Abstract
Hypothesis
Choline transporter-like protein 2 (CTL2), a 68–72 kDa inner ear membrane glycoprotein, is a candidate target antigen in autoimmune hearing loss (AIHL).
Objective
Test recombinant human CTL2 as a potential target for the detection of human autoantibodies in patients with AIHL.
Study Design
In vitro assay development.
Methods
Human inner ear CTL2 mRNA was cloned into baculovirus and used to infect insect cells. Immunofluorescence and western blotting were used to determine optimal expression of recombinant human CTL2 (rHuCTL2) in insect cells. AIHL patient sera of known reactivity with guinea pig inner ear were tested for antibodies to purified rHuCTL2 on western blots. Sera from normal hearing donors were used as controls.
Results
The rHuCTL2 protein migrated as three bands: a core protein of 62kDa, and two N-glycosylated bands at 66 and 70kDa. Sera from 6/12 (50%) of AIHL patients with antibody to the 68–72 kDa inner ear protein or to supporting cells also have antibody to rHuCTL2. Four/4 patients with antibody to rHuCTL2 responded to corticosteroids whereas 4/8 that lacked antibody to rHuCTL2 did not. Among normal human sera 80% were negative; binding was at the limit of detection in 3/15 (20%).
Conclusions
rHuCTL2 can be produced efficiently and used as a substrate for testing human sera. Antibodies to rHuCTL2 were detected in 50% of inner ear reactive AIHL sera. Additionally, circulating antibody to rHuCTL2 is associated response to corticosteroids in some AIHL patients.
Keywords: Antibody, Inner ear antigen, Auditory, Autoimmunity, Cochlea, Hearing loss, Human, Membrane, Virus, Baculovirus, Choline transporter-like protein 2, Solute Carrier Protein 44A2
Introduction
McCabe first proposed that autoimmunity might be a treatable cause of some cases of sensorineural hearing loss1. The discovery that experimental autoimmunity could lead to hearing loss supported this hypothesis2,3 and led to the use of immunosuppressive treatments for suspected autoimmune hearing loss (AIHL). However, as there was no accurate method of diagnosis, many people were treated inappropriately. To address this issue, Harris and Sharp4 investigated sera from suspected AIHL patients and guinea pigs with experimentally induced AIHL for antibodies that bind to bovine inner ear proteins. They found that the guinea pigs and 35% of the hearing loss patients had antibody to a 68 kDa protein from bovine inner ear and proposed this as a potential test for autoimmune hearing loss. Subsequently, Moscicki et al.5 identified a correlation between steroid responsiveness and autoantibodies to a 68 kDa inner ear protein among patients with suspected AIHL. The identity of this antigen has been elusive. Two studies identified heat shock protein 70 (HSP70) as a potential target6–8, but others found evidence that immunization with HSP70 did not cause hearing loss9,10 and that antibodies to HSP70 were not specific for AIHL11.
KHRI-3, a monoclonal antibody that binds to a 68–72kDa glycoprotein expressed on inner ear supporting cells has previously been documented to cause hair cell death and hearing loss in guinea pigs11. The corresponding antigen was subsequently identified as choline transporter-like protein 2 (CTL2). CTL2 is a member of the solute carrier family with the designation SLC44A2. This molecule has 10–11 transmembrane domains, one of which is implicated in lipid transport and metabolism12. The propensity of KHRI-3 antibody to cause hearing loss, along with its binding to a 68–72 kDa protein, suggests that CTL2 might be a target of autoantibodies in human AIHL. This hypothesis is strengthened by the observation that AIHL patients have antibodies that have been demonstrated to bind to inner ear supporting cells with the same distribution pattern as anti-CTL2 antibodies13,14. To examine this hypothesis more directly, we expressed human CTL2 in insect cells using a baculovirus system, which allows for high level expression of proteins, including large membrane proteins like CTL2, in quantities sufficient for development of immunoassays. The construct encodes a 6× histidine tag fused to the N terminus of CTL2 to facilitate isolation and purification of the protein from infected cells. This report describes the production and testing of insect cell-produced CTL2 as a target for diagnosis and potential monitoring of patients with antibody induced AIHL.
Materials and methods
Cloning and production of Recombinant Bacmid DNA
Human vestibular tissues were obtained from patients undergoing ablative surgery of the inner ear for intractable Meniere’s disease or acoustic neuroma removal. All patients signed informed consent for the use of their tissues for this research. RNA was extracted with the RNeasy mini kit (Qiagen) protocol. mRNA was reverse transcribed using Superscript II RT (Invitrogen, Carlsbad, CA) and oligo dT primers. cDNA for the major CTL2 isoforms from promoter 1 (P1) and promoter 2 (P2) was amplified in PCR using the GC rich kit (Roche) and suitable primers. The PCR product for each isoform was inserted into a pGEM-T cloning vector, and individual clones were sequence verified. The Bac-to-Bac® Baculovirus Expression System (Invitrogen Life Technologies, Carlsbad, CA) was used to generate recombinant bacmids. Expression constructs containing CTL2 P1 or P2 isoforms under the control of a baculovirus specific promoter were generated in pFastBac HT donor plasmids. Donor plasmids with the correct inserts were used to transform DH10Bac competent E. coli. Colonies containing recombinant bacmids were selected on LB agar plates containing kanamycin 50ug/ml, gentamicin 7ug/ml, tetracycline 10ug/ml, Bluo-gal 100ug/ml, and IPTG 40ug/ml, isolated and purified from bacterial culture and verified using PCR according to the manufacturer’s protocol.
Production of recombinant baculovirus and expression of recombinant CTL2 protein in Sf9 insect cells
Sf9 insect cells (Spodoptera frugiperda) were cultured in suspension at 27°C in Sf-900 II serum free medium (Invitrogen, Carlsbad, CA) with 1% heat inactivated FBS and 10 ug/ml gentamicin. Cultures were initiated at a density of 0.7 to 1×106 cells/ml and passed when a density of 2–4×106 cells/ml was reached. Sf9 insect cells were transfected with recombinant bacmid using Cellfectin (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Seventy-two hours post-transfection, medium containing virus was collected and centrifuged to remove cells and debris. The viral stock was amplified by re-infecting fresh sf9 cells. Culture supernatants containing amplified virus stock were collected 3–5 days after infection, amplified a second time, clarified by centrifugation, divided into aliquots and stored at 4°C for several months or −80°C for long term storage.
