Abstract
Objective
Daily vitamin D supplementation is often inadequate in treating vitamin D deficiency due to poor compliance. A single, large dose of vitamin D given at timed intervals may be an alternative strategy.
Methods
We conducted a systematic literature review to investigate the efficacy of a single large bolus dose to treat vitamin D deficiency. We identified 2,243 articles in PubMed using the terms “high dose vitamin D,” “single dose vitamin D,” “bolus vitamin D,” or “annual dose vitamin D.” Review articles, cross-sectional studies, nonhuman studies, responses to other articles, and non-English articles were excluded. Manuscripts were also excluded if the study: (1) did not use oral cholecalciferol or ergocalciferol, (2) used vitamin D analogs, (3) enrolled participants under age 18 years, (4) administered doses <100,000 international units (IU) (2.5 mg), or (5) administered >1 dose per year. References of eligible manuscripts and the Cochrane databases were also searched. Two independent reviewers identified eligible manuscripts, and a third reviewer evaluated disagreements. Thirty manuscripts were selected using these criteria.
Results
Large, single doses of vitamin D consistently increased serum/plasma 25-hydroxyvitamin D (25[OH]D) concentrations in several vitamin D-sufficient and -deficient populations. Vitamin D3 doses ≥300,000 IU provided optimal changes in serum/plasma 25(OH)D and parathyroid hormone (PTH) concentrations. Vitamin D supplementation also impacted bone health and extraskeletal endpoints.
Conclusion
This review recommends that vitamin D3 be used for supplementation over vitamin D2 and concludes that single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status and suppressing PTH concentrations for up to 3 months. Lower doses, however, may be sufficient in certain populations. Vitamin D doses >500,000 IU should be used judiciously in order to minimize adverse events.
INTRODUCTION
Vitamin D insufficiency is linked not only to bone disease (1,2) but also to several nonskeletal conditions, including type 2 diabetes mellitus (DM) (3), cardiovascular disease (4–7), chronic lung disease (8–11), tuberculosis (TB) (12–14), and upper respiratory infections (15,16). Vitamin D status is determined by serum 25-hydroxyvitamin D (25[OH]D), the major circulating form of vitamin D (17). Controversy exists as to what serum concentration of 25(OH)D is sufficient; whereas The Endocrine Society Clinical Practice Guidelines on vitamin D have defined sufficiency as >30 ng/mL (18), the Institute of Medicine (IOM) suggests there is no consistent benefit associated with serum 25(OH)D concentrations >20 ng/mL (19,20).
Correction of vitamin D insufficiency is commonly achieved using oral vitamin D supplements. The Endocrine Society guidelines suggest that daily intake of 1,500 to 2,000 international units (IU) of vitamin D is necessary to achieve serum 25(OH)D concentrations consistently >30 ng/mL in adults (18). However, adherence to daily doses has been reported to be low in several large clinical trials (1). Poor adherence has been associated with difficulty swallowing combined vitamin D/calcium tablets, gastrointestinal (GI) side-effects (21), the number of concurrent treatments a patient is receiving, and the patient’s attitude towards vitamin D supplementation (22). Vitamin D given as a large bolus dose has demonstrated higher adherence rates compared with daily and monthly dosing regimens, and has the potential to yield sustained improvements in serum 25(OH)D and parathyroid hormone (PTH) concentrations (23). The sustained effect of high-dose vitamin D may be attributed to its long half-life. Upon ingestion, vitamin D is either converted to 25(OH)D or redistributed into fat, from which it is slowly released over time. By this mechanism, Ish-Shalom et al (24) suggested that daily, weekly, and monthly vitamin D dosing will result in the same circulating concentrations of 25(OH)D over an equivalent period of time. The purpose of this systematic review was to investigate the effects of single, large, bolus doses of vitamin D on serum 25(OH)D concentrations, PTH suppression, and other health outcomes in adults.
METHODS
We searched the terms “high dose vitamin D,” “single dose vitamin D,” “bolus vitamin D,” or “annual dose vitamin D” in PubMed for articles published through September 1, 2012. Limits were preset to manuscripts published in the English language. Titles and abstracts were reviewed. Review articles, cross-sectional studies, non-human studies, and responses to other articles were excluded. Manuscripts were also excluded if the studies: (1) did not use oral cholecalciferol or ergocalciferol, (2) used analog compounds of vitamin D (i.e., calcitriol, doxercalciferol, paricalcitol), (3) study participants were under age 18 years, (4) the study administered doses <100,000 IU (2.5 mg), or (5) vitamin D was given more than once within a year. Manuscripts that could not be excluded by review of title and abstract were examined in their entirety. We also searched the Cochrane databases using the same criteria. Two independent reviewers (J.A., M.K.) identified manuscripts with these criteria, and a third reviewer (V.T.) determined manuscript eligibility when there were disagreements.
Outcomes of interest included: (1) serum/plasma 25(OH)D, (2) serum/plasma PTH, (3) differences between vitamin D2 and D3, and (4) adverse effects.
PubMed Search Results
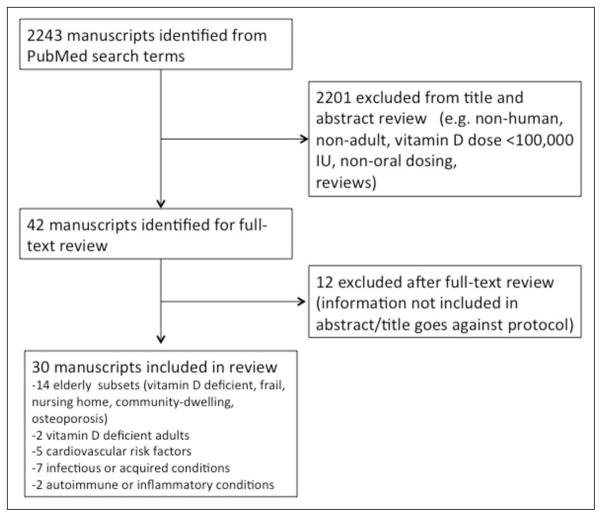
There were 2,243 manuscripts identified from the specified search terms (Fig. 1), and 42 were deemed potentially eligible after applying exclusion criteria to the title and abstract. Following review of these manuscripts, 12 studies were subsequently excluded by criteria not included in the title and abstract. No papers were added from the references of selected manuscripts or the Cochrane databases. A total of 30 studies were included in this review. Of the 30 manuscripts evaluated, three (25–27) provided secondary analyses of data that was published in earlier studies that were also included in this paper (28–30).
