Abstract
OBJECTIVES
To better understand the risk of short-term complications associated with perioperative intravesical mitomycin-C (MMC) therapy for patients undergoing endoscopic management of non-muscle invasive bladder cancer (NMIBC).
METHODS AND MATERIALS
Using an institutional database of patients with bladder cancer, we performed a retrospective case-control study of patients receiving perioperative MMC after tumor resection (2008–2012). MMC cases were matched by clinical stage to controls receiving endoscopic resection alone. Demographic information, clinicopathologic details and outcomes were compared between groups. Outcomes of interest included overall, genitourinary (GU) and major complications. Chi-square tests and multivariable logistic regression were used to evaluate associations between patient characteristics, clinical factors, exposure to MMC and outcomes of interest.
RESULTS
One-hundred sixteen patients treated with MMC were matched to 116 controls. Patients receiving MMC were younger (p=0.04) and more likely to have invasive disease (i.e., T1 or greater) (23% vs. 15%, p=0.02). Complications were more frequent among patients who were treated with MMC (34.5% vs. 19.8%, OR 2.89, 95% CI 1.43–5.81). The most common complication among MMC patients that required medical management was dysuria (17%). Major complications were more common among MMC patients (5.2% vs. 0.9%), but this difference did not reach statistical significance (p=0.11).
CONCLUSIONS
Use of MMC is associated with a greater odds of complications compared to controls. Patients should be counseled regarding both the benefits and potential risks of perioperative intravesical MMC. Continued research is required to understand the safety implications associated with the use of perioperative, intravesical MMC.
Keywords: Mitomycin, bladder cancer, complications, chemotherapy, safety
INTRODUCTION
Bladder cancer is one of the more common malignancies in the United States, with more than an estimated 70,000 cases in 2012.[1] The majority of these bladder cancer patients have non-muscle invasive bladder cancer (NMIBC).[2] Although they rarely experience progression to more invasive disease, patients with NMIBC have frequent recurrences.[3] Surveillance and management of recurrences are responsible for the majority of health care costs in the management of NMIBC.[4] In an attempt to decrease the risk of tumor recurrence, urologists have used intravesical chemotherapy at the time of tumor resection for patients with non-invasive bladder tumors. This treatment strategy is based on findings that show that intravesical chemotherapy, administered at the time of bladder tumor resection, results in a 12% absolute risk reduction in the recurrence of bladder tumors.[5] In addition, as a result of these findings, administration of perioperative intravesical therapy for certain patients with NMIBC is recommended by the European Association of Urology (EAU) guidelines in 2011.[6] Furthermore, American Urological Association (AUA) guidelines recommend that that perioperative intravesical chemotherapy may be given to patients with low-grade Ta tumors, and state that is an option for patients with small lesions of unknown pathology that appear to be Ta (but not CIS). [7]
However, unlike efficacy, the safety profile of intravesical chemotherapy has not been well described in detail. Available literature on the subject is primarily limited to individual reports of severe side effects associated intravesical chemotherapy, including bladder perforation and perivesical fat necrosis.[8–10] The most commonly described side effects in the randomized trials include mild transient irritative bladder symptoms (10%) and allergic skin reactions (3%).[5] Given the relatively modest reduction in recurrence risk of intravesical chemotherapy, any increase risk of complications related to therapy would diminish this clinical benefit, possibly impairing patient quality of life, and decreasing its overall cost-effectiveness.
In order to better understand the risks associated with perioperative intravesical chemotherapy, we performed a retrospective case-control study to assess for complications after administration of single-dose perioperative mitomycin-C (MMC) at the time of bladder tumor resection. Our primary outcome of interest was overall complications within 60 days of treatment. Secondary outcomes of interest included (a) complications specific to the genitourinary (GU) system and (b) major complications. A clearer picture of the risks associated with intravesical MMC at the time of bladder resection will help guide urologists on treatment decision making.
