Abstract
Bariatric surgery is a popular and effective treatment for severe obesity, but may have negative effects on the skeleton. This review summarizes changes in bone density and bone metabolism from animal and clinical studies of bariatric surgery, with specific attention to Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), and sleeve gastrectomy (SG). Skeletal imaging artifacts from obesity and weight loss are also considered. Despite challenges in bone density imaging, the preponderance of evidence suggests that bariatric surgery procedures have negative skeletal effects that persist beyond the first year of surgery, and that these effects vary by surgical type. The long-term clinical implications and current clinical recommendations are presented. Further study is required to determine mechanisms of bone loss after bariatric surgery. Although early studies focused on calcium/vitamin D metabolism and mechanical unloading of the skeleton, it seems likely that surgically-induced changes in the hormonal and metabolic profile may be responsible for the skeletal phenotypes observed after bariatric surgery.
Keywords: bariatric surgery, DXA, biochemical markers of bone turnover, obesity
Introduction
Bariatric surgery is the most effective treatment for severe obesity (as defined by body mass index, BMI>40 kg/m2), leading to sustained weight loss, marked improvements in associated co-morbidities such as diabetes, hypertension, and obstructive sleep apnea, and a decrease in mortality (1, 2). Although rates of obesity may have plateaued in recent years, the subpopulation of severely obese people continues to rise (3) such that one in 20 American adults is now morbidly obese (4). Increasing evidence suggests that patients with lower BMIs may also benefit from these procedures (5). Not surprisingly, the number of bariatric surgeries is steadily increasing, with a doubling in the number of adult bariatric surgeries performed worldwide over the past decade (6). Currently, the most commonly performed procedure is the Roux-en-Y gastric bypass (RYGB), comprising nearly half of all bariatric surgeries, followed in popularity by sleeve gastrectomy (SG) and adjustable gastric banding (AGB) (Figure 1).
Figure 1. Diagram of bariatric surgery procedures and outcomes.
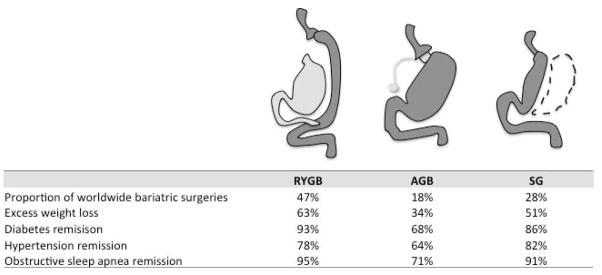
The diagram depicts the surgical procedure for Roux-en-Y gastric bypass (RYGB), adjustable gastric band (AGB), and sleeve gastrectomy (SG) (Adapted with permission from Pories WJ, JCEM 2008, 93(11):S89–S96). Dark grey shading indicates the post-surgical gastrointestinal route for passage of food. Dotted lines represent the excised gastric fundus after SG. The table below summarizes the worldwide popularity (6), average excess weight loss, and improvements in comorbidities associated with the different procedures (1).
Although most long-term metabolic consequences of bariatric surgery are favorable, the effects of bariatric surgery on the skeleton appear to be harmful. Given the increasing popularity of these procedures, and the likelihood for continued expansion to less obese patients (5), it is important to understand potential negative effects on bone metabolism.
This review summarizes currently available data on skeletal changes in clinical and animal studies of bariatric surgery, including a discussion of controversies over skeletal imaging artifacts in obese patients undergoing substantial weight loss. Data from adolescents and older populations are reviewed, along with a discussion of long-term outcomes and clinical implications. Potential mechanisms explaining bone loss after bariatric surgery are briefly considered, although these remain in the realm of hypotheses and require further study. For this review, PubMed articles were reviewed through January 1, 2014 using the search terms ‘bariatric surgery’, ‘gastric bypass’, ‘gastric sleeve’, ‘sleeve gastrectomy’, ‘gastric banding’, ‘bone’ and ‘fracture’. References from the retrieved articles and publications available in the author’s library were also used.
Early studies of intestinal surgery and effects on the skeleton
Initial concerns about skeletal health were based on older studies of post-gastrectomy patients that demonstrated a high prevalence of osteoporosis and increased fracture risk, although it was unclear whether this was a consequence of the surgery or due to underlying comorbidities of the patients (7–9). However, large animal models of gastrectomy revealed calcium malabsorption, secondary hyperparathyroidism and progressive bone loss (10), lending credence to the idea that the surgical manipulation of the gut directly affects bone metabolism. In addition, jejunoileal bypass and biliopancreatic diversion, early versions of bariatric surgery involving more extensive intestinal bypass, were both associated with significant bone loss (11, 12) and histomorphometric changes consistent with osteomalacia and trabecular bone loss (13–16).
Bone outcomes in clinical studies of bariatric surgery
Clinical studies have examined skeletal endpoints after a variety of modern bariatric surgery procedures, although the bulk of the published literature is with RYGB. Longitudinal studies document striking bone loss (Table 1) and increases in bone turnover markers (Table 2) after bariatric surgery. However, these clinical studies suffer from a number of limitations. The majority of the prospective longitudinal studies are small in size; only two cohorts with dedicated spine and hip bone density scans have ≥50 surgical subjects (17, 18). Only a handful of studies (19–21) have a non-surgical comparator group to serve as controls for age-related changes or measurement drifts (19–21). In addition, there are only a few comparative studies to quantify rates of loss between different bariatric surgery procedures (19, 22, 23), of which only one trial involves randomization to remove concerns of referral bias (22). Several longitudinal studies lack pre-operative measurements of bone density and therefore can only describe skeletal changes in the postoperative period (24, 25). Lastly, while there are a few RYGB studies that utilize more advanced bone imaging technology (20, 26, 27), all studies of SG and AGB rely solely on dual-energy x-ray absorptiometry (DXA) bone density measurements that may be affected by soft tissue artifact related to weight loss (see “Controversies regarding skeletal imaging after bariatric surgery”). With these caveats in mind, the results of metabolic bone studies in bariatric surgery patients are summarized below, by surgery type. The effects of bariatric surgery on spine and hip bone mineral density (BMD) are also summarized in Figures 2 and 3.
Table 1.
Summary of longitudinal studies documenting changes in DXA-measured bone mineral density (BMD) before and after bariatric surgery.
| Study | N | Female (%) | Age (yrs) | Timepoint after bariatric surgery | Spine | Total hip | Femoral neck | Whole body | 1/3 radius | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Roux-en-Y Gastirc Bypass (RYGB) | ||||||||||
| Von mach 200419 | 4 | 100% | mean age 45 | 24 months | −12.8% | −3.5% | Comparison group: AGB (n=9); RYGB BMD loss significantly greater than AGB group at both sites; vertebral BMD assessed on whole body scan | |||
| Coates 200435 | 15 | 80% | not specified | 9 months | −3.3% | −7.8% | −5.1% | −1.6% | NS | |
| Johnson 200545 | 23 | 80% | mean age 39 for women, mean age 43 for men | 12 months | −4.5% | −9.3% | −9.3% | Total forearm decline −1.9% at 12 mo; entire cohort n=233 but only 23 had radius/spine/hip DXA; changes at 24 mo (n=14) and 36 mo (n=8) were NS | ||
| Olbers 200622 | 29 | 76% | mean age 37 | 12 months | −2.4%a | Randomized comparison group: VBG (n=46); RYGB BMD loss significantly greater than VBG group; entire RYGB cohort n=37 but only 29 w/DXA | ||||
| Fleischer 200834 | 23 | 78% | age range 20–64 | 12 months | NS | −9.2% | −8.0% | NS | ||
| Mahdy 200839 | 70 | 70% | mean age 45, range 18–51 | 12 months | −3.2% | |||||
| Carrasco 200947 | 42 | 100% | mean age 38, range 19–55 | 12 months | −7.4% | −10.5% | −3.0% | |||
| Carlin 200917 | 30 (randomized to vit D 50,000 IU/week) | 100% | mean age 43 | 12 months | NS | −8.0% | NS | |||
| 30 (randomized to vit D 800 IU/day) | 100% | mean age 43 | 12 months | NS | −12.0% | NS | ||||
| Nogues 201023 | 7 | 100% | mean age 46 | 12 months | −6.3% | −11.1% | −10.8% | NS | ||
| Vilarrasa 201118 | 62 | 100% | mean age 46 | 12 months | −3.2% | −10.2% | −1.9%b | 12 month data also published in Vilarrasa 200946 | ||
| 59 | between 12 and 36 months | −3.1% additional decline | −2.7% additional decline | Rate of decline twice as high in postmenopausal women as compared to premenopausal women | ||||||
| Kaulfers 201148 | 61 | 84% | mean age 17, range 14–24 | 12 months (based on regression modeling) | −5.2%c | Baseline scan occurred from 0–6 months after RYGB; follow-up scan timepoint varied (range 3–27 mo); regression modeling used to predict % change at 12 mo | ||||
| Casagrande 201233 | 22 | 100% | mean age 37 | 12 months | −7.3% | −8.6% | −8.8% | |||
| Yu 201320 | 30 | 87% | mean age 47, range 20–75 | 12 months | −3.3% | −8.9% | −6.1% | NS | NS | Control group: nonsurgical obese (n=20); RYGB BMD loss significantly greater than controls at all sites |
| 12 months | −3.2% (by QCT) | NS (by QCT) | NS (by QCT) | QCT vBMD also obtained in RYGB and controls; RYGB QCT vBMD loss significantly greater than controls at spine, but not different at hip | ||||||
| Stein 201326 | 14 | 100% | mean age 45 | 12 months | NS | −8.1% | −7.9% | NS | Smaller BMD changes in full cohort (n=22) including RYGB (n=14), AGB (n=6) and SG (n=2); data for RYGB group shown here; HR-pQCT data also reported | |
| Adjustable Gastric Banding (AGB) | ||||||||||
| Strauss 200353 | 17 | 88% | mean age 43, range 28–58 | mean 30 months, range 15–39 months | NS | |||||
| Von mach 200419 | 9 | 67% | mean age 41 | 24 months | NS | 3.0% | Comparison group: RYGB (n=6); RYGB BMD loss significantly greater than AGB group at both sites; vertebral BMD assessed on whole body scan | |||
| Giusti 200550 | 37 | 100% | median age 35, range 24–52 | 12 months | 3.5% | −2.3% | NS | 12 month data in subset also published in Pugnale 200351 | ||
| 24 months | NS | −5.8% | NS | |||||||
| Dixon 200721 | 26 | 76% | mean age 42 | 24 months | −2.8%c | Comparison group: medical weight loss (n=27); bone loss in AGB group and medical group were similar | ||||
| Nadler 200954 | 36 | 67% | mean age 16, range 14–18 | 12 months | 5.8%a | |||||
| Di Renzo 201152 | 40 | 70% | mean age 44 | 6 months | −6.1%a | |||||
| Sleeve Gastrectomy (SG) | ||||||||||
| Nogues 201023 | 8 | 100% | mean age 50 | 12 months | −4.6% | −7.1% | −8.3% | NS | ||
| Pluskiewicz 201258 | 29 | 100% | mean age 40 | 6 months | −1.2% | −5.2% | −7.0% | |||
| Ruiz-Tovar 201359 | 42 | 93% | mean age 44, range 20–62 | 12 months | 5.7% | |||||
| 30 | 24 months | 7.9% | ||||||||
Cross-sectional studies and/or longitudinal studies missing pre-operative baseline data are not included. NS = not statistically significant BMD = bone mineral density; BMC = bone mineral content
Percent change estimated from mean values at baseline and follow-up
Percent change estimated from figure
Percent change in BMC shown (BMD not reported in paper)
Table 2.
