Abstract
T-helper-17 (Th17) cells have critical roles in mucosal defense and in autoimmune disease pathogenesis 1-3. They are most abundant in the small intestine lamina propria (SILP), where their presence requires colonization of mice with microbiota 4-7. Segmented Filamentous Bacteria (SFB) are sufficient to induce Th17 cells and to promote Th17-dependent autoimmune disease in animal models 8-14. However, the specificity of Th17 cells, the mechanism of their induction by distinct bacteria, and the means by which they foster tissue-specific inflammation remain unknown. Here we show that the T cell receptor (TCR) repertoire of intestinal Th17 cells in SFB-colonized mice has minimal overlap with that of other intestinal CD4+ T cells and that most Th17 cells, but not other T cells, recognize antigens encoded by SFB. T cells with antigen receptors specific for SFB-encoded peptides differentiated into RORγt-expressing Th17 cells, even if SFB-colonized mice also harbored a strong Th1 cell inducer, Listeria monocytogenes, in their intestine. The match of T cell effector function with antigen specificity is thus determined by the type of bacteria that produce the antigen. These findings have significant implications for understanding how commensal microbiota contribute to organ-specific autoimmunity and for developing novel mucosal vaccines.
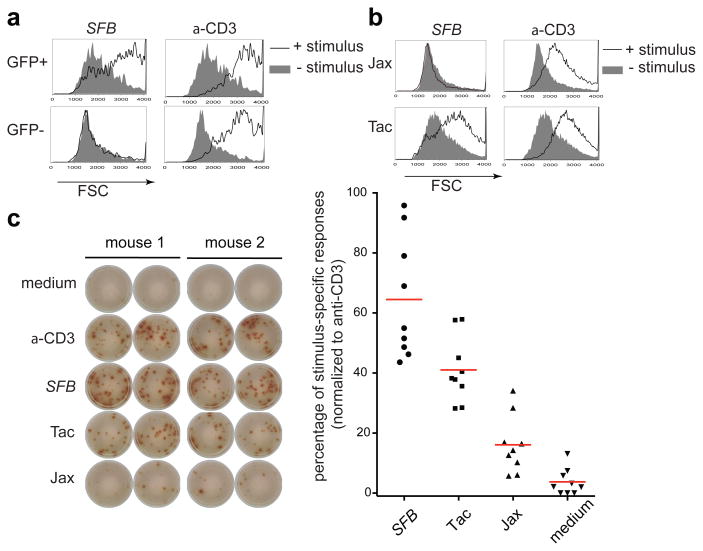
How SFB induces Th17 cells and how these cells contribute to self-reactive pathological responses remain key unanswered questions. A recent study, using mice with monoclonal TCRs, suggested that induction of Th17 cells by SFB or other microbiota is independent of cognate antigen recognition 15. To further evaluate mucosal effector T cell induction in a physiological setting, we undertook an examination of the repertoire and specificity of naturally-arising Th17 cells. To facilitate analyzing live Th17 cells, we used Il-23rGFP reporter mice 16, as among CD4+ T cells, only this subset expresses IL-23R. We first asked if SILP Th17 cells are in general responsive to gut luminal commensal antigens. GFP+ (Th17) and GFP- (non-Th17) CD4+ T cells, purified from Il-23rGFP/+ C57BL/6 (B6) mice that had been colonized with SFB, were incubated with splenic antigen-presenting cells (APCs) and autoclaved small intestinal luminal content of mice from the Jackson laboratory (Jax) and Taconic Farms (Tac). We used the measure of forward scatter (FSC) as a surrogate readout for T cell activation. Intriguingly, only Th17 cells mounted a detectable response to Tac antigens (Extended Data Fig. 1a). SFB is one of the bacteria unique to Taconic flora 8. Thus we repeated the assay with fecal material from SFB-monoassociated mice (SFB-mono antigens) and detected a robust response only among GFP+ cells (Fig. 1a). These cells did not respond to MHCII-deficient APCs loaded with SFB-mono antigens, indicating that the activation was dependent on antigen presentation (Extended Data Fig. 1b). SFB-mono antigens selectively stimulated total CD4+ T cells from B6 Tac mice, but not those from B6 Jax mice, consistent with in vivo priming of SFB-specific Th17 cells (Fig. 1b), and any bystander effect in this assay was negligible (Extended Data Fig. 1c). Next, we used an IL-17A ELISPOT assay to quantify the percentage of Th17 cells from SFB-colonized mice responding to commensal antigens. GFP+ cells had a relatively weak response towards Jax antigens, but had a robust response towards Tac antigens. Significantly, SFB mono-associated mouse fecal antigens stimulated over 60% of the Th17 cells (Fig. 1c). In contrast, there was no response of Th17 cells to fecal material from germ-free mice (data not shown). Thus, the majority of Th17 cells in the SILP of SFB-colonized mice react with SFB-derived antigens, while a small proportion respond to non-SFB antigen, indicating that most Th17 cells are specific for bacteria in the intestinal lumen.
Fig. 1. Intestinal Th17 cells are specific for SFB- and other microbiota-derived antigens.
(a) Selective activation of intestinal GFP+ CD4+ T cells from Il-23rGFP/+ mice by fecal extract from SFB-monoassociated mice. Forward scatter (FSC) was evaluated after 2 days. (b) Activation of SILP CD4+ T cells from B6 Tac mice and B6 Jax mice with fecal extract from SFB-monoassociated mice. (c) IL17A ELISPOT assay of intestinal GFP+ CD4+ T cells from SFB-colonized Il-23rGFP/+ mice treated with indicated stimuli. Left: Representative ELISPOT images. Right: Compilation of results from multiple animals. Each symbol represents cells from a separate animal.
We wished to compare T cell antigen receptor (TCR) repertoires of Th17 cells and those of non-Th17 cells. Using antibodies against a panel of TCR Vβ's, we observed a higher proportion of Vβ14+ T cells in Th17 cells than in non-Th17 cells from the SILP (Extended Data Fig. 2a and 2b). This bias was recapitulated when the CD4+ T cells were stained with antibodies specific for RORγt and IL-17A, two other characteristic markers of Th17 cells (Extended Data Fig. 2c). However, intracellular staining for IFNγ and Foxp3 indicated no Vβ14+ cell bias among Th1 and Treg cells (Extended Data Fig. 2c). To determine if the Vβ14 enrichment of Th17 cells is influenced by microbiota, we compared SFB-free B6 Jax mice with SFB-colonized B6 Tac mice. The Jax mice had few RORγt+ Th17 cells, and there was no enrichment of Vβ14+ cells among them. In contrast, Jax mice cohoused with Tac mice had increased numbers of lamina propria Th17 cells, which were enriched for Vβ14+ TCRs (Extended Data Fig. 2d), indicating that the Th17 repertoire is shaped by specific microbiota.
We chose to focus on Vβ14+ cells to further elucidate the gut CD4+ T cell repertoire. First, we used pyrosequencing to examine the repertoire of Vβ14+ SILP Th17 and non-Th17 cells from SFB-colonized mice. The complementarity determining region 3 (CDR3) of Vβ14 was determined for each cell population from eight Il-23rGFP/+ mice. Each sample contained a minimum of several hundred unique CDR3 sequences (Extended Data Fig. 3a). Interestingly, the ten most frequently used unique CDR3 sequences accounted for 60% of the Th17 and only 40% of the non-Th17 repertoire (Extended Data Fig. 3b). Furthermore, the dominant CDR3 sequences in individual mice exhibited a clear bias towards either Th17 or non-Th17 cells (Supplementary Table 1). Many of these CDR3 sequences were shared between mice and were enriched either in Th17 or in non-Th17 cells in individual mice (Extended Data Fig. 3c).
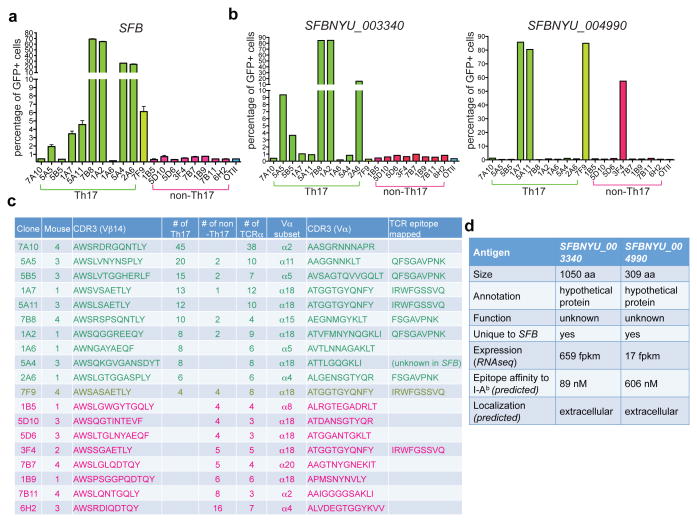
The finding that intestinal Th17 cells have a distinct repertoire prompted us to further determine their antigen specificity. Thus, we sorted single T cells from four mice and sequenced their Vβ14 and paired Vα chains (Extended Data Fig. 4a). Notably, each mouse carried some Vβ14 sequences that were present in multiple sorted cells, and these sequences strongly biased towards Th17 or non-Th17 cells (Extended Data Fig. 4b), corroborating our findings from the high-throughput sequencing analysis. To define the antigen specificity of the TCRs from intestinal Th17 and non-Th17 cell clones, we expressed a cohort of nineteen predominant clonotypic TCRs (ten Th17 clones, eight non-Th17 clones, and one neutral clone) in a NFAT-GFP+ hybridoma that can report on TCR signaling 17. Upon co-culture of the hybridomas with splenic APCs and heat-inactivated mouse intestinal luminal content, several Th17-TCR hybridomas, but not the non-Th17-TCR hybridomas, responded to Tac antigens, but not to Jax antigens (Extended Data Fig. 4c). Furthermore, when SFB-mono antigens were used, we detected responses from 7/10 Th17-TCR and the neutral TCR hybridoma, but none of the non-Th17 cell hybridomas (Fig. 2a). These responses were abrogated if the APCs were from MHCII-deficient mice (Extended Data Fig. 4d).