The initial conditions of recombinant CTL2 P1 and P2 expression described above were further optimized by varying two different parameters; multiplicity of infection (MOI) and time after infection. Sf9 cells (1.5 to 2 ×106 cells/ml) in mid-logarithmic growth phase were infected with passage 3 stock of rHuCTL2P1 and P2 virus at an MOI at 0.01, 0.1, 0.5, 1 and 2. Since similar expression levels were obtained with MOI in the range from 0.1–2, we used an MOI of 0.1 in all subsequent experiments. Cells were harvested at 24, 48 and 72 hours post infection and processed for protein analysis. Expression of recombinant human CTL2 protein from promoter 1 (rHuCTL2P1) and promoter 2 (rHuCTL2P2) was assessed using immunoblotting and immunofluorescence.
Immunoprecipitation, SDS-PAGE and Western blotting
Antibodies to CTL2 were raised in rabbits against synthetic peptides as previously described (Nair et al. 2004). To assist in the analysis and study of this protein, we raised antisera to three highly antigenic domains in the human CTL2 molecule. These include a previously described antibody to the N terminal region (anti CTL2-NT)(Nair et al. 2004), a second antigenic 17 amino acid peptide located in the third outer loop (CTL2-TOL), and a 20 amino acid antigenic peptide located in human CTL2 near the C-terminus (anti CTL2-CT). Monoclonal antibody to the histidine tag was purchased from BD Biosciences, (San Jose CA). Human sera were obtained from AIHL patients and normal volunteers. The study was reviewed and approved by the IRB and all patients and controls signed informed consent for the use of their serum and clinical histories. Cell lysate preparation, immunoprecipitation (IP) and SDS-PAGE were carried out as described previously12. Antibody binding was detected with enhanced chemiluminescence.
Immunofluorescence
Sf9 cells were plated on sterilized coverslips placed in 6-well cluster dishes at a concentration of 2×105 cells in Sf9 growth medium. After 24 hours, cells were infected with baculovirus containing rHuCTL2 P1 or P2 DNA for 48 hours. Cells infected with baculovirus containing no insert were used as a negative control. At 24, 48 and 72 hours infected cells were fixed with 4% paraformaldehyde for 15 minutes and washed with PBS containing 0.1M glycine for 10 minutes. To minimize background staining, rabbit antisera were pre-absorbed with Sf9 cells infected with the empty vector prior to use in immunofluorescence. Following permeabilization (0.1% Triton X-100) and PBS washes, infected Sf9 cells were incubated sequentially with primary and secondary antibodies12. The coverslips were washed and mounted with Prolong antifade mounting media and examined microscopically.
rHuCTL2 P1 Purification
rHuCTL2 P1 purification was carried out at the Michigan State University Research Technology Support Facility using Nickel-NTA columns (Qiagen, Hilden, Germany) or in our laboratory using the Probond™ Nickel column (Invitrogen, Carlsbad, CA). The yield from a 10 L culture was approximately 32mg of rHuCTL2 protein.
Enzymatic deglycosylation
Immunoprecipitation and deglycosylation of rHuCTL2 P1 and P2 isoforms were carried out as previously described12. Samples were subjected to SDS- PAGE and Western blotting.
Human Sera
Sera from 14 patients with documented hearing loss strongly suspected on clinical history to be due to an autoimmune mechanism13, and 15 sera from normal volunteers were selected for assessment of antibody binding to purified rHuCTL2 protein on Western blots. The AIHL sera were from 7 men and 7 women who ranged in age from 22 to 72 years of age. Twelve of the AIHL patients had been treated with corticosteroids as previously described.13 Of these, 9/11 (82%) had antibody that reacted with supporting cells and 11/12 (92%) reacted with a 68–72 kDa protein from guinea pig inner ear. Eight of 12 (67%) had responded to treatment with improved hearing. Two were not treated. The 15 normal donors ranged in age from 24 to 61 years of age and consisted of 11 women and 4 men. These donors were laboratory staff and students who denied hearing loss or other otologic symptoms. The study was reviewed and approved by the University of Michigan Institutional Review Board. All patients and controls signed written informed consent documents for this study.
Results
Expression of rHuCTL2 P1 and P2 in Sf9 insect cells
Immunofluorescence
The rate of infection and expression of rHuCTL2 P1 and P2 in the infected Sf9 cells was confirmed by immunofluorescence. Strong cytoplasmic and membrane expression was observed at 48 hours post infection as shown in Figure 1. Assessment at low magnification indicated that approximately 40% of cells were infected with the P1 construct and 25–30 % of cells were infected with the P2 construct.
Figure 1.
