartilage is not a suitable option for every surgeon.
The expanded two-flap method for auricular reconstruction was described by Dr Park. He believes that with this technique, an erect, highly convoluted auricular framework, including the tragus, can be covered with thin, expanded mastoid skin and fascia flaps in one stage. The anterior surface of the framework is covered with a normal anterior auricular skin-like thin skin, and a posterior surface covered with a thin fascia flap. Furthermore, the deeper concha floor and a hollow simulating the external auditory meatus can be reconstructed. Another advantage is that the coverage flap donor site of the mastoid region is easily closed by advancement of the remaining mastoid skin, and any postoperative scar is confined to the mastoid region. No ensuing baldness in the region is observed. When compared with the subcutaneous expander insertion method, the well-vascularized fasciocutaneous layer protects the embedded expander and minimizes the possibility of its exposure or infection during expansion. Later, the fabricated framework is wrapped with the well-vascularized virgin surfaces of the two split flaps.
One of the drawbacks of this technique is the requirement of frequent outpatient visits. The patient should visit the outpatient department once per week to have the embedded expander inflated. Patients usually visit between 15 and 20 times for such serial expansions. The thickness and degree of vascularity of the mastoid skin layer and fascial layer vary in each patient. In some patients, simultaneous expansion of the two layers causes vascular embarrassment (usually venous congestion) in one of two flaps after final expansion and two-flap elevation. A postoperative salvage procedure and the use of heparin is unavoidable in those cases. The subfascial expansion technique causes more depression of the mastoid bone than the subcutaneous expansion technique due to the high internal pressure of the inflated expander under the tight fascia layer. Although the depression is not permanent, in a severely depressed case, it may be difficult to place the fabricated auricular framework in an optimal position during the second stage operation. Furthermore, after recovery of the depressed region, the axis and projection of the new auricle may be changed.
Auricular reconstruction using Medpor is an alternative option to autologous rib cartilage. Dr Reinisch believes that by using Medpor, the surgeon is able to mimic the delicacy and projection of the normal opposite ear. With this technique, ear reconstruction can be completed in one stage without the need for drains. Furthermore, it enables reconstruction before the child enters school because there is no need to wait for sufficient rib cartilage to grow. This procedure can be performed as an outpatient because there is minimal discomfort. Compared with rib cartilage, the learning curve is shorter. Atresia repair can be performed before or be combined with ear reconstruction.
Because Medpor is a foreign body, any exposure of the implant resulting from overlying soft tissue necrosis requires second surgery because the overlying soft tissue will not heal. The rate of implant fracture in the first five years after ear reconstruction is 1% without canal reconstruction, and 5% in patients with previous or simultaneous atresia repair.
Osseointegrated implant reconstruction is an outpatient surgery, with minimal morbidity, that can be performed in compromised patients and with compromised tissues. This technique allows for tumour surveillance and for salvage of autologous failures. Excellent prosthetic esthetic results can be achieved by osseoauricular implantation.
According to Korus et al (20), for osseointegrated prosthesis reconstruction, a long-term commitment of both patient and prosthetic team is required for optimal results. Hence, the reliability and compliance of the patient and the availability of a multidisciplinary team of caregivers are needed for the success of this procedure. Other considerations include the ongoing expense for maintenance visits and future prostheses every two to five years. It should also be noted that the prosthesis is not one’s own tissue.