Fig. 2. Most Th17 TCR hybridomas recognize SFB-unique proteins.
(a) Responses of the TCR hybridomas, prepared from Th17 and non-Th17 intestinal CD4+ T cells, to fecal material from SFB-monoassociated mice. (b) Responses of the TCR hybridomas to E.coli clones expressing full-length SFBNYU_003340 and SFBNYU_004990. Note that a non-Th17 TCR hybridoma also responded to the clone expressing SFBNYU_004990. (c) Summary of the nineteen dominant clonotypic TCR clones. Ten Th17-biased clones are highlighted in green, and eight non-Th17-biased clones are highlighted in red. (d) Features of the two antigenic proteins of SFB.
We next sought to identify epitopes recognized by Th17 cell TCRs using a whole- genome shotgun cloning and expression screen, an unbiased approach previously used to identify T cell antigens from other bacteria 18 (Extended Data Fig. 5a). One bacterial clone, designated 3F12-E8, stimulated 7B8 and four other Th17-TCR hybridomas (Extended Data Fig. 5b, c and d). Based on the recent annotation of the SFB genome 19,20, we assigned the 672bp 3F12-E8 insert to an SFB gene (SFBNYU_003340 19). We confirmed the specificity by cloning the full-length gene and demonstrating that its product stimulated the aforementioned five TCRs, but not any other TCRs (Fig. 2b, left). We further mapped a minimal epitope that stimulated all five TCRs and a shorter 8 amino acid epitope that stimulated only the 7B8 and 2A6 hybridomas (Extended Data Fig. 5e).
Another expression screen was performed using the 1A7 hybridoma, which along with three other TCRs formed a distinct cluster with an identical Vα and highly similar Vβ14 CDR3 sequences (Extended Data Fig. 6a). A stimulatory clone, designated 2D10-A10 (Extended Data Fig. 6b & c), contained the N-terminal sequence of another SFB gene (SFBNYU_004990 19). We mapped the epitope for the 1A7 hybridoma to 9 amino acids (Extended Data Fig. 6d). Both the full-length gene product and a 9 amino acid peptide stimulated all four TCRs, indicating that these TCRs indeed recognize the same epitope (Fig. 2b, right). However, the single TCR derived from non-Th17 cells (3F4) displayed a much weaker dose-response to peptide antigen than the other TCRs (Extended Data Fig. 6e).
Thus, eight out of eleven Vβ14+ Th17-TCR hybridomas recognized two distinct antigens encoded by SFB (Fig. 2c). Both proteins are unique to SFB, expressed at medium to high level, and predicted to be secreted or at the cell surface (Fig. 2d). Importantly, primary Vβ14+ Th17 cells responded to the two immunodominant SFB epitopes (Extended Data Fig. 7a). Although Vβ14+ cells consistently responded slightly better, Vβ14- Th17 cells were also stimulated by SFB (Extended Data Fig. 7b), suggesting that these cells respond to other SFB epitopes. An in silico search was conducted for potential epitopes within the SFB proteome (Extended Data Fig. 7c and 7d), which yielded several more stimulatory peptides (Extended Data Fig. 7e). Among these, peptide N5, also derived from SFBNYU_003340, was a strong stimulator of intestinal Th17 cells, activating both Vβ14+ cells and Vβ14- cells (Extended Data Fig. 7f). Thus, in the small intestine, SFB is the dominant antigen source for polyclonal Th17 cells, but for few, if any, non-Th17 cells.
We then asked what fate is adopted by T cells expressing SFB-specific TCRs. We generated 7B8 TCR transgenic mice 21, and transferred naïve T cells from these mice into isotype-marked congenic B6 mice 22. After one week, we readily detected donor-derived T cells in the SILP of mice that had been exposed to SFB, whereas they were completely absent in SFB-deficient recipients (Extended Data Fig. 8a). Remarkably, almost all donor-derived cells became positive for RORγt (Fig. 3a). Similar results were obtained upon transfer of T cells from two other TCR (1A2 and 5A11) transgenic strains into SFB-colonized recipient mice (Extended Data Fig. 8b). The donor-derived T cells lacked expression of the transcription factors associated with alternative CD4 T cell programs (e.g. Foxp3, GATA3, and T-bet) (Extended Data Fig. 8c).
Fig. 3. SFB-specific T cells become Th17 cells in the SILP.
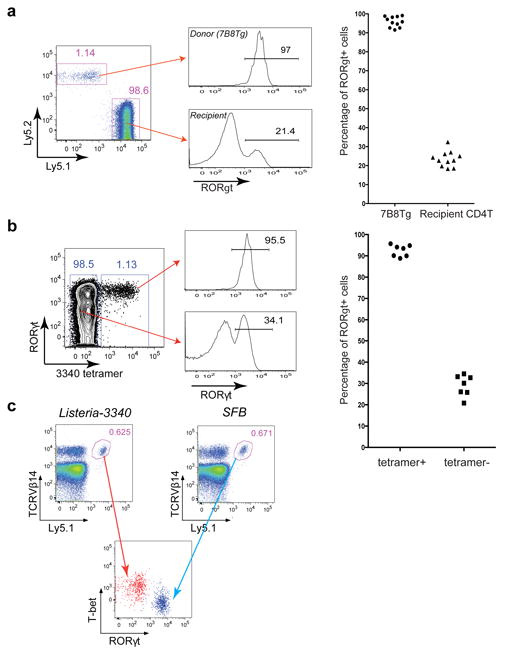
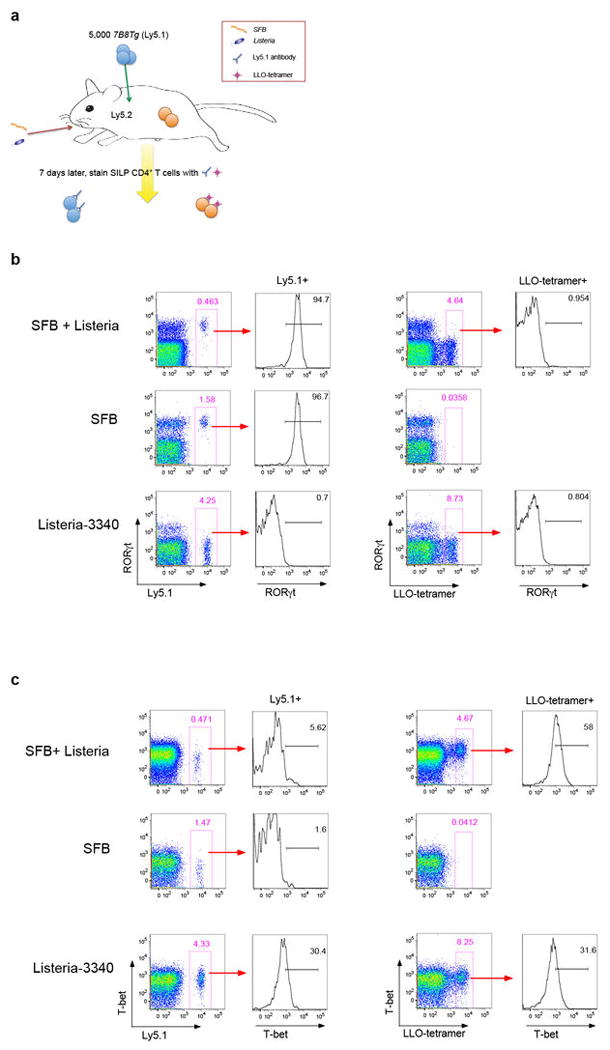
(a) 7B8Tg cells (Ly5.2) were transferred into SFB-colonized mice (Ly5.1), and SILP T cells were analyzed after 8-15 days. Left: Representative FACS plots. Right: Analysis of multiple animals (one symbol/animal). (b) I-Ab/3340-A6 tetramer stain of SILP T cells from SFB-colonized B6 mice. Left: Representative FACS plots. Right: Analysis of multiple animals (one symbol/animal). (c) 7B8Tg cells (Ly5.1) were transferred into Ly5.2 congenic hosts orally colonized with Listeria-3340 or SFB. Seven days after transfer, donor-derived cells in the SILP were analyzed. The results are representative of three experiments.