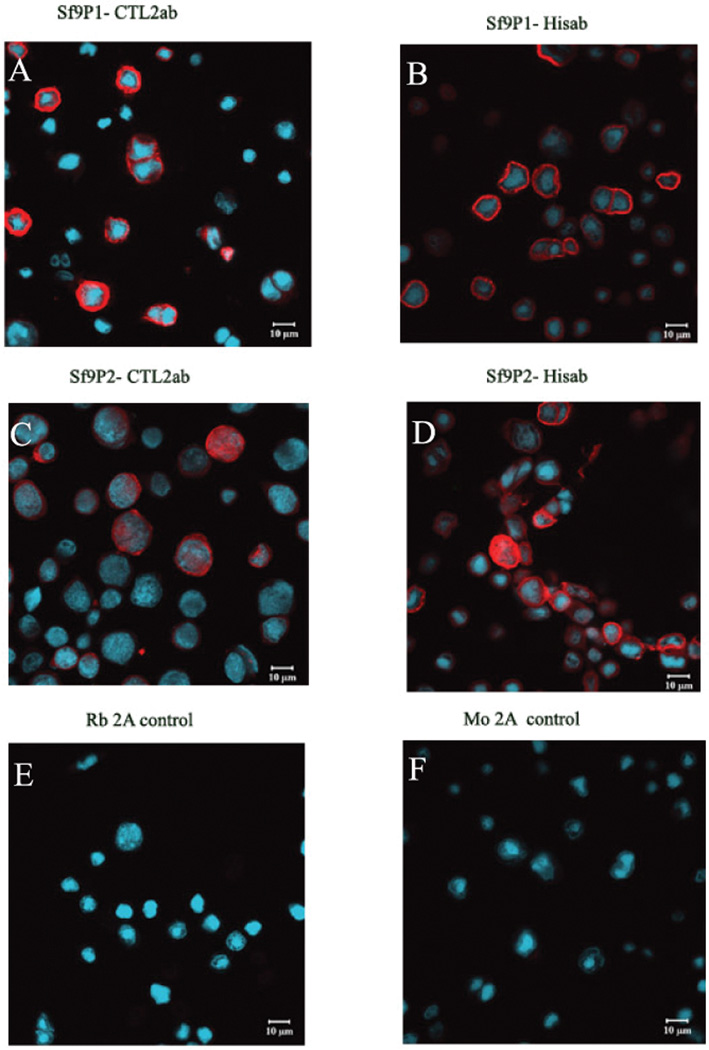
Confocal immunofluorescence photomicrographs of recombinant human CTL2 P1 and P2 isoforms expression in Sf9 insect cells transduced with the Baculovirus expression system. Fixed cells were probed with CTL2-NT rabbit polyclonal antibody and mouse monoclonal anti His-tag antibody. Red staining indicates the expression of rHuCTL2 and blue color indicates the staining of nuclei with Hoechst 33342. Panels A and B show cells expressing CTL2-P1; Panels C and D show cells expressing CTL2-P2; Panel E shows cells expressing CTL2-P1 with anti rabbit Ig second antibody alone. There was also no background staining of cells expressing CTL2-P2. Panel F shows cells representative cells expressing CTL2-P2 and incubated with anti mouse Ig second antibody alone. There was also no background staining of cells expressing CTL2-P1.
Immunoprecipitation and Western Blotting
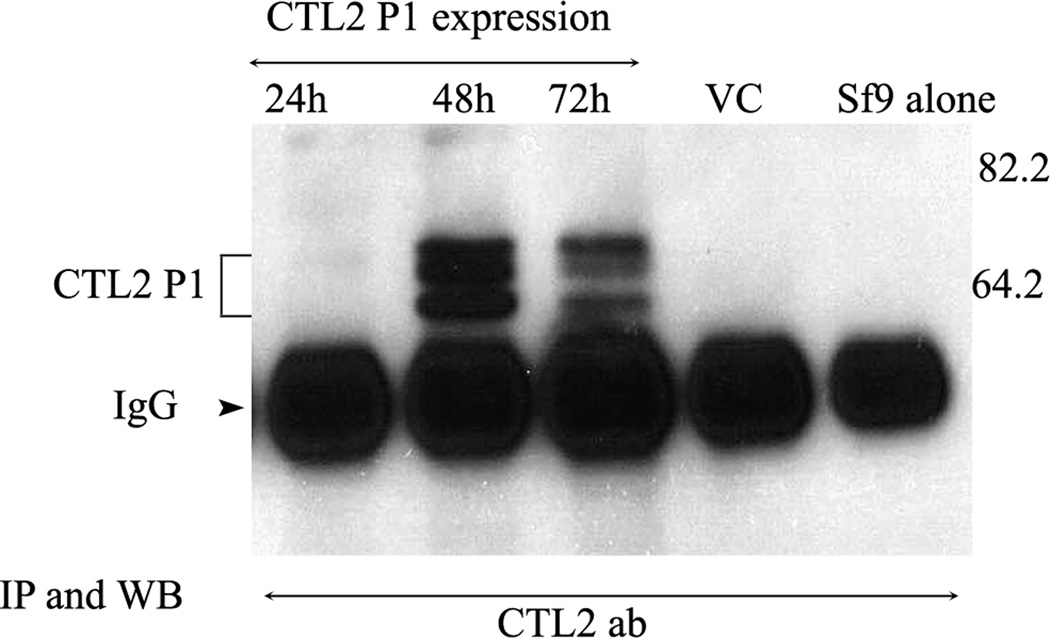
Cells infected in the mid- logarithmic phase of growth were harvested at 24, 48, or 72 hours. Recombinant protein was immunoprecipitated with the CTL2-NT antibody coupled to CNBr beads, western blotted, and probed with the anti-CTL2-NT antibody. Optimal protein expression was observed at the 48 hour time point (Fig. 2). Protein was undetectable at 24 hours, was maximal at 48 hours, and decreased by 72 hours. No CTL2 protein was detected in Sf9 cells or in empty vector infected Sf9 cells. Three distinct bands ranging in molecular mass from 62–70 kDa were present.
Figure 2.
Immunoprecipitation and western blot with the CTL2-NT antibody showing the expression of histidine tagged rHuCTL2 P1 protein in Sf9 extracts at 3 time points after infection. Protein expression was maximal at 48hours (lane 2).