METHODS AND MATERIALS
Data source and patient cohort
We used our prospective, institutional review board-approved database of bladder cancer patients as the data source for this study. The time period of interest was from January 2008 through December 2011. We identified 116 patients who had MMC administered around the time of their bladder tumor resection. All MMC patients were matched 1:1 with control cases based on clinical stage, for a total of 232 patients in our analytic cohort. This included 35 patients who underwent tumor resection at an outside hospital but were then seen in our clinic for further management after their diagnosis of bladder cancer. In general, providers at our institution administer 40mg MMC in 40mL of saline. MMC is administered via indwelling catheter either in the operating room or recovery room. MMC is left to instill for 60 minutes and then drained (unless symptoms dictate earlier drainage). Specific details of MMC administration at other institutions are not available to us.
Data collection
Abstracted data included patient demographics (e.g., age, gender), operative factors (e.g., date, treatment site, procedure type), clinical characteristics (e.g., prior intravesical therapy, patient comorbidity, clinical stage), and pathologic stage. We abstracted all 60-day complications and unanticipated events (e.g., cystoscopy, hospital readmissions, and emergency room visits). Patient comorbidity was tracked and catalogued in the database by using the system described by Charlson et al.[11] Complications were prospectively registered in the database and graded by the Clavien-Dindo classification system.[12] We categorized complications as minor (i.e., grade 1–2) and major (i.e., grade ≥3). A retrospective chart review of all identified patients was carried out to determine specific clinical characteristics (e.g., diagnosis of benign prostatic hypertrophy, administration of new medications) or missing data not captured in the database. A patient was considered to have a diagnosis of benign prostatic hypertrophy or overactive bladder if it was documented in their past medical history or if they had medications listed to treat the respective diagnoses (e.g., 5-α reductase inhibitors, anticholinergics).
Statistical analysis
Bivariate associations between patient, clinical, and pathologic covariates with the receipt of MMC were tested by either chi-square tests or Fisher's exact test (if cell sizes were smaller than 10) for categorical data. Continuous variables were assessed with paired t-test statistics. The same statistical tests were used to evaluate associations between outcomes of interest and receipt of MMC. We then performed conditional multivariable logistic regression to assess the association with (a) overall complications and (b) genitourinary complications. Our model adjusted for variables that were defined a priori, including age (as a continuous variable), year of operation, site of operation, history of pelvic radiation, and pathologic stage. STATA version 11 (College Station, TX) was used to perform all statistical analyses. For sensitivity analyses, we ran models that included covariates not distributed evenly between cases and controls (e.g., BPH/OAB diagnosis). We also ran models excluding 12 patients who underwent re-resection for T1 disease. All analyses were performed to the 5% significance level. Our institutional review board approved the study.
RESULTS
Patients who received perioperative intravesical MMC were relatively similar to controls (Table 1). The groups were similar in terms of gender, prior intravesical chemotherapy, prior pelvic radiation, Charlson comorbidity score, and year of operation (all p>0.05). Patients treated with MMC were younger (67.8 vs. 71.2 years, p=0.04), less likely to have BPH/OAB, more likely to be treated at an outside institution (23% vs. 8%, p<0.01), and more likely to have invasive disease on pathology (23% vs. 16%, p=0.02).
Table 1.
