Abstract
Hair loss is a common problem in captive macaque colonies. A potential factor is the possible influence of stressful environments in the development of hair loss. We examined the relationship between hair loss and chronic hypothalamic–pituitary–adrenal (HPA) axis activity by measuring cortisol in hair. Adult male and female rhesus macaques housed at 3 primate facilities in the United States were screened for degree of hair loss and observed for evidence of hair-plucking behavior. Hair samples and photographic data were obtained from 99 subjects, none of which were hair-pluckers. Macaques with greater than 30% hair loss (alopecia group) showed higher concentrations of hair cortisol than did those with less than 5% hair loss (control group), a finding that was unrelated to age, body weight, or the month in which the sample was collected. Hair loss scores were positively correlated with hair cortisol levels across all monkeys and within the alopecic group alone. In addition, the strong relationship between hair cortisol and alopecia was noted in 2 but not the third facility. Friction with cage surfaces appeared to contribute to hair loss in 18 monkeys. These findings suggest that stress may be one of several factors related to hair loss in some captive nonhuman primates, although whether this relationship is causal or merely correlational is unclear. Moreover, the source of the additional cortisol in the hair of alopecic monkeys (that is, from the circulation or from local synthesis in the skin) remains to be determined.
Abbreviation: HPA, hypothalamic–pituitary–adrenal
Alopecia or hair loss occurs in rhesus macaque populations housed in captivity. Not all nonhuman primates show hair loss, but female animals appear to be more vulnerable to hair loss than are males19,29 of some species.15 Hair loss can occur as a natural consequence of aging,29 for which no treatment is available, or through exposure to a seasonal environment,11 for which no treatment is required. In some cases, hair loss results from various biologic dysfunctions which require specific treatments to reverse the hair loss (see reference 24 for a review). These dysfunctions include endocrine disorders,16 immunologic diseases,20 vitamin and mineral imbalances,30 and allergic reactions producing atopic dermatitis.14 Of recent concern is the role of psychogenic stressors (for example, restricted rearing and housing environments) in inducing hair loss in rhesus macaques.23 Such stressors might lead directly to hair loss or might indirectly induce hair loss as a result of stimulating the abnormal behavior of hair plucking.
A direct relationship of stress exposure to hair loss has been demonstrated experimentally in mice. In depilated mice, exposure to intermittent foot shock led to increased concentrations of plasma corticosterone in comparison to controls and a concomitant alteration of the natural cycle of hair regrowth, that is, prolonged telogen phase and delayed anagen phase induction.1 Foot shock subsequently was shown to produce the same effects on the natural cycle of hair growth in nondepilated mice.13 These findings are supported further by the effects of stress exposure on C3H/HeJ mice, a strain that is predisposed to develop low levels of alopecia areata. In one study, C3H/HeJ mice exposed to a physiologic stressor in the form of heat treatment showed significantly more hair loss than did control mice of the same genetic background.33
If some hair loss is a consequence of exposure to stressors, then pharmacologic treatment might be beneficial. However, few studies have examined the effects of psychoactive drug treatment on stress-induced hair loss. A retrospective study of cats diagnosed with psychogenic overgrooming revealed that most of these animals responded positively to treatment with antidepressant or anxiolytic drugs.26 We know of no reports of successful treatment of alopecia in nonhuman primates with psychoactive medications or of any definitive proof that hair loss is stress-related.
Despite ongoing concerns about hair loss as a marker for stress exposure in captive monkeys, this idea has seldom been subject to scientific scrutiny. In the only nonhuman primate study to date, rhesus monkeys with hair loss had lower concentrations of the cortisol metabolite 11-oxoetiocholanolone in feces than monkeys with normal coats.29 However, because rhesus macaques defecate once or more each day, fecal cortisol levels reflect 1 d of adrenocortical activity at best, unless serial samples are collected over a long period of time. In contrast, an integrated measure of HPA activity over a period of several months can be obtained by assessing cortisol concentrations in hair.3 The objective of the present study was to examine the relationship between hair loss and chronic HPA activity by measuring hair cortisol.
Hair cortisol has recently been used to investigate the influence of major life stressors on chronic HPA activity. In numerous studies, humans undergoing considerable stress showed higher levels of hair cortisol than did either matched controls or the same subjects before stress imposition. Hair cortisol was higher in humans experiencing chronic pain,32 undergoing unemployment,4 engaging in shift work,21 diagnosed with acute myocardial infarction,25 and showing alcohol-dependence28 and in newborn infants who required hospitalization in the neonatal intensive care unit34 as compared with the relevant control groups. In monkeys, hair cortisol has been shown to increase in response to relocation from a familiar to an unfamiliar environment, an effect that has been observed in all species studied to date (rhesus macaques [Macaca mulatta],2 vervet monkeys [Cercopithecus aethiops],6 and bonnet macaques [Macaca radiate]).17 Therefore, a strong association exists between exposure to stressors and chronic changes in the HPA axis as reflected in increased secretion of cortisol.