Summary of longitudinal studies documenting changes in bone turnover markers before and after bariatric surgery.
| Paper | N | Female (%) |
Age (yrs) | Timepoints after bariatric surgery |
Bone formation markers | Bone resorption markers | Notes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| serum P1NP |
serum OC |
serum BSAP |
urinary or serum NTX |
serum CTX |
urinary DPD |
||||||
| Roux-en-Y Gastirc Bypass (RYGB) | |||||||||||
| Von mach 200419 | 4 | 100% | mean age 45 | 0, 3, 6, 12, *24 months | 218%b | 71%b | at 3 mo: DPD 153%c, OC NS; at 6 mo: DPD 58%c, OC 191%c; at 12 mo: DPD 65%c, OC 218%c; significantly different from ABG and controls at 24 mo | ||||
| Coates 200435 | 15 | 80% | not specified | 3, *9 months | NS | NS | 319% urinary | at 3 mo: NTX +174%, OC NS | |||
| El-Kadre 200440 | 30 (premenopausal) | 100% | not specified | 12 months | 53%a | No statistics presented; unclear whether changes in BSAP were statistically significant | |||||
| 30 (postmenopausal) | 100% | not specified | 12 months | 100%a | No statistics presented; unclear whether changes in BSAP were statistically significant | ||||||
| Riedt 200637 | 21 | 100% | mean age 44, range 29–62 | 6 months | 62% serum | 204% | Urinary pyrodinolines 164% | ||||
| Riedl 200838 | 30 | 90% | mean age 43 | 12 months | 183%a | 190%a | Compared to AGB group: change in OC and CTX numerically greater but not statistically different from AGB group | ||||
| Mahdy 200839 | 70 | 70% | mean age 45, range 18–51 | 12 months | NS | ||||||
| Fleischer 200834 | 23 | 78% | range 20–64 | 3, 6, *12 months | 39% | 106% urinary | at 3 mo: uNTX 57%a, OC 86%a; at 6 mo: uNTX 103%a, OC 86%a | ||||
| Carlin 200917 | 30 (randomized to vit D 50,000 IU/week) | 100% | mean age 43 | 12 months | 8% | 55% urinary | |||||
| 30 (randomized to vit D 800 IU/day) | 100% | mean age 43 | 12 months | 11% | 43% urinary | ||||||
| Bruno 201036 | 20 | 50% | mean age 33, range 24–47 | 6*, 18 months | 58%a | 15%a | 83% serum | Different cohort of 19 adults followed for 18 mo: BSAP 29%, sNTX 59% | |||
| Nogues 201023 | 7 | 100% | mean age 46 | 12 months | NS | 111%a urinary | |||||
| Sinha 201143 | 73 (RYGB n=50, AGB n=18, BPD n=5) | 66% | mean age 39 | 3, 6, 9, 12, *18 months | 95%c | NS | 50%c urinary | Data for combined RGYB/AGB/BPD; At 3 mo: uNTX 81%, OC 32%; at 6 mo: uNTX 52%c, OC 93%c; at 9 mo: uNTX 78%c, OC 141%c; at 12 mo: uNTX 67%c, OC 128%c; BSAP NS at all timepoints | |||
| Casagrande 201233 | 22 | 100% | mean age 37 | 12 months | 133%a serum | ||||||
| Yu 201320 | 30 | 87% | mean age 47, range 20–75 | 6, *12 months | 80% | NS | 199% | At 6 mo: P1NP 82%, CTX 220%, OC NS; increases in P1NP and CTX significantly greater than non-surgical controls at 6 and 12 months | |||
| Stein 201326 | 14 | 100% | mean age 45 | 12 months | 20% | 144% | In combined population n=22 of RYGB (n=14) + AGB (n=6) + SG (n=2): CTX 144%, BSAP NS | ||||
| Biagioni 201342 | 22 | 100% | age range 18–40 | 3, *6 months | NS | 285%a | at 3 mo: CTX 174%a, BSAP NS | ||||
| Adjustable Gastric Banding (AGB) | |||||||||||
| Von mach 200419 | 9 | 67% | mean age 41 | 3, 6, 12, *24 months | NS | NS | No change in DPD or OC at any timepoint; Lack of change significantly different than in RYGB group | ||||
| Giusti 200550 | 21 | 100% | median 35 yrs, range 24–52 | 6, 12, 18, *24 months | 62%a urinary | 131%a | at 6 mo: uNTX/Cr 40%a, CTX 100%a; at 12 mo: U NTX/Cr 61%a, CTX 119%a; at 18 mo: uNTX/Cr 38%a, CTX 113%a | ||||
| Riedl 200838 | 10 | 80% | mean age 44 | 12 months | 68%a | 128%a | Compared to RYGB group: change in OC and CTX numerically less but not statistically different from RYGB group | ||||
| Sleeve Gastrectomy (SG) | |||||||||||
| Nogues 201023 | 8 | 100% | mean age 50 | 12 months | −16%a | 140%a urinary | |||||
Cross-sectional studies and/or longitudinal studies missing pre-operative baseline data are not included. NS = not statistically significant
when multiple timepoints were studied, starred timepoint is reported in table
Percent change estimated from mean values at baseline and follow-up
Percent change estimated from figure
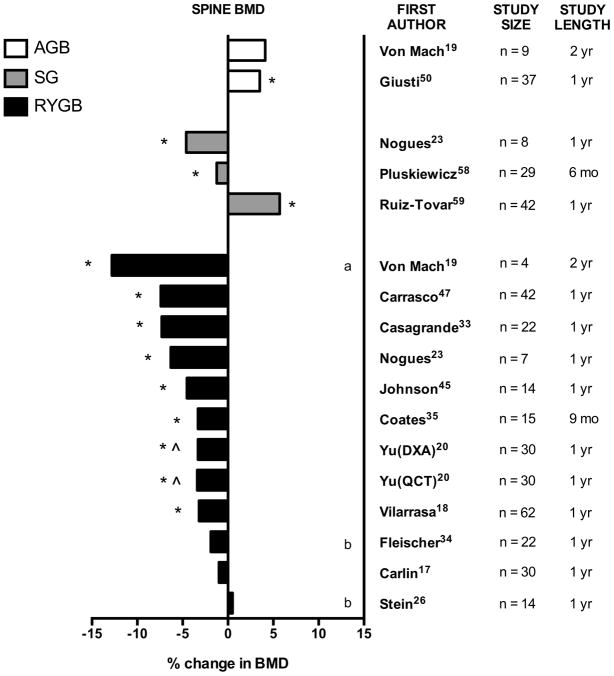
Figure 2. Percent change in spine bone mineral density (BMD) after bariatric surgery.
Graphical summary of data from longitudinal studies of bariatric surgery, by surgery type. Unless otherwise indicated, percent change is measured by DXA at lumbar spine from preoperative baseline to postoperative time-point after RYGB, SG, or AGB. Study size (n) and study length (mo = months, yr = years) are noted. In one study, percent change as measured by quantitative computed tomography (QCT) is also reported.
