Abstract
Objective
St. John’s Wort extract, which is commonly used to treat depression, inhibits the reuptake of several neurotransmitters, including glutamate, serotonin, norepinephrine, and dopamine. Glutamatergic visceral vagal afferents synapse upon neurons of the solitary tract (NST); thus, we evaluated whether St. John’s Wort extract modulates glutamatergic neurotransmission within the NST.
Materials and Methods
We used live cell calcium imaging to evaluate whether St. John’s Wort and its isolated components hypericin and hyperforin increase the excitability of pre-labeled vagal afferent terminals synapsing upon the NST. We used voltage-clamp recordings of spontaneous miniature excitatory postsynaptic currents (mEPSCs) to evaluate whether St. John’s Wort alters glutamate release from vagal afferents onto NST neurons.
Results
Our imaging data show that St. John’s Wort (50 μg/mL) increased the intracellular calcium levels of stimulated vagal afferent terminals compared to the bath control. This increase in presynaptic vagal afferent calcium by the extract coincides with an increase in neurotransmitter release within the nucleus of the solitary tract, as the frequency of mEPSCs is significantly higher in the presence of the extract compared to the control. Finally, our imaging data show that hyperforin, a known component of St. John’s Wort extract, also significantly increases terminal calcium levels.
Conclusion
These data suggest that St. John’s Wort extract can significantly increase the probability of glutamate release from vagal afferents onto the NST by increasing presynaptic calcium. The in vitro vagal afferent synapse with NST neurons is an ideal model system to examine the mechanism of action of botanical agents on glutamatergic neurotransmission.
Keywords: EPSC, NST, electrophysiology, botanical, calcium imaging, vagus nerve, presynaptic
1. Introduction
The nucleus of the solitary tract (NST) is located in the dorsal medulla and controls a number of homeostatic and behavioral functions including gastric motility, feeding, blood pressure, heart rate and respiration. The NST controls these functions through axonal connections with parasympathetic premotor neurons in the medulla, direct and indirect connections with preganglionic sympathetic neurons, projections to oral motor segments of the reticular formation, and projections to the ventral forebrain and hypothalamus as well as the spinal cord [1]. Glutamatergic visceral vagal afferent terminals synapse upon second-order NST neurons [2-4], thereby controlling homeostasis through the connections made by the NST [5, 6].
Over the last few decades, there has been increasing interest in the use of herbal remedies to treat disease. A 2007 National Health Interview Survey (NHIS) indicated that nearly 18 percent of American adults used a nonvitamin or nonmineral natural product in the previous year [7]. St. John’s Wort (Hypericin perforatum) extract has been used for centuries to treat number of disorders and is now available as an over-the-counter compound widely used to treat mild to moderate depression [8, 9]. While the cellular and molecular mechanisms of action of St. John’s Wort in treating depression are not clear, a number of different neurochemical pathways have been proposed to be influenced by the extract. St. John’s Wort extract has been postulated to inhibit the neuronal uptake of neurotransmitters such as serotonin, norepinephrine, dopamine, gamma-aminobutyric acid (GABA), and L-glutamate [10-13], alter noradrenergic and serotonergic receptor expression [14, 15], and inhibit monoamine oxidase enzymatic activity [14].
St. John’s Wort extract is composed of many biologically active compounds, including naphthodianthrones, flavonoids, prenylated phloroglucinols, tannins, phenols, and volatile oils [9, 16, 17]. The phloroglucinol derivative hyperforin is thought to be the major component of St. John’s Wort extract that acts as an antidepressant, although the naphthodianthrone hypericin may also act as an antidepressant [9, 10, 16, 18-20]. Hyperforin is believed to activate the nonselective cation transient receptor potential (TRP) channel TRPC6 to increase intracellular sodium and calcium content, therefore reducing neurotransmitter reuptake [21-24]. Indeed, previous studies on hyperforin suggest that the compound enhances miniature synaptic transmission in hippocampal CA1 and CA3 pyramidal neurons and alters dendritic spine morphology [21].
Because of St. John’s Wort potential effects on neurotransmitter release and synaptic transmission, we evaluated whether the extract influenced the excitability of glutamatergic vagal afferents synapsing upon the NST using live cell calcium imaging. We also evaluated whether St. John’s Wort extract altered the probability of glutamate release from vagal afferents onto the NST neurons by recording mEPSCs. Finally, we evaluated whether hyperforin or hypericin could mediate the effects of St. John’s Wort on glutamatergic neurotransmission.
2. Methods
A total of 12 male and female Long-Evans rats (130-250 g) were used for these studies. Animals were obtained from the breeding colony at Pennington Biomedical Research Center, were maintained in a room with a 12 hour light/dark cycle with constant temperature and humidity, and had access to food and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committees of Pennington Biomedical Research Center and were performed according to the guidelines determined by the National Institutes of Health.