The objective of this study was to examine the relationship between hair loss and hair cortisol levels in monkeys at 3 different facilities across the United States. Hair loss was assessed at all 3 facilities as part of a routine health monitoring program. Monkeys that showed no evidence of hair plucking behavior and that were classified as having either more than 30% hair loss or less than 5% hair loss were identified as subjects for this study. Hair plucking is a syndrome in macaques that includes pulling out fistfuls of hair or single strands or grooming oneself excessively by using hands or teeth. Once removed from the body, the hair is often examined, manipulated, and ingested.23
During routine health exams, hair was collected for cortisol assay, and macaques were photographed in 3 positions. Hair loss then was quantified from photographs by using ImageJ software. In addition, this approach allowed us to confirm the visual classification from the health-monitoring program, examine the relationship between the total surface area of hair loss and hair cortisol concentration, and detect specific patterns of hair loss (symmetrical, friction-induced, and so forth).
Materials and Methods
Subjects.
The subjects were 99 adult rhesus macaques (Macaca mulatta; 36 male; 63 female) from 3 national primate centers (Washington National Primate Research Center, Oregon National Primate Research Center, and Southwest National Primate Center). The subjects ranged in age from 4 to 21 y (mean, 10.2 y), and none of the females was pregnant. At the time of data collection, monkeys were housed in size appropriate cages in rooms where they had visual, auditory, and olfactory contact with other monkeys; 79 (32 male; 47 female) were housed individually; 12 (3 male; 9 female) were paired; and 8 (1 male, 7 female) had physical access to a partner through grooming contact bars. The macaques were maintained in accordance with the Guide for the Care and Use of Laboratory Animals,10 and housing environments were quite similar (Table 1).
Table 1.
Housing and maintenance conditions at the 3 facilities
| Facility A | Facility B | Facility C | |
| Photocycle | 12:12-h cycle; lights on at 0700 | 12:12-h cycle; lights on at 0700 | 12:12-h cycle; lights on at 0600 |
| Frequency of feeding | Twice daily | Twice daily | Twice daily |
| Temperature range | 19–25 °C | 18–27 °C | 22–25 °C |
All rhesus macaques received commercial chow (LabDiet, PMI, St Louis, MO) supplemented with produce, fruits, and grains.
The monkeys received a nutritionally balanced diet supplemented with additional fruit, produce, and grains. All monkeys participated in facility environmental-enrichment programs and were provided with perches, novel toys, foraging devices, and novel food items on a regular basis. The 3 primate facilities are all accredited by AAALAC, and all work was approved by the local IACUC.
At each facility, monkeys that showed hair-plucking behavior directed to either themselves or others as determined by the health-monitoring program were eliminated as subjects. From the resulting pool, monkeys were selected on the basis of hair loss, with subjects showing either 30% or greater hair loss or less than 5% hair loss based on visual assessments. Alopecia is more common in female than male macaques.19,29 At 2 facilities, designated A and B, sufficient numbers of male and female macaques were available for study; at the third facility (facility C), more female than male rhesus were available for study (Table 2). All hair and photographic data were collected during 24 January through 18 July 2012.
Table 2.
Numbers of subjects
| Male macaques |
Female macaques |
Average age (y; [range]) | |||
| Facility | Alopecic | Nonalopecic | Alopecic | Nonalopecic | |
| A | 6 | 6 | 6 | 6 | 11.9 (5–21) |
| B | 10 | 12 | 12 | 16 | 9.2 (5–19) |
| C | 1 | 1 | 10 | 13 | 10.7 (4–14) |
Procedure.
Data were collected during routine health exams conducted by the veterinary staff at each center. After completion of the health portion of the exam, sedated monkeys were positioned on a towel containing a 12-in. (30.5-cm) ruler and photographed in 3 positions: lying on the left side with arms and legs gently separated and extended; on the right side with arms and legs gently separated and extended; and on the stomach with the arms gently positioned above and the legs gently extended below the monkey. After the photographs were obtained, a 2.5-cm2 patch of hair was gently shaved from the nape of the neck of each monkey. Hair samples and photographs were sent to the University of Massachusetts (Amherst, MA), where they were analyzed.
Assessment of alopecia.