* statistically significant compared with baseline (within-group comparison)
^ statistically significant compared with control group (between-group comparison)
a: vertebral BMD was assessed by total body DXA
b: values are estimated from published figures
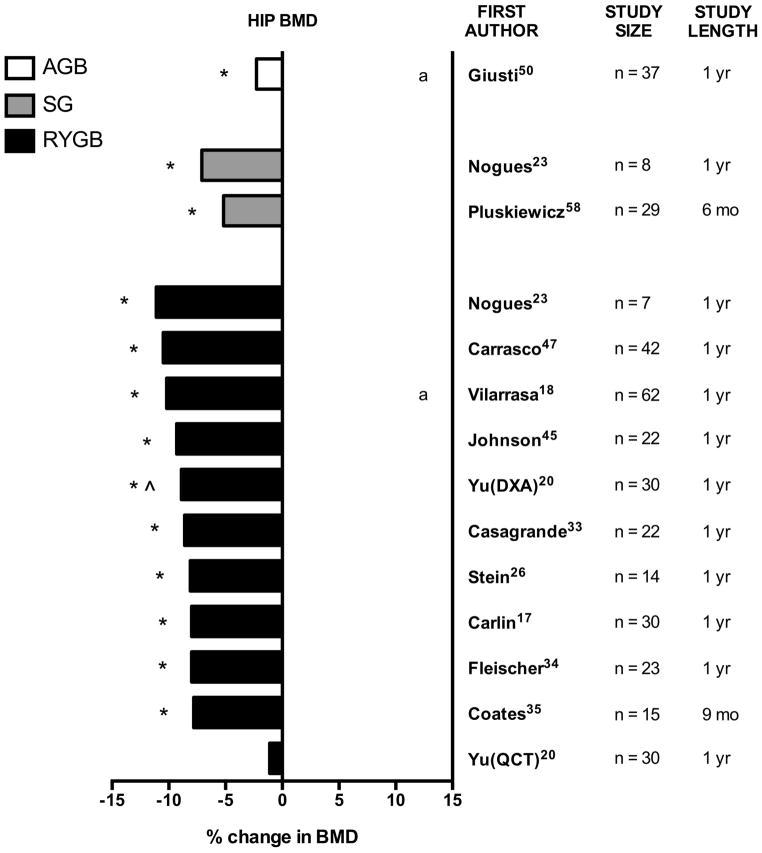
Figure 3. Percent change in hip bone mineral density (BMD) after bariatric surgery.
Graphical summary of data from longitudinal studies of bariatric surgery, by surgery type. Unless otherwise indicated, percent change is measured by DXA at the total hip from preoperative baseline to postoperative time-point after RYGB, SG, or AGB. Study size (n) and study length (mo = months, yr = years) are noted. In selected studies, alternative imaging techniques (QCT = quantitative computed tomography) or alternative hip sites (i.e. femoral neck) are noted.
* statistically significant compared with baseline (within-group comparison)
^ statistically significant compared with control group (between-group comparison)
a: % change in femoral neck BMD is shown as total hip BMD results were not reported
Roux-en-Y gastric bypass (RYGB)
RYGB involves the creation of a 30cc proximal gastric pouch that is anastamosed directly to the proximal jejunum, thus bypassing the greater portion of the stomach and duodenum (Figure 1)(28). RYGB has been the most popular form of bariatric surgery performed worldwide in the past decade (6), and is associated with an average 43 kg weight loss and BMI decrease of 17 kg/m2 (29). Case reports have identified bone pain, height loss, and hypocalcemia (30) as well as histologically confirmed osteomalacia (31) and osteitis fibrosa cystica (32) after RYGB. Numerous studies document elevated urinary and serum markers of bone turnover (17, 19, 20, 23–26, 33–43), beginning as early as 3 months after surgery (19, 34, 35, 42, 43) that remain elevated throughout the 2nd postoperative year (19, 36, 44) (Table 2). The typical increase in bone resorption markers far exceeds the increase in bone formation, consistent with net bone loss.
In the last decade, numerous longitudinal studies describe striking declines in bone density by DXA after RYGB (17–20, 22–26, 33–35, 39, 45–48) (Table 1, Figures 2 and 3). The longest of these studies describes changes in bone density over 3 years in 62 women, a quarter of whom were postmenopausal (18). Women experienced bone loss at the spine (−3%) and femoral neck (−10%) at one year after RYGB, despite unchanged serum 25-hydroxyvitamin D and PTH levels. Between years 1 and 3, there were additional declines in spine (−3%) and femoral neck (−3%) bone density despite mild weight regain.
Multiple studies have reported that DXA-measured hip BMD declines faster than spine BMD in the first year after RYGB, with rates of hip bone loss ranging from 5–11% (17, 20, 23, 26, 33–35, 45–47). Most studies (19, 20, 23, 33, 35, 45–47), but not all (17, 26, 34), have reported that lumbar spine BMD falls by 3–7% at 1 year. Similarly, whole body BMD declines by 2–5% at 1 year in most (19, 22, 35, 39, 46–48) but not all (17, 20) studies. Variable effects on forearm bone density have been observed, with no change at the 1/3 distal radius site (20, 23, 26, 34, 35), and decreases in bone density at the ultradistal (23, 24, 27) and total forearm (45) sites after RYGB.
Nearly all of the aforementioned studies utilized DXA technology to assess bone loss, but DXA may be confounded by artifact in obesity and with weight loss (see “Controversies regarding skeletal imaging after bariatric surgery”). Only one study has evaluated change in axial BMD using alternative bone imaging, namely quantitative computed tomography (QCT) (20). In this study of RYGB patients and matched obese controls, declines in lumbar spine BMD in the first year after surgery were concordant between DXA (−3.3%) and QCT (−3.4%) techniques. However, QCT did not detect any significant changes in total hip and femoral neck BMD measurements after RYGB, despite significant declines in these measurements by DXA (total hip −8.9%, femoral neck −6.1%). Within the trabecular compartment of the hip, QCT did detect bone loss (total hip −4.6%, femoral neck −3.0%), but the magnitude remained smaller than DXA. The reason for this discrepancy is not clear, but certainly highlights that the accuracy of bone density imaging modalities may be adversely affected by changes in body composition.
Two studies have evaluated changes in peripheral bone density after RYGB using high-resolution peripheral QCT (HR-pQCT) (26, 27). HR-pQCT is able to assess changes in cortical and trabecular microarchitecture and volumetric BMD (vBMD). Both HR-pQCT studies found that RYGB led to significant declines in total vBMD at the radius and the tibia, as well as reduced cortical vBMD and cortical thickness in the year after bariatric surgery. Microarchitectural changes were consistent with endocortical resorption and were more pronounced at the tibia than at the radius. This high-resolution technique also permits evaluation of cortical porosity, which increased by 30% after RYGB (27). Lastly, micro-finite element analysis of the HR-pQCT data suggested a decline in estimated bone strength at the tibia (27). Taken together, these central and peripheral QCT studies verify that RYGB induces significant bone loss at the lumbar spine, distal radius, and distal tibia. Further studies are required to determine whether imaging artifacts confound femoral bone loss measurements in the first year after RYGB.
Adjustable gastric banding (AGB)
AGB is a purely restrictive procedure which involves placement of an inflatable band high in the stomach to produce a gastric pouch of ~30cc (Figure 1) (28). AGB is associated with less initial weight loss and more weight regain as compared with RYGB, although long-term weight loss maintenance still far exceeds non-surgical methods (49). AGB is now the 3rd most common bariatric procedure performed worldwide (6).
Though there are fewer studies of this technique, the magnitude of skeletal effects observed after restrictive procedures such as AGB appear to be less than what is observed after RYGB, with less impressive increases in bone resorption markers (19, 38, 50) (Table 2), lower rates of femoral bone loss (50) (Table 1, Figure 3), and a paradoxical sparing or even increase in spine BMD (19, 50) (Table 1, Figure 2).
For example, only one AGB cohort (n=37) has been longitudinally evaluated with DXA scans at spine and hip sites. Results at both 1 year (51) and at 2 years (50) demonstrate decreases in femoral neck BMD (0–1 yr −2.3%; 0–2 yr −5.8%) but no change in lumbar spine BMD at 2 years. These BMD changes were observed in conjunction with increases in both urine N-telopeptide and serum C-telopeptide (+62% and +131%, respectively), and occur despite increases in 25-hydroxyvitamin D and decreases in parathyroid hormone. One additional study found an increase in spine BMD (+3%, as assessed on whole body scan) 2 years after AGB (19). In the absence of a control group, it is unknown whether this increase represents a true increase in spine BMD as a consequence of AGB or an artifact of degenerative change with aging.
There are contradictory data regarding effects of AGB on whole body bone mineral content and density, with several studies finding bone loss within the first 2 years (21, 52), others finding no change in bone density over variable follow-up time (50, 53), and two additional studies finding increased bone density at 2 years (19, 54). In one of the studies, the significant decline in whole body BMD was similar to the decline in a control group randomized to non-surgical weight loss methods (21), suggesting that the observed changes in whole body bone may not be a direct consequence of the AGB procedure.
Interestingly, a similar pattern of mild femoral bone loss with relative sparing of spine and whole body BMD was also observed with vertical banded gastroplasty (VBG), a surgical precursor to AGB (22, 55, 56). This suggests that restrictive bariatric procedures may share a skeletal phenotype that is distinct from other types of bariatric surgeries.
Sleeve gastrectomy (SG)
SG involves creation of a narrow gastric tube through excision of the body of the stomach (Figure 1) (28). It produces weight loss effects that are slightly less than RYGB but is associated with similar improvements in metabolic endpoints and lower complication rates (57). Sleeve gastrectomy is a relatively new bariatric procedure but has skyrocketed in popularity, with a four-fold increase in surgical procedures between 2008 and 2011(6). Also known as vertical sleeve gastrectomy or gastric sleeve, this procedure is now the 2nd most commonly performed bariatric procedure worldwide (6). Given the relatively recent rise of SG, only a few studies have examined the effect of this procedure on skeletal endpoints.
