Abstract
In the past decade, the use of genetically engineered rats has increased exponentially; therefore, the ability to perform embryo transfer (ET) in rats to rederive, reanimate, or create mutant rat lines is increasingly important. However, the successful generation of pseudopregnant female rats for ET represents a limiting factor. We here evaluated the subcutaneous administration of 40 µg luteinizing hormone releasing hormone agonist (LHRHa) for estrus synchronization during the development and implementation of a rat ET program. Our first experiment assessed endogenous estrus cycling patterns by examining vaginal cytology without administration of LHRHa in 5-wk-old peripubertal Sprague–Dawley female rats. These rats then received LHRHa at approximately 7 wk of age; 57% of the rats were synchronized in proestrus or estrus as assessed by vaginal cytology 96 h later. In a second experiment, 8-wk-old virgin, unmanipulated Sprague–Dawley female rats received LHRHa; 55% were synchronized in proestrus or estrus 96 h later. Copulatory plugs were confirmed in 28% and 82% of the rats that had been synchronized in the first and second experiments, respectively, and mated with vasectomized male rats. Embryo transfer surgery was performed, and live pups were born from both fresh and cryopreserved transgenic rat embryos. Our results indicate that subcutaneous administration of 40 µg LHRHa followed by examination of vaginal cytology 96 h later is an effective technique to generate multiple pseudopregnant recipient rats for use in an ET program.
Abbreviation: ET, embryo transfer; GER, genetically engineered rat; LHRHa, luteinizing hormone releasing hormone agonist
The generation of transgenic rats is a relatively recent event, with the first successful genetically engineered rat (GER) produced in the early 1990s.8,20 However, the technology only recently has advanced sufficiently to predictably generate GER.7,18 The difficulty in producing GER has been due, in part, to attempts to directly extrapolate procedures, including stem cell and embryo culture, from in vivo mouse techniques.14,22,29
Assisted reproductive technologies often involve the manipulation of the estrous cycle and are performed frequently in rodent transgenic core facilities. The use of embryo transfer (ET) plays a crucial role in generating genetically modified animals and improving rodent colony health status.6,30 ET entails the surgical transplantation of embryos into a pseudopregnant recipient. To maximize the efficiency of personnel and minimize animal numbers, transgenic core facilities routinely generate multiple pseudopregnant recipients at the same postcoital stage. Numerous methods to generate pseudopregnant recipients have been published for both mice and rats and range from mating male rodents with female rodents in spontaneous estrus to evaluating the electrical impedance of the vagina to select candidates for mating.11,24,28 In rats, increased electrical resistance in the vagina has been shown to correlate with the estrus phase.11 However, other authors have concluded that there is poor correlation between the hormonal cycle and vaginal impedance readings; therefore the use of electrical impedance in rats that have not been evaluated by vaginal cytology is suboptimal.28
Examination of vaginal cytology has been used in dogs, ferrets, and mice and is considered the ‘gold standard’ to accurately evaluate the phase of the estrous cycle.23,28,334 Vaginal cytology first was used to characterize the estrous cycle of rats in 1922, and this technique has continued to be applied during the past century.9,15,17,19 Similar to other species, rats have predominant cell types that correlate with specific phases of the estrous cycle, which lasts approximately 4 to 5 d. Proestrus in rats lasts approximately 12 h and is characterized by a predominance of nucleated epithelial cells.14 Estrus, with a duration of 9 to 15 h, is considered the receptive phase and is characterized by anucleated, cornified epithelial cells.15,17 Early diestrus (also referred to as metestrus) has a duration of approximately 14 to 18 h and is characterized by a mixed cell population consisting of cornified and nucleated epithelial cells in the presence of leukocytes. Late diestrus has a duration of 60 to 70 h in rats and is characterized by the predominance of leukocytes.15-17
Estrus synchronization with exogenous hormones has been used for decades in the food animal industry to maximize reproductive performance and production. These exogenous hormones allow the breeding of animals within a shortened, predefined interval, often streamlining and maximizing the time and effort to generate offspring. Extrapolating from estrus synchronization in other species, namely cattle, early work in estrus synchronization of rats was performed in the 1980s by using subcutaneous implants of the exogenous luteinizing hormone releasing hormone agonist (des-Gly,10 D-Ala6-LH-RH ethalamide; LHRHa).31 LHRHa is a synthetic analog of the hypothalamic neurohormone GnRH and functions at the GnRH receptor, causing the release of FSH and LH from the anterior pituitary.5 By using this technique and according to vaginal cytology, 9 of 10 female albino rats (stock not specified) were in synchronized estrus 4 d after the removal of 25 µg of pelleted LHRHa that had been implanted subcutaneously for 7 d.31 An adaptation to this protocol using 50 µg LHRHa as a single subcutaneous injection to adult Long–Evans female rats generated copulation rates of 75% to 94% at 4 d after LHRHa administration.32 However, limitations of the cited study31 include the lack of evaluation of the phase of the estrous cycle before mating and the mating of female rats to intact male rats.