To visualize endogenous SFB-antigen-specific T cells, we produced MHCII-tetramers containing peptide A6 from SFBNYU_003340 (3340-A6 tetramer) 23. The I-Ab/3340-A6 tetramer specifically stained GFP+ SILP CD4+ T cells from SFB-colonized IL-23RGFP/+ mice (Extended Data Fig. 8d). Furthermore, a sizable population of I-Ab/3340-A6 tetramer-positive cells was present in B6 Tac, but not in B6 Jax mice (Extended Data Fig. 8e), and these cells were uniformly RORγt positive, indicating that they were SFB-elicited Th17 cells (Fig. 3b).
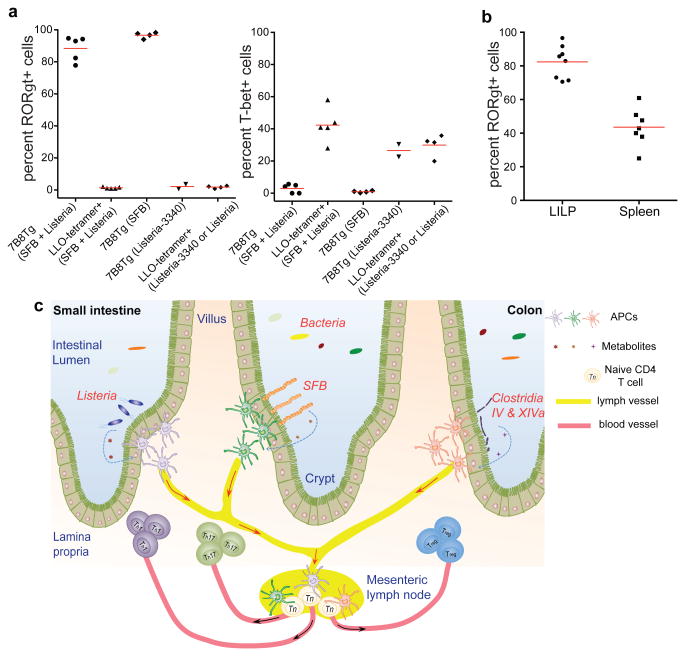
We next aimed to determine whether polarization of the antigen-specific Th17 cells in response to SFB colonization is dictated by the nature of the antigenic protein or properties of the microbe. Listeria monocytogenes, an enteric pathogenic bacterium that also colonizes the small intestine, typically elicits a Th1 response 24. Mice were orally infected with L. monocytogenes expressing SFBNYU-003340 (Listeria-3340) (Extended Data Fig. 8f) or SFB before intravenous transfer of 7B8Tg T cells. 7B8Tg T cells accumulated in the SILP of both sets of mice, but, importantly, they expressed T-bet rather than RORγt when the hosts were colonized with Listeria-3340 (Fig. 3c).
To further investigate a relationship between the fate of SILP T helper cells and the bacterial origins of antigens, we transferred 7B8Tg T cells into mice that were colonized with both SFB and Listeria and simultaneously tracked CD4+ T cell responses specific for both bacteria in the SILP using the Ly5.1+ congenic marker for 7B8Tg cells and LLO-tetramers that stain endogenous Listeria-specific T cells derived from the host (Extended Data Fig. 9a). In the presence of both Th17- and Th1-inducing bacteria, 7B8Tg T cells expressed RORγt, but not T-bet, whereas LLO-tetramer+ cells expressed T-bet, but not RORγt (Fig. 4a and Extended Data Fig. 9b and c). This result is in contrast to the Th1 polarization of TCR transgenic T cells specific for the commensal CBir1 flagellin antigen observed upon infection with the protozoan parasite Toxoplasma gondii 25, a Th1-inducing intestinal pathogen. This suggests that, unlike CBir1-encoding Clostridia, SFB is endowed with the ability to direct a dominant signal specialized for induction of Th17 cells.
Fig. 4. TCR specificity for distinct luminal bacteria underlies divergent T helper cell differentiation in the SILP.
(a) Th17 (RORγt) versus Th1 (T-bet) differentiation of SFB- (7B8Tg) and Listeria (LLO-tetramer)-specific CD4+ T cells in mice colonized with either or both bacteria. Each symbol represents cells from one animal. (b) Proportions of donor-derived 7B8Tg T cells that express RORγt in the colon and spleen of SFB-colonized mice. (c) Model for intestinal niches that promote diverse microbiota-dependent CD4+ effector T cell programs. Microbial signals, may induce polarizing cytokines or preformed niche-specific antigen presenting cells may interact with different T cell-inducing bacteria.
SFB colonization of the small intestine is potentially beneficial, attenuating pathogenic bacteria-induced colitis 8, but it can also trigger or exacerbate systemic autoimmune disease 10,11, raising the question as to whether SFB-specific Th17 cells can circulate beyond the small intestine. We examined the colons and spleens of SFB-positive recipients of 7B8Tg naïve T cells, and found these cells in both organs. Importantly, more than 80% of these SFB-specific T cells in colon and 40% in spleen expressed RORγt (Fig. 4b). Consistent with this result, staining of endogenous T cells from Tac mice revealed 3340-A6 tetramer-positive cells in the large intestine and most of these cells expressed RORγt (Extended Data Fig. 10a and b).
Thus, our results indicate that intestinal antigen-specific CD4+ T cells differentiate to become either Th1 or Th17 cells, depending on which luminal bacterium delivers the antigen. We would like to propose a deterministic model for T helper cell differentiation whereby the bacterial context of cognate antigen delivery dictates the fate of the antigen-specific T cells (Fig. 4c). Our work opens the way towards elucidating the mechanisms of Th17 cell induction by microbiota and of how gut-induced Th17 cells can contribute to distal organ-specific autoimmune disease. In addition, it serves as a guide for future studies of human commensal-specific pro-inflammatory T cells that are thought to contribute to autoimmune diseases such as rheumatoid arthritis 26. Finally, the demonstration of controlled polarized T cell responses towards commensal bacteria offers the potential for novel approaches towards mucosal vaccination.
Methods Summary
Mice
All mice were housed in the animal facility of The Skirball Institute of Biomolecular Medicine at the New York University School of Medicine. Experimental protocols were approved by the Institutional Animal Care and Use Committee. C57BL/6 mice were purchased from Taconic Farm (B6 Tac) or the Jackson Laboratory (B6 Jax). Il-23rGFP mice 16, a gift from M. Oukka (Seattle, Children's Hospital), were maintained by breeding with B6 Tac mice. 7B8Tg, 1A2Tg and 5A11Tg SFB-specific TCR transgenic (Tg) mice were generated as previously described 21 and kept with SFB-minus flora. For adoptive transfer, naive Tg T cells (CD62Lhi CD44lo Vβ14+ CD4+ CD3+) were sorted from the spleen and were injected intravenously into congenic recipient mice 22.
Generation of TCR hybridomas
Retroviruses carrying an expression cassette encoding TCRα, TCRβ, and CD4 were used to infect the NFAT-GFP 58α-β- hybridoma cell line 17.
Construction and screen of whole-genome shotgun library of SFB
The shotgun library was prepared with a procedure modified from a previous study 18. The library is estimated to contain 104 clones. The expression of exogenous proteins was induced by IPTG for 4 hours. For antigen screening, pools of heat-killed bacteria (∼30 clones per pool) were added to a co-culture of APCs and hybridomas.
MHCII tetramer production and staining
MHCII/3340-A6 tetramer was produced as previously described 23. SILP T cells were incubated at room temperature for 60 min with fluorochrome-labeled tetramer (10 nM) before staining with relevant antibodies at 4°C.
Heterologous expression of SFBNYU_003340 in Listeria monocytogenes
The entire coding region of SFBNYU_003340, including its predicted signal sequence, was sub-cloned into the Listeria expression vector pIMK2 27. The resultant plasmid was transformed into electrocompetent Listeria monocytogenes strain 10403S-inlAm and plated on selective medium containing kanamycin (50 μg/ml) 28.
Methods
Mice
C57BL/6 mice were purchased from Taconic Farm (B6 Tac) or Jackson Laboratory (B6 Jax). Il-23rGFP mice 16 were kindly provided by Dr. Mohammed Oukka (Seattle, Children's Hospital) and maintained by breeding with B6 Tac mice. Ly5.1 mice (B6.SJL-Ptprca Pepcb/BoyJ) and MHCII-deficient mice (B6.129S2-H2dlAb1-Ea/J) were from Jackson Laboratory.