2.1. Vagal afferent labeling for calcium imaging
Visceral vagal afferents were labeled as previously described [25]. Briefly, a glass microinjection pipette pulled from 1.8 mm OD starbore capillary tubing (Radnoti Glass Technologies) using a Narishige Model 1D puller was filled with 20% CalciumGreen 1-dextran 3000 molecular weight conjugate (CG) (Life Technologies) reconstituted in 1% Triton X-100 and distilled water. Rats were anesthetized using 2.5-5% isoflurane. Using sterile technique, the right nodose ganglion was accessed through a ventral incision in the neck. Approximately 500 nL of the calcium reporter dye (CG) was microinjected through the sheath of the exposed ganglion using the filled micropipette connected to a Picospritzer (General Valve). The cervical wound was then closed, and the animal was housed in its home cage for 4-5 days to allow for anterograde transport of the dye to the vagal varicosities in the NST.
2.2. Brainstem slice preparation
The pre-labeled animals were deeply anaesthetized using ethyl carbamate (urethane; 3 g/kg; Sigma). The brainstem was rapidly removed and glued to the stage of a vibrating microtome (Leica VT1200); the chamber was filled with cold (4°C) carbogenated (95% O2 / 5% CO2) cutting solution [26]. The brainstem was cut into coronal sections (300 μm thick), which were incubated at 32-34°C in the cutting solution for 10-15 min. The brainstem sections subsequently were incubated at room temperature (22-24°C) for 1-5 hr in carbogenated Krebs recording solution supplemented with 5 mM sodium ascorbate, 3 mM sodium pyruvate, and 2 mM thiourea and titrated to pH 7.4 with HCl [26].
2.3. Live cell calcium imaging
Live cell calcium imaging of CG-labeled vagal varicosities was performed as previously described [27]. Briefly, slices are placed in the recording chamber of a Nikon F1 fixed stage upright microscope and perfused with carbogenated Krebs recording solution at 33°C with a 2.5 mL/min flow rate. A Nikon Fast Scan laser confocal head with a Luca EMCCD camera (Andor Technology) was used to perform time-lapse laser confocal calcium imaging. The CG-labeled varicosities were visualized using a 488 nm excitation/509 nm long pass emission filter, and images were collected a rate of three frames per second. ATP (100 μM) was applied in the bath for 60 s to activate P2X3 ligand-gated cation channels on vagal afferent varicosities and test for the ability of the terminals to produce calcium signals [25]. Following a 10 min bath application of Krebs alone or SJW prepared in Krebs solution, ATP was reapplied for 60 s; thus, each varicosity acted as its own control.
2.4. Patch-clamp recording from NST neurons
During the whole cell voltage-clamp recordings (VHOLD = -60 mV), the slices were placed in the recording chamber of an upright microscope and were perfused with normal Krebs solution at 33°C with a 2.5 mL/min flow rate. Thin-walled borosilicate glass (Warner Instruments) was used to form recording electrodes, which were filled with (in mM) 120 Cs-methanesulfonate, 15 CsCl, 10 tetraethylammonim chloride, 10 HEPES, 8 NaCl, 3 Mg-ATP, 1.5 MgCl2, 0.3 Na-GTP, and 0.2 EGTA at pH 7.3 [28]. Recordings were made using a Multiclamp 700B amplifier (Molecular Devices), filtered at 8 kHz, and were digitized at 20 kHz using Axon pClamp10 software.
2.5. In vitro solutions and drugs
The cutting solution contained (in mM) 92 N-methyl-D-glucamine, 30 NaHCO3, 25 glucose, 20 HEPES, 10 MgSO4-7H2O, 5 sodium ascorbate, 3 sodium pyruvate, 2.5 KCl, 2 thiourea, 1.25 NaH2PO4, and 0.5 CaCl2, titrated to pH 7.4 with HCl [26]. The Krebs recording solution contained (in mM) 124 NaCl, 25 NaHCO3, 10 glucose, 3 KCl, 2 CaCl2, 1.5 NaH2PO4, and 1 MgSO4-7H2O. The recording solution was supplemented with 10 μM bicuculline and 0.5 μM TTX, and the CaCl2 was increased to 4 mM during the patch-clamp recordings.
The St. John’s Wort extracts were prepared at the Rutgers University Botanical Research Center. The flowering herb Hypericum perforatum L. was greenhouse grown from seed and harvested at the flowering stage, freeze dried, and stored at -20°C. The dried herb was extracted in 80% ethanol (1:20 w/v) at 50°C with sonication for 1 hour followed by shaking at room temperature for 24 h. The solid material was removed by centrifugation at 3000 g, and the solvent was subsequently removed by evaporation. The extracts were resuspended in 1000X (50 mg/mL) stocks in DMSO [29].