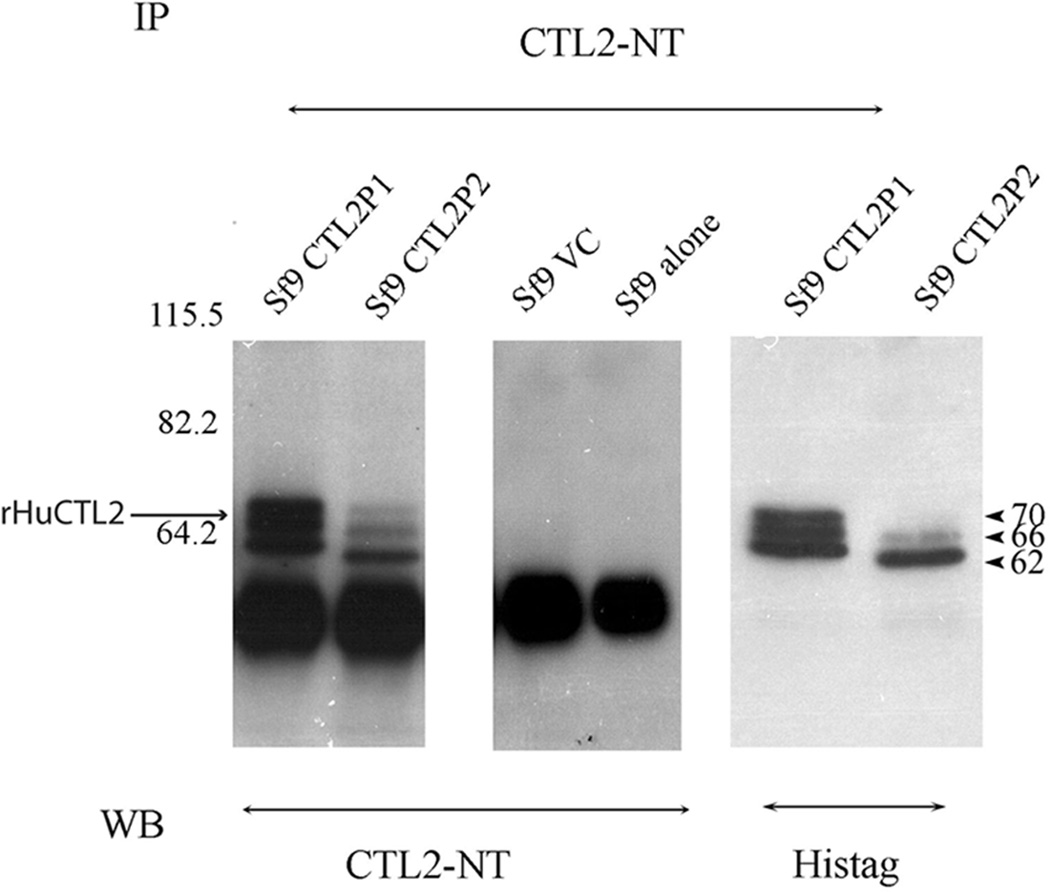
To investigate the expression of rHuCTL2 protein from cells infected with either the P1 or the P2 constructs, lysates were immunoprecipitated with anti CTL2-NT and the western blots were probed with both the anti-CTL2-NT and anti His-tag antibodies (Figure 3). Both antibodies identified three bands of 62, 66 and 70 kDa. Protein expression was lower with the P2 construct and the P2 recombinant protein migrated slightly faster than the P1 protein. No CTL2 bands were detectable in uninfected and vector control samples.
Figure 3.
Immunoprecipitation and western blotting of rHuCTL2 P1 and P2 isoforms from Sf9 insect cell lysates probed with rabbit anti CTL2 NT and anti His-tag mouse monoclonal antibodies. Lanes 1 and 5 contain immunoprecipitates of rHuCTL2 P1; lanes 2 and 6 contain immunoprecipitates of rHuCTL2-P2; lanes 3 and 4 are Sf9 containing the vector control and Sf9 extract alone respectively. Lanes one to four are probed with CTL2-NT antibody. Lanes 1–4 are probed with anti CTL2-NT, 5 and 6 are probed with anti His-tag antibody.
Large Scale rHuCTL2 P1 Protein production, purification and glycosylation
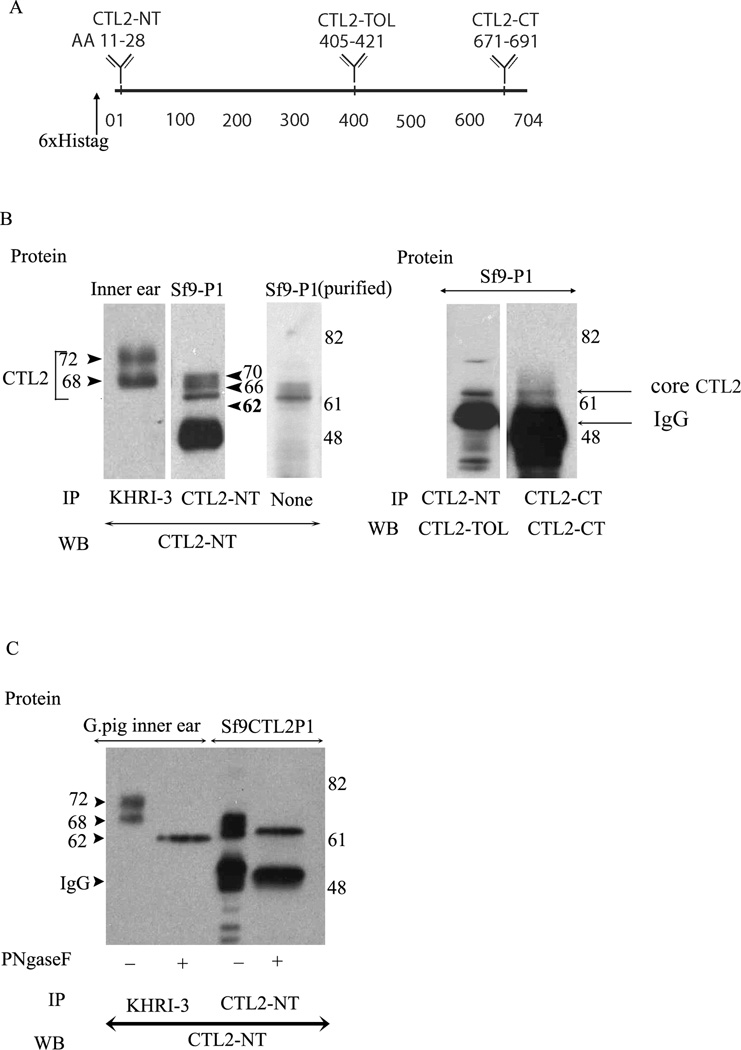
Since the P1 isoform yielded more rHuCTL2 protein and since the P1 isoform is predominant in the guinea pig and human inner ear, optimization and characterization of the protein was performed using the P1 construct. The conditions established for small scale cultures were applied to large scale preparations. Bulk production of cell culture and purification yielded 3.2 mg purified protein / liter of infected cells, which is consistent with high yields previously reported for other proteins produced in Sf9 cells15. To analyze the recombinant protein, three rabbit antisera were used. These are the CTL2-NT antiserum described above, as well as CTL2-TOL and CTL2-CT which react to other highly antigenic regions of the human CTL2 molecule. The locations of the CTL2 peptides used to raise all three rabbit antisera to CTL2 are shown graphically on a line representing the 704 amino acids in the protein (Fig 4A). In Figure 4B, CTL2 immunoprecipitated from guinea pig inner ear by the KHRI-3 monoclonal antibody is compared to the rHuCTL2. The guinea pig CTL2 migrated as two protein bands of 68 and 72 kDa, whereas the recombinant protein migrated as three bands of 62, 66, and 70kDa, whether immunoprecipitated by CTL2-NT antibody (second lane Fig 4B) or purified on the nickel affinity column (third lane Fig 4B). The differences in migration are believed to be related to post-translational modifications, as CTL2-TOL binds poorly to the mature CTL2 proteins obtained from guinea pig inner ear. However, it binds well to the recombinant 62 kDa band (Figure 4B lane 4) as does CTL2-CT (Fig 4B, Lane 5). The binding of the CTL2-TOL to the 62 kDa band but not the mature glycosylated forms is consistent with steric hindrance by N-linked carbohydrate modification of CTL2 by the insect cells within the antigenic domain. When either guinea pig inner ear CTL2 or column purified insect cell derived rHuCTL2 samples were enzymatically deglycosylated, western blotted, and probed with anti CTL2 NT, (Fig. 4C lanes 2 and 4) only a single 62 kDa core protein band was observed for both the guinea pig and recombinant proteins. There is a small difference in the molecular weight of the recombinant protein and the native protein that is due to the His tag on the recombinant protein. Thus, both the insect cell derived rHuCTL2-P1 and native CTL2 protein from the guinea pig inner ear are N-glycosylated, although the molecular mass of glycosylated bands are different. The failure of the CTL2-TOL to bind to the glycosylated recombinant protein indicates that the same N-glycosylation sites are active in both mammals and insects.