Two longitudinal studies found significant declines in bone density at axial sites, with average DXA-measured femoral bone loss (range −5.2 to −8.3%) exceeding average spine bone loss (range −1.2% to −4.6%) within the first year after SG (23, 58) (Table 1, Figures 2 and 3). In contrast, a third study found significant increases in spine BMD over 2 years (+7.9%)(59). The reason for the discrepant results at the lumbar spine are unclear, although the last study had remarkable improvements in vitamin D deficiency (prevalence of 95% preoperatively to 2% postoperatively) (59) as compared with most other studies that have documented either stability or worsening of vitamin D deficiency and secondary hyperparathyroidism after bariatric surgery.
One small study compared SG (n=8) to RYGB (n=7) and found that bone loss at all sites appeared to be less after SG as compared with RYGB, but the analysis lacked power to find statistically significant differences between groups (23). One cross-sectional study found that lumbar spine and femoral neck BMD were similar in SG and RYGB groups 12 months after surgery (60). Unfortunately this analysis lacked pre-operative DXA scans to interpret differences in rates of bone loss. Coupled with data from animal studies (61), it appears that the rate of bone loss after SG is slightly less than that observed with RYGB, though additional data are needed.
Bariatric surgery and bone health: adolescent and older populations
The majority of the studies published to date focus on a premenopausal female population. This is a reflection of the population that had been seeking bariatric surgery up until the early 2000s. In a meta-analysis published in 2004, 73% of bariatric surgery patients were female, with an average age of 39 years (29). In recent years, bariatric surgery procedures have become increasingly utilized at both ends of the age spectrum, each of whom have unique considerations with regards to skeletal health. There has been a steep increase in the number of adolescent bariatric surgery procedures performed in the last decade (62–64). Yet, only two studies have examined the effect of bariatric surgery on skeletal health in adolescents (Table 1). The first of these was a retrospective study that estimated a 7.4% decline in whole body bone mass two years after RYGB (48). This decline exceeds the typical rate of whole body bone loss described after RYGB in adult studies. Nevertheless, the average BMD Z-score remained above 0, indicating that average BMD remained higher than age-matched controls. In contrast, another longitudinal study of adolescents who had undergone AGB found an increase in whole body bone mass over 1 year, similar to adult studies that suggest lesser skeletal effects after AGB (19, 52, 54). There have been no studies examining changes in hip and spine BMD in adolescents after bariatric surgery. Ultimately, the long-term implications of altering bone metabolism in a young population that has yet to achieve peak bone mass are unclear.
On the other end of the age spectrum, the percentage of patients aged 60 or older now exceeds 10% of the bariatric surgery population (65). Given expected age-related declines in bone density, the clinical significance of surgically-induced bone loss in older adults may be greater. Not surprisingly, postmenopausal women have higher post-operative rates of osteopenia and osteoporosis as compared with premenopausal women (66). Furthermore, bone markers (40, 67) and rates of bone loss (18) are twice as high in postmenopausal women as compared with premenopausal women. If confirmed in additional studies, these results suggest that advanced age and/or a low-estrogen state may compound the risk of bariatric surgery-induced bone loss.
Controversies regarding skeletal imaging after bariatric surgery
One practical limitation to the evaluation of skeletal health after bariatric surgery is the difficulty in obtaining accurate and reproducible bone density scans in severely obese patients and during weight loss. Challenges include both logistic and technical problems, and may be specific to certain techniques and bone sites. For example, measurements at axial sites (e.g. spine, hip) may be more difficult to obtain and to interpret than appendicular measurements (e.g. radius, tibia, calcaneus), which are not subject to weight requirements and have less overlying soft tissue to cause artifact.
Logistic hurdles involve practical limitations in obtaining usable DXA scans in obesity due to weight and/or body size. Until recently, standard DXA scanners only had a table weight capacity of 350 lbs (160 kg). Although newer models now support weights up to 450 lbs (205 kg), severely obese patients may also exceed table widths, thus requiring offset scanning or manual imputation to calculate whole body measurements (68). Furthermore, in our personal experience, many spine and hip scans in patients >400 lbs (182 kg) are unreadable due to decreased penetration of photons through soft tissue, and as evidenced by tissue thickness scores above manufacturer-recommended thresholds.
Amongst those scans that are obtainable and readable, there remain technical issues related to the unpredictable impact of soft tissue artifact on bone density imaging techniques. It is well known that DXA bone density measurements are subject to accuracy errors due to changing body composition, as studied in both phantom-based (69) and human clinical studies of fat layering (70–74). The magnitude and direction of the BMD artifacts can be unpredictable, and vary by pencil-beam or fan-beam technology and by DXA manufacturer. Furthermore, the precision error of BMD measurements by DXA increases with increasing BMI (75). These technical artifacts pose complications for both cross-sectional studies of obese patients and for longitudinal studies that involve significant weight loss. Given that bariatric surgery procedures are accompanied by an average 88 lb (40 kg) weight loss (29), it is possible that these large weight changes may be adversely affecting DXA measurements.
These technical difficulties stem from several potential sources of error in DXA measurements, including magnification artifact, the “two-component limitation”, and changing marrow adiposity. Magnification artifact, also known as projection artifact, is a consequence of modern fan-beam scanners. Changing the distance from the x-ray source to the bone following substantial weight loss may alter the measurement of bone area, similar to how an object casts a larger shadow as it gets closer to a light source. Several studies indeed report physiologically implausible changes in bone area after bariatric surgery (19, 20, 39)), consistent with magnification artifact. Importantly, magnification artifact will not affect assessment of BMD, which is measured independently of bone area (76). Nevertheless, erroneous bone area measurements will lead to incorrect values for bone mineral content (BMC), which is calculated from bone area and bone mineral density (BMC = BMD * area). Another potential source of measurement error in obese patients is the “two-component limitation” of DXA, whereby assumptions about fat:lean tissue ratios are made to calculate the three densities of fat, lean tissue, and bone. These assumptions may be inaccurate in obesity and in the setting of profound weight loss. For example, DXA measurements significantly underestimate loss of body fat after RYGB as compared with deuterium-based measurements (77). In addition, as discussed earlier, QCT measurements of BMD after RYGB are discordant with DXA at femoral sites, with DXA demonstrating significantly greater declines in total hip and femoral neck BMD at 1 year (20). It should be noted, however, that QCT may also be subject to beam hardening and other imaging artifacts in obesity, although the impact is thought to be less than that of DXA (70). Lastly, both CT-based and DXA-based measurements of BMD may be affected by changes in marrow adiposity independent of changes in bone density (69). However, little is currently known about how marrow adiposity might be affected by bariatric surgery.
Nevertheless, other clinical data suggest that bone loss after bariatric surgery is indeed occurring, even if BMD assessments are imperfect. As discussed earlier, QCT-based measurements have confirmed bone loss at the lumbar spine, radius, and tibia after RYGB (20, 26, 27). DXA imaging also suggests bone loss at the ultradistal radius (23, 24, 27), a peripheral site that should be less subject to changing body composition. In addition, there is evidence of continued bone loss in the 2nd and 3rd years after bariatric surgery, after weight has stabilized and should not be further affecting DXA measurements (18, 19, 25, 45, 50). Multiple studies confirm that markers of bone turnover are markedly elevated after bariatric surgery (Table 2). The typical increase in bone resorption markers exceeds that observed during the menopause transition (78) or even during prolonged space flight (79). Lastly, bone loss has also been documented in animal models of bariatric surgery. Therefore, it is clear that bariatric surgery does cause a notable negative impact on the skeleton.
Animal studies of bariatric surgery and bone
Animal models of modern bariatric surgery procedures have been developed, largely to study the mechanisms underlying the metabolic effects of weight-loss surgery (80, 81). Initial efforts in this area focused on evaluating surgical techniques in large animals such as in dogs (82) and pigs (83). More recently, rodent models have been developed, including rat models of RYGB (84, 85), gastric banding (86, 87), and sleeve gastrectomy (88–90). Mouse models of bariatric surgery have also been developed (91–93) to take advantage of the power of genetically altered mice to delineate the mechanisms underlying metabolic improvements subsequent to bariatric surgery.
While numerous studies have used animal models to explore the mechanisms underlying the metabolic outcomes after bariatric surgery, few have examined skeletal outcomes (61, 94–96). In general these studies confirm bone loss after bariatric surgery, yet they are limited to date by small sample sizes, use of non-obese models, and inadequate control groups. For example, non-obese rats subjected to gastric bypass had lower in vivo DXA measurements of whole body and femoral BMD as compared with sham-operated controls over a 12-month period (94). Non-obese type 2 diabetic rats that underwent gastro-jejunal bypass had reduced femoral cortical and trabecular BMD 8 weeks after surgery compared with non-operated controls (95). However, interpretations of both of these studies are significantly limited by the lack of weight-matched control groups.
Another study compared skeletal outcomes after different bariatric surgery approaches in adult rats with diet-induced obesity (61). Specifically, rats that underwent gastric bypass had decreased bone volume compared with sham-operated controls, despite dietary supplementation to normalize vitamin D and calcium. In contrast, despite a similar degree of weight loss, rats that underwent sleeve gastrectomy did not exhibit bone loss compared with sham-operated groups. These results suggest that there may be physiologic changes specific to the gastric bypass procedure that induce bone loss.
Finally, a study in obese adult rats suggests that physiologic changes, and not weight loss, may be responsible for bone loss (96). Obese rats that underwent RYGB had lower vertebral BMD than sham-operated controls that were weight-matched by calorie-restriction. These findings were apparent by imaging as early as 2 weeks, and were confirmed by histomorphometry at 14 weeks, providing evidence that the bone loss after RYGB in obese rats is not directly caused by body weight loss.
To date, despite availability of several surgical models, there have been no studies examining the skeletal effects of bariatric surgery in murine models.