Antibodies and flow cytometry
The following antibodies were from eBiosciences, BD Pharmingen or BioLegend: Vβ2 (B20.6), Vβ3 (KJ25), Vβ4 (KT4), Vβ5 (MR9-4), Vβ6 (RR4-7), Vβ7 (TR310), Vβ8 (F23.1), Vβ8.1/8.2 (MR5-2), Vβ8.3 (8C1), Vβ10 (B21.5), Vβ11 (CTVB11), Vβ12 (MR11-1), Vβ14 (14-2), CD3 (145-2C11), CD4 (RM4-5), CD25 (PC61), Ly5.1 (A20), Ly5.2 (104), MHCII (M5/114), RORγt (AFKJS-9 or B2D), FOXP3 (FJK-16s), T-bet (eBio4B10), GATA3 (TWAJ), IL-17A (eBio17B7) and IFNγ (XM61.2). Flow cytometric analysis was performed on an LSR II (BD Biosciences) or an Aria II (BD Biosciences) and analyzed using FlowJo software (Tree Star). DAPI (Sigma) was used to exclude dead cells.
T cell preparation and staining
Small intestine lamina propria were minced and then incubated for 30 min at 37°C with collagenase D (1mg/ml; Roche), dispase (0.05U/ml; Worthington) and DNase I (100μg/ml; Sigma). Lymphocytes were collected at the interface of a 40%/80% Percoll gradient (GE Healthcare). Cells were stained for surface markers, followed by fixation and permeabilization (eBioscience).
Calculating enrichment scores
An enrichment score for a given Vβ in IL-23R (GFP)+ cells is defined as the equation of (% of Vβ+ cells in the GFP-positive fraction) / (% of Vβ+ cells in the GFP-negative fraction). e.g. for Extended Fig. 2b, Vβ14 enrichment was calculated as (7.45/ (7.45+26.2))/ (4.48/ (4.48+61.8)) or 3.3. A score > 1 means a positive enrichment and a score ≈ 1 means no enrichment.
High throughput TCR sequencing
The SILP cells from Il-23rGFP/+ mice were stained for surface markers and Vβ14+ CD4+ T cells were sorted on the Aria II. For each sample, we collected about 2 × 104 cells (2.17±0.43 × 104 cells for GFP+ Th17 cells and 2.38±0.54 × 104 cells for GFP- non-Th17 cells). Cells were lysed in Trizol reagent (Invitrogen) and RNA was extracted following the manufacture's instruction. RNA precipitation was aided with GlycoBlue (Invitrogen). cDNAs were prepared with a reverse transcription kit (USB). Vβ14 PCRs were performed using barcoded oligos. PCR products from 16 samples were quantified on Nanodrop. Equal amounts of barcoded PCR product were mixed and sequenced using a 454 GS Junior system (Roche). The raw sequencing data was first aligned using the high-throughput analysis tool provided by IMGT 29. We obtained 6647±954 reads for Th17 cells and 5573±889 reads for non-Th17 cells. CDR3 usage was further computed with Perl-based scripts developed in-house. The Th17 samples had 340-772 unique Vβ14 CDR3 sequences and the non-Th17 samples had 849-2148 unique Vβ14 CDR3 sequences.
Single cell TCR sequencing
The SILP cells from Il-23rGFP/+ mice were stained for surface markers. GFP+ and GFP- Vβ14+ CD4+ T cells were sorted on the BD Aria II and deposited at one cell per well into 96-well PCR plates preloaded with 5μl reverse transcription mix (USB). Immediately after sorting, whole plates were incubated at 50°C for 60 min for cDNA synthesis. Half of the cDNA was used for Vβ14 PCR using forward primer 5′- ACGACCAATTCATCCTAAGCAC -3′ and reverse primer 5′- AAGCACACGAGGGTAGCCT -3′. To retrieve Vα sequences, the other half of cDNA was preamplified for 16 cycles using a mix of twenty-one forward primers 30 (each modified by adding a 5′ extended anchor sequence: TAATACGACTCACTATAGGG) and a reverse primer 5′- CATGTCCAGCACAGTTTTGTCAGT -3′. The primary Vα PCR products were diluted and subjected to a second round PCR using forward primer 5′- TAATACGACTCACTATAGGG -3′ and reverse primer 5′- GTCAAAGTCGGTGAACAGGC -3′. PCRs were performed in a Lightcycler 480 (Roche). PCR products were cleaned up with ExoSap-IT reagent (USB) and Sanger sequencing was performed by Macrogen. In nearly all cases, for cells with the same Vβ14 sequence, we retrieved a single unique Vα sequence, indicating that these cells were clonotypically identical.
Generation of TCR hybridomas
The NFAT-GFP 58α -β- hybridoma cell line 17 was kindly provided by Dr. Kenneth Murphy (Washington University, St. Louis). To reconstitute TCRs, we used a self-cleavage sequence of 2A to link cDNAs of TCRα and TCRβ generated from annealing of overlapping oligos (TCRα-p2A-TCRβ) and shuttled the cassette into a modified MigR1 retrovector in which IRES-GFP was replaced with IRES-mCD4. Then retroviral vectors were transfected into Phoenix E packaging cells using Lipofectamine 2000 (Invitrogen). Hybridoma cells were transduced with viral supernatants in the presence of polybrene (8μg/ml) by spin infection for 90 min at 32°C. Transduction efficiencies were monitored by checking mCD3 surface expression on day 2. We generated nineteen hybridomas for predominant clonotypic TCRs whose Vα and Vβ sequences were retrieved from single-cell TCR sequencing (# of Vβ ≥ 6 for Th17 biased clones, and # of Vβ ≥4 for Non17 biased clones, # of Vα ≥ 3. Note that a single unique Vα was identified for every Vβ). We also generated the OTII hybridoma using TCR sequences kindly provided by Dr. Francis Carbone (chicken ovalbumin antigen-specific, I-Ab restricted).
Assay for hybridoma activation
To prepare antigen-presenting cells, splenocytes from B6 mice that were injected i.p. with 8 × 106 FLT3-B16 melanoma cells 10 days before were positively enriched for CD11c+ cells using MACS LS columns (Miltenyi). 1 × 104 hybridoma cells were incubated with 2 × 105 APCs and autoclaved antigens from intestinal luminal contents or fecal material for two days. GFP induction in the hybridomas (CD3+ fraction) was analyzed by flow cytometry.
Construction and screen of whole-genome shotgun library of SFB
The shotgun library was prepared with a procedure modified from a previous study 18. In brief, genomic DNA was purified from the feces of SFB-monoassociated mice by phenol:choloroform extraction. DNA was subjected to whole-genome amplification with the REPLI-g kit (Qiagen) following the manufacturer's instructions. Amplified materials were partially digested with Sau3A (NEB), then ligated with the BamHI-linearized pGEX-4T1 expression vector (GE Healthcare). Ligation products were introduced into competent Stbl3 cells (Invitrogen). To ensure the quality of the library, we sequenced the inserts of randomly picked colonies. All the sequences were mapped to the SFB genome. The library is estimated to contain 104 clones. We grew bacteria in 96-well deepwell plates (VWR) with AirPort microporous cover (Qiagen). The expression of exogenous proteins was induced by IPTG for 4 hours. Then bacteria were heat killed by incubating at 70°C for 1 hour, and stored at -20°C until use. For antigen screens, pools of bacterial clones (∼30 clones per pool) were added to a co-culture of APCs and hybridomas. Clones within the positive pools were screened individually against the hybridoma bait. Finally, the inserts of positive clones were subjected to Sanger sequencing. The sequences were blasted against the SFB genome and aligned to annotated open reading frames.
Epitope mapping
We expressed overlapping fragments spanning the active ORF using the pGEX-4T1 bacterial expression system, and colonies were used to stimulate the relevant hybridoma. This process was repeated until we identified minimal fragments conferring antigenicity. The mapping was further verified by stimulating hybridomas with synthetic peptides (Genescript).
RNA-seq analysis of the SFB transcriptome
Wild-type B6 mice from Jackson Laboratory or Taconic Farm, confirmed for the presence or absence of SFB by qPCR 19, were used for microbiome transcriptome analysis. Within five minutes after sacrifice, the terminal ileum of each mouse was resected and luminal contents were squeezed with sterile forceps into a mortar cooled with liquid nitrogen. 1 ml nuclease-free TE was washed through the ileum into the mortar. The total luminal contents and washing were then ground to a fine powder with a pestle cooled with liquid nitrogen and kept on dry ice. The powder was then transferred to 15 mL Trizol (Life Technologies) in a 50 mL falcon tube and vortexed. The manufacturer's protocol was then used to obtain RNA. The resulting RNA was extracted twice with acid phenol-chloroform, precipitated, treated with Ambion Turbo DNA-free, and cleaned-up with an RNeasy column to yield RNA with an undetectable concentration of DNA by Qubit. A portion of this RNA was treated once with Epicentre's Ribo-Zero rRNA removal kit, using equal volumes of specific oligos from the Meta-bacteria and Human/mouse/rat kits. An Illumina RNA-seq library was prepared from these samples using a previously-described strand-specific Nextera protocol. The resulting reads were aligned to the SFBNYU genome with Bowtie 31 and transcript abundance was estimated using Cufflinks 32 with default parameters.