Characteristics of analytic cohort.
| Covariate | MMC (n=116) | NoMMC (n=116) | p |
|---|---|---|---|
| Age (mean years (SD)) | 67.8 (12.4) | 71.2 (12.1) | 0.04 |
| Age (categorical) (n, %) | |||
| <50 years old | 9 (7.8) | 5 (4.3) | 0.16 |
| 50–59 years old | 23 (19.8) | 16 (13.8) | |
| 60–69 years old | 34 (29.3) | 27 (23.3) | |
| 70–79 years old | 25 (21.6) | 39 (33.6) | |
| 80+ years old | 25 (21.6) | 29 (25.0) | |
| Gender (n, %) | 0.21 | ||
| Male | 86 (74.1) | 94 (81.0) | |
| Female | 30 (25.9) | 22 (19.0) | |
| Prior intravesical chemotherapy (n, %) | 34 (29.6) | 46 (39.7) | 0.10 |
| Diagnosis of BPH/OAB (n, %) | 21 (18.3) | 34 (29.3) | 0.04 |
| Prior pelvic radiation (n, %) | 9 (7.8) | 12 (10.3) | 0.65 |
| Charlson comorbidity score (n, %) | |||
| 0 | 55 (47.4) | 47 (40.5) | 0.53 |
| 1–2 | 40 (34.5) | 43 (37.0) | |
| 3+ | 21 (18.1) | 26 (22.4) | |
| Year of operation (n, %) | 0.20 | ||
| 2008 † | 25 (21.6) | 26 (22.4) | |
| 2009 | 16 (13.8) | 26 (22.4) | |
| 2010 | 38 (32.8) | 26 (22.4) | |
| 2011 ‡ | 37 (31.9) | 38 (32.8) | |
| Location (n, %) | <0.01 | ||
| Home institution | 89 (77.4) | 107 (92.2) | |
| Other institution | 26 (22.6) | 9 (7.8) | |
| Clinical stage (n, %) | 1.00 | ||
| T0 | 2 (1.7) | 2 (1.7) | |
| Ta/Tis | 84 (72.4) | 84 (72.4) | |
| T1 | 13 (11.2) | 13 (11.2) | |
| T2 | 1 (0.9) | 1 (0.9) | |
| Not described (Tx) | 16 (13.8) | 16 (13.8) | |
| Pathologic stage (n, %) | 0.02 | ||
| T0 | 9 (7.8) | 25 (21.6) | |
| Ta/Tis * | 79 (68.7) | 72 (62.1) | |
| T1 | 15 (13.0) | 11 (9.5) | |
| T2 | 12 (10.4) | 8 (6.9) | |
| Type of operation (n, %) | 0.46 | ||
| Transurethral resection | 91 (79.1) | 87 (75.0) | |
| Cold cup biopsy | 24 (20.9) | 29 (25.0) |
Abbreviations: MMC – mitomycin-C; SD – standard deviation; BPH – benign prostatic hyperplasia; OAB – overactive bladder
Two cases prior to 2008
Three cases from 2012
Includes nine patients with CIS
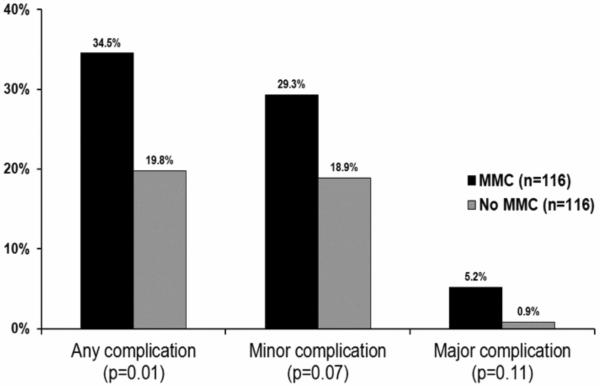
Overall, complications were more frequent among patients who were treated with MMC at the time of tumor resection (34.5% vs. 19.8%, p=0.01, Figure 1). Although minor (29.3% vs. 18.9%, p=0.07) and major complications (5.2% vs. 0.9%, p=0.11) were more common after perioperative MMC had been administered, these findings did not reach statistical significance. As expected, the majority of complications (55/63, 87%) were related to the genitourinary system (Table 2). Patients who received intravesical MMC had more dysuria (17% vs. 4%, p<0.01) and irritative voiding symptoms (7% vs. 1%, p=0.04) that required medical management. Nearly twice as many MMC patients required new prescriptions for medication to manage complications (23.3% vs. 12.9%, p=0.04). The most common new medications were antibiotics (13% vs. 7%, p=0.13) and anticholinergics (7% vs. 1%, p=0.02). Unplanned cystoscopy occurred more frequently among MMC patients (4% vs. 0%, p=0.03). Episodes of emergency room visits, unplanned operations, unplanned clinic visits, and unplanned hospital readmissions were rare and similar between groups (all p>0.25). Patients with pathologic T1 disease who underwent re-resection were more likely to receive MMC (10/12, 83%) than those not re-resected (5/9, 36%, p=0.02) but not more likely to experience complications (25% vs. 21%, p=1.00).
Figure 1.
Complications associated with perioperative intravesical mitomycin-C (MMC), stratified by severity.
This figure demonstrates the proportion of patients that experienced complications, based on whether or not they received perioperative MMC at the time of resection. The y-axis is the percentage of cases that experienced complications. The x-axis portrays the subgroups of complication types (i.e., overall, minor, and major). Minor complications are those with Clavien classification less than or equal to two. Major complications were defined as those with Clavien classification three or greater. The black bars represent patients who received MMC, and the grey bars signify the patients who were not treated with MMC.