Fig. 1.
Flow diagram of studies identified for review.
RESULTS
Study Design
The 30 studies that met eligibility criteria of this paper were published after 1990 and evaluated adult populations receiving single, oral vitamin D doses >100,000 IU. Elderly populations were sampled in 14 studies (26,27,29–40), and vitamin D-deficient adults were observed in 2 studies (41,42). Five studies evaluated cardiovascular risk factors (DM, insulin resistance, peripheral artery disease [PAD], and stroke history) (3,43–46). Two studies evaluated populations with autoimmune and inflammatory conditions (primary dysmenorrhea and rheumatologic patients) (47,48). Seven studies looked at populations with infectious or acquired conditions (alcoholic liver cirrhosis [49], cystic fibrosis [CF] [25,28], TB [50,51], intensive care unit [ICU] placement [52], and pregnancy [53]).
Table 1 summarizes the 21 studies that provided information on serum 25(OH)D or PTH before and after vitamin D dosing compared to a control group. Three studies (25–27) not included in Table 1 provided additional analysis of previously published studies that were already included in the table. The remaining 6 studies (32,37,42,46,47,49) are discussed below when relevant to adverse events or secondary measures.
Table 1.
Summary of Studies Investigating Single, High-Dose Vitamin D
| Author/year | Dose (IU) | Population | Baseline/Posttreatment 25(OH)D (ng/mL)a,b |
Baseline/Posttreatment PTH (pg/mL)a,b |
Other Outcomes |
|---|---|---|---|---|---|
|
| |||||
| D2 and D3: 300,000 IU | |||||
|
| |||||
| Romagnoli et al 2008 (31) | 32 vitamin D-deficient elderly females | ↑ 25(OH)D most rapid in PO groups. ↑ 25(OH)D from baseline at 30 days only in D3 groups. D3 is 2× as potent as D2. ↓ PTH in D3 group only at 60 days. | |||
| 300,000 D2 vs. 300,000 D3 | n = 8; mean age 78.5 (7.5) yr | Baseline: 12.6 (9.1) Day 30: +17.3 (4.7)c Day 60: +10.19 (6.75)c |
Baseline: 32.5 (20.3) Day 60: +0.96 (7.51) |
||
| 300,000 D3 vs. 300,000 D2 | n = 8; mean age 80.6 (5.0) yr | Baseline: 13.3 (9.9) Day 30: +47.8 (7.3)c,e Day 60: +28.06 (8.33)c,e |
Baseline: 43.8 (24.5) Day 60: −22.8 (16)c,e |
||
|
| |||||
| Leventis and Kiely 2009 (41) | 300,000 D3 PO vs. 300,000 D2 IM | 69 vitamin D-deficient adults; mean age 43 yr | Baseline: 10.8 Week 6: 53.84 (26–85.6)c,e,h Week 12: 32.72 (18.8–47.6)c,e,h Week 24: 17.12 (9.2–31.2)c,e,h |
Baseline: 52.45 (24.44–83.66)h Week 12: 40.51 (16.92–54.52)c,h Week 24: 41.08 (26.32–55.46)h |
↑ 25(OH)D in D3>D2. ↓ PTH at 12 weeks in 42% (D2) and 89% (D3 of subjects with elevated) PTH. Vomiting after dose (n = 1). |
|
| |||||
| D2: 100,000 IU | |||||
|
| |||||
| Martineau et al 2007 (50) | 100,000 D2 vs. placebo | 192 patients with tuberculosis; mean age 30.1 yr | Baseline: 14.08 Week 6: 26.96 (10.52–35.48)c,f |
unlisted | ↓ in vitro bacterial growth. ↔ IFN-γ response. |
|
| |||||
| Martineau et al 2009 (51) | 100,000 D2 vs. placebo | 81 patients with tuberculosis; mean age 38.7 (12.4) yr | Baseline: 9.28 (7.4) Week 1: +43.8 (28.84–58.72)c,e,f Week 8: +8.72 (0.56–12.96)c,e,f |
unlisted | ↑ 25(OH)D in TB patients>healthy controls after 1 week. Difference attributed to larger BMI in healthy group. |
| 100,000 D2 | 81 healthy controls; mean age 33.5 (12.7) yr | Baseline: 13.76 (11.12) Week 1: +27.05 (23.96–30.12)c,f Week 8: unlisted |
|||
|
| |||||
| Witham et al 2012 (43) | 100,000 D2 vs. placebo | 58 patients with stroke; mean age 66.2 (13.0) | Baseline: 15.48 (7.04) Week 8: 21.6 (6)c Week16: 20.4 (8.8) |
Baseline: 58.19 (26.88) Week 8: 49.82 (17.86) Week 16: 49.82 (16.92) |
↑ endothelial function at 8 but not 16 weeks. ↔ BP. |
|
| |||||
| D2: 200,000 IU–300,000 IU | |||||
|
| |||||
