Abstract
Objective
We analyzed the vaginal fluid proteome to identify biomarkers of intraamniotic infection among women in preterm labor.
Study Design
Proteome analysis was performed on vaginal fluid specimens from women with preterm labor, using multidimensional liquid chromatography, tandem mass spectrometry, and label-free quantification. Enzyme immunoassays were used to quantify candidate proteins. Classification accuracy for intraamniotic infection (positive amniotic fluid bacterial culture and/or interleukin-6>2 ng/mL) was evaluated using receiver-operator characteristic curves obtained by logistic regression.
Results
Of 170 subjects, 30 (18%) had intraamniotic infection. Vaginal fluid proteome analysis revealed 338 unique proteins. Label-free quantification identified 15 proteins differentially expressed in intraamniotic infection, including acute-phase reactants, immune modulators, high-abundance amniotic fluid proteins and extracellular matrix–signaling factors; these findings were confirmed by enzyme immunoassay. A multi-analyte algorithm showed accurate classification of intraamniotic infection.
Conclusion
Vaginal fluid proteome analyses identified proteins capable of discriminating between patients with and without intraamniotic infection.
Keywords: intraamniotic infection, preterm labor, proteomics, vaginal fluid
INTRODUCTION
Preterm birth is a major unsolved problem in the United States, where in 2004, 1 in 8 births occurred at 37 weeks gestation.1 Preterm birth, which is a leading cause of neonatal morbidity and death, has actually increased in recent years, despite concerted prevention efforts. A substantial proportion of bacterial pathogens or the presence of high concentrations of proinflammatory cytokines such as interleukin-6 in amniotic fluid.2,3 The diagnosis is difficult because most women with intraamniotic infection do not have fever, uterine tender ness, or other clinical signs of infection other than preterm labor2,4 and because they tend to deliver within 48 hours of examination.5,6 Evidence is increasing that neonates who are exposed to intraamniotic infection have an increased risk in 2004, 1 in 8 births occurred at <37 weeks gestation.1 Preterm birth, which is a leading cause of neonatal morbidity and death, has actually increased in recent years, despite concerted prevention efforts. A substantial proportion of spontaneous preterm births are associated with intraamniotic infection, which is defined by the recovery of adverse outcomes (such as neonatal sepsis, respiratory distress syndrome, and intraventricular hemorrhage) when compared with nonexposed infants of similar birthweight.4,7
Currently, the definitive diagnosis of intraamniotic infection requires a transabdominal amniocentesis to perform a direct examination of amniotic fluid. However, many health care providers are reluctant to perform amniocentesis because of perceived risks such as bleeding, rupture of the fetal membranes, or increased uterine contractions.
Recent developments in proteomic analysis provide the opportunity to examine the vaginal fluid proteome as a less invasive predictor of intraamniotic infection and preterm birth.8 To date, this technique has been examined in a nonhuman primate model9 and in a small series of women in spontaneous preterm labor.10 We hypothesized that systematic analysis of the vaginal fluid proteome would identify sensitive, novel markers of intraamniotic infection among women in spontaneous preterm labor with intact membranes. If successful, such markers could limit the role of amniocentesis as a confirmatory test among cases with high suspicion of intraamniotic infection. A proteomic approach from vaginal fluid samples ultimately might lead to the development of less invasive tests for intraamniotic infection among women in preterm labor and potentially influence clinical treatment.
MATERIALS AND METHODS
We conducted a secondary analysis of 170 archived vaginal fluid samples from a prospective observational cohort of women in spontaneous preterm labor.11 Participants were at gestational ages of 20–34 weeks by obstetric estimate, which was determined from menstrual dating or from the earliest available ultrasound scan. Preterm labor was defined as regular uterine contractions at a frequency of <10 minutes with either documented cervical change or a cervical dilation of >1 cm or effacement of >50%. All participants had intact membranes at study enrollment that was confirmed by sterile speculum examination. Women with cervical dilation >4 cm or ruptured membranes at admission were not eligible for study inclusion. The University of Washington Institutional Review Board approved the original study protocol, and the subjects provided written informed consent at the time of original study enrollment. The present analyses were approved administratively by the University of Washington Human Subjects Division and considered exempt from further review because they involved secondary analysis of existing, deidentified data and specimens.