Figure 4.
Immunoprecipitation and western blot comparative analysis of the mature and de-glycosylated forms of guinea pig inner ear Sf9 produced CTL2. Panel A. Schematic representation of CTL2 protein showing the location of the N-terminus His-tag (6×) and location of the three antigenic peptides used to raise rabbit antibodies. CTL2-NT was raised to a 19 amino acid peptide in the N-terminal domain of human CTL2. CTL2-TOL was raised against 17 amino acid peptide in the third outer loop. CTL2-CT was made against a 20-amino acid antigenic peptide located near the C-terminus. Panel B. Comparison of CTL2 from guinea pig to insect cell-produced rHuCTL2. left panel: immunoprecipitation and western blot of CTL2 from guinea pig inner ear tissue (Lane 1), rHuCTL2 P1 from Sf9 insect cells (lane 2). Lane 3 shows Nickel -NTA column purified rHuCTL2 P1 probed with CTL2-NT antibody. Lane 4: rHuCTL2- P1 precipitated from Sf9 cells with CTL2-NT and probed with CTL2-TOL. Lane 5: rHuCTL2 P1 precipitated from Sf9 cells with CTL2-CT antibody and probed with CTL2-CT. Both antibodies bind to the un-glycosylated 62 kDa core protein. Panel C. Deglycosylation of native CTL2 and rHuCTL2-P1. Immunoprecipitated CTL2 from guinea pig inner ear or rHuCTL2 from Sf9 cells was treated with PNGase F. Lane 1: Untreated guinea pig CTL2. Lane 2: deglycosylated guinea pig CTL2. Lane 3: glycosylated rHuCTL2-P1. Lane 4: Deglycosylated rHuCTL2-P1. The difference in the molecular mass between native and recombinant deglycosylated protein, is due to the histidine tag and linker. Both guinea pig and rHuCTL2 have a core protein of ~62 kDa.
Binding of human serum antibodies to rHuCTL2 P1 Protein
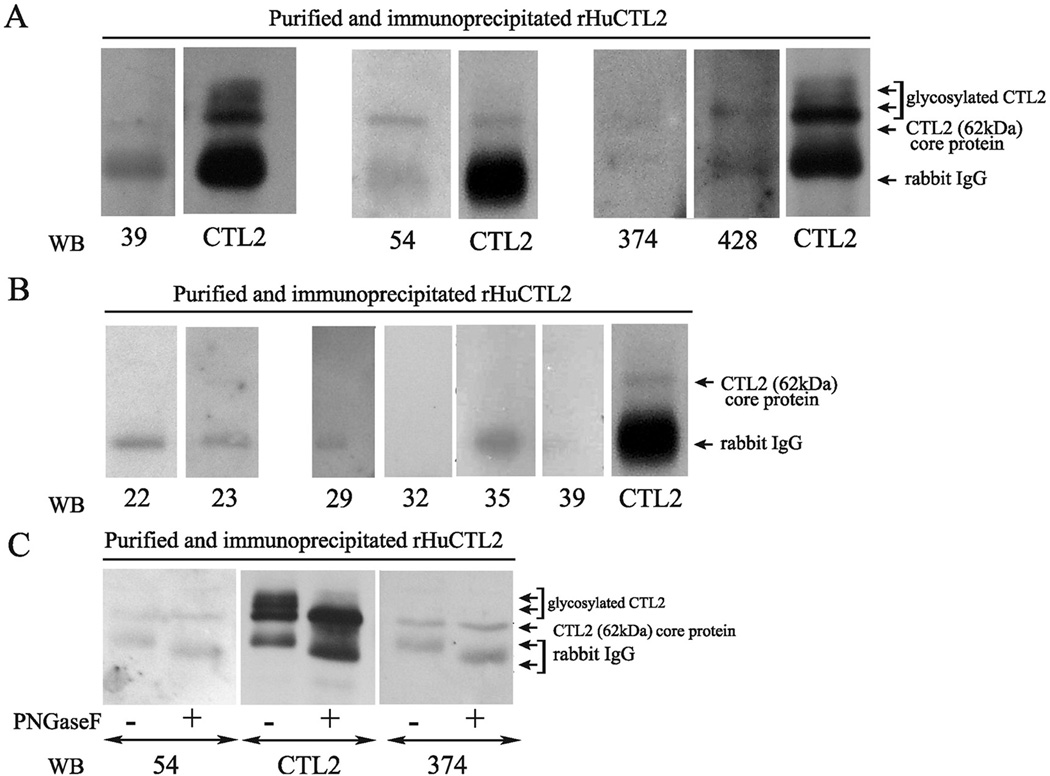
To test the role of rHuCTL2 protein as a substrate for detecting autoantibodies, the purified protein was assessed for reactivity with a set of human autoimmune hearing loss and control sera in western blot assays. Sera from fourteen AIHL patients, 12 known to have antibody reactive with guinea pig inner ear tissues and two known to be negative for such antibody (Table 1A and 1B), were tested and compared to sera from fifteen normal hearing donors (Table 2). The patient sera included eleven sera previously reported in Zeitoun et al..13 Of the 14 AIHL sera 12 were positive for antibody to the 68–72 kDa inner ear antigen or to supporting cells and two were negative for antibody. Of the twelve antibody positive cases three were previously unreported. The previously reported patients are shown by an asterisk in Table 1. Nickel-column purified rHuCTL2 protein that was further purified by immunoprecipitation with CTL2-NT antibody coated beads was used as a substrate in western blots as shown in Figure 5. Panel A shows representative blots from three experiments with sera from AIHL patients P-39, P-54, P-374, P-428 who have antibody to supporting cells. In each case the patient antibodies bind to the rHuCTL2 62 kDa core protein. In Figure 5 panel B sera from AIHL patients P-22 and P-23 who lacked antibody to supporting cells (Table 1B), and sera from normal hearing controls N-29, N-32, N-35, and N-39 (Table 2). Both patients who lacked antibody to supporting cells also lacked antibody to the rHuCTL2. Serum from normal donor N29 exhibited weak binding to rHuCTL2 (Table 2). This was barely detectable on the films of the chemiluminescence exposures but was too faint to be illustrated on the scan of the film. The other three normal donor sera were negative for binding to the rHuCTL2. Positive control lanes on each blot were probed with the CTL2-NT antibody to provide a direct comparison of molecular mass. To confirm the reactivity of patient sera with the core protein, representative patient sera were also tested against enzymatically deglycosylated rHuCTL2 protein. Figure 5C shows reactivity of sera from patients P-54 and P-374 with untreated rHuCTL2 and protein that was incubated with PNGase enzyme to remove N-glycosylated carbohydrates. As shown, both sera recognize the core protein.