Production of anti-SFB antibody and immunostaining
The cDNA fragments corresponding to amino acids 43-359 (3340N) and 734-1060 (3340C) of SFBNYU_003340 were cloned into the pGEX6p1 expression vector. Recombinant proteins fused to N-terminal GST were expressed in E.coli BL21, purified with Glutathione sepharose 4B (GE), and were released with PreScission protease (GE). The flowthrough fractions containing polypeptides without the GST tag were collected as immunogen. Rabbit polyclonal antibodies against both polypeptides were raised by Covance. For immunostaining, bacteria were fixed with 2% paraformaldehyde, followed by washing with 0.5% TritonX-100. Bacteria were incubated sequentially with primary antibody (1:1 mix of the two rabbit-anti-3340 antibodies) and PE-conjugated goat anti-rabbit antibody.
Activation of polyclonal SILP Th17 cells
GFP+ and GFP- SILP CD4+ T cells sorted from Il-23rGFP/+ mice were incubated with 2 × 105 APCs (CD11c+ cells purified from the spleen) and indicated stimuli in complete RPMI medium supplemented with IL-2 (10u/ml) and IL-7 (5ng/ml) for 2∼3 days. Cells were harvested and stained with Vβ-specific antibodies. Forward scatter increment, as readout for cell activation, was analyzed by FACS.
IL-17A ELISPOT assay
IL-17A ELISPOT was performed with a Mouse/Rat IL-17A ELISPOT Ready-SET-Go! kit (eBioscience). Dots were automatically enumerated with ImmunoSpot software (Version 5.0)
MHCII tetramer production and staining
I-Ab/3340-A6 tetramer was produced as previously described 23. Briefly, QFSGAVPNKTD, an immunodominant epitope from SFBNYU_0033400, covalently linked to I-Ab via a flexible linker, was produced in Drosophila S2 cells. Soluble pMHCII monomers were purified, biotinlyated, and tetramerized with PE- or APC- labeled streptavidin. To stain endogenous cells, SILP cells were first resuspended in FACS buffer with FcR block, 2% mouse serum and 2% rat serum. Then tetramer was added (10 nM) and incubated at room temperature for 60 min. Cells were washed and followed by regular staining at 4°C. I-Ab/2W and I-Ab/LLO tetramers were previously described 23, 33.
Generation of Th17-TCRTg mice
TCR sequences of 7B8, 1A2 and 5A11 were cloned into the pTα and pTβ vectors kindly provided by Dr. Diane Mathis 21. TCR transgenic animals were generated by the Rodent Genetic Engineering Core at the New York University School of Medicine. Positive pups were genotyped by PCR and kept on SFB-minus flora.
Adoptive transfer
Spleens from 7B8Tg mice were harvested and disassociated. Red blood cells were lysed using ACK lysis buffer (Lonza). Naive Tg T cells (CD62Lhi CD44lo Vβ14+ CD4+ CD3+) were sorted on a BD Aria II. Cells were transferred into congenic Ly5.1 recipient mice by retro-orbital injection. In some experiments, we used Ly5.1/Ly5.2 TCRTg mice as donor and transferred naive Tg T cells to congenic Ly5.2 recipient mice.
Heterologous expression of SFBNYU_003340 in Listeria monocytogenes
To generate strains of L. monocytogenes that express the SFBNYU_003340 antigen, the entire coding region including its predicted signal sequence was PCR-amplified from a plasmid containing the SFBNYU_003340 gene. The resultant PCR product was digested and sub-cloned into the Listeria expression vector pIMK2 (provided by Colin Hill), allowing the gene to be expressed under the synthetic promoter Phelp (High expression promoter in L.M.) 27. The resultant plasmid designated pIMK2-3340 was transformed into electrocompetent Listeria monocytogenes strain 10403S-inlAm (provided by Nancy E. Freitag) and plated on selective medium containing Kanamycin (50 μg/ml) 28. pIMK2 is a derivative of the plasmid pPL2 and stably integrates in single copy within the tRNAArg gene following electroporation 34. The integrity of the SFBNYU_003340 gene was validated by PCR and expression confirmed by coomassie staining of L. monocytogenes exoproteins.
Oral infection with SFB and L. monocytogenes
For SFB colonization, we dissolved in sterile PBS fresh fecal pellets collected from Il-23rGFP/GFP RAG2-/- mice that have highly elevated levels of SFB, and infected mice by oral gavage. For L. monocytogenes colonization, we grew Listeria-3340 and Listeria-empty in brain heart infusion medium and infected mice orally with 1×109 c.f.u.
Bioinformatic analysis
Protein predictions were made by bioinformatic tools, including Psort (Version 3.0) 35 and Cello (Version 2.5) 36 for localization prediction, and IEDB (Immune Epitope Database) for MHCII binding affinity prediction.
Statistical analysis
All analyses were performed using GraphPad Prism (Version 6.0). Differences were considered to be significant at P values <0.05.
Extended Data Legends
Extended Data Fig. 1. Stimulation of SILP Th17 cells requires intestinal microbiota antigen presentation.
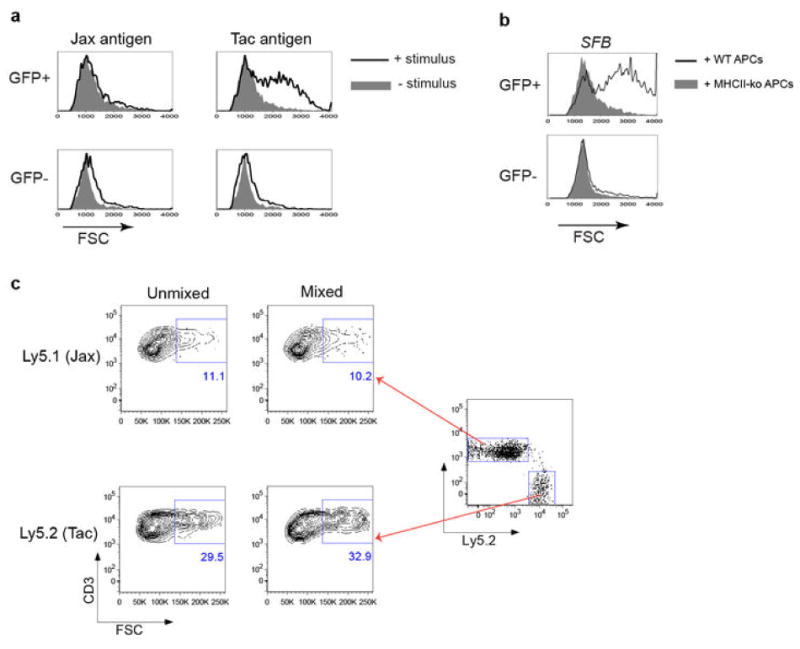
(a) Intestinal GFP+ CD4+ T cells from Il-23rGFP/+ mice stimulated with fecal material from Jax and Tac mice in the presence of syngeneic splenic APCs. Forward scatter was evaluated after 2 days. (b) Th17 cell activation by fecal material from SFB-monoassociated mice in the presence of APCs sufficient (WT) or deficient (KO) for MHC class II. (c) Evaluation of potential activation of bystander CD4+ T cells upon stimulation with SFB antigen. SILP CD4+ T cells from mice with Jax flora (Ly5.1) and Taconic flora (Ly5.2) were co-cultured or stimulated separately with APCs and SFB-monoassociated fecal material, and FSC was evaluated.
Extended Data Fig. 2. Microbiota-dependent TCR usage bias among SILP Th17 cells.
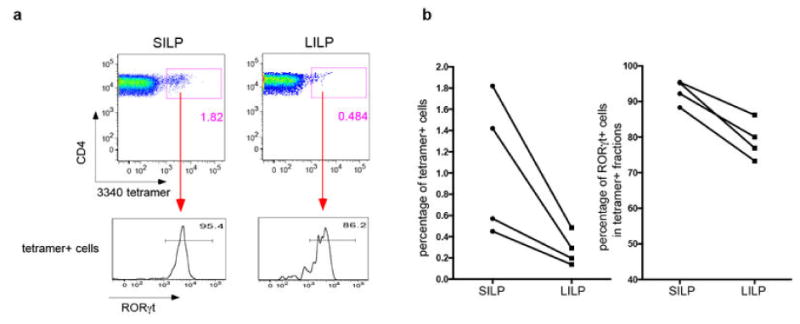
(a) SILP CD4+ T cells from Il-23rGFP/+ mice were analyzed for utilization of Vβ's in Th17 cells versus non-Th17 cells. Ratios of the percentage of each TCR Vβ in GFP+ vs. GFP- cells are shown. Each symbol represents one mouse. (b) Relative expression of Vβ14 and Vβ6 TCRs by SILP Th17 versus non-Th17 CD4+ T cells from Il-23rGFP/+ mice. Left: Representative FACS plots; Right: Analysis of multiple animals. (c) Specific enrichment of Vβ14 TCRs in CD4+ T cells expressing RORγt and IL-17A, but not FOXP3 or IFNγ. Left: Representative FACS plots. Right: Analysis of multiple animals. Each symbol represents one mouse. (d) Correlation of Vβ14 enrichment in Th17 cells with the presence of specific commensal microbiota. B6 Jax mice were housed alone or cohoused with B6 Tac mice for two weeks. Left: Representative FACS analyses. Right: Analysis of multiple animals.