At study entry (after speculum and cervical digital examination), amniotic fluid from all participants was obtained by transabdominal amniocentesis; vaginal fluid was obtained by the saturation of a Dacron swab with fluid from the posterior vaginal fornix. Amniotic and vaginal fluid specimens were stored at −70°C in pyrogen-free containers until assayed. Amniotic and vaginal fluid bacterial cultures, demographic and reproductive history data, and pregnancy outcomes were available from the original study cohort. Women received tocolytics, corticosteroids, and antibiotics according to the judgment of their clinical providers. Amniotic fluid interleukin-6 concentrations were determined by commercial enzyme immunoassay (Genzyme Diagnostics, Cambridge, MA). Intraamniotic infection was defined by an “expanded gold standard” as a positive amniotic fluid bacterial culture and/or interleukin-6 >2 ng/mL, as previously reported.12 Early preterm birth was defined as delivery at ≤34 weeks gestation, because most neonatal morbidity occurs at this gestational age.
Of 220 archived vaginal fluid samples that were available from the original study cohort, 43 samples had insufficient protein (0.17 μg/μL), and 7 samples did not have detectable human albumin and/or detectable concentrations for at least 8 of the 15 biomarkers of interest, which left 170 samples to be included in these analyses. There were no clinically important differences in subject characteristics or pregnancy outcomes between included and excluded samples. The 170 samples that were included were from 30 subjects with intraamniotic infection (12 subjects with a positive amniotic fluid bacterial culture and 18 subjects with a negative bacterial culture and interleukin- 6 concentration >2 ng/mL) all of whom had an early preterm birth (intraamniotic infection group), 55 subjects who had an early preterm birth without intraamniotic infection (early preterm birth group), and 85 subjects without intraamniotic infection who had symptoms of preterm labor but delivered at >34 weeks gestation (preterm labor group).
Previously published approaches that were used for mass spectrometric analysis8–10 are briefly described later. Pools of vaginal fluid samples were created from 100 μL each of 21 randomly selected samples from each group or 63 total samples (intraamniotic infection, early preterm birth, and preterm labor).
Pooled samples were subjected to 2-dimensional liquid chromatography with tandem mass spectrometry and label free quantification to identify differentially expressed proteins between the groups.8–10
For mass spectrometry, 600 μg protein from each pooled sample was digested with trypsin, and the resulting peptides were first separated with the use of strong cation exchange column into 32 fractions.9 These fractions were analyzed with an Agilent 1100 liquid chromatographer that was connected to a tandem mass spectrometer (Thermo Finnegan, San Jose, CA). A total of 101,377 mass spectra were collected that represented the 3 subject groups.
Peptides that were present in each sample were identified by a search of the corresponding mass spectra against a protein database that contained forward and reverse entries of the Swiss-Prot human database (version 46.6) with the use of 2 independent search engines: TurboSequest (Thermo Finnegan) and X! Tandem (The Global Proteome Organization; www.thegpm.org/tandem/index. html).13 Peptide identifications from a sample were assembled into protein identifications with Scaffold software (version1.3.2; Proteome Software, Portland, OR). Protein identifications that had at least 2 independent peptide identifications were considered to be present in the sample.
The total number of mass spectra that were matched to a particular protein was used to assess the relative abundance of a protein in a sample with the use of a label-free quantification method that had been described previously.9,14 This method compares the spectral counts of a protein between 2 samples with either an independent 2 × 2 χ2 test or a Fisher’s Exact test. Proteins that passed the quantification method with a probability value of ≤.05 were considered to be expressed differentially between the samples. Fold changes of differentially expressed proteins were determined with a reference formula for calculating spectral count ratios.15
Confirmation of the presence of differentially expressed biomarkers of interest was validated by enzyme-linked immunosorbent assays for 15 candidate biomarkers on all 170 individual samples, by previously described techniques.8–10 Available commercial antibodies and antigens were purchased from various vendors to prepare immunoassays. Standard curves were developed with the use of known quantities of recombinant proteins or standards provided by manufacturer to reference sample concentrations. All assays were performed on 100 μL samples of vaginal fluid in triplicate. Interassay and intraassay coefficient of variations ranged from 3–7%. The mean protein concentration across triplicates was used in subsequent analyses.