More recently, an alternative method for estrus synchronization by using LHRHa and vasectomized male rats has been described.4 In the cited study,4 94% of Sprague–Dawley female rats were in early estrus or estrus at 4 d after subcutaneous administration of 40 µg LHRHa, and 75% of female rats selected for mating had copulatory plugs.4 Because of this high rate of female rats in estrus, the authors concluded that cytologic staging of the estrous cycle of rats that had received LHRHa 4 d earlier was unnecessary.1,4 Despite our attempts to follow this previously described protocol using 40 µg LHRHa to synchronize estrus in Sprague–Dawley female rats, we experienced much lower rates of females in estrus and could not recapitulate the authors’ results. Although numerous environmental, genetic and intrinsic species factors might have led to the differences between our findings and those of the previous study,4 we decided to pursue an evaluation of the natural estrous cycle of Sprague–Dawley rats by using vaginal cytology and to evaluate the effects of LHRHa on estrus synchronization, to the end of generating pseudopregnant recipients for use in an ET program.
Materials and Methods
Animals.
Male and female NTac:SD rats were obtained from Taconic Farms (Germantown, NY) and singly housed in static filter-top microisolation caging (Allentown, PC10198HT, Allentown, NJ) under a 12:12-h light:dark cycle. These SPF rats tested negative for all major rodent bacterial and viral pathogens including Kilham rat virus, rat coronavirus, rat minute virus, rat parvovirus, sialodacryoadenitis virus, Toolan H1 parvovirus, encephalomyelitis virus, pneumonia virus of mice, respiratory enteric virus III, Sendai virus, Hantaan virus, mouse adenovirus, β-hemolytic Streptococcus, Streptococcus pneumoniae, Corynebacterium kutscheri, Klebsiella spp., Mycoplasma spp., Pasturella spp., Pseudomonas aeruginosa, Salmonella spp., cilia-associated respiratory bacillus, Helicobacter spp., Clostridium piliforme and were negative for all internal and external parasites tested. Male rats underwent surgical vasectomies at approximately 6 wk of age and were allowed to recover for more than 4 wk before being used in matings. Female rats were obtained at approximately 5 wk of age (body weight, approximately 75 to 100 g) or 8 wk of age (body weight, approximately 175 to 200 g) and were individually housed. The Lee–Boot effect, a phenomenon in which cohoused females become anestrus, is well characterized in mice but less so in rats26,27 and was not considered a variable in our experimental design because all female rats for this experiment were singly housed for the duration of all experiments. Food (Prolab RMH 3000, PMI, St Louis, MO) and water were provided ad libitum. Housing conditions were maintained at 68 to 75 °F (20.0 to 23.9 °C) and 30% to 70% relative humidity. Animals were housed in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals.10 All rats were acclimated for a minimum of 72 h before any manipulations began. All experiments were performed under the approval of the IACUC at the Massachusetts Institute of Technology. Embryos for rederivation were harvested at the 2-cell stage from superovulated transgene-carrying or wild-type rats after being mated with transgene-carrying stud male rats 36 h previously. All embryos used in these experiments belonged to various institutional investigators. Cryopreservation and reanimation of selected 2-cell stage embryos were performed per investigator request by using standard rapid-freeze and thaw methods.25
Examination of vaginal cytology to determine the estrous cycle phase in rats.