Extended Data Fig. 3. Th17 TCR repertoire analysis by pyrosequencing.
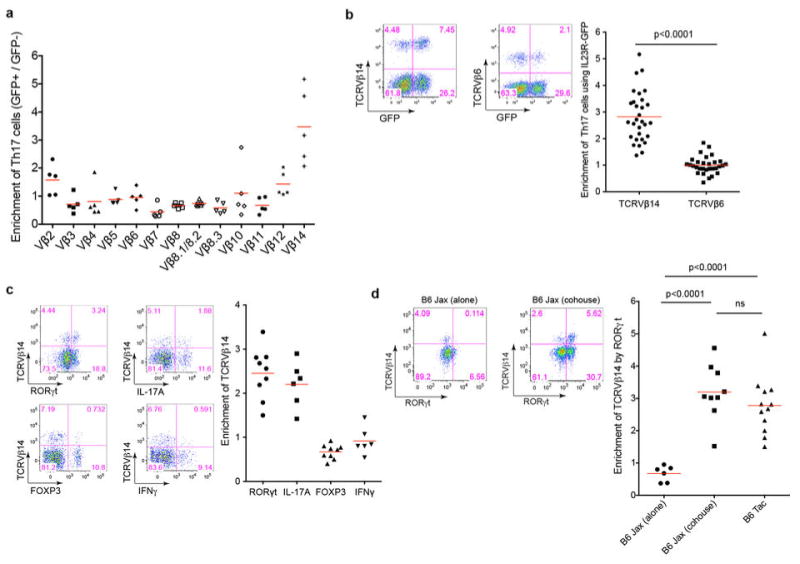
(a) Numbers of unique Vβ14 CDR3 sequences of individual SILP Th17 and non-Th17 samples. The sequences were normalized for numbers of cells and total reads. (b) Preferential expansion of Vβ14+ clones in the Th17 compartment in the SILP. The proportions of the 10 most abundant Vβ14 CDR3 sequences from Th17 and non-Th17 cells from 8 mice are shown. (c) Th17-non Th17 bias of unique Vβ14 CDR3 sequences in the SILP of multiple mice.
Extended Data Fig. 4. Single-cell TCR cloning and TCR hybridoma screen.
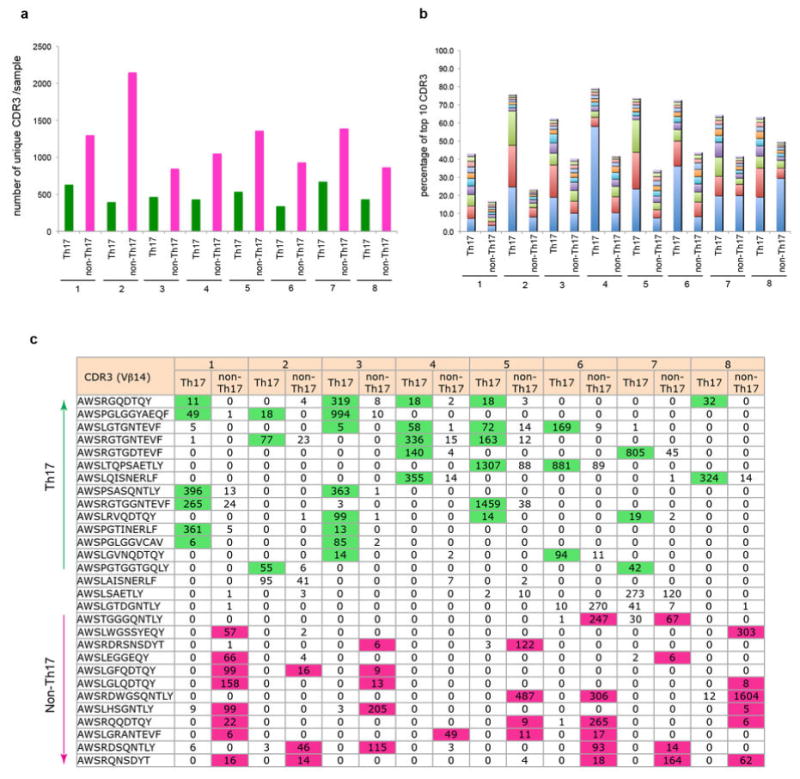
(a) Efficiency of single-cell Vβ14 cloning from SILP Th17 and non-Th17 cells of multiple mice. (b) Distributions of unique Vβ14 sequences in Th17 and non-Th17 cells within the SILP. Each plot represents one mouse shown in (a). y and x axes represent numbers of Th17 cells and non-Th17 cells for each unique Vβ14 sequence. Numbers of unique sequences are shown in colored circles. (c) Responses of Th17 and non-Th17 TCR hybridomas to small intestinal luminal contents from B6 Tac and B6 Jax mice. (d) Stimulation of Th17 TCR hybridomas by SFB-monoassociated antigens in the presence of APCs sufficient (WT) or deficient (KO) for MHC class II.
Extended Data Fig. 5. Identification of SFBNYU_003340 epitopes recognized by a subset of the Th17 TCR hybridomas.
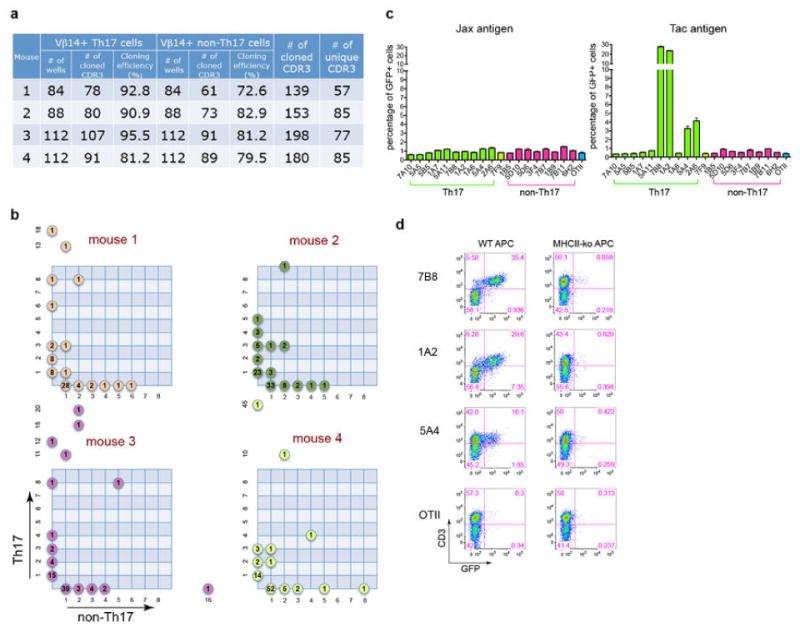
(a) Schematic representation of the antigen screen using a whole-genome shotgun SFB library. (b) Stimulation of the 7B8 hybridoma by bacterial pool 3F12. (c) Reactivity of 7B8 and four other TCR hybridomas with bacterial clone 3F12-E8. (d) Diversity of the CDR3 sequences of TCRs specific for 3F12-E8. Note that they belong to different Vα subsets and have distinct Vβ14 CDR3 sequences. (e) Responses of the 3F12-E8-specific TCR hybridomas to core epitopes encoded by minigenes expressed in E.coli.
Extended Data Fig. 6. Identification of SFBNYU_004990 epitopes recognized by related TCRs.