One-way analyses of variance were conducted to compare natural log-transformed enzyme immunoassay values of samples from women in 2 groups: those with intraamniotic infection vs those without infection (early preterm birth and preterm labor groups combined). For presentation, we transformed the mean log value back to original units (geometric mean and geometric standard deviation). We applied the Bonferroni correction to account for multiple comparisons (15 proteins were compared). We evaluated the classification performance of several different combinations of 2, 3, or 4 proteins using logistic regression models. Predicted values (ie, risk scores) were computed for each woman from each multianalyte model, and receiver operating characteristic (ROC) curves were plotted. Areas under the ROC curves with 95% confidence intervals (CIs) were calculated with bootstrap methods. Proteins were selected for multianalyte models based on their ability to discriminate individually between patients with intraamniotic infection and no intraamniotic infection and/or based on the improvement in classification that was observed by adding them to the models. Descriptive and inferential statistics were computed with SAS software (version 9.1: SAS Institute Inc, Cary, NC); ROC curves were produced and compared with customized STATA modules (Stata Corp, College Station, TX).16
RESULTS
Maternal and pregnancy characteristics that were stratified by infection status and timing of delivery are presented in Table 1. Maternal age and race and occurrence of other pregnancy complications (such as twin gestation) were comparable across groups. The 30 subjects with intraamniotic infection were seen and delivered at an earlier gestational age, were more likely to have bacterial vaginosis, and had a substantially shorter time between enrollment and delivery than the 140 subjects without infection.
Table 1.
Maternal and pregnancy characteristics by intraamniotic infection and timing of birth
| Intraamniotic infection (n=30) |
No intraamniotic infection | ||||
|---|---|---|---|---|---|
| Characteristic | All (n=140) | Early preterm birth (n=55) | Preterm labor (n=85) | P valuea | |
| Median maternal age, y | 25 | 24 | 26 | 24 | .47 |
| Maternal race, n (%) | .38 | ||||
| White | 20 (67) | 88 (63) | 37 (67) | 51 (60) | |
| African American | 7 (23) | 24 (17) | 8 (15) | 16 (19) | |
| Other | 3 (10) | 28 (20) | 10 (18) | 18 (21) | |
| Parity, n (%) | 12 (40) | 83 (59) | 33 (60) | 50 (59) | .05 |
| Previous delivery ≤34 wk, n (%)b | 2 (17) | 29 (35) | 13 (39) | 16 (33) | .32 |
| Parity, n (%) | 12 (40) | 83 (59) | 33 (60) | 50 (59) | .05 |
| Twin gestation, n (%) | 4 (13) | 19 (14) | 10 (18) | 9 (11) | .97 |
| Bacterial vaginosis, n (%) | 7 (24) | 17 (12) | 6 (11) | 11 (13) | .009 |
| Current cigarette smoker, n (%) | 3 (10) | 24 (17) | 17 (32) | 7 (8) | .42 |
| Mean gestational age at enrollment, wk | 28 | 32 | 30 | 32 | .0004 |
| Mean gestational age at delivery, wk | 28 | 35.5 | 32 | 37 | <.0001 |
| Days between enrollment and delivery, n | 3 | 21 | 6 | 32 | <.0001 |
Compares subjects with intraamniotic infection (n=30) with those with no intraamniotic infection (n=140); values were based on χ2 tests for categoric variables, on independent samples t tests for normally distributed variables (mean presented), and on Wilcoxon (nonparametric) tests for nonnormally distributed variables (median presented);
Among parous women only (n=95).