2.6. Data Analysis
Nikon Elements AR software was used to analyze the confocal live cell fluorescent signal as previously described [25]. The relative changes in cytoplasmic calcium were expressed as changes in fluorescence [(ΔF/F)%] of the CG reporter dye, where F is the intensity of the baseline fluorescence signal before stimulation, and ΔF is the difference between the peak fluorescence intensity and the baseline signal. Briefly, the afferent fiber and varicosity regions of interest (ROI) were outlined, and the background fluorescence was subtracted from the fluorescence signal before the relative changes in cytoplasmic calcium is calculated. Each varicosity acted as its own control, and data were evaluated for statistical significance using the paired t-test; significance was set at p < 0.05. Data are reported as mean ± S.E.M.
Spontaneous miniature EPSCs from the whole cell voltage-clamp recordings from NST neurons were analyzed as previously described using the Mini Analysis Program (Synaptosoft, Inc., Decatur, GA) [30]. The mEPSCs were collected over 2 min periods and were detected automatically. Only events with amplitudes greater than 2.5 times the RMS noise and rise times more rapid than 10 ms were included in the analysis. Selected mEPSCs from each recording were scaled and averaged, and the deactivation time constants were calculated by fitting the following single exponential equation to the data:
| (1) |
where τ is the deactivation time constant, and Amp is the current amplitude of the deactivation component.
Statistical significance of the distribution of mEPSC inter-event intervals was determined using the Kolmogorov-Smirnov nonparametric analysis, while the paired t-test was used to evaluate amplitudes and deactivation time constants. Significance was set at p < 0.05. Data are reported as mean ± S.E.M.
3. Results
3.1. St. John’s Wort enhances vagal afferent terminal excitability
Because previous studies have indicated that St. John’s Wort extract and its isolated components can alter neurotransmitter reuptake and synaptic transmission [10-15, 21], we first evaluated whether St. John’s Wort extract modulated the excitability of glutamatergic visceral vagal afferents terminating in the NST using calcium imaging of CG-labeled terminals. Bath application of ATP was used to stimulate the vagal terminals, as ATP causes a wave-like monotonic rise in calcium within the terminals by activating P2X3 channels [31, 32]. In order to establish time controls for each varicosity, slices were exposed to ATP (100 μM; 60 s) two times, separated by a 10 min interval (Fig. 1). We first evaluated “time control” applications of ATP, whereby the slices were exposed to two 60 s applications of ATP (100 μM) separated by 10 minutes in bath alone (Table 1). The first ATP application caused a relative increase in CG fluorescence [(ΔF/F)%] of 29 ± 1.3% (n = 172; Table 1), while the second ATP application resulted in a relative increase in CG fluorescence [(ΔF/F)%] of 29 ± 1.5% (n = 172; Table 1). Thus, there is no significant difference in CG fluorescence between the first and second ATP applications (p > 0.05; paired t-test). We subsequently evaluated how St. John’s Wort extract modulated calcium influx. The first ATP application resulted in a relative increase in CG fluorescence [(ΔF/F)%] of 27 ± 0.86% (Table 1), after which the slices were perfused with recording solution containing 50 μg/mL St. John’s Wort for 10 min. Finally, the slices were exposed to ATP again (100 μM; 60 s; Fig. 1). Terminal excitability was significantly increased following the 10 min SJW exposure, as the relative increase in CG fluorescence [(ΔF/F)%] was 33 ± 1.0% (n = 213; p < 0.05; paired t-test; Table 1; Fig. 1E). These data indicate that St. John’s Wort extract evokes a significant increase in the calcium signal of vagal afferent terminals synapsing upon neurons in the NST.
Figure 1.
