Abstract
Although efficient transcervical transfer of embryos in mice would provide many advantages over a surgical method, the low success rate of transcervical transfer has hampered its acceptance and use. Here, we describe a novel device and protocol for transcervical embryo transfer in mice. Blastocysts from CD1 female mice were transferred into the uteri of 2.5-d pseudopregnant CD1 mice by using this method resulted in the successful development of 66.7% to 73.5% of the transferred blastocysts into live-born fetuses. Our method is as efficient as surgical embryo transfer yet is much simpler, easier, and markedly less traumatic to the recipient. In addition, our method provides hygienic and economic advantages and conforms to the principles of humane experimental technique. More importantly, our method provides a model for studying transcervical embryo transfer in cattle, other large animals, and humans.
The vast numbers of embryos produced, manipulated, and cryopreserved for research involving genetically modified animals require the completion of numerous embryo transfer procedures in mice. The rapid transcervical transfer of embryos from donors into the uteri of recipient mice is preferable to use of surgical techniques, but the low success rate has dissuaded researchers from adopting this method,1-4,6 and effective procedures are not yet available. We recently developed a method for the transcervical transfer of embryos in mice that we believe will assist many researchers.
Materials and Methods
Transcervical embryo transfer device.
Devices for transcervical embryo transfer were generated by using 10-μL pipette tips (model T-300; Axygen, CA) and arched polytetrafluoroethylene tubes (outside diameter, 0.6 mm; inside diameter, 0.3 mm; model 30L, Woer, Shenzhen, China). Each tube was inserted into a pipette tip until the tube protruded 25 mm from the exit end of the pipette tip; the joint was sheathed by using a heat-shrinkable tube (outer diameter, 0.8 mm; catalog no. RSFR-125H, Woer). The devices were autoclaved at 120 °C and then dried at 80 °C. Domestic-use polypropylene drinking straws (outside diameter, 4.0 mm) were cut into 10-mm lengths to serve as vaginal speculums. Both the ends of the speculums and devices were blunted over an alcohol flame.
Mice.
CD1 mice (6 to 8 wk) were purchased from Vital River Laboratories (Beijing, China) and housed at 25 °C under 50% to 60% relative humidity and a 12:12-h light:dark photoperiod. Mice were fed commercial pelleted food and water ad libitum. All experimental protocols were approved by the Laboratory Animal Care and Use Committee of the Agricultural University of Hebei.
Embryo collection, in vitro culture, and blastocyst transfer.
CD1 female mice each were injected with 7.5 U pregnant mare serum gonadotropin (Tianjin Laboratory Animal Center, China) and 48 h later with 7.5 U human chorionic gonadotropin to induce ovulation; injected female mice then were mated with CD1 male mice. The presence of a vaginal plug the next morning (that is, day 1 of gestation) was taken as evidence of mating. On the morning of day 1, 2, or 3 of gestation, donor female mice were killed by cervical dislocation, and 1-celled, 2-celled, or blastocyst embryos, respectively, were collected as previously described.5 The 1- and 2-celled embryos were cultured to blastocysts in microdrops on standard bacterial culture dishes (Nalge Nunc, Roskilde, Denmark) under mineral oil (Sigma, St Louis, MO) at 37 °C in an atmosphere of 5% CO2 in air. Potassium simplex optimized medium was prepared freshly for embryo culture;5 M2 medium was used for procedures at room temperature. We transferred 6 to 8 blastocysts into a single uterine horn of 2.5-d pseudopregnant female mice, which had been anesthetized by intraperitoneal injection of 1.25% Avertin solution (0.02 mL per gram of body weight) before embryo transfer.5 Live pups after natural birth were counted.
Procedure for transcervical embryo transfer.
Fifty blastocysts were placed in a 50-μL drop of M2 medium (catalog no. M7167, Sigma) in groups of 5 to 10 embryos on the lid of a 60-mm culture dish. The transcervical transfer device (Figure 1) was attached to a 2.5-μL pipette (Eppendorf Research Plus) that had been set to 1.0 μL previously. The pipette plunger was pressed to half the distance to the first stop, the tip was lowered into the blastocysts-containing drop of M2 medium, and (with the assistance of a dissecting microscope) a group of embryos was pulled slowly into the tip of the device. If the first attempt failed, the device was exchanged for a fresh one, because embryos were difficult to pull into an already damp device. Once the embryos were drawn into the device, the pipette was withdrawn immediately from the M2 medium. The length of M2 liquid column in the device did not exceed 3 mm. The pipette tip was patted carefully to create a small (about 1 mm long) air bubble; if the attempt was unsuccessful, the pipetter was reset to 1.1 μL and the tip patted again.
Figure 1.