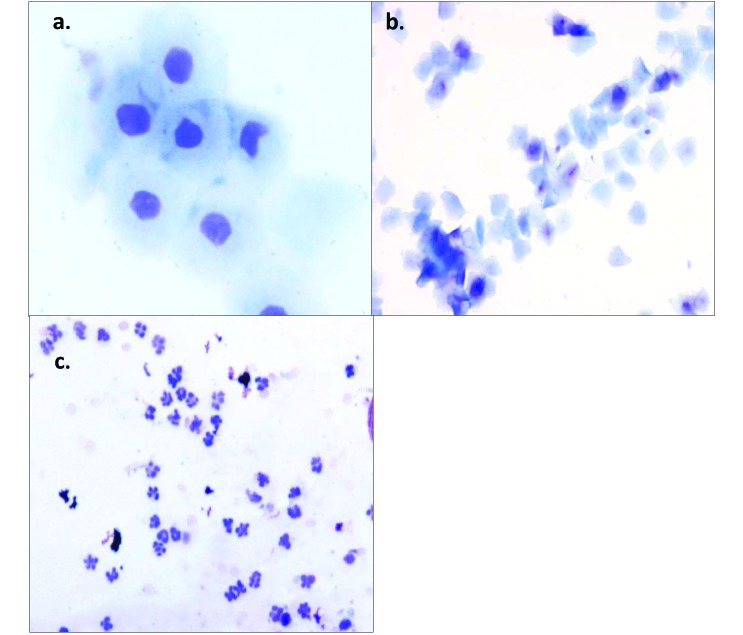
Samples for vaginal cytologic evaluation were obtained daily between 0800 and 1000 for all experiments performed. Sterile cotton-tipped applicators were moistened with sterile water, and the vaginal mucosa was swabbed. Samples taken were applied to Unifrost microscope slides (Azer Scientific, Morgantown, PA), air-dried, and stained using Wright–Giemsa (Richard Allan Scientific Three-Step Stain Kit, Thermo Scientific, Kalamazoo, MI). Notations of animal behavior and gross descriptions of vaginal fluids were made. Two veterinarians with experience in cytologic evaluation (TMB and JP) independently scored slides and had greater than 96% agreement on all samples evaluated, according to assigned characteristics, and representative of the phase of the estrous cycle2 (Figure 1).
Figure 1.
(A) The proestrus phase is characterized by nucleated epithelial cells, which are often found clustered together or in sheets. Magnification, 400×. (B) The estrus phase of the rat is indicated by anucleated cornified cells, which often have angular edges. Magnification, 100×. (C) Diestrus is characterized by an abundance of leukocytes, which often are mixed with cellular debris. Magnification, 200×. Wright–Giemsa stain.
Estrus synchronization and pseudopregnancy.
Experiment 1 (Figure 2).
Figure 2.
Evaluation of estrous cycle and timeline of experiment 1.