Longterm outcomes and fractures after bariatric surgery
The long-term consequences of the observed bone loss after bariatric surgery remain in dispute. It is clear that many of the early bariatric procedures were associated with calcium and vitamin D deficiencies, which led to case reports of histologically confirmed osteomalacia, osteoporosis, osteitis fibrosa cystica, and brown tumors after bariatric surgery (32, 97–99). Since then there has been a shift towards surgeries with less malabsoprtive sequelae coupled with more aggressive vitamin and mineral supplementation, and the incidence of these case reports has declined.
Several longitudinal studies have reported that bone markers remain elevated (16, 19, 36, 44, 50, 100, 101) and bone loss may continue into the second and third years after surgery (18, 19, 25, 45, 48, 50). Cross-sectional studies also suggest that bone markers are higher than expected even 3 years after bariatric surgery (24, 102). However, morbidly obese patients tend to have a higher BMD pre-operatively(103), and therefore the clinical significance of bone loss after bariatric surgery is unclear. There are contradictory studies regarding the prevalence of osteopenia/osteoporosis after bariatric surgery, with some studies suggesting lower BMD than expected (104–106) and others finding no difference compared with age-matched controls (16, 18, 24, 102).
Only two studies have examined the risk of fractures in a bariatric surgery population (107, 108). The first retrospective cohort study utilized the United Kingdom General Practice Research Database (GPRD) and examined 2079 patients who had undergone bariatric surgery (107). This study did not find an increase in fracture risk for patients in the first two years after bariatric surgery as compared with weight-matched obese controls. However, 2/3 of the cohort had undergone AGB, the procedure associated with the least amount of bone loss in longitudinal studies. In addition, this study was limited by the young age of the cohort (44.6 years old) and relatively short follow-up time (2.2 years). While there was a trend towards increased risk of fracture three to five years after surgery, there were limited data at these later time-points and these results were not statistically significant.
In contrast, a retrospective study from the Rochester Epidemiology Project determined that bariatric surgery patients had a two-fold increased risk of fracture compared with community-based incidence rates (standardized incidence ratio [95% CI], 2.3 [1.8–2.8]), (108) including an increase in vertebral (3.1 [1.4–5.9]), femoral (5.5 [1.5–14]), proximal humerus (5.0 [2.2–9.0]) and leg fractures (2.4 [1.5–3.7]). Important differences from the UK GPRD study include a smaller number of subjects (n=258) but longer follow-up time (8.9 years) and a predominance of RYGB procedures (75%) in the Rochester cohort. In addition, this study did not compare the bariatric surgery cohort to a weight-matched cohort, and thus was unable to determine whether the increased fracture risk was a direct consequence of the bariatric surgery or was due to underlying obesity, which often persists even after surgical weight loss. Some studies have suggested that obesity may be independently associated with increased fracture risk at certain sites (109–111), although others studies have not found this association (112, 113).
Clinical implications and management of skeletal health in bariatric surgery patients
There are important practical considerations regarding the prevention of bone loss and fractures in patients who have undergone bariatric surgery. Despite considerable weight loss, many morbidly obese patients continue to have body mass indices in the obesity range after surgery (1). Obese patients and those who have undergone bariatric surgery may be at higher risk of falls, and may also be at higher risk of injury as a consequence of falls (114) (115). In addition, obese patients tend to fracture at higher bone density (116) and lower FRAX scores (117) than their normal-weight peers, suggesting that different treatment cut-offs may be necessary to identify obese patients at risk of fracture. Despite these difficulties in bone density interpretation, experts have generally recommended DXA scans at baseline and every 1–2 years after bariatric surgery until BMD measurements stabilize (118–120).
Calcium and vitamin D supplements are recommended for all patients who have undergone bariatric surgery, with recent guidelines suggesting 1200–1500 mg/d of calcium citrate and 3000 IU/day of vitamin D (118). Note, however, that supplementation amounts vary significantly between patients,(118, 121) with many patients requiring significantly higher doses (as much as 50,000 IU/day) to maintain vitamin D sufficiency and avoid secondary hyperparathyroidism. The reason for this discrepancy in requirements remains unclear, but accentuates the importance of regular monitoring of calcium, 25-hydroxyvitamin D, and parathyroid hormone levels in bariatric surgery patients. As for osteoporosis medications, there is currently no consensus on who should receive treatment but it is generally advisable to consider treatment in osteoporotic patients and those with high fracture risk. Importantly, there are no studies that have specifically evaluated the efficacy of osteoporosis treatments in a bariatric surgery population. Post-hoc analyses suggest that some (122, 123) but not all (124) anti-resorptive agents may have reduced efficacy in overweight and obese patients. After bariatric surgery, the bioavailability of oral osteoporosis medications may be further reduced, and concerns have been raised about posssible negative effects of oral bisphosphonates on erosion of surgical gastrointestinal anastamoses. Most importantly, until the mechanisms of bone loss after bariatric surgery have been elucidated, it remains unclear whether standard osteoporosis treatments will be efficacious to prevent bone loss and/or fracture. If anti-resorptive treatments are utilized, it is important to note that bariatric surgery patients receiving bisphosphonates or denosumab may be at higher risk of developing hypocalcemia (125). In general, those who are at higher risk of complications from bone loss should be monitored more aggressively; notably adolescents, older populations, and those starting with low bone density or who have other risks for fracture at baseline.
Mechanisms of bone loss after bariatric surgery
The mechanisms of bone loss after bariatric surgery are currently unknown, but are likely multifactorial. Secondary hyperparathyroidism due to vitamin D deficiency and mechanical unloading due to weight loss are among the most commonly cited potential mechanisms underlying bone loss after bariatric surgery, yet data do not support these hypotheses. New hypotheses involving crosstalk between the skeleton and gastrointestinal, adipocytic and neurohormonal systems are now being explored.
Early explanations of bariatric surgery-induced bone loss centered on calcium and vitamin D malabsorption. RYGB bypasses the duodenum and proximal jejunum, the primary sites of calcium absorption, and may lead to malabsorption of fat-soluble vitamins such as vitamin D (126). Indeed, calcium absorption declines after gastric bypass surgery, but pre-operative absorption efficacy is relatively high such that post-operative values remain within the normal range (37). Importantly, both animal (61) and clinical (17, 20, 25, 33, 35, 39, 46) studies have documented striking declines in BMD and increases in bone turnover markers even in the absence of significant changes in circulating vitamin D or PTH. These data clearly indicate that other mechanisms must explain the majority of the bone loss seen after bariatric surgery.
Another commonly cited mechanistic hypothesis for metabolic bone changes is mechanical unloading of the skeleton due to drastic weight loss after bariatric surgery. Some (26, 34, 47), but not all (20) studies have found an association between bone loss and weight loss, and two studies have found that bone loss and lean mass loss are correlated (18, 35). While these associations might reflect the impact of mechanical unloading on the skeleton, it is also possible that it might be due to limitations of DXA-based BMD measurements in the setting of changing body composition. Furthermore, as discussed earlier, bone loss and elevations in bone markers persist despite weight stabilization in subsequent years after bariatric surgery. Lastly, animal studies have found that the rapid bone loss seen after RYGB is not observed in weight-matched calorie-restricted controls (96), strongly suggesting that weight loss is not the underlying mechanism of skeletal changes.
Other regulatory mechanisms are now being explored to explain the observed bone loss after bariatric surgery. Indeed, there is a push to rename bariatric procedures as “metabolic surgeries”, to highlight that improvements extend beyond simple weight loss and are likely mediated by changes in the hormonal profile after surgery. Bariatric surgery is associated with dramatic changes in gut-derived hormones, such as ghrelin, GLP-1, and PYY, as well as changes in bile acid metabolism (127). Similarly, the large alterations in body composition after bariatric surgery are accompanied by changes in estradiol and adipocytic hormones (e.g. leptin, adiponectin, visfatin, resistin) (128, 129). There are data to suggest that many of these hormones may have direct effects upon bone homeostasis (130, 131). Lastly, increased energy expenditure has been documented after bariatric surgery (132), as well as metabolic changes such as metabolic acidosis (96). Many of these hormonal and metabolic effects may contribute to the observed changes in bone after bariatric surgery, although these hypotheses remain exploratory at this time. It is also possible that the discrepancies in bone metabolism after the various bariatric procedures may be in part explained by the different impact of these surgeries on the neurohormonal and metabolic profile. For a more in depth consideration of exploratory hypotheses relating bone and hormonal changes after bariatric surgery, please refer to published reviews (133–135).
Conclusion
The worldwide obesity epidemic has led to increasing utilization of bariatric surgery procedures. While these procedures have beneficial effects on many cardiometabolic outcomes, the possible negative effects on bone metabolism and long-term skeletal health must be examined. Despite challenges in bone density imaging, the preponderance of evidence suggests that modern bariatric surgery procedures have negative effects on bone homeostasis that persist for at least several years, and that these effects vary by surgical type. In particular, the negative skeletal effects of RYGB and SG appear to be much greater than for purely restrictive procedures such as AGB. The clinical implications for osteoporosis and fracture risk are still unclear, but treatment recommendations for all patients undergoing bariatric surgery include aggressive calcium and vitamin D supplementation and serial bone density monitoring.
Future directions for research include further utilization of multiple bone density imaging modalities to verify the magnitude of bone loss after bariatric surgery, longitudinal studies to evaluate long-term effects of bariatric surgery on bone metabolism and risk of fractures, and additional focus on susceptible populations who are increasingly seeking bariatric surgery, including adolescents and the elderly. Lastly, it is imperative to understand the mechanisms by which bariatric surgery leads to bone loss, and whether these mechanisms vary according to the specific surgical intervention. This information will not only lead to a better understanding of potential treatments for bone loss, but will also be an important step towards unraveling the fascinating and complex interactions between bone, gut, fat, muscle, and brain.