| Yu et al 2009 (53) | 200,000 D2 vs. 800 D2 daily | 180 pregnant women; ages 18–45 yrs | Week 27 of pregnancy: 10.4 (8.4– 16.4)i Delivery: 13.6 (12–18.4)c,i |
Week 27 of pregnancy: 41.36 (24.44–63.92)i delivery: 31.02 (13.16–156.04)c,i |
↑ Cord 25(OH)D. Only 9% of infants sufficient postsupplement. GI upset (n = 3). |
|
| |||||
| Lantham et al 2003 (33) | 300,000 D2 vs. placebo, w/ or w/ out high-intensity exercises | 243 frail elderly; mean age 79 (77–80) yr | Baseline: 15 (14–18) Month 3: +9 (7–11)c,g |
unlisted | ↔ Frailty, physical health or falls. ↑ Risk of injury w/ exercise. |
|
| |||||
| D3: 100,000 IU | |||||
|
| |||||
| Khaw et al 1994 (30) | 100,000 D3 vs. placebo | 189 healthy elderly adults; mean age 69.4 (2.9) yr | Baseline: 14.6 (6.2) Week 5: +7.76 (4.64)c |
Baseline: 29.89 Week 5: −2.54 (7.33)c |
|
|
| |||||
| Ilahi et al 2008 (40) | 100,000 D3 vs. placebo | 40 healthy elderly; ages 61–84 yr | Baseline: 27.1 (7.7) Day 7: 42.0 (9.1)c Day 84: 32.1 |
Baseline: 22.1 (7.41) Day 60: 23.6 (9.22) |
Better ↑ 25(OH)D with younger age. |
|
| |||||
| Sugden et al 2008 (44) | 100,000 D3 vs. placebo | 34 patients with type 2 DM; mean age 64 yr | Baseline: 16.08 (4.12) Week 8: +9.16 (6.64)c |
Baseline: 40.33 (16.83) Week 8: −1.32 (9.31) |
↑ Endothelial function in those with low 25(OH)D. ↓ systolic BP. ↔ IR |
|
| |||||
| Witham et al 2010 (3) | 100,000 D3 vs. placebo | 61 adults with type 2 DM; mean age 65.3 (11) yr | Baseline: 16.4 (5.6) Week 8: 25.2 (8)c Week16: 23.6 (7.2) |
Baseline: 42.3 (16.92) Week 8: 37.6 (14.1) Week 16: 38.94 (18.8) |
↓ BP at 8 weeks. ↔ Endothelial function, glycosylated hemoglobin, IR. |
|
| |||||
| Stricker et al 2012 (45) | 100,000 D3 vs. placebo | 62 patients with peripheral artery disease; mean age 72.9 (8.7) yr | Baseline: 16.3 (6.7) Day 30: 24.3 (6.2)c |
Baseline: 50.76 (30.08) Day 30: unlisted (NS) |
8 ng/mL ↓ 25(OH)D in winter vs. summer. ↔ Endothelial function, coagulation, inflammation. |
|
| |||||
| D3: 200,000 IU–300,000 IU | |||||
|
| |||||
| Witham et al 2010 (3) | 200,000 D3 vs. placebo | 61 adults with type 2 DM; mean age 63.3 (9.6) yr | Baseline: 19.2 (8.4) Week 8: 31.6 (12.4)c Week16: 30.4 (12)c |
Baseline: 41.36 (17.86) Week 8: 43.24 (22.56) Week 16: 36.66 (15.98) |
↓ BP at 8 weeks. ↓ BNP. ↔ Endothelial function, glycosylated hemoglobin, IR. |
|
| |||||
| Grossman et al 2012 (25,28) | 250,000 D3 vs. placebo | 30 patients with cystic fibrosis; age >18 yr | Baseline: 30.6 (3.2) Week 1: 58.1 (3.5)c Week12: 36.7 (2.6) |
Baseline: 44.6 (9.2) Week 1: 39.8 (12.8) Week 12: 32.4 (6.0) |
↑ 1-year survival and hospital-free days. ↓ TNF-α concentration at 12 weeks. Trend towards ↑ IV antibiotic-free days. |
|
| |||||
| Premaor et al 2008 (35) | 300,000 D3 vs. 800 IU daily | 28 low-income elderly with hyperparathyroidism; mean age 80.8 (8.7) yr | Baseline: 12.4 (6.7) Month 1: 35c,e,j Month 2: 28c,e,j Month 3: 24j Month 6: 14j Month 9: 18j |
Baseline: 74.5 (26.2) Month1: 50j Month 2: 46c,j Month 3: 60j Month 6: 58j Month 9: 55j |
Single-dose vitamin D improved 25(OH)D better than daily 800 IU vitamin D. GI upset (n = 2). |
|
| |||||
| Sakalli et al 2012 (38) | 300,000 D3 oral vs. 300,000 D3 IM vs. placebo | 120 vitamin D-deficient elderly; mean age 70.1 (4.3) yr | Baseline: 20.9 (9.5) Week 6: 27.0 (12.0)c |
Baseline: 82.7 (32.5) Week 6: 50.8 (23.4)c |
Improved Timed Up and Go, visual analog scale tests, physical functioning, and fulfillment of physical roles. ↑ Urine calcium. |
|
| |||||
| Stoll et al 2012 (49) | 300,000 D3 vs. placebo | 124 Rheumatologic patients; mean age 49.2 (13.1) yr | Baseline: 21 (1.5–45.9)h Month 3: 28.6 (7.5–56.5)c,h |
unlisted | 1 or 2 oral doses ↑ 25(OH)D in 50% of participants. |
|
| |||||
| D3: >300,000 IU | |||||
|