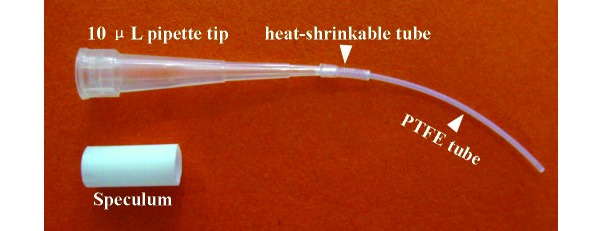
Transcervical embryo transfer device for mice.
The anesthetized recipient female mouse was restrained on the top of a cage. The base of the tail was grasped by using the thumb and forefinger, and the tail was angled upward as the researcher lightly pressed the base of the tail with the opposite edge of the hand. An appropriate speculum was inserted gently into the vagina. Using an adjustable Multiposition Fiber Optic Illuminator System (Beijing Fukai Instrument, Beijing, China) to shine into the speculum facilitated the identification of the cervical opening.
Performing a mock embryo transfer immediately before the actual transfer greatly diminished the incidence of difficult transfers and increased implantation rates. A mock transfer device (no pipette tip, no embryos) was gently inserted into the speculum, through the cervix, and into the uterus. The mock device was withdrawn slowly when the heat-shrinkable tube reached the cervical opening. If traces of blood were seen in the tip of the device, the female mouse could not be used as a recipient. The mock procedure was thought to work by cleaning cervical mucus and directing the actual transfer.
After the mock procedure, embryos were transferred as described; once the heat-shrinkable tube reached the cervical opening, the pipette plunger was pressed to the first stop to expel the embryos, and the device was slowly withdrawn as the plunger was pressed to the second stop. The dissecting microscope was used to confirm that the device was empty of embryos, and the recipient female mice were to their cages. The transcervical embryo transfer procedure is shown in Figure 2.
Figure 2.
Procedure of transcervical embryo transfer.. A, An anesthetized recipient female was held on the top of a cage. Grasp the base of the tail using thumb and forefinger, and angle the tail upward while lightly pressing the base of the tail with the opposite edge of the hand. B, An appropriate speculum was gently inserted into vagina. C and D, The device of transcervical embryo transfer were inserted into a uterine horn using an adjustable multiposition fiber optic illuminator system.
Results
To test whether embryos transferred by using our device developed to term, 1- and 2-celled embryos were allowed to develop in vitro to the blastocyst stage before transcervical transfer into pseudopregnant recipients. By using this protocol, 72.7% (80 of 110) of 1-celled embryos and 93.8% (90 of 96) of 2-celled embryos developed to blastocysts when cultured in vitro for 3 d and 2 d, respectively. Of the blastocysts from 1-celled embryos, 48 blastocysts were transferred transcervically to 8 pseudopregnant CD1 mice, of which 7 had pups. A total of 32 pups were born, representing 66.7% of the transferred blastocysts. As a control, 30 blastocysts were surgically transferred into uteri of 4 pseudopregnant recipients, and all 4 of these had pups. A total of 19 pups were born, representing 63.3% of the transferred blastocysts (Table 1).
Table 1.
Success rate of blastocyst transfer by transcervical and surgical methods
| Type of blastocysts | Method of transfer | No. of blastocysts transferred | No. of recipients | No. of pregnant recipients | No. of live pups | Success ratea |
| Blastocysts in vitro from 1-celled embryos | Transcervical | 48 | 8 | 7 | 32 | 66.7% |
| Surgical | 30 | 4 | 4 | 19 | 63.3% | |
| Blastocysts in vitro from 2-celled embryos | Transcervical | 53 | 7 | 7 | 38 | 71.7% |
| Surgical | 30 | 5 | 5 | 22 | 73.3% | |
| Natural blastocysts | Transcervical | 230 | 30 | 28 | 169 | 73.5% |
| Surgical | 150 | 15 | 13 | 113 | 75.3% |
No. of live pups divided by the no. of blastocysts transferred. The success rate was analyzed by using the Student t test. Differences were considered significant at P < 0.05. For the same type of blastocysts, the success rate did not differ between the transcervical and surgical methods.
Regarding blastocysts from 2-cell embryos, 53 blastocysts were transferred transcervically to 7 pseudopregnant CD1 mice, and 7 of these had pups. A total of 38 pups were born, representing 71.7% of the transferred blastocysts. As a control, 30 blastocysts were transferred surgically into the uteri of 5 pseudopregnant mice, all of which had pups. A total of 22 pups were born, representing 73.3% of the transferred blastocysts (Table 1).
Regarding donor blastocysts, 230 blastocysts were transferred into 30 pseudopregnant mice, of which 28 (93.3%) had pups. A total of 169 pups were born, representing 73.5% of the transferred blastocysts. As a control, 150 blastocysts were transferred surgically into the uteri of 15 pseudopregnant mice, of which 13 (86.7%) had pups. A total of 113 pups were born, representing 75.3% of the transferred blastocysts (Table 1).