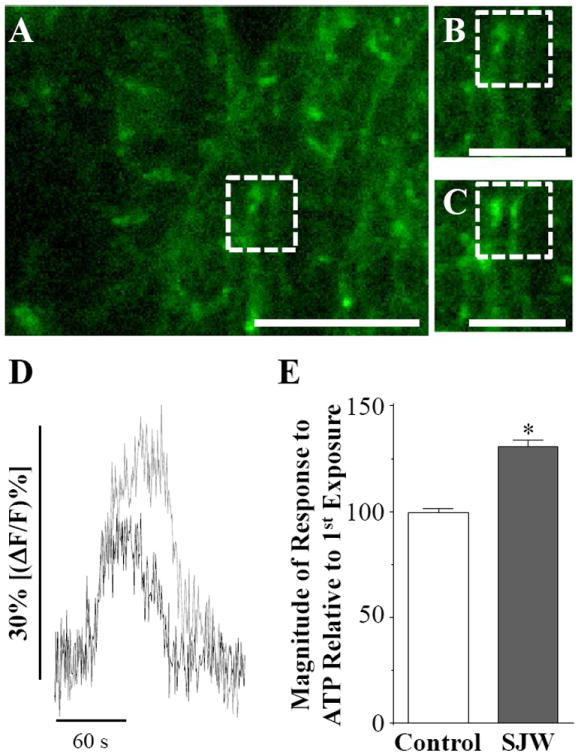
St. John’s Wort extract increases vagal afferent excitability. We evaluated how St. John’s Wort extract modulates vagal afferent excitability using live cell calcium imaging of prelabeled vagal terminals. A, The complete field view of a brainstem slice is given with a region of interest (ROI) outlined by a dotted box. The ROI is shown before (B) and at the peak (C) of ATP stimulation following a 10 min incubation in St. John’s Wort extract. D, Plot of change in fluorescence for the above varicosity in response to application of ATP before (black) and after (gray) treatment with St. John’s Wort. E, A plot of normalized changes in calcium flux for the second ATP application relative to the first application of ATP are given for the time control and for the afferent terminals treated with St. John’s Wort (unpaired t-test; * p < 0.05). Scale bars: A = 10 μm; B and C = 6 μm.
Table 1. St. John’s Wort extract and hyperforin increase vagal afferent excitability.
We evaluated whether St. John’s Wort extract and its isolated components increased the calcium signal in prelabeled vagal afferent terminals. Slices were stimulated with ATP (100 μM; 60 s) twice, separated by a 10 min interval in which the slices were perfused with normal recording solution (Time Control) or St. John’s Wort extract (100 μg/mL), hyperforin (10 μM), or hypericin (1 μM) prepared in recording solution. Statistical significance was determined for each condition using a paired t-test.
| Modulator | (ΔAF/F)% 1st ATP | (ΔAF/F)% 2nd ATP | n |
|---|---|---|---|
| Time Control | 29 ± 1.3% | 29 ± 1.3% | 172 |
| St. John’s Wort | 27 ± 0.86% | 33 ± 1.0%* | 213 |
| Hyperforin | 21 ± 1.2% | 25 ± 1.6%* | 60 |
| Hypericin | 24 ± 1.5% | 23 ± 1.2% | 48 |
p < 0.05.
3.2. St. John’s Wort increases the synaptic activity of the NST
We next investigated whether the increase in afferent calcium signal caused by St. John’s Wort extract led to an increase in quantal glutamate neurotransmitter release from vagal afferents synapsing upon the neurons in the NST. We recorded miniature excitatory postsynaptic currents (mEPSCs) from NST neurons in the presence of 0.5 μM TTX to block sodium channels and prevent action potentials and 10 μM bicuculline to inhibit GABA receptors. We then evaluated how St. John’s Wort modulated mEPSC frequency, amplitude, and deactivation kinetics (Fig. 2). Bath application of 50 μg/mL St. John’s Wort significantly increased the frequency of the NST mEPSCs, as the cumulative probability plot of inter-event intervals shows a significant leftward shift in the presence of St. John’s Wort compared to the control (p < 0.05; Kolmogorov-Smirnov; Fig. 2B). Indeed, the mEPSC frequency increased from 15 ± 8.5 Hz in the control to 20 ± 11 Hz in the presence St. John’s Wort extract (n = 7). St. John’s Wort extract did not influence mEPSC amplitude (21 ± 2.3 pA vs 21 ± 2.6 pA; p > 0.05; paired t-test; Fig. 2C). The deactivation time courses of the mEPSCs were best fit by a single exponential function and also were not significantly altered by St. John’s Wort (4.8 ± 0.26 ms vs 4.8 ± 0.54 ms; p > 0.05; paired t-test; Fig. 2D). These data suggest that St. John’s Wort acts on presynaptic vagal afferents to increase the frequency of glutamate release onto the NST neurons, similar to what has been observed in CA1 and CA3 hippocampal pyramidal neurons [21].
Figure 2.