Table 2.
Outcomes of cases with and without administration of intravesical MMC.
| Covariate | MMC (n=116) | No MMC (n=116) | P |
|---|---|---|---|
| Any genitourinary complication (n, %) | 32 (27.6) | 23 (19.8) | 0.17 |
| Dysuria | 20 (17.2) | 5 (4.3) | <0.01 |
| Bladder/pelvic/urethral pain | 3 (2.6) | 1 (0.9) | 0.62 |
| Gross hematuria | 5 (4.3) | 4 (3.5) | 1.00 |
| Urinary retention | 6 (5.2) | 8 (6.9) | 0.78 |
| Urinary frequency/urgency/incontinence | 8 (6.9) | 1 (0.9) | 0.04 |
| UTI † | 5 (4.3) | 4 (3.5) | 1.00 |
| Other | 1 (0.9) | 2 (1.7) | 1.00 |
| Any new medication (n, %) | 27 (23.3) | 15 (12.9) | 0.04 |
| Steroids | 3 (2.6) | 0 (0.0) | 0.12 |
| Antibiotics | 15 (13.0) | 8 (6.9) | 0.13 |
| NSAIDs | 4 (3.5) | 1 (0.9) | 0.21 |
| Benadryl | 3 (2.6) | 0 (0.0) | 0.12 |
| Anticholinergics | 8 (7.0) | 1 (0.9) | 0.02 |
| Alpha-blockers | 4 (3.5) | 3 (2.6) | 0.72 |
| Unplanned cystoscopy (n, %) | 5 (4.4) | 0 (0.0) | 0.03 |
| Foley catheter placement (n, %) | 6 (5.2) | 6 (5.2) | 1.00 |
| Emergency room visit (n, %) | 8 (7.0) | 6 (5.2) | 0.59 |
| Unplanned return to clinic (n, %) | 8 (7.0) | 4 (3.5) | 0.25 |
| Unplanned return to operating room ‡ (n, %) | 2 (1.7) | 1 (0.9) | 1.00 |
| Unplanned readmission (n, %) | 2 (1.7) | 1 (0.9) | 1.00 |
Abbreviations: MMC – mitomycin-C; UTI – urinary tract infection; NSAID – non-steroidal anti-inflammatory drug
Includes one case of prostatitis
Excluding definitive treatment or restaging
For analyses based on clinical and pathologic characteristics, complications were most common among patients who had Ta/Tis bladder tumors (34% Ta/Tis vs. 23% T1 vs. 9% T0, p<0.01) (Table 3). Though not statistically significant, there was a trend for complications being more common among patients with prior intravesical chemotherapy (31% with prior therapy vs. 21% without, p=0.13). This was also the case with prior pelvic radiation (29% with prior radiation vs. 14% without, p=0.20) and site of operation (30% home institution vs. 14% outside institution, p=0.07). For genitourinary complications, the only significant association was noted with pathologic stage, where they were again most common among patients with Ta/Tis disease (p=0.02).
Table 3.
Patient covariates and overall and GU complications.