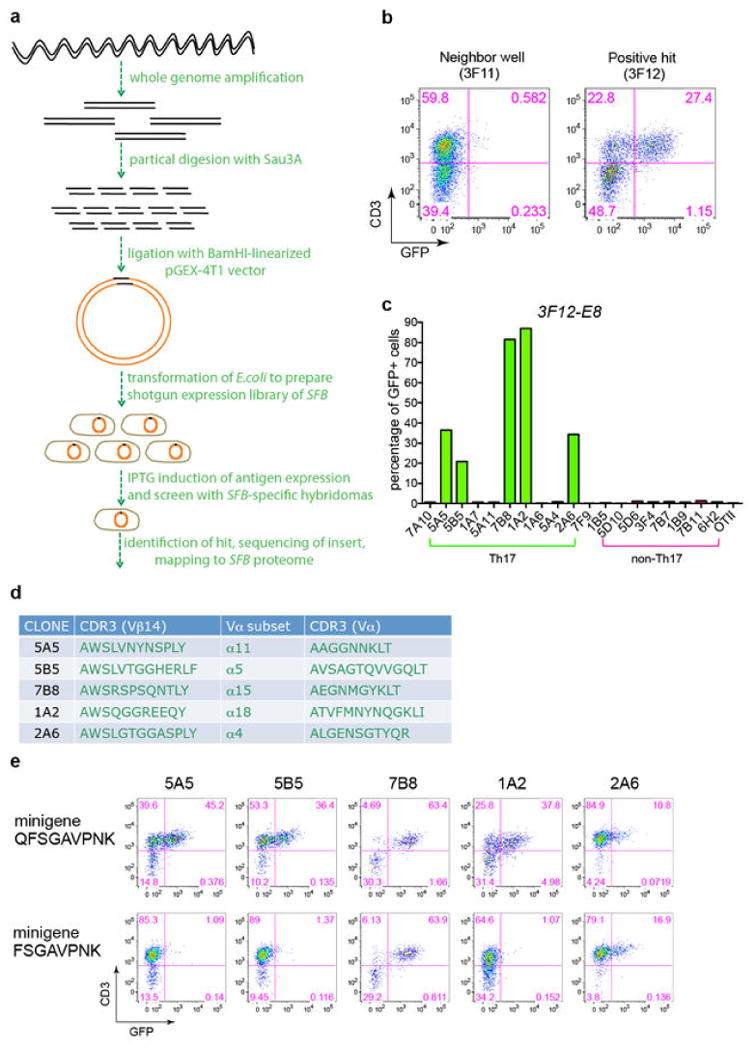
(a) Top: The distribution in Th17 and non-Th17 cells of four TCRs that share an identical TCRα chain. Bottom: Amino acid alignment of the Vβ14 CDR3 sequences. The green box highlights the sequence differences. (b) Stimulation of the 5A11 hybridoma by bacterial pool 2D10 in the SFB antigen screen. (c) Responses of 4 TCR hybridomas, including a non-Th17 hybridoma, to bacterial clone 2D10-A10. (d) Responses of the 2D10-A10-specific TCR hybridomas to core epitopes encoded by minigenes expressed in E.coli. (e) TCR hybridoma responses to titrated synthetic peptide (IRWFGSSVQKV) in the presence of APCs.
Extended Data Fig. 7. SFB epitopes recognized by diverse Th17 cell TCRs.
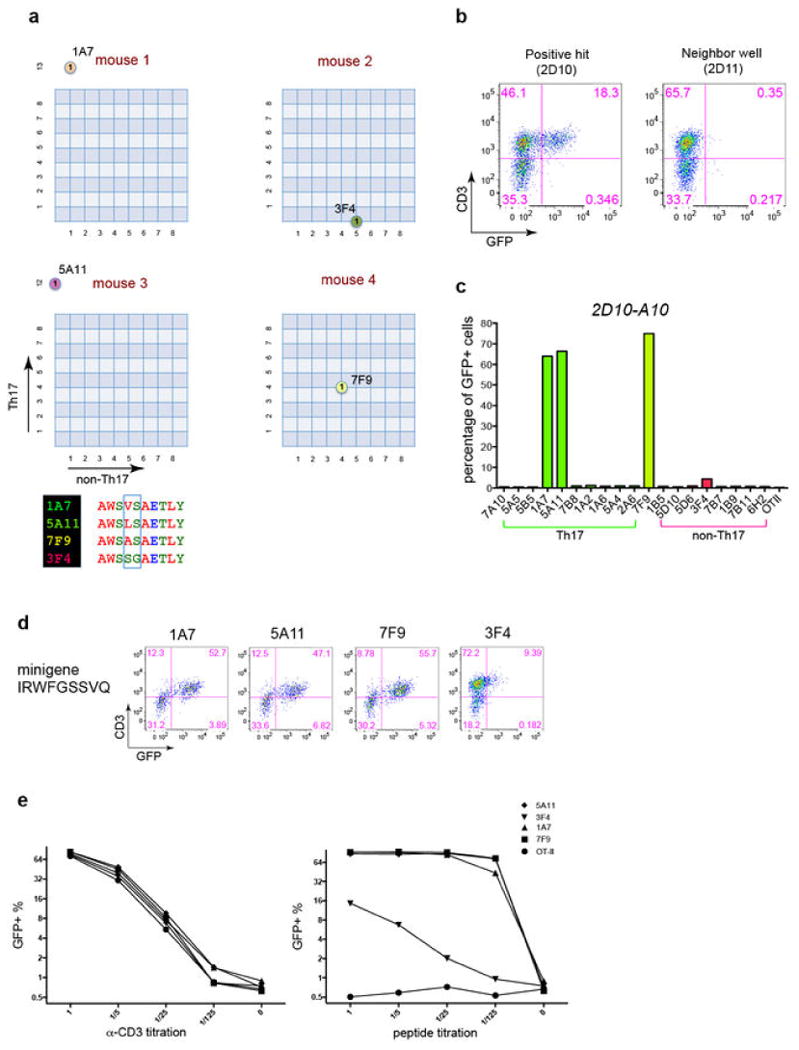
(a) The epitopes recognized by the Vβ14+ TCR hybridomas stimulate only Vβ14+ Th17 cells from the SILP. Th17 cells sorted from Il-23rGFP/+ mice were stimulated with indicated peptides (listed in (d)) in the presence of APCs. Left: Representative IL-17A ELISPOT assay with triplicates. Right: Normalized peptide-specific Th17 responses. Each dot represents one mouse. (b) Polyclonal responses of Vβ14+ and Vβ14- SILP Th17 cells to SFB antigens. Representative FACS plots from five experiments are shown. (c) Bioinformatics filtering approach to select candidate SFB epitopes. (d) Summary of newly-selected and the known A6 and A15 SFB peptides. (e) IL-17A ELISPOT screen for indicated peptides using SILP Th17 cells sorted from SFB-colonized Il-23rGFP/+ mice. The A6 peptide from SFBNYU_003340 and anti-CD3 served as positive controls. (f) Vβ14 usage in Th17 cells specific for peptide N5. Left: Representative IL-17A ELISPOT assay with triplicates for peptide N5, using Vβ14+ and Vβ14- SILP Th17 cells sorted from Il-23rGFP/+ mice. Right: Normalized N5-specific Th17 responses. Each dot represents one mouse.
Extended Data Fig. 8. SFB-specific T cells become Th17 cells in SFB-colonized mice.
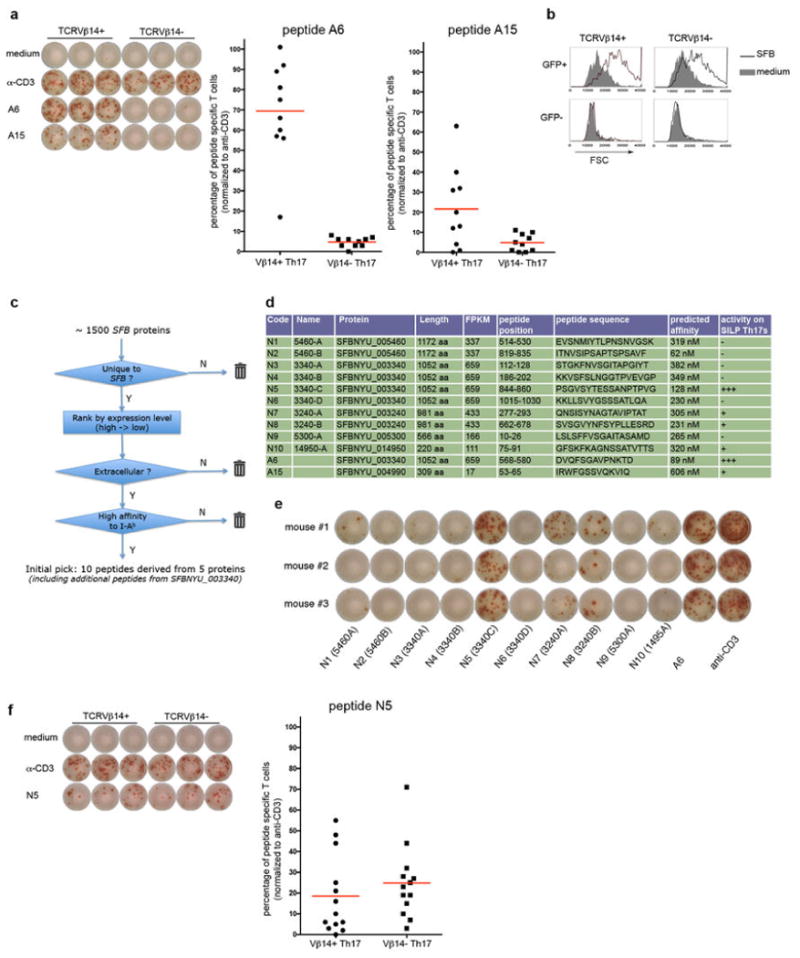
(a) SFB-dependent 7B8Tg T cell accumulation in the SILP. 2×104 naive 7B8Tg T cells were transferred into congenic Ly5.1 recipient mice that were SFB-colonized or SFB-free. CD4+ T cells in the SILP were examined for donor and recipient isotype markers after 13 days. (b) Top: Strategy for co-transfer of congenic 1A2Tg and 5A11Tg T cells into SFB-colonized recipient mice. Bottom: FACS analysis of RORγt expression in host- and donor-derived CD4+ T cells in the SILP at 7 days after transfer. (c) FACS analysis of transcription factors in host- and donor- derived SILP CD4+ T cells after transfer of naïve 7B8Tg T cells as in (a). (d) FACS analysis of SILP T cells from Il-23rGFP/+ mice, stained with I-Ab/3340-A6 tetramer and control tetramer (2W). (e) FACS analysis of SILP T cells of B6 mice from colonies with different microbiota, stained with I-Ab/3340-A6 tetramer and intracellular RORγt antibody. (f) Expansion of 7B8Tg T cells in mice colonized with Listeria monocytogenes expressing SFBNYU_003340. Top: Immunofluorescence microscopic visualization of the expression of SFB protein by L. monocytogenes. Listeria-3340 and Listeria-empty were stained with anti-3340 rabbit polyclonal antibody. Red: anti-3340 antibody staining. Blue: DAPI staining. Bottom: Naive Ly5.1+ 7B8Tg cells were transferred into congenic mice infected with Listeria-3340 or Listeria-empty. Seven days after transfer, donor derived CD4+ T cells in the SILP were analyzed by FACS.