We detected 338 unique proteins in vaginal fluid. Functional annotation of the vaginal fluid proteome using Gene Ontology terms (DAVID; version 2.1; david.abcc.ncifcrf.gov/)17 revealed that most of these proteins were associated with metabolism (34%) and immune response (18%). Significant pair-wise differences were seen in relative abundance between pooled samples from women with intraamniotic infection, compared with the early preterm birth or preterm labor groups, for the 26 vaginal fluid proteins by spectral count analysis (Table 2). Twenty proteins were expressed differentially between those patients with intraamniotic infection and those with preterm labor, and 18 proteins were expressed differentially between the intraamniotic infection and early preterm birth or preterm labor groups, for the 26 vaginal fluid proteins by spectral count analysis (Table 2). Twenty proteins were expressed differentially between those patients with intraamniotic infection and those with preterm labor, and 18 proteins were expressed differentially between the intraamniotic infection and early preterm birth groups. These differentially abundant proteins included acute-phase reactants (such as alpha-1-acid glycoprotein), immune modulators (such as calgranulin C and cystatin A), high abundance amniotic fluid proteins (such as insulin-like growth factor binding protein-1 and vitamin D binding protein), and extracellular matrix–signaling factors (such as fatty acid binding protein). In general, acute-phase reactants and amniotic fluid proteins were more abundant and extracellular matrix–signaling proteins less abundant among those with intraamniotic infection (Table 2). Immune modulators such as calgranulin C were more abundant with intraamniotic infection, and some such as cystatin A were relatively depleted in the context of intraamniotic infection. Although changes in protein abundance were in a similar direction for both the preterm labor and the early preterm birth groups, when compared with those with intraamniotic infection, total fibronectin was less abundant among those with intraamniotic infection when compared with those with early preterm birth and more abundant among those with intraamniotic infection when compared with those with preterm labor.
Table 2.
Vaginal fluid proteins with significant changes in abundance associated with intraamniotic infection, by spectral count
| Intraamniotic infection vs preterm labor | Intraamniotic infection vs early preterm birth | ||||
|---|---|---|---|---|---|
| Swiss-Prot accession | Protein name | Fold change | P value | Fold change | P value |
| Acute-phase reactants | |||||
| P02763 | Alpha-1-acid glycoprotein 1a | 4.01 | .004 | 1.99 | .12 |
| P01009 | Alpha-1-antitrypsina | 3.34 | .004 | 1.98 | .01 |
| P04217 | Alpha-1B-glycoprotein | 7.94 | .001 | 2.49 | .09 |
| Immune modulators | |||||
| P01024 | Complement C3a | −1.05 | .74 | 1.76 | .007 |
| P80511 | Calgranulin Ca,b | 1.96 | .44 | 3.94 | .13 |
| P61626 | Lysozyme Ca | 2.48 | <.0001 | 2.07 | <.0001 |
| P02788 | Lactotransferrin | −1.54 | <.0001 | 1.01 | .96 |
| P01040 | Cystatin Aa | −1.34 | .21 | −3.28 | <.0001 |
| P14780 | Matrix metalloproteinase-9a | 1.74 | .07 | 1.27 | .44 |
| P31151 | S100 calcium-binding protein A7 | −13.27 | <.0001 | −18.15 | <.0001 |
| P59665 | Neutrophil defensin 1a | 1.36 | .13 | −1.57 | .02 |
| Amniotic fluid proteins | |||||
| P08833 | Insulin-like growth factor binding protein 1a | 10.47 | .001 | 1.07 | .87 |
| P02774 | Vitamin D-binding proteina | 5.37 | <.0001 | 2.67 | <.0001 |
| P00738 | Haptoglobina | 2.5 | <.0001 | 3.00 | <.0001 |
| Extracellular matrix–signaling proteins | |||||
| Q9UBC9 | Small proline-rich protein 3 | −1.26 | .04 | −1.97 | <.0001 |
| Q01469 | Fatty acid-binding proteina | −2.01 | <.0001 | −1.24 | .04 |
| O60437 | Periplakin | −6.66 | <.0001 | −10.28 | <.0001 |
| P07476 | Involucrina | −1.57 | .08 | −4.84 | <.0001 |
| P29508 | Squamous cell carcinoma antigen 1a | −8.98 | <.0001 | −2.57 | .002 |
| P02545 | Lamin A/C | −9.59 | <.0001 | −5.33 | .001 |
| P15924 | Desmoplakin | −10.75 | <.0001 | −8.18 | <.0001 |
| P02751 | Fibronectina | 9.42 | <.0001 | −2.07 | <.0001 |
| P32926 | Desmoglein-3 | −2.61 | .003 | −3.47 | <.0001 |
| P18206 | Vinculin | −4.2 | .001 | −5.34 | <.0001 |
| Q9UKR3 | Kallikrein 13 | −4.49 | <.0001 | −2.28 | .11 |
| Q9UIV8 | Serpin B13 | −5.97 | .001 | −3.43 | .06 |
Proteins that had immunoassay validation;
Notable fold changes demonstrated for Calgranulin C; however, comparisons did not reach statistical significance because of a small number of spectral counts.