| Covariate | Complications (n=63) (n, row %) | p | GU Complications (n=55) (n, row %) | p |
|---|---|---|---|---|
| Age (categorical) | 0.22 | 0.29 | ||
| <50 years old | 6 (42.9) | 5 (35.7) | ||
| 50–59 years old | 13 (33.3) | 10 (25.6) | ||
| 60–69 years old | 19 (31.2) | 18 (29.5) | ||
| 70–79 years old | 12 (18.8) | 10 (15.6) | ||
| 80+ years old | 13 (24.1) | 12 (22.2) | ||
| Gender | 0.69 | 0.22 | ||
| Male | 50 (27.8) | 46 (25.6) | ||
| Female | 13 (25.0) | 9 (17.3) | ||
| Prior intravesical chemotherapy | 0.13 | 0.32 | ||
| Yes | 17 (21.3) | 16 (20.0) | ||
| No | 46 (30.5) | 39 (25.8) | ||
| Diagnosis of BPH/OAB | 1.00 | 0.97 | ||
| Yes | 15 (27.3) | 13 (23.6) | ||
| No | 48 (27.2) | 42 (23.9) | ||
| Prior pelvic radiation | 0.20 | 0.42 | ||
| Yes | 3 (14.3) | 3 (14.3) | ||
| No | 60 (28.6) | 52 (24.8) | ||
| Charlson comorbidity score | 0.47 | 0.36 | ||
| 0 | 25 (24.5) | 20 (19.6) | ||
| 1–2 | 22 (26.5) | 21 (25.3) | ||
| 3+ | 16 (34.0) | 14 (29.8) | ||
| Year of operation | 0.06 | 0.18 | ||
| 2008 † | 18 (35.2) | 13 (25.5) | ||
| 2009 | 16 (38.1) | 15 (35.7) | ||
| 2010 | 12 (18.8) | 12 (18.8) | ||
| 2011 ‡ | 17 (22.7) | 15 (20.0) | ||
| Site of operation | 0.07 | 0.20 | ||
| Home institution | 58 (29.6) | 30 (25.5) | ||
| Outside institution | 5 (14.3) | 5 (14.3) | ||
| Pathologic stage | <0.01 | 0.02 | ||
| T0 | 3 (8.8) | 3 (8.8) | ||
| Ta/Tis * | 52 (34.4) | 45 (29.8) | ||
| T1 | 6 (23.1) | 5 (19.2) | ||
| T2 | 2 (10.0) | 2 (10.0) |
Abbreviations: GU – genitourinary; BPH – benign prostatic hyperplasia; OAB – overactive bladder
Two cases prior to 2008
Three cases from 2012
Includes nine patients with CIS
Our multivariable model showed that patients receiving perioperative MMC had nearly three times the odds of experiencing a complication within 60 days from surgery (OR 2.89, 95% CI 1.43ȓ5.81) (Table 4). Patients who had no evidence of malignancy on pathology (OR 0.15, 95% CI 0.04 – 0.55) and who received treatment at an outside institution (OR 0.23, 95% CI 0.07 – 0.73) were less likely to experience complications. Though it did not reach statistical significance, there was also a trend towards increased odds of genitourinary complications associated with receipt of MMC therapy (OR 1.82, 95% CI 0.92 – 3.61). Sensitivity analyses did not reveal any significant changes to our findings (data available upon request).
Table 4.
Regression models assessing odds of overall and GU-specific complications after MMC therapy (values bolded if p < 0.05)
| Total Complications (n=63) | GU Complications (n=55) | |||
|---|---|---|---|---|
| Covariate | Unadjusted OR (95% CI) | Adjusted OR† (95% CI) | Unadjusted OR (95% CI) | Adjusted OR† (95% CI) |
| Administration of MMC | 2.12 (1.17 – 3.86) | 2.89 (1.43–5.81) | 1.54 (0.84 – 2.84) | 1.82 (0.92 – 3.61) |
| Pathologic stage ‡ | ||||
| T0 | 0.18 (0.05 – 0.63) | 0.15 (0.04 – 0.55) | 0.23 (0.07 – 0.78) | 0.21 (0.06 – 0.75) |
| T1 | 0.57 (0.22 – 1.51) | 0.62 (0.22 – 1.78) | 0.56 (0.20 – 1.58) | 0.60 (0.20 – 1.75) |
| T2 | 0.21 (0.05 – 0.95) | 0.22 (0.04 – 1.13) | 0.26 (0.06 – 1.18) | 0.32 (0.07 – 1.57) |
| Prior radiation therapy | 0.42 (0.12 – 1.47) | 0.51 (0.13 – 1.95) | 0.51 (0.14 – 1.79) | 0.62 (0.17 – 2.32) |
| Treatment at outside institution | 0.40 (0.15 – 1.07) | 0.23 (0.07 – 0.73) | 0.49 (0.18 – 1.32) | 0.36 (0.12 – 1.09) |
Abbreviations: GU – genitourinary; OR – odds ratio; CI – confidence interval; MMC – mitomycin-C
Model includes the above covariates and adjusts for age and year of operation
Reference group is stage Ta/Tis
Details regarding the six major complications associated with perioperative MMC therapy are available in a supplemental table (Table 5). These patients were significantly younger than MMC patients who did not experience major complications (53.1 vs. 68.6 years old, p<0.01). These patients were predominantly male and had Ta/Tis disease. Five of six complications were primarily characterized symptoms related to a chemical cystitis requiring cystoscopic evaluation and/or intervention. The most significant complication involved a protracted course for one patient that ultimately required radical cystectomy with ileal conduit urinary diversion.