| |||||
| Bacon et al 2009 (39) | 500,000 D3 vs. 500,000 D3 + 50,000 D3/month vs. 50,000 D3/month | 63 frail elderly; mean age 82 (7) yr | Baseline: 23.2 (12.8) Month 1: +23.2 (11.2)c Month 3: +4.4 (0.8) |
Baseline: 47.94 (22.56) Month 1: −9.4c |
Plateau in 25(OH)D at 3–5 month with 50,000 IU/month following 500,000 IU start dose. |
|
| |||||
| Sanders et al 2010 (29) | 500,000 IU D3 vs. placebo | 137 elderly females at risk for hip fracture; mean age 76 yr | Baseline: 21.2 (16–26)h Month 1: 48c,d,j Month 3: 36c,j Month 12: 29.6 (22–29.6)c,h |
Baseline: 40.42 (27.26–65.8) Month 1: unlisted (NS) Month 12: unlisted (NS) |
Fall rate ↑ in vitamin D group. Trend towards ↑ fracture risk. 41% ↑ 25(OH)D 12-months after dose (received in 2–5 consecutive years). |
|
| |||||
| Amrein et al 2011 (52) | 540,000 D3 vs. 200 IU/day | 25 ICU patients; mean age 62 (16) yr | Baseline: 13.1(2.0) Day1: 20.5c Day 2: 33.1c Day 3: 35.1 (15.2)c Day 7: 38.2 (16.5)c |
Baseline: 73.7 Day 1: 65.1 Day 2: 77.3 Day 3: 100.4 Day 7: 52.0c |
↑ 25(OH)D >30 ng/mL 2 days after dose (range 1–47 ng/mL). |
|
| |||||
| Rossini et al 2012 (34) | 600,000 D3 vs. placebo | 36 elderly women with osteoporosis; mean age 76 (3) yr | Baseline: 21.7 (5.6) Day1: 46.8 (7.5)c Day 3: 67.1 (17.1)c Day 7: 62.2 (12.5)c Day 14: 60.9 (13.3)c Day 30: 51.6 (11.9)c Day 60: 43.1 (10.3)c Day 90: 35.2 (5.8)c |
Baseline: 35.0 (8.7) Day 1: 32.0 (9.5) Day 3: 25.5 (7.4)c Day7: 23.4 (6.4)c Day 14: 15.8 (7.8)c Day 30: 27.0 (9.8)c Day 60: 29.3 (6)c Day 90: 28.3 (6.1)c |
↑ sCTX and sNTX; ↔ ALP (markers of bone metabolism). ↑ 1,25(OH)2D (25–50% from baseline). |
|
| |||||
| Telligolu et al 2012 (36) | 600,000 D3 oral vs. 600,000 D3 IM | 66 vitamin D-deficient, elderly, nursing home residents; mean age 75.3 (7.5) yr | Baseline: 14.87 (6.9) Week 6: 47.57 (12.7)c,e Week12: 42.94 (13.4)c |
Baseline: 52.03 (22.5) Week 12: 40.58c |
↑ 25(OH)D IM > oral at 12 weeks. 25(OH)D >30 ng/mL in 100% IM vs. 83.3% oral. ↑ Balance and quadriceps strength with supplements. Hypercalciuria (n = 6). |
Abbreviations: ↑ = increase; ↓ = decrease; ↔ = no change; 25(OH)D = 25-hydroxyvitamin D; ALP = alkaline phosphatase; BNP = B-type natriuritic peptide; CI = confidence interval; ICU = intensive care unit; IM = intramuscular; IQR = interquartile range; IR = insulin resistance; IU = international units; NS = not significant; PTH = parathyroid hormone; sCTX = collagen type 1 cross-linked C-telopeptide; sNTX = collagen type 1 cross-linked N-telopeptide; TNF-α = tumor necrosis factor-α.
Mean (± SD) (unless otherwise noted).
Data provided for single, oral doses of vitamin D2 or D3 only.
P<.05 (change from baseline).
Median (range).
P<.05 (difference from other group).
Mean (95% CI).
Median (95% CI).
Mean (range).
Median (IQR).
Value is estimated from tables in paper.
Vitamin D on Serum/Plasma 25(OH)D and PTH Concentrations
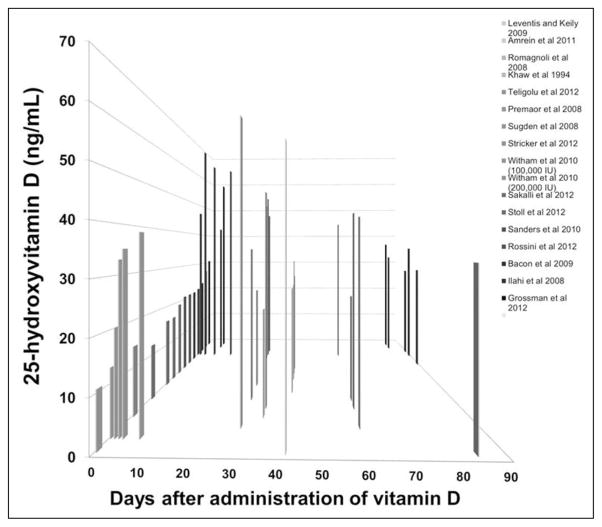
Oral doses of vitamin D2 and D3 (100,000 to 600,000 IU) significantly increased serum 25(OH)D concentrations from baseline in all reviewed studies. The greatest increases in serum 25(OH)D consistently occurred between days 1 and 30 (Fig. 2); peak levels were measured at 3 days (34) and 7 days (25,40,49) following dosing, although concentrations >30 ng/mL were noted as soon as 1 day following 600,000 IU of D3 (34) and 540,000 IU of D3 (52).
Fig. 2.
Relationship between single, high-dose vitamin D and serum/plasma 25(OH)D concentration within the 90 days following the dose. Serum/plasma 25(OH)D increased significantly from baseline in all studies that administered vitamin D (P<.05). A majority of data points were confined to the first 90 days following the dose of vitamin D. 25(OH)D = 25-hydroxyvitamin D.