Acknowledgments
I thank Dr. Mary Bouxsein, PhD and Dr. Joel Finkelstein, MD, of the Endocrine Unit of Massachusetts General Hospital, for their expert review of this article.
Footnotes
Disclosures: This work was supported by NIH grant K23 DK093713 (EWY).
References
- 1.Chang S-H, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. The Effectiveness and Risks of Bariatric Surgery: An Updated Systematic Review and Meta-analysis, 2003–2012. JAMA Surgery. 2013 doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA: The Journal of the American Medical Association. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 3.Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2009–2010 2012. 2014 Jan 5; Available from: http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.pdf.
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. JAMA: The Journal of the American Medical Association. 2012 doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA: The Journal of the American Medical Association. 2013;309(21):2250–61. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Oien DM. Metabolic/Bariatric Surgery Worldwide 2011. Obesity Surgery. 2013;23(4):427–36. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 7.Mellstrom D, Rundgren A. Long-term effects after partial gastrectomy in elderly men. A longitudinal population study of men between 70 and 75 years of age. Scand J Gastroenterol. 1982;17(3):433–9. doi: 10.3109/00365528209182082. [DOI] [PubMed] [Google Scholar]
- 8.Zittel TT, Zeeb B, Maier GW, Kaiser GW, Zwirner M, Liebich H, et al. High prevalence of bone disorders after gastrectomy. Am J Surg. 1997;174(4):431–8. doi: 10.1016/s0002-9610(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ, 3rd, Crowson CS, Khosla S, O’Fallon WM. Fracture risk after surgery for peptic ulcer disease: a population-based cohort study. Bone. 1999;25(1):61–7. doi: 10.1016/s8756-3282(99)00097-6. [DOI] [PubMed] [Google Scholar]
- 10.Maier GW, Kreis ME, Zittel TT, Becker HD. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg. 1997;225(2):181–92. doi: 10.1097/00000658-199702000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krolner B, Ranlov PJ, Clemmesen T, Nielsen SP. Bone loss after gastroplasty for morbid obesity: side-effect or adaptive response to weight reduction? Lancet. 1982;1(8278):956–7. doi: 10.1016/s0140-6736(82)91949-3. [DOI] [PubMed] [Google Scholar]
- 12.Tsiftsis DDA, Mylonas P, Mead N, Kalfarentzos F, Alexandrides TK. Bone Mass Decreases in Morbidly Obese Women after Long Limb-Biliopancreatic Diversion and Marked Weight Loss Without Secondary Hyperparathyroidism. A Physiological Adaptation to Weight Loss? OBES SURG. 2009;19(11):1497–503. doi: 10.1007/s11695-009-9938-z. [DOI] [PubMed] [Google Scholar]
- 13.Parfitt AM, Miller MJ, Frame B, Villanueva AR, Rao DS, Oliver I, et al. Metabolic bone disease after intestinal bypass for treatment of obesity. Ann Intern Med. 1978;89(2):193–9. doi: 10.7326/0003-4819-89-2-193. [DOI] [PubMed] [Google Scholar]
- 14.Parfitt AM, Podenphant J, Villanueva AR, Frame B. Metabolic bone disease with and without osteomalacia after intestinal bypass surgery: a bone histomorphometric study. Bone. 1985;6(4):211–20. doi: 10.1016/8756-3282(85)90003-1. [DOI] [PubMed] [Google Scholar]
- 15.Compston JE, Vedi S, Gianetta E, Watson G, Civalleri D, Scopinaro N. Bone histomorphometry and vitamin D status after biliopancreatic bypass for obesity. Gastroenterology. 1984;87(2):350–6. [PubMed] [Google Scholar]
- 16.Marceau P. Does Bone Change After Biliopancreatic Diversion? Journal of Gastrointestinal Surgery. 2002;6(5):690–8. doi: 10.1016/s1091-255x(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 17.Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2009;5(4):444–9. doi: 10.1016/j.soard.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Vilarrasa N, San José P, García I, Gómez-Vaquero C, Medina Miras P, Gordejuela AGR, et al. Evaluation of Bone Mineral Density Loss in Morbidly Obese Women After Gastric Bypass: 3-Year Follow-Up. Obesity Surgery. 2011;21(4):465–72. doi: 10.1007/s11695-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 19.von Mach M-A, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism: clinical and experimental. 2004;53(7):918–21. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Yu EW, Bouxsein M, Roy AE, Baldwin C, Cange A, Neer RM, et al. Bone loss after bariatric surgery: Discordant results between DXA and QCT bone density. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JB, Strauss BJG, Laurie C, O’Brien PE. Changes in Body Composition with Weight Loss: Obese Subjects Randomized to Surgical and Medical Programs*. Obesity. 2007;15(5):1187–98. doi: 10.1038/oby.2007.639. [DOI] [PubMed] [Google Scholar]
- 22.Olbers T, Björkman S, Lindroos A, Maleckas A, Lönn L, Sjöström L, et al. Body Composition, Dietary Intake, and Energy Expenditure After Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Vertical Banded Gastroplasty. Annals of Surgery. 2006;244(5):715–22. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogues X, Goday A, Peña MJ, Benaiges D, de Ramón M, Crous X, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cirugía española. 2010;88(2):103–9. doi: 10.1016/j.ciresp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40–7. doi: 10.1038/oby.2004.7. [DOI] [PubMed] [Google Scholar]
- 25.Pereira FA, de Castro JA, dos Santos JE, Foss MC, Paula FJ. Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Braz J Med Biol Res. 2007;40(4):509–17. doi: 10.1590/s0100-879x2007000400009. [DOI] [PubMed] [Google Scholar]
- 26.Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, et al. Bariatric Surgery Results in Cortical Bone Loss. Journal of Clinical Endocrinology & Metabolism. 2013;98(2):541–9. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu EW, Putman M, Bouxsein M, Roy A, Derrico N, Finkelstein J. Endosteal resorption and worsening cortical porosity after Roux-en-Y gastric bypass surgery. American Society for Bone and Mineral Research Conference; October 2013; Baltimore, MD. 2013. [Google Scholar]
- 28.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S89–96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 30.Crowley LV, Seay J, Mullin G. Late effects of gastric bypass for obesity. Am J Gastroenterol. 1984;79(11):850–60. [PubMed] [Google Scholar]
- 31.Al-Shoha A, Qiu S, Palnitkar S, Rao DS. Osteomalacia with bone marrow fibrosis due to severe vitamin D deficiency after a gastrointestinal bypass operation for severe obesity. Endocr Pract. 2009;15(6):528–33. doi: 10.4158/EP09050.ORR. [DOI] [PubMed] [Google Scholar]
- 32.Shaker JL, Norton AJ, Woods MF, Fallon MD, Findling JW. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos Int. 1991;1(3):177–81. doi: 10.1007/BF01625450. [DOI] [PubMed] [Google Scholar]
- 33.Casagrande DS, Repetto G, Mottin CC, Shah J, Pietrobon R, Worni M, et al. Changes in Bone Mineral Density in Women Following 1-Year Gastric Bypass Surgery. Obesity Surgery. 2012;22(8):1287–92. doi: 10.1007/s11695-012-0687-z. [DOI] [PubMed] [Google Scholar]
- 34.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 36.Bruno C, Fulford AD, Potts JR, Mcclintock R, Jones R, Cacucci BM, et al. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95(1):159–66. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14(11):1940–8. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedl M, Vila G, Maier C, Handisurya A, Shakeri-Manesch S, Prager G, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93(6):2307–12. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- 39.Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. OBES SURG. 2008;18(12):1526–31. doi: 10.1007/s11695-008-9653-1. [DOI] [PubMed] [Google Scholar]
- 40.El-Kadre LJ, Rocha PRS, de Almeida Tinoco AC, Tinoco RC. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. OBES SURG. 2004;14(8):1062–6. doi: 10.1381/0960892041975505. [DOI] [PubMed] [Google Scholar]
- 41.DiGiorgi M, Daud A, Inabnet WB, Schrope B, Urban-Skuro M, Restuccia N, et al. Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. OBES SURG. 2008;18(9):1144–8. doi: 10.1007/s11695-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 42.Biagioni MF, Mendes AL, Nogueira CR, Paiva SA, Leite CV, Mazeto GM. Weight-Reducing Gastroplasty with Roux-en-Y Gastric Bypass: Impact on Vitamin D Status and Bone Remodeling Markers. Metab Syndr Relat Disord. 2013 doi: 10.1089/met.2013.0026. [DOI] [PubMed] [Google Scholar]
- 43.Sinha N, Shieh A, Stein EM, Strain G, Schulman A, Pomp A, et al. Increased PTH and 1.25(OH)2D Levels Associated With Increased Markers of Bone Turnover Following Bariatric Surgery. Obesity. 2011;19(12):2388–93. doi: 10.1038/oby.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granado-Lorencio F, Simal-Antón A, Salazar-Mosteiro J, Herrero-Barbudo C, Donoso-Navarro E, Blanco-Navarro I, et al. Time-Course Changes in Bone Turnover Markers and Fat-Soluble Vitamins After Obesity Surgery. Obesity Surgery. 2010;20(11):1524–9. doi: 10.1007/s11695-010-0257-1. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9(8):1106–10. doi: 10.1016/j.gassur.2005.07.012. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 46.Vilarrasa N, Gómez JM, Elio I, Gómez-Vaquero C, Masdevall C, Pujol J, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. OBES SURG. 2009;19(7):860–6. doi: 10.1007/s11695-009-9843-5. [DOI] [PubMed] [Google Scholar]