Table 5.
Major complications associated with perioperative intravesical MMC therapy.
| Age | Clinical stage | Pathologic stage | Outside institution | Complication(s) | Medication(s) | Intervention(s) | Miscellaneous |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 52 | Ta | Ta | No | Dysuria, prostatitis, chemical cystitis | Phenazopyridine, antibiotics, tolterodine | Cystoscopy | • Empiric antibiotics for prostatitis by PCP |
| • Seen in emergency room for intractable pelvic and urethral pain | |||||||
| • Underwent unplanned cystoscopy and diagnosed with chemical cystitis | |||||||
|
| |||||||
| 66 | T1 | T2 | No | Urinary retention, syncope, fall with head laceration | None | Closure of head laceration | • Syncopal event on day following surgery |
| • Seen at local emergency room where head laceration was closed primarily | |||||||
| • Also had urinary retention requiring Foley catheter placement | |||||||
|
| |||||||
| 38 | Ta | Ta | Yes | Dysuria, chemical cystitis | Phenazopyridine, oxybutynin, hydrocodone | Cystoscopy, resection of eschar; urodynamic evaluation | • Underwent repeat cystoscopy and resection for intractable pain 4 weeks after initial resection |
| • Ultimately treated with steroids and diphenhydramine for suspected eosinophilic cystitis three months after initial resection | |||||||
|
| |||||||
| 50 | T1 | Ta | No | Dysuria, gross hematuria, chemical cystitis | Antibiotics, acetaminophen | Cystoscopy, bladder biopsies | • Underwent unplanned cystoscopy in clinic for persistent pain six weeks after mitomycin therapy, which demonstrated diffuse cystitis |
| • Went to operating room for cystoscopy and bladder biopsies, which were negative for malignancy | |||||||
|
| |||||||
| 49 | Ta | Ta | No | Dysuria, gross hematuria, chemical cystitis | Phenazopyridine | Cystoscopy | • Had intractable symptoms six weeks after receipt of mitomycin |
| • Underwent unplanned cystoscopy in clinic which confirmed chemical cystitis | |||||||
|
| |||||||
| 63 | Ta | Ta | Yes | UTI, pelvic pain, chemical cystitis, bladder perforation, necrotizing fasciitis | Morphine SR, silodosin, prednisone, diphenhydramine , antibiotics | Cystoscopy; debridement of necrotizing fasciitis of right thigh; placement of percutaneous nephrostomy tubes; skin grafting; radical cystectomy with ileal conduit diversion | • Had urinary retention and persistent pelvic pain after initial resection |
| • Cystoscopy demonstrated chemical cystitis, contracted bladder, and necrotic bladder wall | |||||||
| • Presented to emergency room two weeks later with bladder perforation and necrotizing fasciitis of thigh | |||||||
| • Eventually required radical cystectomy with ileal conduit urinary diversion | |||||||
DISCUSSION
In a contemporary sample of bladder cancer patients treated with perioperative MMC, one third of patients experienced complications, with nearly a 15% absolute increased risk of complications compared to patients who did not receive MMC. After adjusting for various clinical characteristics, this represents a nearly three-fold increase in odds of a complication associated with treatment with MMC. It is reassuring that nearly 85% of complications after MMC therapy were minor, only requiring medical management. Dysuria and urinary frequency/urgency were the most common complications. However, over 5% of MMC patients had a major complication requiring procedural testing and/or intervention, compared to around 1% of those who did not receive MMC at the time of their resection.