TABLE I.
Antibody Reactivity With Purified Sf9 rHuCTL2 P1 Protein Using Sera From UMHL Patients With Antibody to a 68–72 kDa Inner Ear Protein.
| UMHL Patient No./Sex/Age, y |
rHuCTL2 Binding |
Previous WB Result* |
Previous IF Result* |
Pretreatment* PTA, R/L |
Post-treatment* PTA, R/L |
Improvement After Corticosteroid* |
Type of Hearing Loss |
Side Affected |
Other Autoimmune Diseases |
|---|---|---|---|---|---|---|---|---|---|
| 20/F/51* | − | + | + | 81/45 | 81/21 | + | Rapidly progressive | Left | - |
| 25/F/72 | +/− | + | NT | NA | NA | +(R ear only) | Hearing loss/Tinnitus | Left | - |
| 33/M/48* | − | + | + | 53/45 | 46/35 | + | Rapidly progressive | Bilateral | - |
| 37/F/65* | +/− | + | + | 25/52 | 21/25 | + | Sudden | Left | - |
| 39/M/70* | + | + | + | 75/20 | 72/5 | + | Rapidly progressive | Left | - |
| 43/F/60* | − | − | + | 53/00 | 43/00 | + | Rapidly progressive | Right | - |
| 54/M/58* | + | + | + | 60/70 | 35/50 | + | Rapidly progressive | Bilateral | - |
| 55/M/58* | − | + | + | 38/100 | 36/60 | + | Rapidly progressive | Left | - |
| 56/M/54* | − | + | + | 33/47 | 27/38 | − | Rapidly progressive | Bilateral | - |
| 151/M/22* | − | + | + | 45/55 | 43/51 | − | Rapidly progressive | Bilateral | - |
| 374/F/64 | + | + | − | 39/42 | NA | No Treatment | Progressive | Bilateral | Unknown |
| 428/F/43 | + | + | − | 49/57 | NA | No treatment | Lifelong progressive | Bilateral | Unknown |
| Totals, N=12 | 6/12 (50%) | 11/12 (92%) | 9/11(82%) | 8/12 (67%) | None |
UMHL = University of Michigan Hearing Loss; WB = western blot; IF = immunofluorescence; PTA = pure tone average; R = right; L = left; F = female; NT = not tested; NA = not available; M = male
Previous tests as reported in Zeitoun et al. 200513 (except for patients 25, 374 and 428 who were not previous studied).
TABLE II.
Antibody Reactivity With Purified Sf9 rHuCTL2 P1 Protein Using Sera From UMHL Patients Negative For Antibody to a 68–72 kDa in Inner Ear Protein.
| UMHL Patient No/sex/age |
rHuCTL2 binding |
Previous WB Result* |
Previous IF Result* |
Pretreatment* PTA, R/L |
Post-treatment* PTA, R/L |
Improvement After Corticosteroid* |
Type of Hearing Loss |
Side Affected |
Other Autoimmune Diseases |
|---|---|---|---|---|---|---|---|---|---|
| 22/M/39* | - | - | - | 25/00 | 20/00 | - | Rapidly progressive | Left | - |
| 23/F/65* | - | - | - | 25/28 | 25/22 | - | Rapidly progressive | Bilateral | - |
| Totals, N=2 | 0/2 | 0/2 | 0/2 | 0/2 | None |
UMHL = University of Michigan Hearing Loss; WB = western blot; IF = immunofluorescence; PTA = pure tone average; R = right; L = left; M = male; F = female
Previous tests as reported in Zeitoun et al. 200513
Figure 5.
Western blots of rHuCTL2 purified by nickel affinity column and by immunoprecipitation with rabbit anti CTL2 tested with sera from autoimmune hearing loss patients and normal hearing controls (see Tables 1 and 2). Panel A. Examples of patient sera (patients P-39, P-54, P-374, and P-428) that bind to the CTL2 core protein. Each of these sera were known to have antibodies to inner ear supporting cells or a 68–72 kDa inner ear antigen. For each blot a rabbit anti CTL2 positive control was used. Panel B. Western blots with sera from two AIHL patients who did not have antibody to supporting cells or the 68–72 kDa antigen (patients P-22, and P-23) and sera from 4 normal hearing controls (N-29, N-32, N-35 and N-39). Panel C. Western blot assessment of antibody binding to either the glycosylated rHuCTL2 or rHuCTL2 that had been enzymatically deglycosylated with PNGaseF. Note the antibodies in the sera of patient P- 54 and P-374 bind well to the deglycosylated core protein. Anti CTL2 binds to both the core protein and the glycosylated forms.