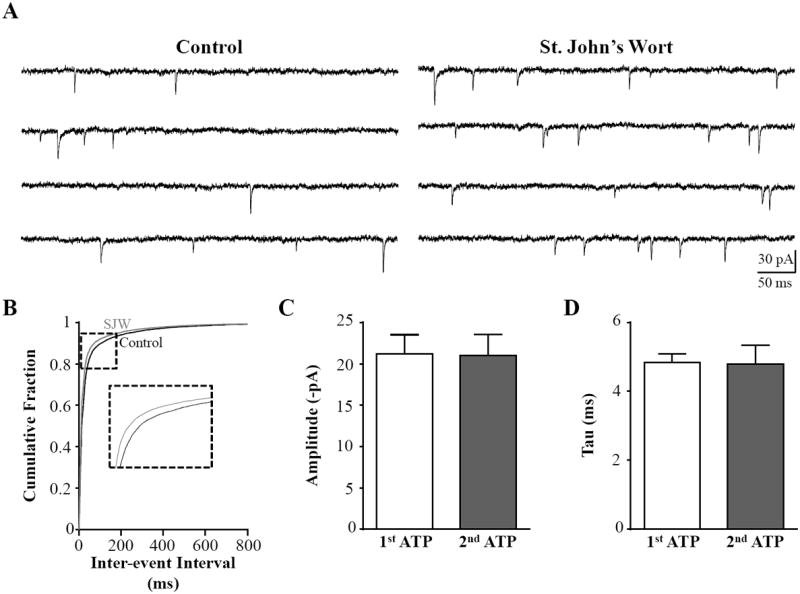
St. John’s Wort extract increases mEPSC frequency in NST neurons. A, We recorded mEPSCs (VHOLD=-60 mV) from NST neurons to evaluate whether St. John’s Wort extract caused increased quantal glutamate release from vagal afferent terminals. A representative control recording (left) was obtained in the presence of 10 μM bicuculline and 0.5 μM TTX. St. John’s Wort extract increased the frequency of events (right) in the same representative recording. B, The distribution of inter-event intervals was shifted significantly to the left in the presence of St. John’s Wort compared to control (Kolmogorov-Smirnov test, p < 0.05), suggesting that frequency of mEPSC events was increased by the extract. Neither peak amplitude (C) nor deactivation time course (D) was impacted by St. John’s Wort extract (paired t-test, p > 0.05). These data suggest that St. John’s Wort increases the probability of glutamate release from presynaptic vagal afferent terminals.
3.3. Hyperforin mediates the effects of St. John’s Wort on terminal excitability
Finally, we used live cell calcium imaging of prelabeled vagal afferent terminals to determine whether hypericin and/or hyperforin, two biologically active compounds within St. John’s Wort extract thought to mediate its effects on depression, modulate vagal afferent calcium signaling. Hyperforin ranges in content from 2 to 5% in the dried plant [9, 19], while hypericin ranges in content from 0.02 to 2.5% [9, 16, 20]. As with our experiments using St. John’s Wort extract, slices were exposed to ATP (100 μM; 60 s) two times, separated by a 10 min interval in which the slices were exposed to hyperforin or hypericin. We first evaluated whether hyperforin had similar effects on vagal afferent excitability as St. John’s Wort. The first ATP application produced a relative increase in CG fluorescence [(ΔF/F)%] of 21 ± 1.2%. Following a 10 min application of 10 μM hyperforin, CG fluorescence [(ΔF/F)%] evoked by application of ATP significantly increased to 25 ± 1.6% (n = 60; p < 0.05; paired t-test; Fig. 3A; Table 1). However, hypericin (1 μM; 10 min) did not alter the terminal calcium responses, as there was no significant difference between the first and second applications of ATP (24 ± 1.5% vs. 23 ± 1.2%; n = 48; p > 0.05; paired t-test; Fig. 3B; Table 1). These data suggest that hyperforin within St. John’s Wort contributes to the extract’s effects on afferent excitability and neurotransmission.
Figure 3.
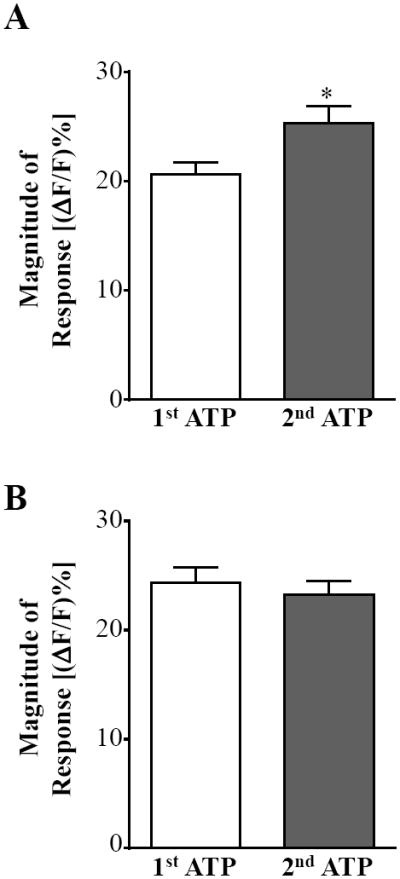
Hyperforin mediates St. John’s Wort extract’s effects on vagal afferent excitability. A, Bath application of 10 mM hyperforin significantly increased the calcium signal of prelabeled afferent terminals evoked by the second application of ATP compared to the first in our live cell calcium imaging recordings. B, Hypericin (1 mM) did not significantly increase the calcium signal evoked by the second ATP application compared to the first (paired t-test, * p < 0.05).