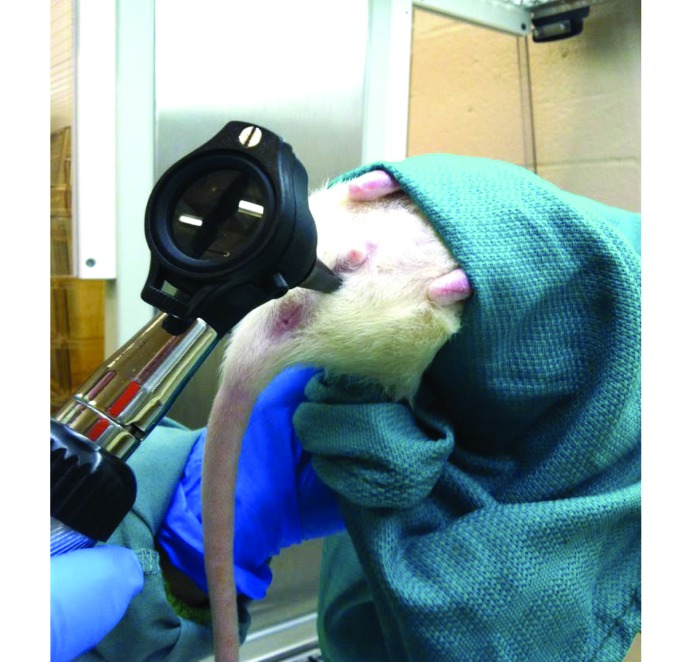
Vaginal cytologies from 14 Sprague–Dawley rats (age, 5 wk) were obtained daily for a total of 20 d, starting on day 0. For 14 d, peripubertal data was recorded to evaluate endogenous estrous cycling patterns without exogenous hormonal influence (group 1). On the morning of day 14, all rats in group 1 subcutaneously received 40 µg LHRHa diluted in 1.0 mL sterile water (Sigma, St Louis, MO). On day 18 (that is, 96 h after injection), female rats found to be in late proestrus, early estrus, or estrus according to vaginal cytology were paired 1:1 with vasectomized adult Sprague–Dawley male rats for 20 to 24 h. The presence of copulatory plugs was determined by using an illuminated otoscope with a 2.5-mm disposable pediatric speculum (Innovative Innovations, Laramie, WY) on the morning of day 19 (Figure 3).13 Bilateral oviduct transfer was not performed due to lack of transferrable rat embryos.
Figure 3.
Using an illuminated otoscope with a pediatric speculum to assess copulatory plugs in restrained rats.
Female rats in group 1 that lacked copulatory plugs on day 18 were randomly divided into 2 groups (groups 1a and 1b). After a rest period of 7 d, rats in group 1a received 40 µg LHRHa on the morning of day 26; rats in group 1b received no injection. The half-life of synthetic gonadotropin releasing hormone analogs, including LHRHa, is approximately 3 h.35 Therefore, we postulated female rats would have no residual effects from LHRHa when it was readministered on day 26. On day 30, any rats that were in late proestrus to estrus according to cytology were paired 1:1 with vasectomized Sprague–Dawley male rats for 20 to 24 h. On the morning of day 31, the presence of copulatory plugs was determined by using an illuminated otoscope. Bilateral oviduct transfer of cryopreserved 2-cell staged transgenic rat embryos was performed.
Experiment 2.
Unmanipulated virgin Sprague–Dawley female rats (n = 20; age, 8 wk; group 2) received 40 µg LHRHa subcutaneously, and vaginal cytology was performed 96 h later. Only rats in late proestrus to estrus were paired 1:1 with vasectomized male rats on the same day for approximately 20 to 24 h. Female rats were evaluated for copulatory plugs the morning after pairing by using an illuminated otoscope. This experiment was repeated by using an additional 20 virgin, 8-wk-old unmanipulated Sprague–Dawley female rats (group 3), and results were pooled for analysis. Bilateral oviduct transfer of fresh 2-cell transgenic rat embryos was performed.
Results
Experiment 1.
Vaginal cytologic examination to determine the phase of the estrous cycle in rats.