We next selected 15 of these potential biomarkers for further validation by immunoassay, based on statistical significance and potential clinical relevance. As summarized in Table 3, statistically significant differences occurred in concentrations of 13 of the 15 candidate proteins among women with and without intraamniotic infection (which consisted of the combined early preterm birth and preterm labor groups). Both of the acute-phase reactants, most of the immune modulators and all of the high abundance amniotic fluid proteins that were tested had significantly higher concentrations in the vaginal fluid of women with intraamniotic infection, compared with women without intraamniotic infection. However, most of the extracellular matrix–signaling proteins were relatively depleted among women with intraamniotic infection, compared with the women without intraamniotic infection. Although trends that were shown by spectral counting and immunoassay were in a similar direction for most biomarkers, some biomarkers (such as complement C3) showed apparently contradictory results. Bacterial vaginosis was associated with increases in some vaginal fluid biomarkers that included complement C3, vitamin D binding protein, fatty acid binding protein, squamous cell carcinoma antigen, and involucrin. However, a subset analysis that was restricted to women with normal vaginal flora by Gram stain showed the same significant associations between these biomarkers and intraamniotic infection.
Table 3.
Candidate vaginal fluid protein biomarker concentrations (geometric mean and geometric standard deviation, both in nanograms per milliliter)
| Variable | Infection (n=30) |
No infection (n=140) |
P valuea | Area under receiver operating characteristic curve | 95% CI |
|---|---|---|---|---|---|
| Acute-phase reactants | |||||
| Alpha-1-acid glycoprotein | 1998±12.6 | 65±26.0 | <.0001 | 0.85 | 0.79–0.90 |
| Alpha-1-antitrypsin | 44,355±3.8 | 10,404±4.6 | <.0001 | 0.77 | 0.70–0.83 |
| Immunomodulators | |||||
| Complement component 3 | 2059±10.5 | 268±11.6 | <.0001 | 0.81 | 0.73–0.86 |
| Calgranulin C | 174±20.5 | 18±10.7 | <.0001 | 0.74 | 0.66–0.79 |
| Lysozyme | 1620±4.0 | 578±6.3 | .0043 | 0.69 | 0.58–0.79 |
| Cystatin A | 372±2.8 | 498±2.4 | .1223 | 0.58 | 0.50–0.65 |
| Matrix metalloproteinase-9 | 608±8.8 | 123±18.2 | .0075 | 0.7 | 0.60–0.81 |
| Human neutrophil defensin 1 | 10±34.8 | 7±25.0 | .5439 | 0.54 | 0.46–0.62 |
| Amniotic fluid proteins | |||||
| Insulin-like growth factor binding protein 1 | 240±4.4 | 12±16.3 | <.0001 | 0.82 | 0.75–0.89 |
| Vitamin D binding protein | 498±8.2 | 110±6.1 | <.0001 | 0.78 | 0.70–0.83 |
| Haptoglobin | 1394±4.3 | 194±8.6 | <.0001 | 0.8 | 0.73–0.85 |
| Extracellular matrix–signaling proteins | |||||
| Fatty acid binding protein | 699±151.4 | 7187±21.8 | .0027 | 0.68 | 0.60–0.75 |
| Involucrin | 25±12.3 | 67±7.9 | .0285 | 0.64 | 0.56–0.71 |
| Squamous cell carcinoma antigen | 358±13.7 | 796±5.8 | .0520 | 0.60 | 0.52–0.67 |
| Fibronectin | 1652±2.7 | 523±8.2 | .0118 | 0.70 | 0.59–0.81 |