- 47.Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, et al. Changes in Bone Mineral Density, Body Composition and Adiponectin Levels in Morbidly Obese Patients after Bariatric Surgery. OBES SURG. 2009;19(1):41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 48.Kaulfers A-MD, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011;127(4):e956–61. doi: 10.1542/peds.2010-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. The New England journal of medicine. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 50.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burckhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow-up. Int J Obes Relat Metab Disord. 2005;29(12):1429–35. doi: 10.1038/sj.ijo.0803040. [DOI] [PubMed] [Google Scholar]
- 51.Pugnale N, Giusti V, Suter M, Zysset E, Heraief E, Gaillard RC, et al. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(1):110–6. doi: 10.1038/sj.ijo.0802177. [DOI] [PubMed] [Google Scholar]
- 52.Di Renzo L, Carbonelli MG, Bianchi A, Iacopino L, Fiorito R, Di Daniele N, et al. Body composition changes after laparoscopic adjustable gastric banding: what is the role of −174G>C interleukin-6 promoter gene polymorphism in the therapeutic strategy? International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2011;36(3):369–78. doi: 10.1038/ijo.2011.132. [DOI] [PubMed] [Google Scholar]
- 53.Strauss BJG, Marks SJ, Growcott JP, Stroud DB, Lo CS, Dixon JB, et al. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta Diabetologica. 2003;40(S1):s266–s9. doi: 10.1007/s00592-003-0083-1. [DOI] [PubMed] [Google Scholar]
- 54.Nadler EP, Reddy S, Isenalumhe A, Youn HA, Peck V, Ren CJ, et al. Laparoscopic adjustable gastric banding for morbidly obese adolescents affects android fat loss, resolution of comorbidities, and improved metabolic status. Journal of the American College of Surgeons. 2009;209(5):638–44. doi: 10.1016/j.jamcollsurg.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Cundy T, Evans MC, Kay RG, Dowman M, Wattie D, Reid IR. Effects of vertical-banded gastroplasty on bone and mineral metabolism in obese patients. British Journal of Surgery. 1996;83(10):1468–72. doi: 10.1002/bjs.1800831046. [DOI] [PubMed] [Google Scholar]
- 56.Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. OBES SURG. 2003;13(3):383–8. doi: 10.1381/096089203765887705. [DOI] [PubMed] [Google Scholar]
- 57.Li JF, Lai DD, Ni B, Sun KX. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56(6):E158–64. doi: 10.1503/cjs.026912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pluskiewicz W, Bužga M, Holéczy P, Bortlík L, Šmajstrla V, Adamczyk P. Bone Mineral Changes in Spine and Proximal Femur in Individual Obese Women after Laparoscopic Sleeve Gastrectomy: A Short-Term Study. Obesity Surgery. 2012;22(7):1068–76. doi: 10.1007/s11695-012-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz-Tovar J, Oller I, Priego P, Arroyo A, Calero A, Diez M, et al. Short- and Mid-term Changes in Bone Mineral Density After Laparoscopic Sleeve Gastrectomy. Obesity Surgery. 2013:1–6. doi: 10.1007/s11695-013-0866-6. [DOI] [PubMed] [Google Scholar]
- 60.Vilarrasa N, Gordejuela AGR, Gómez-Vaquero C, Pujol J, Elio I, San José P, et al. Effect of Bariatric Surgery on Bone Mineral Density: Comparison of Gastric Bypass and Sleeve Gastrectomy. Obesity Surgery. 2013;23(12):2086–91. doi: 10.1007/s11695-013-1016-x. [DOI] [PubMed] [Google Scholar]
- 61.Stemmer K, Bielohuby M, Grayson BE, Begg DP, Chambers AP, Neff C, et al. Roux-en-Y Gastric Bypass Surgery But Not Vertical Sleeve Gastrectomy Decreases Bone Mass in Male Rats. Endocrinology. 2013;154(6):2015–24. doi: 10.1210/en.2012-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161(3):217–21. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 63.Schilling PL, Davis MM, Albanese CT, Dutta S, Morton J. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008;206(1):1–12. doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 64.Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg. 2013;48(12):2401–7. doi: 10.1016/j.jpedsurg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Flum DR, Belle SH, King WC, Wahed AS, Berk P, et al. Consortium LAoBSL. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papapietro K, Massardo T, Riffo A, Diaz E, Araya AV, Adjemian D, et al. Bone mineral density disminution post Roux-Y bypass surgery. Nutr Hosp. 2013;28(3):631–6. doi: 10.3305/nh.2013.28.3.6400. [DOI] [PubMed] [Google Scholar]
- 67.Balsa JA, Botella-Carretero JI, Peromingo R, Caballero C, Muñoz-Malo T, Villafruela JJ, et al. Chronic increase of bone turnover markers after biliopancreatic diversion is related to secondary hyperparathyroidism and weight loss. Relation with bone mineral density. OBES SURG. 2010;20(4):468–73. doi: 10.1007/s11695-009-0028-z. [DOI] [PubMed] [Google Scholar]
- 68.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17(6):1281–6. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41(1):138–54. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27(1):119–2. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Binkley N, Krueger D, Vallarta-Ast N. An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom. 2003;6(3):199–204. doi: 10.1385/jcd:6:3:199. [DOI] [PubMed] [Google Scholar]
- 72.Evans EM, Mojtahedi MC, Kessinger RB, Misic MM. Simulated change in body fatness affects Hologic QDR 4500A whole body and central DXA bone measures. J Clin Densitom. 2006;9(3):315–22. doi: 10.1016/j.jocd.2006.04.117. [DOI] [PubMed] [Google Scholar]
- 73.Tothill P, Laskey MA, Orphanidou CI, van Wijk M. Anomalies in dual energy X-ray absorptiometry measurements of total-body bone mineral during weight change using Lunar, Hologic and Norland instruments. Br J Radiol. 1999;72(859):661–9. doi: 10.1259/bjr.72.859.10624323. [DOI] [PubMed] [Google Scholar]
- 74.Svendsen OL, Haarbo J, Hassager C, Christiansen C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr. 1993;57(5):605–8. doi: 10.1093/ajcn/57.5.605. [DOI] [PubMed] [Google Scholar]
- 75.Knapp KM, Welsman JR, Hopkins SJ, Fogelman I, Blake GM. Obesity increases precision errors in dual-energy X-ray absorptiometry measurements. J Clin Densitom. 2012;15(3):315–9. doi: 10.1016/j.jocd.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Blake GM, Parker JC, Buxton FM, Fogelman I. Dual X-ray absorptiometry: a comparison between fan beam and pencil beam scans. Br J Radiol. 1993;66(790):902–6. doi: 10.1259/0007-1285-66-790-902. [DOI] [PubMed] [Google Scholar]
- 77.Levitt DG, Beckman LM, Mager JR, Valentine B, Sibley SD, Beckman TR, et al. Comparison of DXA and water measurements of body fat following gastric bypass surgery and a physiological model of body water, fat, and muscle composition. J Appl Physiol. 2010;109(3):786–95. doi: 10.1152/japplphysiol.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sowers MR, Zheng H, Greendale GA, Neer RM, Cauley JA, Ellis J, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98(7):2854–63. doi: 10.1210/jc.2012-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith SM, Wastney ME, O’Brien KO, Morukov BV, Larina IM, Abrams SA, et al. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res. 2005;20(2):208–18. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 80.Ashrafian H, Bueter M, Ahmed K, Suliman A, Bloom SR, Darzi A, et al. Metabolic surgery: an evolution through bariatric animal models. Obesity Reviews. 2010;11(12):907–20. doi: 10.1111/j.1467-789X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 81.Rao RS, Rao V, Kini S. Animal Models in Bariatric Surgery—A Review of the Surgical Techniques and Postsurgical Physiology. Obesity Surgery. 2010;20(9):1293–305. doi: 10.1007/s11695-010-0135-x. [DOI] [PubMed] [Google Scholar]
- 82.Odaibo SK, Lee KY, Chey WY. Motility abnormality in dogs with gastrojejunostomy. Scandinavian journal of gastroenterology Supplement. 1986;124:203–7. doi: 10.3109/00365528609093805. [DOI] [PubMed] [Google Scholar]
- 83.Nocca D, Gagner M, Abente FC, Del Genio GM, Ueda K, Assalia A, et al. Laparoscopic gastric bypass with silicone band in a pig model: prevention of anastomotic dilatation -- feasibility study. OBES SURG. 2005;15(4):523–7. doi: 10.1381/0960892053723303. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, Ohinata K, Meguid MM, Marx W, Tada T, Chen C, et al. Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. The Journal of surgical research. 2002;107(1):56–63. doi: 10.1006/jsre.2002.6508. [DOI] [PubMed] [Google Scholar]
- 85.Meguid MM, Ramos EJ, Suzuki S, Xu Y, George ZM, Das UN, et al. A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg. 2004;8(5):621–30. doi: 10.1016/j.gassur.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Monteiro MP, Monteiro JD, Aguas AP, Cardoso MH. A rat model of restrictive bariatric surgery with gastric banding. Obes Surg. 2006;16(1):48–51. doi: 10.1381/096089206775222159. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. OBES SURG. 2009;19(3):357–62. doi: 10.1007/s11695-008-9688-3. [DOI] [PubMed] [Google Scholar]
- 88.Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. The Journal of surgical research. 2009;157(2):243–50. doi: 10.1016/j.jss.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Liu J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced obese rats. OBES SURG. 2009;19(7):921–5. doi: 10.1007/s11695-008-9663-z. [DOI] [PubMed] [Google Scholar]
- 90.Pereferrer FS, Gonzalez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Dejardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. OBES SURG. 2008;18(1):97–108. doi: 10.1007/s11695-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 91.Liu W, Zassoko R, Mele T, Luke P, Sun H, Garcia B, et al. Establishment of duodenojejunal bypass surgery in mice: a model designed for diabetic research. Microsurgery. 2008;28(3):197–202. doi: 10.1002/micr.20454. [DOI] [PubMed] [Google Scholar]