Of the 14 peripubertal Sprague–Dawley female rats under endogenous hormonal control that underwent serial vaginal cytology sampling (group 1), 12 (85.7%) had abnormal estrous cycles characterized by multiple, continuous days in a particular phase of the estrous cycle. Two rats in group 1 had a single interval of a normal 4-d estrous cycle during the 20 d observed; however, these rats had estrous cycle irregularities before and after this single normal cycle; these irregularities were characterized by multiple days in proestrus or by complete absence of estrus for longer than 5 sampling days.
Administration of LHRHa to synchronize estrus in rats.
Of the 14 SD female rats in group 1 that received LHRHa on day 14, 8 (57%) were in late proestrus, early estrus, or estrus at 96 h (day 18) after LHRHa administration, as determined by vaginal cytology. We selected 7 female rats to mate with vasectomized males; 2 of the 7 (28.6%) female rats had copulatory plugs on day 19, and no rat embryos were available to perform ET on day 19. One female rat that was in estrus after LHRHa administration was removed from further experimentation because of injuries and abrasions sustained during mating with a vasectomized male rat; 6 wk later, this female rat was diagnosed with pyometra and septic peritonitis.
Readministration of LHRHa to female rats.
Estrus, as determined by cytologic evaluation, occurred in 2 of the 6 (33%) rats in group 1b (no LHRHa) and in 2 of the 6 (33%) in group 1a (LHRHa redose). All female rats in estrus from both groups 1a and 1b that were paired with vasectomized males for 20 to 24 h had confirmed copulatory plugs. Surgical bilateral oviduct ET was performed with cryopreserved 2-cell transgenic rat embryos to 2 female rats from group 1a on day 31. In addition, 30 thawed embryos were transferred to 2 plugged recipients, which delivered a total of 6 pups, for a live-born rederivation rate of 20% (6 of 30).
Experiment 2.
Of the 40 female rats in experiment 2 that received 40 µg LHRHa (groups 2 and 3), 22 (55%) were in late proestrus, early estrus, or estrus according to vaginal cytology. In addition, 18 of these 22 (82%) female rats had copulatory plugs 20 to 24 h after being paired with a vasectomized male rat. Of the 18 remaining female rats that were not paired with vasectomized males, 13 (72.2%) were in early diestrus and the remaining 5 (27.8%) were in late diestrus or early proestrus at 96 h after LHRHa. Surgical bilateral oviduct ET was performed with fresh 2-cell transgenic rat embryos. Of the 122 fresh embryos transferred to 11 plugged recipient females, 39 pups were born to 10 dams, for a live-born rederivation rate of 31.9% (39 of 122).
Discussion
In this study, we characterized the estrous cycle of rats according to cytologic evaluation of vaginal swabs to reproducibly generate pseudopregnant female recipient rats for use in an ET program. By accurately determining the phase of the estrous cycle via vaginal cytology after estrus synchronization with LHRHa, we found 55% to 57% of Sprague–Dawley rats were in stages ranging from late proestrus to estrus. Our results contrast with a previously published study, in which 94% of Sprague–Dawley rats given LHRHa underwent estrus synchronization 96 h later; the authors therefore concluded that staging the estrous cycle of rats receiving LHRHa was unnecessary.1,4 The differences between these studies may reflect the younger female rats that we used or differences in outbred stock genetics, husbandry, or environment.21 Despite the lower percentages of rats in estrus after LHRHa administration, using vaginal cytologic assessments in conjunction with a synchronization protocol, like LHRHa, enables more accurate selection of female rats in early estrus or estrus to mate with vasectomized male rats, thereby generating precise estimates for copulatory plugs. In our study, 82% of female rats had copulatory plugs 20 to 24 h after mating. By pairing female rats in late proestrus or estrus (when they are considered to be receptive)15,17 with vasectomized males, our method eliminates the pairing of nonreceptive female rats.