Table 3 summarizes the area under the entire ROC curve and 95% CI for the 15 potential biomarkers for intraamniotic infection. Alpha-1-acid glycoprotein had the best classification performance (area under ROC curve, 0.85; 95% CI, 0.79–0.90; Figure). Several other individual proteins classified well, with areas under ROC curve of approximately ≥0.80 and included insulin-like growth factor binding protein-1, complement component C3, and haptoglobin. Multianalyte models incrementally improved test performance. A 2-analyte model that included alpha-1-acid glycoprotein and insulin-like growth factor binding protein- 1 had an improved area under ROC curve of 0.86 (95% CI, 0.78–0.94), and the addition of fatty acid binding protein as a negative predictor resulted in a slight additional improvement in classification performance (area under ROC curve, 0.87; 95% CI, 0.78–0.95; Figure). A 4-analyte model that contained alpha-1-acid glycoprotein, insulin-like growth factor binding protein-1, calgranulin C, and cystatin A had the strongest classification performance of all combinations that were evaluated (area under ROC curve, 0.89; 95% CI, 0.86–0.95; Figure).
Figure. Receiver-operator curve for the prediction of intraamniotic infection.
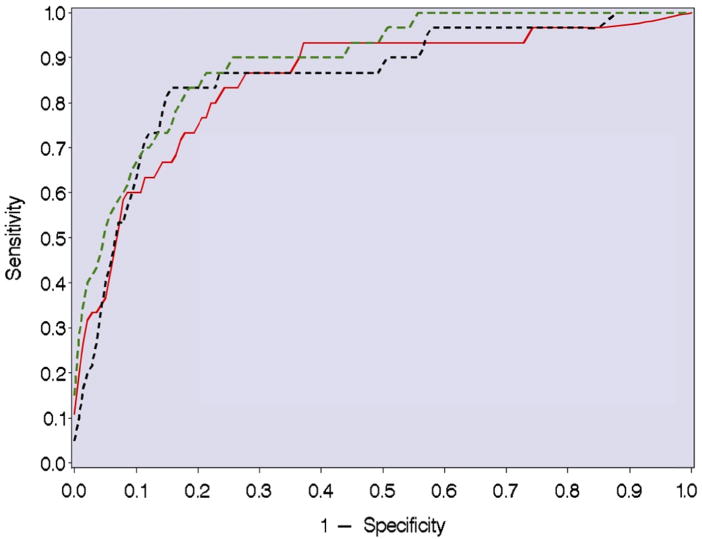
The red solid line shows the performance of alpha-1-acid glycoprotein alone; the black dotted line shows the performance of alpha-1-acid glycoprotein in combination with insulin-like growth factor binding protein-1 and fatty acid binding protein; and the green broken line shows the performance of alpha-1-acid glycoprotein in combination with insulin-like growth factor binding protein-1, calgranulin C, and cystatin A.
COMMENT
In this cohort of pregnant women with symptoms of preterm labor and intact membranes, a comprehensive analysis of the vaginal fluid proteome identified multiple novel predictors of intraamniotic infection. The specific classes of potential biomarkers of intraamniotic infection included acute-phase reactants and immune modulators, as one might predict. Of interest, high-abundance amniotic fluid proteins were also increased substantially in the intraamniotic infection group, compared with the women without intraamniotic infection. The presence of increased vaginal concentrations of amniotic fluid proteins (such as insulin-like growth factor binding protein-1 and vitamin D binding protein) suggests that a transudative process across the chorioamnion occurs with intraamniotic infection. In contrast, the relative depletion of extracellular matrix–signaling proteins (such as fatty acid binding protein) among pregnancies with intraamniotic infection (compared with the early preterm birth group) may relate to infection-mediated cervical effacement and dilation, which suggests that the mechanism of preterm birth with intraamniotic infection may differ from preterm birth without infection. These data illustrate the possibility that proteomic analyses might highlight potential alternative mechanisms that contribute to the pathophysiologic findings of both intraamniotic infection and non–infection-related early preterm birth and could provide a foundation on which to develop novel preventive and therapeutic strategies.