Improvement in vitamin D status was associated with lowering of PTH concentration in a majority of the studies (30,31,34–36,38,39,41,52,53); significant decreases (P<.001) were noted as soon as day 3 in studies using 600,000 IU of vitamin D3 (34) and remained significantly decreased for as long as 12 months (following 600,000 IU of vitamin D3) (36). However, lower single doses of vitamin D in the range of 100,000 to 500,000 IU did not significantly lower PTH concentrations in several studies (3,25,28,29,40,43–45).
Data regarding PTH and 25(OH)D modulation is stratified below by: vitamin D formulation (D2 versus D3), dose (100,000, 200,000 to 300,000, and >300,000 IU), and relative baseline 25(OH)D concentration (>20 ng/mL or <20 ng/mL).
Supplementation of 100,000 IU Vitamin D: Baseline Serum 25(OH)D <20 ng/mL
A 100,000 IU dose of vitamin D3 in subjects with serum 25(OH)D <20 ng/mL failed to increase serum 25(OH)D concentrations to >30 ng/mL. However, serum 25(OH)D concentrations >20 ng/mL were sustained at: 4 weeks in patients with PAD (45), 5 weeks in healthy adults (27,30), and 8 (44) and 26 weeks (3) in populations with type 2 DM.
Two studies evaluated doses of 100,000 IU vitamin D2 in patients with TB (50,51). Martineau et al (51) demonstrated that subjects reached a mean serum 25(OH)D concentration >30 ng/mL at 1 week following the vitamin D dose but were unable to maintain the serum 25(OH)D concentration above 30 ng/mL at 8 weeks. Both studies (50,51) maintained serum 25(OH)D concentrations >20 ng/mL at 6 weeks (50) and 8 weeks (51).
The dose of 100,000 IU of vitamin D was only associated with a significant lowering of PTH concentration in the study by Khaw et al (30), which had a much larger sample size (N = 189) than the other studies that evaluated PTH lowering at this dose (N = 34 [44], N = 61 [3], N = 62 [45]).
Supplementation of 100,000 IU Vitamin D: Baseline Serum 25(OH)D >20 ng/mL
Only Ilahi et al (40) dosed 100,000 IU of vitamin D3 in a relatively vitamin D-sufficient population, observing an increase in 25(OH)D concentration that peaked at 1 week and remained >30 ng/mL at week 12. This study observed no significant decrease in PTH concentration.
Supplementation of 200,000–300,000 IU of Vitamin D: Baseline Serum 25(OH)D <20 ng/mL
A dose of 200,000 IU of vitamin D3 increased mean 25(OH)D concentrations to >30 ng/mL for up to 16 weeks in adults with type 2 DM (3), whereas 300,000 IU of vitamin D3 increased serum 25(OH)D concentrations to >30 ng/mL after 4 weeks (not significant at 12 weeks) (35), 8 weeks (31), and 12 weeks (not significant at 24 weeks) (41) in elderly adults.
In contrast, vitamin D2 (ergocalciferol) in the dose range of 200,000 to 300,000 IU consistently failed to achieve 30 ng/mL concentrations of serum 25(OH) D (31,33,43,53), although concentrations >20 ng/mL occurred at: 8 weeks in vitamin D-deficient adults (31), 12 weeks in frail elderly (33), and 16 weeks in stroke patients (43). Yu et al (53) failed to achieve average 25(OH) D concentrations >20 ng/mL in a group of pregnant participants.
Vitamin D doses in the range of 200,000 to 300,000 IU were associated with significantly lower plasma PTH concentrations at 8 weeks in elderly adults (31,35) and 24 weeks in vitamin D-deficient adults (41). Only Witham et al (3), who used a dose of 200,000 IU of vitamin D3, failed to observe a significant decrease in PTH over a 16-week study. Baseline 25(OH)D was relatively high (19.2 ± 8.4 ng/mL) in this population relative to other groups (range, 10.8 to 13.3 ± 9.9 ng/mL) (31,35,41).
Three of four studies failed to show PTH lowering using 200,000 to 300,000 IU vitamin D2 (31,33,43); only Yu et al (53) showed a significant decrease in PTH in pregnant women at delivery, following administration of 200,000 IU of vitamin D in the 27th week of pregnancy. This population exhibited a high prevalence (27%) of secondary hyperparathyroidism (53).
Supplementation of 200,000–300,000 IU Vitamin D: Baseline Serum 25(OH)D >20 ng/mL
Two studies (28,48) achieved 25(OH)D concentrations >30 ng/mL at: 12 weeks following a dose of 300,000 IU vitamin D3 in patients with rheumatologic conditions (48) and 1 week (not significant at 12 weeks) following a dose of 250,000 IU vitamin D3 in patients with CF (28). Sakalli et al (38) did not show serum concentrations of 25(OH)D >30 ng/mL at 6 weeks in an elderly population; this study population only reached 27 ± 12 ng/mL.
PTH suppression was inconsistent between studies. Grossman et al (28) showed no suppression in PTH concentration following a 250,000 IU dose of vitamin D3, whereas Sakalli et al (38) observed a significant decrease in PTH concentration at 6 weeks (82.7 ± 32.5 pg/mL to 50.8 ± 23.4 pg/mL). This study population had the highest PTH concentration at baseline of all studies evaluated.
Supplementation of >300,000 IU vitamin D: Baseline Serum 25(OH)D <20 ng/mL
Following a dose of 540,000 IU of vitamin D3, mean serum 25(OH)D concentrations were >20 ng/mL by day 1 and peaked at 38.2 ± 16.5 ng/mL at 1 week in a population of ICU patients (52). Similarly, a dose of 600,000 IU of vitamin D3 raised serum 25(OH)D to >30 ng/mL by 12 weeks in elderly subjects (36).