The majority of our rats that underwent estrous cycle assessment between 5 and 8 wk of age had abnormal endogenous cycles. The first estrus of Sprague–Dawley rats occurs after vaginal opening, which occurs at approximately 35 and 36 d of age.3 At this age, the female Sprague–Dawley rats are considered to be functionally mature. We obtained vaginal cytology samples from female Sprague–Dawley rats starting at approximately 35 d of age and continuing through 54 d of age (Figure 2). Although it is well recognized in the literature that pubertal rats undergo irregularities in their estrous cycle, we expected to see most rats transition into a reproducible, 4- to 5-d estrous cycle over the 2-wk period during which we sampled the rats.12 However, the majority of our rats had a prolonged diestrus or proestrus, which lasted as long as 12 continuous days in some rats. This finding may be due in part to the physical nature of vaginal swabbing, given that stimulation of the vagina and cervix may trigger the neuroendocrine reflex, thereby causing rats to become pseudopregnant and exhibit prolonged diestrus; this effect may present a complication when performing serial vaginal cytologic sampling in rats.33 Although we were careful to sample only the vaginal mucosa, excess stimulation cannot be ruled out as a cause of the estrous cycle abnormalities we observed. A previous study that compared the cytology of vaginal flushes with vaginal wall impedance in Lewis rats suggested that female rats may require 8 to 14 d to reach the stimulation threshold which triggers pseudopregnancy, and rats may retain this state for up to 15 d.11 From our experience, abnormal cyclicity in group 1 occurred as early as the second day of vaginal cytologic sampling and persisted throughout most of the experiment, suggesting that the perturbations we noted could be attributed to endogenous hormonal factors, as previously published and may verify this phenomenon.12 We obtained vaginal flushes in pilot animals but discontinued this practice after we deemed that reproducibility and quality were unsuitable for experimental use. However, the use of vaginal cytologic sampling obtained by swabbing appeared to have no direct influence on the estrus-inducing effects of LHRHa after day 26 in experiment 1, because estrus occurred at equal rates in groups 1a and 1b.
To evaluate LHRHa's influence on the estrous cycle of Sprague–Dawley rats and its ability to synchronize estrus and eliminate any possible influence on presynchronization pseudopregnancy due to repeated vaginal swabbing, we chose to administer 40 µg LHRHa to acclimated, unmanipulated, virgin rats and to collect vaginal cytologic samples once, 96 h after injection. In the 2 trials performed during experiment 2, we again observed an estrus synchronization rate much lower (55%, 22 of 40) than the previously published 94%.4 However, 18 of these 22 (82%) female rats that were paired 1:1 with vasectomized males in our study had copulatory plugs as determined by otoscope evaluation, suggesting that the use of vaginal cytologic sampling in conjunction with LHRHa more precisely generates reproducible numbers of pseudopregnant female rats for use in an ET program, because nonreceptive female rats are not mated. Differences between copulatory plugging rate between experiments 1 and 2 may reflect the inexperience of the vasectomized male, given that their first exposure to female rats occurred during experiment 1. Variability in copulatory plug location and size in rats has been previously documented.1,13 Similar to the practice in mice, visual inspection of rats for larger, externally visible vaginal plugs should be performed first, including inspection of the bedding. However, in our experience, copulatory plugs in rats were small, occurred deep within the vagina, were not easily visible, and required an otoscope to perform the assessment.1,13 Nevertheless, live pups were obtained from both fresh and cryopreserved surgically transferred 2-cell embryos of various genetic backgrounds in both experiments 1 and 2.
During the past decade, the use of GER models in research has increased exponentially; the ability to perform ET in rats to rederive, reanimate, or create mutant rat lines is of utmost importance. Challenges often arise when implementing new, unfamiliar techniques or when experimental outcomes do not recapitulate previously published data. Our current study refines a previous estrus synchronization method for Sprague–Dawley rats and allows the streamlining of ET procedures that can maximize the generation of GER models. When establishing a new ET program, we recommend the administration of 40 µg LHRHa subcutaneously to Sprague–Dawley female rats, with vaginal cytology evaluated 96 h after administration. Cytologic evaluation should be performed on the same day as vaginal swabbing, and female rats that are in late proestrus or estrus should be paired 1:1 with vasectomized male rats and allowed to mate for approximately 20 to 24 h. If copulatory plugs are not readily visible externally, we recommend examination by using an illuminated otoscope with a pediatric speculum. Pseudopregnant female rats obtained by this method can then be used for ET procedures.