4. Discussion
There are several key findings in our study on St. John’s Wort modulation of vagal afferents synapsing upon neurons in the NST. First, using live cell calcium imaging of prelabeled vagal afferent terminals we observed that St. John’s Wort extract significantly increased the excitability of visceral vagal afferents synapsing in the NST. Second, the increased excitability of the vagal afferents leads to an increase in the frequency of glutamate release onto the NST neurons, as NST mEPSC frequency is increased in the presence of St. John’s Wort extract. Finally, both St. John’s Wort extract and hyperforin, one component of the extract, elicited the same increased effects on vagal afferent excitability.
Depression is a major psychiatric disorder that affects about 20% of the population of the United States [33]. Common treatments for depression include selective serotonin reuptake inhibitors, tricyclic antidepressants, norepinephrine-reuptake inhibitors, and monoamine oxidase inhibitors [33, 34]. However, these classical antidepressants are not effective in as many as half of depressive patients, necessitating the development of new treatments for depression [35, 36]. Clinical trials suggest that St. John’s Wort extract and hyperforin both have efficacy in treating depression when compared to placebos or other antidepressants [9, 10, 37]. However, both St. John’s Wort and hyperforin diminish the therapeutic efficacies of other drugs, likely due to their effects on drug metabolism, as St. John’s Wort extract and hyperforin induce the expression of cytochrome P450 enzymes, induce the expression of P-glycoprotein, and activate the pregnane X receptor [38-41]. Thus, use of St. John’s Wort in conjunction with certain pharmaceuticals is not recommended [9].
One major side effect of St. John’s Wort extract is gastrointestinal malaise [9, 16, 42]. Previous studies have shown that signaling molecules that act on vagal afferent terminals synapsing upon the NST can evoke significant changes in gastrointestinal functions associated with illness [31, 43-48]. Tumor necrosis factor-α (TNF), a cytokine that is released from macrophages and microglia, is an example of a molecule capable of positively modulating vagal afferent excitability. TNF can sensitize visceral vagal afferents synapsing upon the NST, increasing the calcium signal in in vitro live cell imaging [25, 27], which may be responsible for the reduction in gastric motility, malaise, nausea, and emesis caused by peripherally generated TNF [49, 50]. Given that our data show that St. John’s Wort extract increases the excitability of vagal afferents and increases the synaptic transmission of NST neurons, it is possible that the extract is acting on the same neurocircuitry of the dorsal vagal complex to elicit the “side effect” of this gastrointestinal reaction.
In conclusion, our data show that St. John’s Wort extract is a powerful mediator of glutamatergic neurotransmission within the NST. St. John’s Wort increases the excitability of vagal afferents, leading to an increase in glutamate release onto neurons of the NST. Thus, St. John’s Wort may be a useful agent to treat diseases in which the underlying cause is a glutamatergic hypofunction.
Acknowledgments
The authors thank Elizabeth Sell for excellent technical assistance. Funding was provided by NIH T32-AT004094 (KMV), NS60664 (RCR), and the Botanical Research Center grant P50AT002776-01 (DMR).
Abbreviations
- AMPA
2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid
- ATP
adenosine triphosphate
- CG
CalciumGreen 1-dextran 3000 molecular weight conjugate
- DL-AP5
DL-amino-5-phosphonovaleric acid
- DNQX
7-dinitroquinoxaline-2,3-dione
- GABA
gamma-aminobutyric acid
- mEPSC
miniature excitatory postsynaptic current
- NMDA
N-methyl-D-aspartate
- NST
nucleus of the solitary tract
- SJW
St. John’s Wort
- TTX
tetrodotoxin
Footnotes
Author Contributions. KMV, GEH, and RCR designed the study and conducted the experiments. D. Ribnicky purified the St. John’s Wort extract. All authors participated in writing the manuscript.