Discussion
Although transcervical transfer of embryos in mice was first described in 1951,1 the low implantation and pregnancy rates hampered its adoption as an alternative to surgical embryo transfer.1-4,6 We encountered similarly poor implantation and pregnancy rates during our early attempts, which used a straight, thick, rigid tube.2 We found that uterine scratching and puncturing and embryo expulsion from the uterine horn contributed to the poor results associated with transcervical transfer.2 Furthermore, all cases of uterine scratching affected the antimesometrial side of the uterine horn, which is the initial attachment site of the embryo, such that this damage inevitably disturbs embryo implantation and development. To eliminate these problems, we incorporated the following major technical improvements into our procedure. First, we developed a device comprising an arched, thin, long tube with blunt ends that was linked to a 10-μL pipette tip, which then could be used with a 2.5-μL pipette (Eppendorf Research plus, Hamburg, Germany) to accomplish transcervical transfer of embryos. Our revised devices displayed an excellent ability to transverse the cervix without scratching the endometrium or puncturing the uterine horn.
Second, we incorporated a mock embryo transfer process immediately before the actual transfer. This adjustment not only diminished the incidence of difficult transfers but also markedly decreased embryo expulsion from the uterine horn and increased implantation rates. We consider that the mock procedure helps to remove excess cervical mucus and guide the actual transfer. In addition, the status of uteri can be evaluated according to the mucus samples obtained during the mock process, so that embryos aren't transferred into recipients in which embryo development is unlikely to occur. If excess mucus or traces of blood appear during the mock process, those recipients should not be used.
Third, we strongly suggest that the recipient mouse should be anesthetized before embryo transfer. Anesthesia helps to minimize uterine scratching and contraction, which can lead to expulsion of embryos from uteri. Fourth, limiting the volumes of transferred medium (to less than 0.3 μL) and air (to less than 1.2 μL) injected into the uterine horn also was beneficial in preventing embryos from being expelled. The number of embryos transferred (5 to 14) did not influence the implantation rate. In light of the described modifications, the success rate (66.7% to 73.5%) of transcervical embryo transfer device was markedly higher than that in any other previous report, in which fewer than 45% of the transferred blastocysts developed to term.1-4,6 Our devices and protocol are highly similar to a previous nonsurgical embryo transfer device, the NSET. Although the NSET is already on the market worldwide, its users are few, likely because the associated success rate is low (33% for in vitro blastocysts and 45% for natural blastocysts).3,6 Based on our a great refinement in both technique as well as an improvement to the NSET, the success rate was significantly increased (66.7% to 71.7% for in vitro blastocysts and 73.5% for natural blastocysts).
To suppress the autologous interference of manipulated embryos and specifically test effects of the device on embryo transfer, we transferred only natural and cultured blastocysts with normal morphologic features into uteri of 2.5-d pseudopregnant recipients. Manipulated embryos (for example, chimeric blastocysts containing embryonic stem cells or induced pluripotent stem cells, cryopreserved, genetically modified blastocysts) were not used in the current study. Nonmanipulated and manipulated blastocysts had the same transfer success rate by transcervical embryo transfer in a previous study.2
This transcervical embryo transfer method is as efficient as surgical procedures yet it is simpler, faster, and easier and eliminates the possibility of surgery-associated trauma to the recipient mouse. In addition, surrogate dams for embryo transfer can be used repeatedly. Our method offers hygienic and economic advantages and conforms to the concept of the 3Rs (replacement, reduction, refinement) with the stated goal of improving humane treatment of experimental animals. More importantly, this method provides a model to study potential improvements in transcervical embryo transfer in cattle, other large animals, and humans.
Acknowledgments
We thank Professor F Anthony Lai (Cardiff University School of Medicine) for his critical remarks and editing this manuscript. This work was supported by the National Natural Science Foundation of China (no. 30871790) and Natural Science Foundation of Hebei Province (no. C2012204047 and C2014201186).
The authors have no conflicts of interest.
References
- 1.Beatty RA. 1951. Transplantation of mouse eggs. Nature 168:995. [DOI] [PubMed] [Google Scholar]
- 2.Davis T. 1981. Nonsurgical transfer of mouse embryos. Bios 52:127–133 [Google Scholar]
- 3.Green M, Bass S, Spear BT. 2009. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential postoperative complications. Biotechniques 47:919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsk L, Larsson KS. 1974. A simple method for nonsurgical blastocyst transfer in mice. J Reprod Fertil 37:393–398 [DOI] [PubMed] [Google Scholar]
- 5.Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the mouse embryo: a laboratory manual. New York (NY): Cold Spring Harbor Press [Google Scholar]
- 6.Steele K, Stone B, Hester J, Spear B, Fath-Goodin A. [Internet]. 2012. Nonsurgical embryo transfer with the NSETTM device is a 3Rs refinement technique that reduces stress in CD1 mice. [Cited 2012 May 30. Available at: http://www.paratechs.com/wp-content/uploads/2012/11/AALAS-poster-BS-2012-final.pdf