These findings go beyond previous work that showed that individual biomarkers in cervicovaginal fluid (such as fetal fibronectin,18,19 matrix metalloprotease-9,20 and human neutrophil defensins21) can predict intraamniotic infection and/or preterm birth. All 3 of these proteins also were expressed differentially between women with and without intraamniotic infection in this data set, as indicated in Table 3. However, these analyses allowed the selection of other biomarkers with a more optimal discriminant capability to detect intraamniotic infection.
For biomarker discovery, this investigation used pooled specimens from subjects in each of the 3 study groups. The pooled approach is used commonly in the discovery phase proteomic analysis to conserve resources and laboratory effort. One potential drawback of this approach is that important differences between subjects might be masked. This could introduce bias toward negative results but is not likely to skew results in the direction of a positive finding.
Importantly, the data in this report go beyond discovery to include immunoassay confirmation of the candidate biomarkers for intraamniotic infection from all 170 individual samples. The high concordance between our findings from pooled samples by 2-dimensional liquid chromatography/mass spectrometry and individual sample immunoassay validation lend credibility that these potential biomarkers truly differ in concentration among the intraamniotic infection, early preterm birth, and preterm labor groups. Only a few peptides were discordant in expression when mass spectrometry results were compared with immunoassay (eg, complement C3). These seemingly contradictory results likely reflect differences in methods. Mass spectrometry techniques that were used in this study identify proteins based on amino acid content of digested unique peptides, not molecular integrity, and are less quantitatively precise than immunoassay. In contrast, immunoassay is potentially more quantitatively precise but depends on an immunologically recognized intact peptide and may be subject to degradation in the vagina. Thus, we chose to use both complementary techniques in our studies to detect potential biomarkers optimally. Further, the detection of candidate proteins by immunoassay demonstrates the feasibility to develop a straightforward, rapid test in the future to diagnose intraamniotic infection accurately among women in preterm labor.
Differential expression of proteins from several distinct functional groups that include acute-phase reactants, amniotic fluid proteins, extracellular matrix proteins, and immune modulators suggests a potential to further improve the detection of intraamniotic infection with the use of a multianalyte model that is composed of biomarkers from different functional classes. In our data set, classification of subjects based on risk scores from models that contain ≥2 proteins with different mechanistic functions and the inclusion of both positive and negative predictor molecules resulted in improved classification accuracy. For example, a 3-protein model that contained alpha-1-acid glycoprotein plus insulin-like growth factor binding protein-1 and fatty acid binding protein (a vaginal protein that is associated negatively with intraamniotic infection) resulted in an area under the ROC curve of 0.87. A 4-analyte model that included alpha-1-acid glycoprotein plus insulin-like growth factor binding protein-1, calgranulin C (more abundant with intraamniotic infection), and cystatin A (depleted with intraamniotic infection) that represented different functional groups resulted in an area under the ROC curve of 0.89. Some markers that did not perform well individually did contribute significantly to multiprotein models. Multianalyte logistic regression models take into account these potentially complex relationships and facilitate the identification of vaginal fluid protein combinations that classify pregnant women with and without intraamniotic infection as accurately as possible. Thus, several biomarker combinations classified subjects with intraamniotic infection better than individual proteins alone. Certainly other potential combinations of biomarkers across functional groups might perform equally well.