Disclosure Statement. The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogers RC, Hermann GE. Brainstem control of gastric function. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Academic Press; 2012. pp. 861–92. [Google Scholar]
- 2.Leone C, Gordon FJ. Is l-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius. J Pharmacol Exp Ther. 1989;250(3):953–62. [PubMed] [Google Scholar]
- 3.Ohta H, Talman WT. Both nmda and non-nmda receptors in the nts participate in the baroreceptor reflex in rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1994;267(4):R1065–R70. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- 4.Aylwin ML, Horowitz JM, Bonham AC. Non-nmda and nmda receptors in the synaptic pathway between area postrema and nucleus tractus solitarius. Am J Physiol-Heart Circul Physiol. 1998;275(4):H1236–H46. doi: 10.1152/ajpheart.1998.275.4.H1236. [DOI] [PubMed] [Google Scholar]
- 5.Blessing W. The lower brainstem and body homeostasis. New York: Oxford UP; 1997. [Google Scholar]
- 6.Rogers RC, Hermann GE. Tumor necrosis factor (tnf) positively modulates vagal afferent excitability within the solitary nucleus: An in vitro live-cell imaging study. Gastroenterology. 2006;130(4):A246–A. [Google Scholar]
- 7.Barnes P, Bloom B, Nahin R. CDC National Health Statistics Report #12. Dec 10, 2008. Complementary and alternative medicine use among adults and children: United states, 2007. [PubMed] [Google Scholar]
- 8.Butterweck V. Mechanism of action of st john’s wort in depression - what is known? Cns Drugs. 2003;17(8):539–62. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- 9.Barnes J, Anderson LA, Phillipson JD. St john’s wort (hypericum perforatum l.): A review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53(5):583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 10.Muller WE. Current st. John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47(2):101–9. doi: 10.1016/s1043-6618(02)00266-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaehler ST, Sinner C, Chatterjee SS, et al. Hyperforin enhances the extracellular concentrations of catecholamines, serotonin and glutamate in the rat locus coeruleus. Neurosci Lett. 1999;262(3):199–202. doi: 10.1016/s0304-3940(99)00087-7. [DOI] [PubMed] [Google Scholar]
- 12.Neary JT, Bu YR. Hypericum li 160 inhibits uptake of serotonin and norepinephrine in astrocytes. Brain Research. 1999;816(2):358–63. doi: 10.1016/s0006-8993(98)01126-3. [DOI] [PubMed] [Google Scholar]
- 13.Wonnemann M, Singer A, Siebert B, et al. Evaluation of synaptosomal uptake inhibition of most relevant constituents of st. John’s wort. Pharmacopsychiatry. 2001;34:S148–S51. doi: 10.1055/s-2001-15465. [DOI] [PubMed] [Google Scholar]
- 14.Muller WE, Rolli M, Schafer C, et al. Effects of hypericum extract (li 160) in biochemical models of antidepressant activity. Pharmacopsychiatry. 1997;30:102–7. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- 15.TeufelMayer R, Gleitz J. Effects of long-term administration of hypericum extracts on the affinity and density of the central serotonergic 5-ht1 a and 5-ht2 a receptors. Pharmacopsychiatry. 1997;30:113–6. doi: 10.1055/s-2007-979530. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Landa JF, Contreras CA. A review of clinical and experimental observations about antidepressant actions and side effects produced by hypericum perforatum extracts. Phytomedicine. 2003;10(8):688–99. doi: 10.1078/0944-7113-00340. [DOI] [PubMed] [Google Scholar]
- 17.Butterweck V, Nahrstedt A. What is known about st. John’s wort? Phytochemistry and pharmacology. Pharmazie in unserer Zeit. 2003;32(3):212–9. doi: 10.1002/pauz.200390067. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee SS, Bhattacharya SK, Wonnemann M, et al. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sciences. 1998;63(6):499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 19.Orth HCJ, Rentel C, Schmidt PC. Isolation, purity analysis and stability of hyperforin as a standard material from hypericum perforatum 1. J Pharm Pharmacol. 1999;51(2):193–200. doi: 10.1211/0022357991772132. [DOI] [PubMed] [Google Scholar]
- 20.Mulinacci N, Bardazzi C, Romani A, et al. Hplc-dad and tlc-densitometry for quantification of hypericin in hypericum perforatum l. Extracts. Chromatographia. 1999;49(3-4):197–201. [Google Scholar]
- 21.Leuner K, Kazanski V, Mueller M, et al. Hyperforin - a key constituent of st. John’s wort specifically activates trpc6 channels. Faseb Journal. 2007;21(14):4101–11. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- 22.Leuner K, Li W, Amaral MD, et al. Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating ca2±-permeable trpc6 channels. Hippocampus. 2013;23(1):40–52. doi: 10.1002/hipo.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh WL, Davies JA. The involvement of sodium and calcium ions in the release of amino acid neurotransmitters from mouse cortical slices elicited by hyperforin. Life Sciences. 2002;71(22):2645–55. doi: 10.1016/s0024-3205(02)02104-5. [DOI] [PubMed] [Google Scholar]