Vaginal cytology is an invaluable tool for programs that use assisted reproductive techniques in rats and should be incorporated when establishing a new ET program, attempting to increase copulatory plug rate, or validating estrus synchronization. Vaginal cytology is easily performed and accomplished once personnel learn the common cytologic characteristics of the estrous cycle. In our study, collecting vaginal swabs from 20 female rats took less than 30 min, and cytologic assessment of slides, when performed by experienced personnel, took approximately 45 to 60 s per slide. We believe the extra time invested in these procedures (less than 1 h), in conjunction with estrus synchronization, is extremely beneficial when establishing a rat ET program. By using this protocol, estimating the number of pseudopregnant rats is more accurate and reduces possible wastage of embryos.
Acknowledgments
We thank Alyssa Terestre for assistance with manuscript preparation and Philip Damiani for technical advice.
References
- 1.Cozzi J, Anegon I, Braun V, Gross AC, Merrouche C, Cherifi Y. 2009. Pronuclear DNA injection for the production of transgenic rats. Methods Mol Biol 561:73–88 [DOI] [PubMed] [Google Scholar]
- 2.Devall AJ, Lovick TA. 2010. Differential activation of the periaqueductal gray matter by mild anxiogenic stress at different stages of the estrous cycle in female rats. Neuropsychopharmacology 35:1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans AM. 1986. Age at puberty and first-litter size in early- and late-paired rats. Biol Reprod 34:322–326 [DOI] [PubMed] [Google Scholar]
- 4.Filipiak WE, Saunders TL. 2006. Advances in transgenic rat production. Transgenic Res 15:673–686 [DOI] [PubMed] [Google Scholar]
- 5.Fink G. 1979. Feedback actions of target hormones on hypothalamus and pituitary with special reference to gonadal steroids. Annu Rev Physiol 41:571–585 [DOI] [PubMed] [Google Scholar]
- 6.Fray MD, Pickard AR, Harrison M, Cheeseman MT. 2008. Upgrading mouse health and welfare: direct benefits of a large-scale rederivation programme. Lab Anim 42:127–139 [DOI] [PubMed] [Google Scholar]
- 7.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. 2009. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. 1990. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell 63:1099–1112 [DOI] [PubMed] [Google Scholar]
- 9.Hartman CG. 1944. Some new observations on the vaginal smear of the rat. Yale J Biol Med 17:99–112 [PMC free article] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 11.Jaramillo LM, Balcazar I, Duran C. 2012. Using vaginal wall impedance to determine estrous cycle phase in Lewis rats. Lab Anim (NY) 41:122–128 [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Shin JH, Moon HJ, Kim TS, Kang IH, Seok JH, Kim IY, Park KL, Han SY. 2002. Evaluation of the 20-day pubertal female assay in Sprague–Dawley rats treated with DES, tamoxifen, testosterone, and flutamide. Toxicol Sci 67:52–62 [DOI] [PubMed] [Google Scholar]
- 13.Krinke G. 2000. The laboratory rat (the handbook of experimental animals). London (UK): Academic Press [Google Scholar]
- 14.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL. 2008. Germline competent embryonic stem cells derived from rat blastocysts. Cell 135:1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long JA, Evans HML. 1922. The oestrous cycle in the rat and its associated phenomena. Berkeley (CA): University of California Press [Google Scholar]