- 92.Yin DP, Gao Q, Ma LL, Yan W, Williams PE, McGuinness OP, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254(1):73–82. doi: 10.1097/SLA.0b013e3182197035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KLJ, et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. The Journal of clinical endocrinology and metabolism. 2012;97(6):E1023–31. doi: 10.1210/jc.2011-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.STENSTROM B, FURNES M, TOMMERAS K, Syversen U, ZHAO C, CHEN D. Mechanism of Gastric Bypass–Induced Body Weight Loss: One-Year Follow-up After Micro–Gastric Bypass in Rats. Journal of Gastrointestinal Surgery. 2006;10(10):1384–91. doi: 10.1016/j.gassur.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Pérez-Castrillón JL, Riancho JA, Luis D, Caeiro JR, Guede D, González-Sagrado M, et al. The Deleterious Effect of Bariatric Surgery on Cortical and Trabecular Bone Density in the Femurs of Non-obese, Type 2 Diabetic Goto-Kakizaki Rats. Obesity Surgery. 2012;22(11):1755–60. doi: 10.1007/s11695-012-0732-y. [DOI] [PubMed] [Google Scholar]
- 96.Abegg K. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. 2013:1–43. doi: 10.1152/ajpregu.00038.2013. [DOI] [PubMed] [Google Scholar]
- 97.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329(2):57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. OBES SURG. 2002;12(5):685–92. doi: 10.1381/096089202321019693. [DOI] [PubMed] [Google Scholar]
- 99.Benhalima K, Mertens A, Van den Bruel A, Laga K, Vanderschueren D, Samson I, et al. A brown tumor after biliopancreatic diversion for severe obesity. Endocr J. 2009;56(2):263–8. doi: 10.1507/endocrj.k08e-199. [DOI] [PubMed] [Google Scholar]
- 100.Moreiro J, Ruiz O, Perez G, Salinas R, Urgeles JR, Riesco M, et al. Parathyroid hormone and bone marker levels in patients with morbid obesity before and after biliopancreatic diversion. Obesity Surgery. 2007;17(3):348–54. doi: 10.1007/s11695-007-9063-9. [DOI] [PubMed] [Google Scholar]
- 101.Alcalde OL, Duce AM, Bustos FA, Torres RF, Huarte MG, González JG, et al. Ultrasonic Value is Not Useful to Detect Bone Changes Following a Biliopancreatic Diversion. Obesity Surgery. 2010;21(2):173–8. doi: 10.1007/s11695-010-0323-8. [DOI] [PubMed] [Google Scholar]
- 102.Valderas J, Velasco S, Solari S, Liberona Y, Viviani P, Maiz A, et al. Increase of Bone Resorption and the Parathyroid Hormone in Postmenopausal Women in the Long-term after Roux-en-Y Gastric Bypass. Obesity Surgery. 2009 doi: 10.1007/s11695-009-9890-y. [DOI] [PubMed] [Google Scholar]
- 103.Holbrook TL, Barrett-Connor E. The association of lifetime weight and weight control patterns with bone mineral density in an adult community. Bone and mineral. 1993;20(2):141–9. doi: 10.1016/s0169-6009(08)80023-2. [DOI] [PubMed] [Google Scholar]
- 104.Duran de Campos C, Dalcanale L, Pajecki D, Garrido AB, Halpern A. Calcium intake and metabolic bone disease after eight years of Roux-en-Y gastric bypass. OBES SURG. 2008;18(4):386–90. doi: 10.1007/s11695-007-9393-7. [DOI] [PubMed] [Google Scholar]
- 105.Ott M, Fanti P, Malluche H, Ryo U, Whaley F, Strodel W, et al. Biochemical Evidence of Metabolic Bone Disease in Women Following Roux-Y Gastric Bypass for Morbid Obesity. OBES SURG. 1992;2(4):341–8. doi: 10.1381/096089292765559936. [DOI] [PubMed] [Google Scholar]
- 106.Bano G, Rodin DA, Pazianas M, Nussey SS. Reduced bone mineral density after surgical treatment for obesity. Int J Obes Relat Metab Disord. 1999;23(4):361–5. doi: 10.1038/sj.ijo.0800827. [DOI] [PubMed] [Google Scholar]
- 107.Lalmohamed A, De Vries F, Bazelier MT, Cooper A, van Staa T-P, Cooper C, et al. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ (Clinical research ed) 2012;345(aug03 1):e5085-e. doi: 10.1136/bmj.e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakamura KM, Haglind EGC, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporosis Int. 2013:1–8. doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nielson CM, Marshall LM, Adams AL, Leblanc ES, Cawthon PM, Ensrud K, et al. BMI and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2010 doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. The American Journal of Medicine. 2011;124(11):1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prieto-Alhambra D, Premaor MO, Fina Avilés F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, et al. The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 112.Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, et al. A meta-analysis of the association of fracture risk and body mass index in women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 doi: 10.1002/jbmr.2017. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 113.Premaor MO, Compston JE, Fina Avilés F, Pagès-Castellà A, Nogues X, Díez-Pérez A, et al. The association between fracture site and obesity in men: A population-Based cohort study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(8):1771–7. doi: 10.1002/jbmr.1878. [DOI] [PubMed] [Google Scholar]
- 114.Himes CL, Reynolds SL. Effect of Obesity on Falls, Injury, and Disability. Journal of the American Geriatrics Society. 2011;60(1):124–9. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 115.Berarducci A, Haines K, Murr MM. Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity. Applied nursing research : ANR. 2009;22(1):35–41. doi: 10.1016/j.apnr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Premaor MO, Pilbrow L, Tonkin C, Parker R, Compston J. Obesity and Fractures in Postmenopausal Women. J Bone Miner Res. 2009 doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 117.Premaor M, Parker RA, Cummings S, Ensrud K, Cauley JA, Lui L-Y, et al. Predictive value of FRAX for fracture in obese older women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;28(1):188–95. doi: 10.1002/jbmr.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient - 2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013:e1–e36. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams SE. Metabolic Bone Disease in the Bariatric Surgery Patient. Journal of Obesity. 2011;2011:1–9. doi: 10.1155/2011/634614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heber D, Greenway F, Kaplan L, Livingston E, Salvador J, Still C. Endocrine and Nutritional Management of the Post-Bariatric Surgery Patient: An Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism. 2010;95(11):4823. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 121.Flores L, Martínez Osaba MJ, Andreu A, Moizé V, Rodriguez L, Vidal J. Calcium and Vitamin D Supplementation after Gastric Bypass Should Be Individualized to Improve or Avoid Hyperparathyroidism. Obesity Surgery. 2010;20(6):738–43. doi: 10.1007/s11695-010-0138-7. [DOI] [PubMed] [Google Scholar]
- 122.McClung MR, Boonen S, Torring O, Roux C, Rizzoli R, Bone HG, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res. 2012;27(1):211–8. doi: 10.1002/jbmr.536. [DOI] [PubMed] [Google Scholar]
- 123.McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, et al. Ten-year fracture probability identifies women who will benefit from clodronate therapy--additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int. 2009;20(5):811–7. doi: 10.1007/s00198-008-0786-9. [DOI] [PubMed] [Google Scholar]
- 124.Eastell R, Black DM, Boonen S, Adami S, Felsenberg D, Lippuner K, et al. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab. 2009;94(9):3215–25. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosen CJ, Brown S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N Engl J Med. 2003;348(15):1503–4. doi: 10.1056/NEJM200304103481521. [DOI] [PubMed] [Google Scholar]
- 126.Slater G. Serum fat-soluble vitamin deficiency andabnormal calcium metabolism after malabsorptivebariatric surgery. Journal of Gastrointestinal Surgery. 2004;8(1):48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 127.Quercia I, Dutia R, Kotler DP, Belsley S, Laferrere B. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2013 doi: 10.1016/j.diabet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ballantyne GH, Gumbs A, Modlin IM. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: role of the adipocytokines, leptin, adiponectin and resistin. OBES SURG. 2005;15(5):692–9. doi: 10.1381/0960892053923789. [DOI] [PubMed] [Google Scholar]
- 129.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91(4):1578–81. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 130.Wong IP, Baldock PA, Herzog H. Gastrointestinal peptides and bone health. Current opinion in endocrinology, diabetes, and obesity. 2010;17(1):44–50. doi: 10.1097/MED.0b013e3283344a05. [DOI] [PubMed] [Google Scholar]
- 131.Wucher H, Ciangura C, Poitou C, Czernichow S. Effects of weight loss on bone status after bariatric surgery: association between adipokines and bone markers. OBES SURG. 2008;18(1):58–65. doi: 10.1007/s11695-007-9258-0. [DOI] [PubMed] [Google Scholar]
- 132.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y Gastric Bypass Enhances Energy Expenditure and Extends Lifespan in Diet-induced Obese Rats. Obesity. 2009;17(10):1839–47. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2012;36(11):1373–9. doi: 10.1038/ijo.2012.115. [DOI] [PubMed] [Google Scholar]
- 134.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obesity Reviews. 2013;14(1):52–6. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 135.Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporosis Int. 2013:1–17. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]