The seminal meta-analysis describing the efficacy of intravesical perioperative chemotherapy reported dysuria, urinary frequency, or gross hematuria in approximately 10% of patients, and described systemic toxicity as rare.[5] Our findings provide a unique, in-depth look into the specific complications that can occur from receipt of intravesical chemotherapy after bladder tumor resection. MMC is thought to be safer and better tolerated that other agents (e.g., thiotepa) due to its larger molecular size. The first published randomized trial assessing the efficacy of perioperative intravesical MMC described dysuria or frequency in <1% of patients.[13] Rates of chemical cystitis ranged between 3–4% of patients in the other MMC studies included in the meta-analysis.[14] Our findings are discordant from what has been published previously, with over 17% of MMC patients in our cohort requiring medical management for dysuria, and 7% experiencing new onset frequency and/or urgency. Toxicity associated with induction and maintenance courses of intravesical MMC has been previously described in detail. The most common complications in this context include chemical cystitis (in up to 41% of cases), dermatitis, and reduced bladder capacity.[15] Hematologic disturbances, like leukopenia and thrombocytopenia, were rare.[15] Unfortunately, these prior studies do not categorize the severity or interventions required for complications associated with mitomycin therapy beyond stating that they resolved after cessation of treatment. Descriptions of major complications associated with perioperative intravesical MMC are limited to case reports, but include eosinophilic cystitis[16], bladder perforation[17], perirectal abscesses[10], and chronic cystitis and ureteral stenosis.[18]
Our findings must be interpreted with consideration of the limitation of this study design. First, minor complications that were managed by local primary care physicians or urologists may not have been catalogued in our database. Although our chart review of individual patient records (including available records from outside institutions) helped minimize this bias, there is likely still an element of underreporting of complications. Nevertheless, in the absence of rigorous methods of complication identification—like those that are standard in clinical trials—it is more likely that our complication rates are a conservative estimate. Second, the frequency of major complications was uncommon for both cases and controls in our analytic cohort. This limited our ability to evaluate (and adjust for) measured confounding factors with a multivariable model with major complication as an outcome of interest. Furthermore, without randomization there are likely unmeasured confounding factors contributing to our findings. In fact, selection bias associated with our study design is a major limitation. Important unmeasured confounding factors include methods of MMC administration (e.g., length of instillation time), depth/breadth of resection, multifocality of disease, and perforation. We also acknowledge that, as a tertiary center, there may also be a referral bias to evaluate and manage the worst complications associated with intravesical therapy. However, patients who underwent resection at an outside hospital did not have more complications than patients treated at our institution. Finally, as we were interested in intermediate-term (>30 day) complications, our results are biased towards finding more complications with a longer time-frame (compared to the 30-day windows in existing clinical trials).
Despite the limitations described above, the findings may be generalizable to most patients undergoing resection of bladder tumors at tertiary care centers. Our findings may reflect the consequences of inappropriate use of MMC (e.g., in setting of perforation or wide resection), or variation in how aggressively complications were managed at our institution. Nevertheless, our findings have important implications for patients and providers involved in management of NMIBC. First, the 10–15% absolute reduction of the risk of recurrence associated with perioperative intravesical MMC is partially offset by our observed 5% risk of major complications. This information related to the increased risk for minor complications can play a pivotal role in the decision-making for patients and providers. That is, a patient may prefer an increased risk of recurrence (resulting in repeat TURBT) than experiencing intractable lower urinary tract symptoms after the primary resection (however small the risk). For providers, our findings highlight and emphasize the potential risks related to administering intravesical MMC at the time of bladder tumor resection. Specifically, the benefit of this study for urologists is two-fold. For one, this information can potentially allow for more thorough counseling of patients on the specific risks of MMC therapy. Furthermore, our findings can help heighten awareness for providers and elevate suspicion for complications after perioperative MMC administration.
To date, the utilization of perioperative intravesical chemotherapy has been supported by its efficacy demonstrated by meta-analysis data.[5] Guidelines generally recommend perioperative MMC[6] or describe it as an option[7,19] for patients that have low-grade appearing tumors. Nevertheless, in general practice, administration of perioperative chemotherapy is uncommon, with less than 33% of urologists using mitomycin at the time of non-muscle invasive bladder tumor resection.[20,21] Reasons for this vary, including logistical difficulty in obtaining the medication, uncertainty of malignancy, suspected bladder perforation, and toxicity.[20,22] More research (through multi-institutional studies using validated metrics assessing LUTS and quality of life, evaluation of nationally-representative administrative claims databases, and quality collaboratives[22,23]) will be required to further characterize the safety implications of perioperative MMC therapy on a broader scale.