Tables 1A and 1B summarize prior data from each AIHL patient and compare the results of serum reactivity on inner ear substrates with that on rHuCTL2. A summary for the normal hearing controls is given in Table 2. Age corresponds to donor age at the time of the serum draw. Information from hearing test results before and after corticosteroid treatment for eleven previously studied patients (Table 1A and 1B) is reproduced from Zeitoun et al.13 (with permission from Arch Otolaryngol Head & Neck Surgery). In summary, 6/12 (50%) AIHL patients who had antibody reactive with the 68–72 kDa inner ear antigen or inner ear supporting cells also have antibody to the rHuCTL2 core protein. Four/4 patients with antibody to rHuCTL2 responded to corticosteroids whereas 4/8 that lacked antibody to rHuCTL2 did not (Table 1A and 1B).
Discussion
Following the work of McCabe1, Harris et al.4 and Moscicki et al.5, the search for the inner ear antigen(s) of autoimmune hearing loss has been a research goal of many labs. Two labs identified a 68 kDa antigen by sequencing proteins that migrate at 68 kDa6–8,12,16,17. Both identified HSP70, a chaperone protein that is up-regulated in cells exposed to heat stress, as the potential target of AIHL antibodies. A western blot analysis was developed that used bovine kidney cells exposed to heat shock as the substrate. Some studies have reported that this test has value in diagnosis of AIHL18,19, whereas others found poor correlations between anti-HSP antibodies and clinical manifestations of AIHL20. Anti-HSP antibodies have also been reported in patients with Meniere’s disease21. However, we found that HSP antibodies are not more common in affected individuals than in normal controls11 and others showed that immunization with HSP70 does not result in hearing impairment in animal models9. There are other molecules that have been suggested as potential target antigens in autoimmune hearing loss, such as P0, collagen, cochlin, etc. It is still unknown whether any or all of these may be important targets, or if different patients make antibody to different antigens. The frequent identification of antibodies to supporting cells in sera from patients with AIHL13,14,22 and the characterization of CTL2 as a primary supporting cell antigen target suggest that this molecule is likely one of the most important targets in this disorder. Further, evidence that the monoclonal antibody KHRI-3 that binds to this protein in vivo and causes damage to hair cells resulting in hearing loss, strongly supports this conclusion. CTL2 is a member of the solute carrier family of transporter proteins with the designation SLC44A2. Although the transport function of this protein is still unknown, we suspect that antibody binding in vivo blocks its transport function leading to a change in the microenvironment of the inner ear that is toxic to hair cells. The development of an in vitro system to produce and purify rHuCTL2 in quantity is an important prerequisite needed for development of an assay that can quickly and specifically identify patients with anti-CTL2 antibodies. Typically recombinant proteins are produced in E. coli, however, preparing recombinant human CTL2 was difficult, since its expression is toxic to bacteria and yeast. Expression of rHuCTL2 in transfected mammalian cells can be achieved but at levels that are insufficient for use as a test substrate. In this report we demonstrate a robust means of producing relatively large quantities of human CTL2 protein in vitro, making it feasible to develop a potentially useful diagnostic system. The use of a purified recombinant protein will increase the reliability and sensitivity of the assay system, decrease the cost, reduce the use of animals, and lessen the possibility that the antibodies being measured are directed against contaminating inner ear proteins that happen to migrate with a similar mass on electrophoretic gels.
The western blot results suggest that many AIHL patients have antibody that binds to the rHuCTL2 core protein, since the reactivity of these sera was the same with the whole protein and with deglycosylated protein. We have begun testing protein production in infected Sf9 cells treated with tunicamycin, an inhibitor of glycosylation, since this may be a more productive strategy to enrich the sample for the un-glycosylated form. Although good reactivity of patient sera was observed with the core protein, it is possible that some patients might have antibodies directed against the carbohydrate moiety. However, since humans, guinea pigs and insect cells all have different glycosylation enzymes, developing rHuCTL2 with human glycosylation will require insect cells engineered to express the appropriate human glycosyltransferases. This is theoretically possible since such enzymes have been successfully introduced into yeast expression systems23,24 and could also be transferred to the insect cells in a similar manner.
CONCLUSION
Objective diagnostic criteria for autoimmune sensorineural hearing loss remain elusive. Although an assay for HSP70 was widely adopted for clinical use, the test has shown poor performance characteristics and this molecule has been largely discredited as a valid target antigen. This current report builds upon prior work that strongly suggests that CTL2 is a target antigen in many cases of AIHL. Furthermore, it appears that clinical testing for autoantibodies to this molecule is feasible in the near future. The 50% positive results in this sample of suspect AIHL patients exceeded our expectations for what is almost surely a heterogeneous condition. This suggests that with improved sensitivity this assay could become a reliable and useful clinical assay. Moreover, none of the corticosteroid non-responders had antibody to the rHuCTL2 protein, suggesting that this assay may be predictive of response to steroid treatment. Additional testing of clinical samples from larger numbers of patients suspected of having AIHL and normal controls is forthcoming, and will allow for measurement of the performance characteristics of the assay and its clinical utility in diagnosis and monitoring of treatment.
TABLE III.
Antibody Reactivity With Purified Sf9 rHuCTL2 P1 Protein Using Serum From Normal Hearing Donors.
| UMNS No./Sex/Age |
rHuCTL2 Binding |
Self Assessment Hearing Loss |
Autoimmune Diseases |
|---|---|---|---|
| 11/F/35 | - | - | - |
| 12/M/52 | - | - | - |
| 15/F/46 | −/+ | - | - |
| 26/M/36 | - | - | - |
| 27/F/56 | - | - | Lupus |
| 28/F/24 | - | - | - |
| 29/F/48 | −/+ | - | - |
| 32/M/37 | - | - | - |
| 33/F/25 | −/+ | - | Joint inflammation |
| 34/F/27 | - | - | - |
| 35/F/50 | - | - | - |
| 36/F/61 | - | - | - |
| 37/F/36 | - | - | - |
| 38/F/49 | - | - | - |
| 39/M/51 | - | - | - |
| Totals: N=16 | 3/15 (20%) | none | 2/15 |
UMNS = University of Michigan normal subject; F = female; M = male
Acknowledgements
Supported by: Autoimmune Sensorineural Hearing Loss Research fund, The Ruth and Lynn Townsend Family Fund, NIH NIDCD (R01 DC03686), the NIDCD Research Center Core grant (P30 DC05188) and the NIH Rheumatic Core Diseases Center Grant (1P30 AR048310). PK was supported by NIDCD training grant T32 DC00011.