- 24.Singer A, Wonnemann M, Muller WE. Hyperforin, a major antidepressant constituent of st. John’s wort, inhibits serotonin uptake by elevating free intracellular na±. J Pharmacol Exp Ther. 1999;290(3):1363–8. [PubMed] [Google Scholar]
- 25.Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci. 2006;26(49):12642–6. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao SL, Ting JT, Atallah HE, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8(9):745–U91. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers RC, Hermann GE. Tumor necrosis factor activation of vagal afferent terminal calcium is blocked by cannabinoids. J Neurosci. 2012;32(15):5237–41. doi: 10.1523/JNEUROSCI.6220-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzman JN, Sanchez-Padilla J, Chan CS, et al. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29(35):11011–9. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amini Z, Boyd B, Doucet J, et al. St. John’s wort inhibits adipocyte differentiation and induces insulin resistance in adipocytes. Biochemical and Biophysical Research Communications. 2009;388(1):146–9. doi: 10.1016/j.bbrc.2009.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CJ, Mannaioni G, Yuan H, et al. Astrocytic control of synaptic nmda receptors. J Physiol. 2007;581(3):1057–81. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin YH, Bailey TW, Li BY, et al. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24(20):4709–17. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers RC, Nasse JS, Hermann GE. Live-cell imaging methods for the study of vagal afferents within the nucleus of the solitary tract. Journal of Neuroscience Methods. 2006;150(1):47–58. doi: 10.1016/j.jneumeth.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adell A, Castro E, Celeda P, et al. Strategies for productring faster acting antidepressants. Drug Discovery Today. 2005;10(8):578–85. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- 35.Insel TR. Beyond efficacy: The star*d trial. American Journal of Psychiatry. 2006;163(1):5–7. doi: 10.1176/appi.ajp.163.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in star*d: Implications for clinical practice. American Journal of Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 37.Nathan PJ. Hypericum perforatum (st john’s wort): A non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. Journal of Psychopharmacology. 2001;15(1):47–54. doi: 10.1177/026988110101500109. [DOI] [PubMed] [Google Scholar]
- 38.Obach RS. Inhibition of human cytochrome p450 enzymes by constituents of st. John’s wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294(1):88–95. [PubMed] [Google Scholar]
- 39.Roby CA, Anderson GD, Kantor E, et al. St john’s wort: Effect on cyp3a4 activity. Clinical Pharmacology & Therapeutics. 2000;67(5):451–7. doi: 10.1067/mcp.2000.106793. [DOI] [PubMed] [Google Scholar]
- 40.Johne A, Brockmoller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from st john’s wort (hypericum perforatum) Clinical Pharmacology & Therapeutics. 1999;66(4):338–45. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 41.Moore LB, Goodwin B, Jones SA, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane x receptor. Proc Natl Acad Sci U S A. 2000;97(13):7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woelk N, Burkard G, Grunwald J. Evaluation of the benefits and risks of the hypericum extract li-160 based on a drug-monitoring study with 3250 patients. Nervenheilkunde. 1993;12(6A):308–13. [Google Scholar]
- 43.Emch GS, Hermann GE, Rogers RC. Tnf-alpha activates solitary nucleus neurons responsive to gastric distension. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2000;279(3):G582–G6. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- 44.Hermann GE, Hebert SL, Van Meter MJ, et al. Tnf alpha-p55 receptors: Medullary brainstem immunocytochemical localization in normal and vagus nerve-transected rats. Brain Research. 2004;1004(1-2):156–66. doi: 10.1016/j.brainres.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 45.Page AJ, Young RL, Martin CM, et al. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128(2):402–10. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 46.Hermann G, Rogers RC. Tumor-necrosis-factor-alpha in the dorsal vagal complex suppresses gastric-motility. Neuroimmunomodulation. 1995;2(2):74–81. doi: 10.1159/000096874. [DOI] [PubMed] [Google Scholar]
- 47.Doyle MW, Bailey TW, Jin YH, et al. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22(18):8222–9. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doyle MW, Bailey TW, Jin YH, et al. Strategies for cellular identification in nucleus tractus solitarius slices. Journal of Neuroscience Methods. 2004;137(1):37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Hermann GE, Holmes GM, Rogers RC. Tnf alpha modulation of visceral and spinal sensory processing. Current Pharmaceutical Design. 2005;11(11):1391–409. doi: 10.2174/1381612053507828. [DOI] [PubMed] [Google Scholar]
- 50.Hermann GE, Tovar CA, Rogers RC. Lps-induced suppression of gastric motility relieved by tnfr : Fc construct in dorsal vagal complex. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283(3):G634–G9. doi: 10.1152/ajpgi.00412.2001. [DOI] [PubMed] [Google Scholar]