CONCLUSIONS
Administration of perioperative MMC therapy is associated with an increased risk of (typically minor) post-operative complications. Although there was an increased number of major complications associated with intravesical MMC, these events were rare, and the increase was not statistically significant. For policymakers, a better understanding of postoperative outcomes of intravesical chemotherapies (outside of the controlled environment of randomized clinical trials) will assist in development of future treatment protocols and guidelines.
Acknowledgments
This research was supported in part by the National Institutes of Health Training in Clinical Investigation in Urology grant (NIH-T32-DK007782).
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Pashos CL, Botteman MF, Laskin BL, Redaelli A. Bladder cancer. Cancer Pract. 2002;10:311–322. doi: 10.1046/j.1523-5394.2002.106011.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee RR, Droller MJ. The natural history of bladder cancer. Urol Clin North Am. 2000;27:1–13. doi: 10.1016/s0094-0143(05)70229-9. [DOI] [PubMed] [Google Scholar]
- 4.Avritscher EBC, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, Oosterlinck W, van der Meijden APM. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta-T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 Update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Cliff AM, Romaniuk CS, Parr NJ. Perivesical inflammation after early mitomycin C instillation. BJU Int. 2000;85:556–557. doi: 10.1046/j.1464-410x.2000.00539.x. [DOI] [PubMed] [Google Scholar]
- 9.Doherty AP, Trendell-Smith N, Stirling R, Rogers H, Bellringer J. Perivesical fat necrosis after adjuvant intravesical chemotherapy. BJU Int. 1999;83:420–423. doi: 10.1046/j.1464-410x.1999.00951.x. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuijzen JA, Bex A, Horenblas S. Unusual complication after immediate postoperative intravesical mitomycin C instillation. Eur Urol. 2003;43:711–712. doi: 10.1016/s0302-2838(03)00151-9. [DOI] [PubMed] [Google Scholar]
- 11.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolley DA, Hargreave TB, Smith PH, et al. Effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: interim report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party) Br Med J. 1988;296:1759–1761. doi: 10.1136/bmj.296.6639.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solsona E, Iborra I, Ricos JV, Monros JL, Casanova J, Dumont R. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long term follow-up. J Urol. 1999;161:1120–1123. [PubMed] [Google Scholar]
- 15.Thrasher JB, Crawford ED. Complications of intravesical chemotherapy. Urol Clin North Am. 1992;19:529–539. [PubMed] [Google Scholar]
- 16.Clark T, Chang SS, Cookson MS. Eosinophilic cystitis presenting as a recurrent symptomatic bladder mass following intravesical mitomycin C therapy. J Urol. 2002;167:1795. [PubMed] [Google Scholar]
- 17.Racioppi M, Porreca A, Foschi N, Delicato G, Destito A, D'Addessi A. Bladder perforation: a potential risk of early endovesical chemotherapy with mitomycin C. Urol Int. 2005;75:373–375. doi: 10.1159/000089179. [DOI] [PubMed] [Google Scholar]
- 18.Oehlschlager S, Loessnitzer A, Froehner M, Hakenberg OW, Manseck A, Wirth MP. Distal ureteral stenosis after early adjuvant intravesical mitomycin C application for superficial bladder cancer. Urol Int. 2003;70:74–76. doi: 10.1159/000067698. [DOI] [PubMed] [Google Scholar]
- 19.Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology. 2005;66:108–125. doi: 10.1016/j.urology.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 20.Cookson MS, Chang SS, Oefelein MG, Gallagher JR, Schwartz B, Heap K. National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol. 2012;187:1571–1576. doi: 10.1016/j.juro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 21.Chamie K, Saigal CS, Lai J, et al. Compliance with guidelines for patients with bladder cancer. Cancer. 2011;117:5392–5401. doi: 10.1002/cncr.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burks FN, Liu AB, Suh RS, et al. Understanding the use of immediate intravesical chemotherapy for patients with bladder cancer. J Urol. 2012;188:2108–2113. doi: 10.1016/j.juro.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Miller DC, Murtagh DS, Suh RS, Knapp PM, Dunn RL, Montie JE. Establishment of a Urological Surgery Quality Collaborative. J Urol. 2010;184:2485–2490. doi: 10.1016/j.juro.2010.08.015. [DOI] [PubMed] [Google Scholar]