References
- 1.McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585–589. doi: 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 2.Harris JP. Experimental autoimmune sensorineural hearing loss. Laryngoscope. 1987;97:63–76. doi: 10.1288/00005537-198701000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Harris JP. Immunology of the inner ear: response of the inner ear to antigen challenge. Otolaryngol Head Neck Surg. 1983;91:18–32. doi: 10.1177/019459988309100105. [DOI] [PubMed] [Google Scholar]
- 4.Harris JP, Sharp PA. Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. The Laryngoscope. 1990;100:516–524. doi: 10.1288/00005537-199005000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Moscicki RA, San Martin JE, Quintero CH, Rauch SD, Nadol JB, Jr, Bloch KJ. Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA. 1994;272:611–616. [PubMed] [Google Scholar]
- 6.Bloch DB, San Martin JE, Rauch SD, Moscicki RA, Bloch KJ. Serum antibodies to heat shock protein 70 in sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 1995;121:1167–1171. doi: 10.1001/archotol.1995.01890100075013. [DOI] [PubMed] [Google Scholar]
- 7.Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol. 1995;104:181–188. doi: 10.1177/000348949510400302. [DOI] [PubMed] [Google Scholar]
- 8.Billings PB, Shin SO, Harris JP. Assessing the role of anti-hsp70 in cochlear impairment. Hear Res. 1998;126:210–213. doi: 10.1016/s0378-5955(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 9.Trune DR, Kempton JB, Mitchell CR, Hefeneider SH. Failure of elevated heat shock protein 70 antibodies to alter cochlear function in mice. Hear Res. 1998;116:65–70. doi: 10.1016/s0378-5955(97)00198-6. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson AK, Hyden D, Roberg M, Skogh T. Evaluation of anti-hsp70 antibody screening in sudden deafness. Ear Hear. 2003;24:233–235. doi: 10.1097/01.AUD.0000069230.36940.AC. [DOI] [PubMed] [Google Scholar]
- 11.Nair TS, Prieskorn DM, Miller JM, Mori A, Gray J, Carey TE. In vivo binding and hearing loss after intracochlear infusion of KHRI-3 antibody. Hear Res. 1997;107:93–101. doi: 10.1016/s0378-5955(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 12.Nair TS, Kozma KE, Hoefling NL, et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci. 2004;24:1772–1779. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeitoun H, Beckman JG, Arts HA, et al. Corticosteroid response and supporting cell antibody in autoimmune hearing loss. Arch Otolaryngol Head Neck Surg. 2005;131:665–672. doi: 10.1001/archotol.131.8.665. [DOI] [PubMed] [Google Scholar]
- 14.Disher MJ, Ramakrishnan A, Nair TS, et al. Human autoantibodies and monoclonal antibody KHRI-3 bind to a phylogenetically conserved inner-ear-supporting cell antigen. Ann N Y Acad Sci. 1997;830:253–265. doi: 10.1111/j.1749-6632.1997.tb51896.x. [DOI] [PubMed] [Google Scholar]
- 15.Klaassen CH, Bovee-Geurts PH, Decaluwe GL, DeGrip WJ. Large-scale production and purification of functional recombinant bovine rhodopsin with the use of the baculovirus expression system. Biochem J. 1999;342(Pt 2):293–300. [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch DB, Gutierrez JA, Guerriero V, Jr, Rauch SD, Bloch KJ. Recognition of a dominant epitope in bovine heat-shock protein 70 in inner ear disease. The Laryngoscope. 1999;109:621–625. doi: 10.1097/00005537-199904000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Shin SO, Billings PB, Keithley EM, Harris JP. Comparison of anti-heat shock protein 70 (anti-hsp70) and anti-68-kDa inner ear protein in the sera of patients with Meniere's disease. The Laryngoscope. 1997;107:222–227. doi: 10.1097/00005537-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Bonaguri C, Orsoni JG, Zavota L, et al. Anti-68 kDa antibodies in autoimmune sensorineural hearing loss: are these autoantibodies really a diagnostic tool? Autoimmunity. 2007;40:73–78. doi: 10.1080/08916930601119377. [DOI] [PubMed] [Google Scholar]
- 19.Hirose K, Wener MH, Duckert LG. Utility of laboratory testing in autoimmune inner ear disease. The Laryngoscope. 1999;109:1749–1754. doi: 10.1097/00005537-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Berrocal JR, Ramirez-Camacho R, Arellano B, Vargas JA. Validity of the Western blot immunoassay for heat shock protein-70 in associated and isolated immunorelated inner ear disease. The Laryngoscope. 2002;112:304–309. doi: 10.1097/00005537-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Rauch SD, San Martin JE, Moscicki RA, Bloch KJ. Serum antibodies against heat shock protein 70 in Meniere's disease. Am J Otol. 1995;16:648–652. [PubMed] [Google Scholar]
- 22.Rauch SD. Clinical management of immune-mediated inner-ear disease. Ann N Y Acad Sci. 1997;830:203–210. doi: 10.1111/j.1749-6632.1997.tb51891.x. [DOI] [PubMed] [Google Scholar]
- 23.Shimma Y, Jigami Y. Expression of human glycosyltransferase genes in yeast as a tool for enzymatic synthesis of sugar chain. Glycoconj J. 2004;21:75–78. doi: 10.1023/B:GLYC.0000043752.89729.bb. [DOI] [PubMed] [Google Scholar]
- 24.Lattard V, Fondeur-Gelinotte M, Gulberti S, et al. Purification and characterization of a soluble form of the recombinant human galactose-beta1,3-glucuronosyltransferase I expressed in the yeast Pichia pastoris. Protein Expr Purif. 2006;47:137–143. doi: 10.1016/j.pep.2005.10.012. [DOI] [PubMed] [Google Scholar]