PTH concentrations were significantly lowered in both of the studies that evaluated PTH lowering in this subset of studies (36,52).
Supplementation of >300,000 IU vitamin D: Baseline Serum 25(OH)D >20 ng/mL
Vitamin D3 doses >300,000 IU were similarly effective in patients with 25(OH)D concentrations >20 ng/mL; all 3 studies (29,34,39) observed mean concentrations >30 ng/mL at 4 weeks, though the results peaked at day 3 (reaching 67.1 ± 17.1 ng/mL from 21.7 ± 5.6 at baseline) in the study of Rossini et al (34). Sanders et al (29) showed long-term efficacy of a 500,000 IU dose; the 25(OH)D concentration remained >30 ng/mL at 12 weeks and was significantly increased at 1 year in a cohort of women with osteoporosis. Bacon et al (39) did not sustain a mean 25(OH)D concentration >30 ng/mL at 12 weeks in a frail elderly population.
PTH concentrations were found to be significantly lower in both studies that evaluated this measure; Rossini et al (34) and Bacon et al (39) both showed significant suppression of PTH, which was significant 3 days following the dose (34) and was sustained at 4 weeks (34,39). Sanders et al (29) did not show a significant decrease in PTH.
Vitamin D2 Versus Vitamin D3
Two studies compared single, large doses of vitamin D2 and D3. Romagnoli et al (31) found serum 25(OH)D concentrations >30 ng/mL to be achieved consistently only by those taking oral vitamin D3. Similarly, Leventis and Kiely (41) found 100% of participants receiving 300,000 IU of vitamin D3 to have sustained serum 25(OH)D concentrations >20 ng/mL by 6 weeks, compared with 0% of those receiving vitamin D2. Vitamin D3 also enabled greater PTH suppression than vitamin D2 (31,41); Leventis and Kiely (41) found that 300,000 IU of vitamin D3 suppressed secondary hyperparathyroidism in 100% of participants by 12 weeks, compared with 42% of participants receiving vitamin D2. The superiority of vitamin D3 compared with vitamin D2 in suppressing PTH was evident within 3 days (P<.01) and persisted for >60 days (P<.01) (31). Taken together, the results of these studies indicate that single large doses of vitamin D3 appear to be superior to vitamin D2 in achieving higher and more sustained serum 25(OH)D concentrations. However, vitamin D2, as illustrated by its positive effects in several studies, including that of Rossini et al (32) on reducing fracture risk, may have disease-specific indications.
Adverse Effects
Few studies have documented complications following high-dose vitamin D supplementation. Three studies reported subjects with GI complaints, including an episode of vomiting following administration of 300,000 IU of vitamin D3 in a vegetable-oil solution (41) and various GI complaints following ingestion of 300,000 IU of vitamin D3 and 200,000 IU of vitamin D2 in tablet form (n = 2 and n = 3, respectively) (35,53). Rossini et al (34) showed an increase in several bone turnover markers (collagen type 1 cross-linked N-telopeptide and collagen type 1 cross-linked C-telopeptide) following 600,000 IU of vitamin D3. von Restorff (37) documented 2 participants with mild hypercalcemia (>10.76 mg/dL) that normalized by 6 months following a 300,000 IU dose of vitamin D3. Hypercalciuria immediately following ingestion of 300,000 IU of vitamin D3 (38) and within 12 weeks of ingesting 600,000 IU of vitamin D3 (36), in addition to increased urine magnesium 3 days after 600,000 IU of vitamin D3 (43), has also been reported. The reports of hypercalciuria were not linked to any significant clinical complications (36,38). The clinical significance of increased urine magnesium was also unclear, as serum calcium and magnesium remained normal in these subjects (42).
DISCUSSION
This systematic review demonstrated the consistent efficacy and safety of single, large, oral doses of vitamin D in adults. All studies evaluated report a significant increase in serum/plasma 25(OH)D concentration relative to baseline, which tended to peak between days 7 and 30 (Fig. 2). Mean serum/plasma 25(OH)D concentration surpassed IOM guidelines for vitamin D sufficiency (25[OH]D concentration >20 ng/mL) in all but 1 study (53). However, the formulation and dose of vitamin D appeared to impact the ability for certain doses to meet Endocrine Society Guidelines (25[OH]D concentrations >30 ng/mL).
Although many groups receiving vitamin D3 (cholecalciferol) formulations achieved mean 25(OH)D concentrations >30 ng/mL, only 1 study using vitamin D2 (ergocalciferol) surpassed that benchmark (51). Thus, vitamin D2 was consistently less effective than vitamin D3 in achieving optimal serum 25(OH)D concentrations. In head-to-head studies, vitamin D3 was almost twice as potent as equimolar vitamin D2 (31) and elicited a greater, more sustained, and more rapid serum 25(OH)D response than vitamin D2 (31,41,52). Thus, vitamin D3 should be the formulation of choice for high doses of vitamin D.
The dose of vitamin D also affected the increase of 25(OH)D concentration observed. A vitamin D3 dose of 100,000 IU was found to be insufficient to meet Endocrine Society Guidelines for sufficiency in populations with baseline 25(OH)D concentrations <20 ng/mL; Ilahi et al (40), who reported a mean baseline 25(OH)D concentration of 27.1 ± 7. 7 ng/mL, were the only investigators who found that 100,000 IU of vitamin D3 was sufficient to achieve 25(OH)D concentrations >30 ng/mL. Generally, doses of ≥200,000 IU of vitamin D3 were required to sustain mean 25(OH)D concentrations >30 ng/mL (3,28,29,31,34–36,39,41,48,52). Only Sakalli et al (38) narrowly failed to reach this benchmark, reaching 25(OH) D concentrations of 27 ± 12 ng/mL at 6 weeks.