CONCLUSION
The major methods of ear reconstruction include the use of autologous costal cartilage grafts, tissue expansion, implants, osseointegration and prostheses. All techniques have their associated advantages and disadvantages, which should be discussed with the patient and family before confirming a surgical plan for ear reconstruction. The experience of the surgeon is another important factor. The esthetic results of each of these techniques can be excellent when performed by an experienced surgeon in appropriately selected patients.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Jaffe B. The incidence of ear diseases in the Navajo Indians. Laryngoscope. 1968;79:2126–34. doi: 10.1288/00005537-196912000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Luquetti DV, Heike CL, Hing AV, Cunningham ML, Cox TC. Microtia: Epidemiology and genetics. Am J Med Genet A. 2011;158A:124–39. doi: 10.1002/ajmg.a.34352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horlock N, Vögelin E, Bradbury ET, Grobbelaar AO, Gault DT. Psychosocial outcome of patients after ear reconstruction: A retrospective study of 62 patients. Ann Plast Surg. 2005;54:517–24. doi: 10.1097/01.sap.0000155284.96308.32. [DOI] [PubMed] [Google Scholar]
- 4.Tanzer RC. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. 1959;23:1–15. doi: 10.1097/00006534-195901000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brent B. Auricular repair with autogenous rib cartilage grafts: Two decades of experience with 600 cases. Plast Reconstr Surg. 1992;90:355–74. [PubMed] [Google Scholar]
- 6.Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993 Aug;92(2):187–201. doi: 10.1097/00006534-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Marx H. Die Missbildungen des ohres. In: Denker AK, editor. Handbuch der Spez Path Anatomie Histologie. Berlin: Springer; 1926. p. 131. [Google Scholar]
- 8.Meurman Y. Congenital microtia and meatal atresia; observations and aspects of treatment. AMA Arch Otolaryngol. 1957;66:443–63. doi: 10.1001/archotol.1957.03830280073008. [DOI] [PubMed] [Google Scholar]
- 9.Walton RL, Beahm EK. Auricular reconstruction for microtia: Part I Surgical techniques. Plast Reconstr Surg. 2002;110:234–49. doi: 10.1097/00006534-200207000-00041. [DOI] [PubMed] [Google Scholar]
- 10.Brent B. Microtia repair with rib cartilage grafts: A review of personal experience with 1000 cases. Clin Plast Surg. 2002;29:257–71. doi: 10.1016/s0094-1298(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 11.Nagata S. Total auricular reconstruction with a three-dimensional costal cartilage framework. Ann Chir Plast Esthet. 1995;40:371–99. [PubMed] [Google Scholar]
- 12.Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: Part I. Avoidance and prevention of intraoperative and postoperative complications and problems. Plast Reconstr Surg. 2006;117:2011–18. doi: 10.1097/01.prs.0000210015.28620.1c. [DOI] [PubMed] [Google Scholar]
- 13.Kawanabe Y, Nagata S. A new method of costal cartilage harvest for total auricular reconstruction: Part II. Evaluation and analysis of the regenerated costal cartilage. Plast Reconstr Surg. 2007;119:308–15. doi: 10.1097/01.prs.0000244880.12256.7c. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZC, Goh RC, Chen PK, Lo LJ, Wang SY, Nagata S. A new method for the second-stage auricular projection of the Nagata method: Ultra-delicate split-thickness skin graft in continuity with full-thickness skin. Plast Reconstr Surg. 2009;124:1477–85. doi: 10.1097/PRS.0b013e3181babaf9. [DOI] [PubMed] [Google Scholar]
- 15.Park C. Subfascial expansion and expanded two-flap method for microtia reconstruction. Plast Reconstr Surg. 2000;106:1473–87. doi: 10.1097/00006534-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Reinisch JF, Lewin S. Ear reconstruction using a porous polyethylene framework and temporoparietal fascia flap. Facial Plast Surg. 2009;25:181–9. doi: 10.1055/s-0029-1239448. [DOI] [PubMed] [Google Scholar]
- 17.Brånemark B, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38:175–81. [PubMed] [Google Scholar]
- 18.Tjellström A. Osseointegrated implants for replacement of absent or defective ears. Clin Plast Surg. 1990;17:355–66. [PubMed] [Google Scholar]
- 19.Granström G, Bergström K, Odersjö M, Tjellström A. Osseointegrated implants in children: Experience from our first 100 patients. Otolaryngol Head Neck Surg. 2001;125:85–92. doi: 10.1067/mhn.2001.116190. [DOI] [PubMed] [Google Scholar]
- 20.Korus LJ, Wong JN, Wilkes GH. Long-term follow-up of osseointegrated auricular reconstruction. Plast Reconstr Surg. 2011;127:630–6. doi: 10.1097/PRS.0b013e3181fed595. [DOI] [PubMed] [Google Scholar]