Extended Data Fig. 9. Transcription factor expression in SFB-specific and Listeria-specific T cells in co-infected mice (representative of data plotted in Fig. 4b).
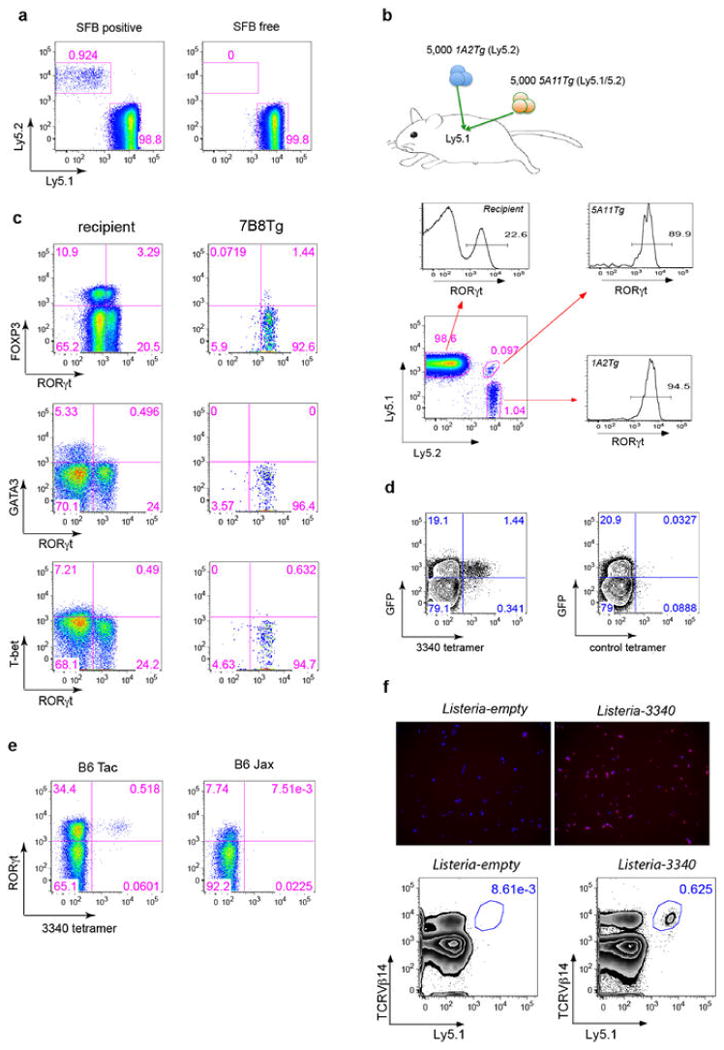
(a) Experimental design for tracking both SFB- and Listeria- specific CD4+ T cells following intestinal colonization with both bacteria. Ly5.2 B6 mice were colonized with Listeria monocytogenes, SFB, or both bacteria, and 7B8Tg T cells from Ly5.1 mice were injected IV. Expression of Th1 and Th17 transcription factors in the SFB-specific 7B8Tg cells and LLO tetramer-specific recipient T cells was evaluated. (b) Intracellular stain for RORγt. (c) Intracellular stain for T-bet.
Extended Data Fig. 10. SFB-specific Th17 cells are present in both SILP and LILP of SFB-colonized mice.
T cells were stained with I-Ab/3340-A6 tetramer and antibody to intracellular RORγt. (a) Representative FACS plots (gated on CD4+ T cells). (b) Analysis of multiple animals. Left: percent of tetramer-positive cells among total CD4+ T cells in each region of the intestine. Right: percent of RORγt+ cells among the tetramer-positive cells. Each symbol represents cells from a separate animal.
Supplementary Material
Acknowledgments
We thank Dr. Sang Yong Kim in the Rodent Genetic Engineering Core (NYU) for generating TCR transgenic mice; Dr. Debra Morrison in the Immune Monitoring Core (NYU), which is supported in part by grant UL1 TR00038 from the National Center for Advancing Translational Sciences and grant 5P30CA016087-32 from the National Cancer Institute; the NYU Histology Core which is supported in part by grant 5P30CA016087-32 from the National Cancer Institute; Dr. Agnes Viale in the Genomics Core Laboratory (MSKCC) for 454 pyrosequencing; Dr. Richard Myers at the HudsonAlpha Institute of Biotechnology for RNA-seq; Dr. Nancy Freitag (University of Illinois at Chicago) for providing the Listeria strain and expression vector; Dr. Yoshinori Umesaki (Yakult) for SFB samples; and Dr. Kenneth Murphy (Washington University, St. Louis) for providing the 58α-β- hybridoma line. Y.Y. was supported by the Arthritis National Research Foundation. M.X. is supported by the Irvington Institute fellowship program of the Cancer Research Institute. D.R.L. is a Howard Hughes Medical Institute Investigator.
Footnotes
Author contributions: Y.Y. and D.R.L. designed the experiments and wrote the manuscript with input from the co-authors. Y.Y., M.B.T., M.X., C.N., A.C., X.L., and J.L. performed most analyses. M.B.T. constructed TCR hybridomas. M.X. developed SFB-specific antibodies. M.G., H.X. and J.J.L. did TCR pyrosequencing analysis. J.L.L. and M.K.J. developed tetramers. F.A. and V.J.T. generated transgenic Listeria. A.S. performed RNA-seq analysis of SFB.
Author information: Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Seminars in immunology. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 7.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013 doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaboriau-Routhiau V, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell host & microbe. 2012;12:496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnupf P, Gaboriau-Routhiau V, Cerf-Bensussan N. Host interactions with Segmented Filamentous Bacteria: An unusual trade-off that drives the post-natal maturation of the gut immune system. Seminars in immunology. 2013;25:342–351. doi: 10.1016/j.smim.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Lochner M, et al. Restricted microbiota and absence of cognate TCR antigen leads to an unbalanced generation of Th17 cells. J Immunol. 2011;186:1531–1537. doi: 10.4049/jimmunol.1001723. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ise W, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nature immunology. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanderson S, Campbell DJ, Shastri N. Identification of a CD4+ T cell-stimulating antigen of pathogenic bacteria by expression cloning. The Journal of experimental medicine. 1995;182:1751–1757. doi: 10.1084/jem.182.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sczesnak A, et al. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell host & microbe. 2011;10:260–272. doi: 10.1016/j.chom.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakash T, et al. Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of th17 cell differentiation. Cell host & microbe. 2011;10:273–284. doi: 10.1016/j.chom.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. Journal of immunological methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 22.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 23.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 25.Hand TW, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scher JU, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monk IR, Gahan CG, Hill C. Tools for functional postgenomic analysis of listeria monocytogenes. Applied and environmental microbiology. 2008;74:3921–3934. doi: 10.1128/AEM.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xayarath B, Marquis H, Port GC, Freitag NE. Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis. Molecular microbiology. 2009;74:956–973. doi: 10.1111/j.1365-2958.2009.06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alamyar E, Giudicelli V, Li S, Duroux P, Lefranc MP. IMGT/HighV-QUEST: the IMGT(R) web portal for immunoglobulin (IG) or antibody and T cell receptor (TR) analysis from NGS high throughput and deep sequencing. Immunome research. 2012;8:26. [Google Scholar]
- 30.Currier JR, Robinson MA. Spectratype/immunoscope analysis of the expressed TCR repertoire. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 10. Chapter 10. 2001. p. 28. [DOI] [PubMed] [Google Scholar]
- 31.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–2329. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 33.Tubo NJ, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. Journal of bacteriology. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu NY, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu CS, Chen YC, Lu CH, Hwang JK. Prediction of protein subcellular localization. Proteins. 2006;64:643–651. doi: 10.1002/prot.21018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.