The increases in 25(OH)D concentration observed occurred safely in a majority of individuals; no adverse effects were noted at doses <200,000 IU of vitamin D, and many studies found no adverse events at up to 500,000 IU of vitamin D3 (26,29,31) and 540,000 IU of vitamin D3 (52). However, potentially detrimental changes in biochemical markers occurred in all studies evaluating a single dose of 600,000 IU of vitamin D3, indicating the need for greater discretion when administering single doses of >500,000 IU. Overall, whereas vitamin D3 doses of ≥200,000 IU appear to be most effective in promoting vitamin D sufficiency, certain healthy, relatively vitamin D-sufficient populations, such as that in the study of Ilahi et al (40), may benefit from smaller doses and may thus avoid the risk of adverse events with higher doses.
Vitamin D classically influences bone metabolism through its increase in GI tract absorption of calcium and subsequent lowering of PTH. Significant decreases in plasma PTH concentrations were observed in a majority of the studies evaluated, occurring as soon as day 3 in studies using 600,000 IU of vitamin D3 (34) and remaining significantly decreased for as long as 12 months (following 600,000 IU of vitamin D3) (36). However, variability between results was evident. This inconsistency was likely due primarily to the dose of vitamin D administered. Vitamin D3 doses <300,000 IU appeared generally insufficient at decreasing PTH concentrations, regardless of baseline 25(OH)D concentration (3,28,40,44,45); only 1 study (30) showed a significantly decreased PTH concentration using a 100,000 IU dose of vitamin D3. Doses of ≥300,000 IU of vitamin D3 showed more consistent PTH lowering; of studies evaluating PTH concentration, only that of Sanders et al (29) did not elicit a significant decrease in PTH concentration following a dose of 500,000 IU of vitamin D3 in osteoporotic women. Overall, it appears that doses <300,000 IU may not provide an adequate amount of vitamin D to restore vitamin D status and lower plasma PTH concentrations in most populations. In addition, baseline serum 25(OH)D concentration does not appear to have an impact in decreasing PTH concentrations following a single, large dose of vitamin D >100,000 IU.
Lowered PTH concentrations in response to vitamin D supplementation have been associated with lower fracture risk (54,55). However, higher doses of vitamin D, in the range of 300,000 to 600,000 IU, may actually increase fracture risk (29,34), as seen in the study of Rossini et al (34), which showed elevated bone turnover markers following a dose of 600,000 IU of vitamin D3. Rapidly increased calcitriol concentrations may have some osteoclastic activity (56) and may also inhibit osteoblast function in bone mineralization (57). Additional studies are needed to determine the potential fracture risk posed by high-dose vitamin D, particularly in patients at risk for fractures and osteoporotic changes. An optimal therapeutic dose of vitamin D must balance these potential negative impacts on bone mineralization.
In addition to the classical effects on bone outcomes, improving vitamin D status provides extraskeletal benefits for several populations at risk for vitamin D insufficiency. In patients with CF who were hospitalized for pulmonary exacerbation, a single dose of 250,000 IU of vitamin D3 increased 1-year survival and the number of hospital-free days and decreased levels of inflammatory cytokines (25,28). A 100,000 IU dose of vitamin D2 was found to decrease in vitro bacterial growth in a population with active TB and potentially prevent reactivation of latent TB infection (50). Lasco et al (47) suggested that a single 300,000 IU dose of vitamin D3 reduced pain in women with dysmenorrhea. Vitamin D may also affect cardiovascular system factors, although this is inconclusive, as positive results were seen in some (3,43,44,46), but not all (27,45), of the studies reviewed.
The limitations of this review are based largely on the inconsistencies between study populations and vitamin doses, which prevent reliable inter-study comparisons, in addition to the lack of data from healthy, nonelderly, adult populations, which would allow the impact of vitamin D supplementation to be observed without concurrent disease processes. Furthermore, once-yearly doses of vitamin D are nonphysiologic; whereas large doses consistently show better efficacy than daily doses, there may be a more optimal intermittent dosing strategy not evaluated by this review. As discussed in Ilahi et al (40), 100,000 IU of vitamin D3 dosed every 2 to 3 months may provide optimal benefit in people with baseline 25(OH)D concentrations >20 ng/mL. Bacon et al (39) showed similar improvements in the sustainability of 25(OH)D concentrations in the long-term by adding monthly 50,000 IU vitamin D3 doses following an initial 500,000 IU vitamin D3 bolus. Such subannual dosing strategies may strike a balance between the convenience of once-yearly dosing and the poor compliance of daily dosing and thus serve to better maintain 25(OH)D concentrations in deficient populations.
CONCLUSION
In conclusion, a single vitamin D3 dose of ≥100,000 IU offers a consistently efficient means of improving short-term vitamin D concentrations of >20 ng/mL, although vitamin D3 doses of ≥300,000 IU are necessary to achieve 25(OH)D concentrations >30 ng/mL and lowering of plasma PTH concentrations. Although generally safe, bolus doses of >500,000 IU of vitamin D3 must be used with caution due to the potential for increased fracture risks, altered biochemical markers, and issues with tolerability, such as GI upset. Future considerations not addressed specifically by studies in this review include: (1) vitamin D doses to prevent the winter decline of serum 25(OH)D; (2) vitamin D supplementation in healthy, nonelderly adult populations; and (3) the duration of the serum 25(OH)D increase following supplementation.
Acknowledgments
Supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- CF
cystic fibrosis
- DM
diabetes mellitus
- GI
gastrointestinal
- IU
international units
- PTH
parathyroid hormone
- TB
tuberculosis
Footnotes
To purchase reprints of this article, please visit: www.aace.com/reprints.