- 16.Mandl AM. 1951. The phases of the oestrous cycle in the adult white rat. J Exp Biol 28:576–584 [Google Scholar]
- 17.Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614 [DOI] [PubMed] [Google Scholar]
- 18.Michalkiewicz M, Michalkiewicz T, Geurts AM, Roman RJ, Slocum GR, Singer O, Weihrauch D, Greene AS, Kaldunski M, Verma IM, Jacob HJ, Cowley AW. 2007. Efficient transgenic rat production by a lentiviral vector. Am J Physiol Heart Circ Physiol 293:H881–H894 [DOI] [PubMed] [Google Scholar]
- 19.Montes GS, Luque EH. 1988. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 133:192–199 [DOI] [PubMed] [Google Scholar]
- 20.Mullins JJ, Peters J, Ganten D. 1990. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 344:541–544 [DOI] [PubMed] [Google Scholar]
- 21.Pare WP, Kluczynski J. 1997. Differences in the stress response of Wistar–Kyoto (WKY) rats from different vendors. Physiol Behav 62:643–648 [DOI] [PubMed] [Google Scholar]
- 22.Popova E, Bader M, Krivokharchenko A. 2005. Strain differences in superovulatory response, embryo development and efficiency of transgenic rat production. Transgenic Res 14:729–738 [DOI] [PubMed] [Google Scholar]
- 23.Post K. 1985. Canine vaginal cytology during the estrous cycle. Can Vet J 26:101–104 [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos SD, Lee J, Peuler J. 2001. An inexpensive meter to measure differences in electrical resistance in the rat vagina during the ovarian cycle. J Appl Physiol 91:667–670 [DOI] [PubMed] [Google Scholar]
- 25.Renard JP, Babinet C. 1984. High survival of mouse embryos after rapid freezing and thawing inside plastic straws with 1,2-propanediol as cryoprotectant. J Exp Zool 230:443–448 [DOI] [PubMed] [Google Scholar]
- 26.Schank JC. 2001. Do Norway rats (Rattus norvegicus) synchronize their estrous cycles? Physiol Behav 72:129–139 [DOI] [PubMed] [Google Scholar]
- 27.Sharp P. 1998. The laboratory rat. Boca Raton (FL): CRC Press [Google Scholar]
- 28.Singletary SJ, Kirsch AJ, Watson J, Karim BO, Huso DL, Hurn PD, Murphy SJ. 2005. Lack of correlation of vaginal impedance measurements with hormone levels in the rat. Contemp Top Lab Anim Sci 44:37–42 [PMC free article] [PubMed] [Google Scholar]
- 29.Tong C, Li P, Wu NL, Yan Y, Ying QL. 2010. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467:211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Keuren ML, Saunders TL. 2004. Rederivation of transgenic and gene-targeted mice by embryo transfer. Transgenic Res 13:363–371 [DOI] [PubMed] [Google Scholar]
- 31.Vickery BH, McRae G. 1980. Synchronization of oestrus in adult female rats by utilizing the paradoxical effects of an LHRH agonist. J Reprod Fertil 60:399–402 [DOI] [PubMed] [Google Scholar]
- 32.Walton EA, Armstrong DT. 1983. Oocyte normality after superovulation in immature rats. J Reprod Fertil 67:309–314. [DOI] [PubMed] [Google Scholar]
- 33.Westwood FR. 2008. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol 36:375–384 [DOI] [PubMed] [Google Scholar]
- 34.Williams ES, Thorne ET, Kwiatkowski DR, Lutz K, Anderson SL. 1992. Comparative vaginal cytology of the estrous cycle of black-footed ferrets (Mustela nigripes), Siberian polecats (M. eversmanni), and domestic ferrets (M. putorius furo). J Vet Diagn Invest 4:38–44 [DOI] [PubMed] [Google Scholar]
- 35.Wilson AC, Meethal SV, Bowen RL, Atwood CS. 2007. Leuprolide acetate: a drug of diverse clinical applications. Expert Opin Investig Drugs 16:1851–1863 [DOI] [PubMed] [Google Scholar]