Preterm birth continues to be a vexing public health problem in the United States. To date, efforts to identify pregnancies at the highest risk of preterm birth and to construct effective intervention strategies have been largely ineffective. If these findings are validated prospectively in other populations, a rapid test to identify selected vaginal fluid biomarkers could provide a sensitive, noninvasive test to diagnose intraamniotic infection that potentially could lead to early preterm birth. At present, intraamniotic infection frequently is not identified among women in preterm labor, because clinical signs of infection usually are not present and obstetricians have been reluctant to perform an invasive amniocentesis procedure at gestational ages remote from term, when infection is most common. Thus, missed opportunities to detect intraamniotic infection and provide appropriate interventions occur more frequently than is recognized. Ideally, a rapid, sensitive test that would use vaginal fluid biomarkers could increase the detection of intraamniotic infection and limit the use of transabdominal amniocentesis to a group at particularly high risk of intraamniotic infection and early preterm birth and, at the same time, limit the discomfort and potential risks of amniocentesis to only those women suspected of being at the highest risk for these complications.
At present, the diagnosis of intraamniotic infection is not particularly sought by physicians who care for women in preterm labor, despite its association with serious neonatal disease, which includes neonatal sepsis, respiratory distress syndrome, intraventricular hemorrhage, and even cerebral palsy. One reason for a failure to seek intraamniotic infection actively is the absence of adequately powered and designed clinical treatment trials of intraamniotic infection among women in preterm labor. Such treatment trials would be much easier to justify and conduct if the subset of pregnant women who are at the highest risk for intraamniotic infection, preterm birth, and adverse neonatal outcome could be sharpened with a noninvasive test of vaginal fluid. Proteomic methods could contribute to new understandings of the mechanisms that are involved in both intraamniotic infection and early preterm birth and eventually might lead to more focused therapy for both mother and fetus.
Acknowledgments
National Institutes of Health Grant no. AI31871. These analyses were supported by ProteoGenix, Inc.
Footnotes
Disclosures: Oregon Health & Science University and Drs Gravett, Nagalla, and Lapidus have a significant financial interest in ProteoGenix, Inc, which is a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed, and a management plan has been approved by the Oregon Health & Science University Integrity Program Oversight Council and by the University of Washington Office of Technology Transfer. ProteoGenix provided the technical assays and proteomic analysis. Article preparation and submission was independent of the sponsor
References
- 1.Behrman RE. Preterm birth: causes, consequences, and prevention: Institute of Medicine Committee on Understanding Premature Birth and Assuring Health Outcomes. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 4.Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol. 2001;98:1080–8. doi: 10.1016/s0029-7844(01)01567-8. [DOI] [PubMed] [Google Scholar]
- 5.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–8. [PubMed] [Google Scholar]
- 6.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin–6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 7.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 8.Dasari S, Pereira L, Reddy AP, et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res. 2007;6:1258–68. doi: 10.1021/pr0605419. [DOI] [PubMed] [Google Scholar]
- 9.Gravett MG, Thomas A, Schneider KA, et al. Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. 2007;6:89–96. doi: 10.1021/pr060149v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira L, Reddy AP, Jacob T, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007;6:1269–76. doi: 10.1021/pr0605421. [DOI] [PubMed] [Google Scholar]
- 11.Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001;97:211–9. doi: 10.1016/s0029-7844(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 12.Hitti J, Riley DE, Krohn MA, et al. Broadspectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 13.Fenyo D, Beavis RC. A method for assessing the statistical significance of mass spectrometry-based protein identifications using general scoring schemes. Anal Chem. 2003;75:768–74. doi: 10.1021/ac0258709. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 15.Old WM, Meyer-Arendt K, Aveline-Wolf L, et al. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Pepe M. Statistical methods for classification and prediction. Available at: http://labs.fhcrc.org/pepe/book/index.html. Accessed Aug. 27, 2009.
- 17.Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 18.Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669–74. doi: 10.1056/NEJM199109053251001. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth: NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643–8. doi: 10.1016/0029-7844(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 20.Athayde N, Romero R, Gomez R, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8:213–9. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Balu RB, Savitz DA, Ananth CV, et al. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol. 2003;101:862–8. doi: 10.1016/s0029-7844(03)00042-5. [DOI] [PubMed] [Google Scholar]