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Boonen S, Vanderschueren D, Haentjens P, Lips P. Calcium and vitamin D in the prevention and treatment of osteoporosis - a clinical update. J Intern Med. 2006;259:539–552. doi: 10.1111/j.1365-2796.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 2.Fraser DR. Vitamin D. Lancet. 1995;345:104–107. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 3.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 5.Dobnig H, Pils S, Scharnagl H. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 9.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85:1307–1311. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 11.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–381. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M, Schenk K, Papadopoulou C, et al. Serum 25-hydroxy vitamin D and exercise capacity in COPD. Thorax. 2011;66:544–545. doi: 10.1136/thx.2010.152785. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 15.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 16.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–393. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 17.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen CJ, Abrams SA, Aloia JF. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unson CG, Litt M, Reisine S, Mahoney-Trella P, Sheperd T, Prestwood K. Adherence to calcium/vitamin D and estrogen protocols among diverse older participants enrolled in a clinical trial. Contemp Clin Trials. 2006;27:215–226. doi: 10.1016/j.cct.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Sanfelix-Genovés J, Gil-Guillén VF, Orozco-Beltran D, et al. Determinant factors of osteoporosis patients’ reported therapeutic adherence to calcium and/or vitamin D supplements: a cross-sectional, observational study of postmenopausal women. Drugs Aging. 2009;26:861–869. doi: 10.2165/11317070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Stephens WP, Klimiuk PS, Berry JL, Mawer EB. Annual high-dose vitamin D prophylaxis in Asian immigrants. Lancet. 1981;2:1199–1202. doi: 10.1016/s0140-6736(81)91439-2. [DOI] [PubMed] [Google Scholar]
- 24.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–3435. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66:1072–1074. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198:357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 27.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 28.Grossmann RE, Zughaier SM, Kumari M, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol. 2012;4:191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 30.Khaw KT, Scragg R, Murphy S. Single-dose cholecalciferol suppresses the winter increase in parathyroid hormone concentrations in healthy older men and women: a randomized trial. Am J Clin Nutr. 1994;59:1040–1044. doi: 10.1093/ajcn/59.5.1040. [DOI] [PubMed] [Google Scholar]
- 31.Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 32.Rossini M, Alberti V, Flor L, et al. Effect of oral vitamin D2 yearly bolus on hip fracture risk in elderly women: a community primary prevention study. Aging Clin Exp Res. 2004;16:432–436. doi: 10.1007/BF03327397. [DOI] [PubMed] [Google Scholar]
- 33.Latham NK, Anderson CS, Lee A, Bennet DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–299. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 34.Rossini M, Gatti D, Viapiana O, et al. Short-term effects on bone turnover markers of a single high dose of oral vitamin D3. J Clin Endocrinol Metab. 2012;97:E622–E626. doi: 10.1210/jc.2011-2448. [DOI] [PubMed] [Google Scholar]
- 35.Premaor MO, Scalco R, da Silva MJ, Froelich PE, Furlanetto TW. The effect of a single dose versus a daily dose of cholecalciferol on the serum 25-hydroxycholecalciferol and parathyroid hormone levels in the elderly with secondary hyperparathyroidism living in a low-income housing unit. J Bone Miner Metab. 2008;26:603–608. doi: 10.1007/s00774-008-0858-0. [DOI] [PubMed] [Google Scholar]
- 36.Tellioglu A, Basaran S, Guzel R, Seydaoglu G. Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly. Maturitas. 2012;72:332–338. doi: 10.1016/j.maturitas.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 37.von Restorff C, Bischoff-Ferrari HA, Theiler R. High-dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone. 2009;45:747–749. doi: 10.1016/j.bone.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Sakalli H, Arslan D, Yucel AE. The effect of oral and parenteral vitamin D supplementation in the elderly: a prospective, double-blinded, randomized, placebo-controlled study. Rheumatol Int. 2012;32:2279–2283. doi: 10.1007/s00296-011-1943-6. [DOI] [PubMed] [Google Scholar]
- 39.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20:1407–1415. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 40.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–691. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 41.Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38:149–153. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 42.Cipriani C, Romagnoli E, Scillitani A, et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95:4771–4777. doi: 10.1210/jc.2010-0502. [DOI] [PubMed] [Google Scholar]
- 43.Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:864–870. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 45.Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg. 2012;44:307–312. doi: 10.1016/j.ejvs.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 46.Selimoglu H, Duran C, Kivici S, et al. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33:234–238. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 47.Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012;172:366–367. doi: 10.1001/archinternmed.2011.715. [DOI] [PubMed] [Google Scholar]
- 48.Stoll D, Dudler J, Lamy O, Hans D, Krieg MA, Aubry-Rozier B. Can one or two high doses of oral vitamin D3 correct insufficiency in a non-supplemented rheumatologic population? Osteoporos Int. 2013;24:495–500. doi: 10.1007/s00198-012-1962-5. [DOI] [PubMed] [Google Scholar]
- 49.Malham M, Peter Jørgensen S, Lauridsen AL, Ott P, Glerup H, Dahlerup JF. The effect of a single oral mega-dose of vitamin D provided as either ergocalciferol (D2) or cholecalciferol (D3) in alcoholic liver cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:172–178. doi: 10.1097/MEG.0b013e32834d1755. [DOI] [PubMed] [Google Scholar]
- 50.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 51.Martineau AR, Nanzer AM, Satkunam KR, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:119–125. [PubMed] [Google Scholar]
- 52.Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf) 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 54.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heikinheimo RJ, Inkovaara JA, Harju EJ, et al. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int. 1992;51:105–110. doi: 10.1007/BF00298497. [DOI] [PubMed] [Google Scholar]
- 56.Maierhofer WJ, Gray RW, Cheung HS, Lemann J., Jr Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1983;24:555–560. doi: 10.1038/ki.1983.193. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi M, Weitzmann MN. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int J Mol Med. 2012;29:934–938. doi: 10.3892/ijmm.2012.900. [DOI] [PubMed] [Google Scholar]