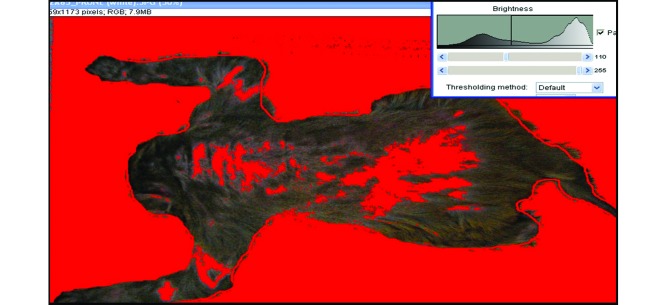
Photographs were analyzed by using ImageJ software (NIH; http://imagej.nih.gov/ij/) to quantify total hair loss. To obtain a whole-body surface area, the Brush tool in the Microsoft Paint program (Microsoft, Redmond, WA) was used to trace the body outline of the 3 orientations (left, right, and prone). Only the dark coat was analyzed for alopecia; the white coat of the inner arms, legs, chest and belly was not assessed, as is typically the case in visual scoring systems. Once the whole body was outlined, we examined areas of alopecia by using the Threshold image adjuster in ImageJ, thus highlighting areas of alopecia in red. The threshold was set to identify patches of alopecia that were larger than 0.5 cm2 on the body. When the cursor clicked on a particular patch, the program automatically calculated its area and maintained a sum for each animal as the cursor was moved from patch to patch (Figure 1). A ruler on each photograph not only ensured that this criterion was applied equally across all subjects but also allowed us to directly compare individual subjects on various morphometric measures. The only part of this process that involved possible subjectivity was the tracing of the body outline. A rescoring of 15 randomly selected images balanced across facilities yielded an average percentage agreement score of 97.5%, with a range across images of 92.3% to 99.7%.
Figure 1.
Alopecia in a female rhesus macaque assessed by using ImageJ software. Areas highlighted in red represent hair loss.
Role of hair plucking.
As stated earlier, monkeys that had been identified as hair-pluckers were excluded from this study. This determination was based on brief assessments of the monkeys, during which animals were examined semiannually or quarterly at each facility for the presence of abnormal behavior, and on comments of the animal care technicians. In addition, we obtained 2 videotapes of all the monkeys in their home cages as a part of conducting the Human Intruder Test for assessing anxious behavior (data not reported here). The test generally was administered in the morning, at least 1 h after the morning feeding period. Each tape contained 10 min of baseline data collection, during which only the camera was in the room (that is, no human observer). At the start of the intruder stage, the intruder entered the room and stood within 2 ft. of each monkey. The intruder then spent 2 min in profile with no direct eye contact, 2 min looking at the monkey, and 2 min with her back turned. The intruder then turned off the camera and carried it out of the room. When these tapes were screened for hair plucking by using Mpeg Streamclip (www.squared5.com), none was observed in any of the animals.
Finally, a comparison of hair loss as a function of body orientation (left side, right side, prone) revealed very high correlations across all 3 orientations. These high correlations argue against a simple hair-pulling interpretation of hair loss in this group of monkeys, given that the animals would not be expected to equalize hair plucking behavior across all 3 body positions, particularly between the back and either side.
Hair cortisol assay.
Hair was collected from individual monkeys during routine health exams at all 3 facilities, stored in aluminum-foil packets, and maintained in a freezer (either at −20 or −80 °F) until shipment. Previous research has established that the cortisol in hair remains highly stable in ambient temperature environments for many months.7 The samples were shipped to the University of Massachusetts at Amherst for processing.3 Briefly, the samples were washed twice with isopropanol to remove external sources of contamination (for example, sweat or sebum) and air-dried. The cleaned hair samples then were powdered by using a ball mill (Retsch, Verder Scientific, Newtown, PA) to break up the hair's protein matrix and increase the surface area for extraction. Subsequently, approximately 50 mg of powdered hair from each sample was extracted overnight with methanol. Extracts were evaporated, reconstituted in assay buffer, and analyzed in duplicate for cortisol by enzyme immunoassay (Salimetrics, State College, PA). The intraassay coefficient of variation was 1.9% and the interassay coefficient of variation was 6.9%.
Data analysis.
Data were analyzed using Systat Software (Systat Software, San Jose, CA). Because the collection of hair samples was spread across 6 mo, we first determined whether there was a relationship between hair cortisol and time of year (by converting dates to months: 1, January; 2, February; and so forth). Hair cortisol concentrations were unrelated to time of year (r = 0.09, P = 0.38); therefore this variable was not considered further. Relatively equal numbers of male and female macaques with and without alopecia were sampled in each month.
Facility C had a low number of male macaques (n = 2), and as a result, hair cortisol data initially were analyzed for female macaques only by ANOVA, with facility (A, B, C) and alopecia status (yes, no) as between-subjects variables and body weight (measured during the health exam) and age as covariates. An ANOVA was also used to examine potential differences in total hair loss at each facility. The hair cortisol data from facilities A and B were then examined for sex differences using ANOVA with facility (A, B), alopecia status (Y, N) and sex (M, F) as between subjects variables with body weight and age as significant covariates. Finally, Pearson correlations were used to examine the relationship between hair cortisol levels and the digitized alopecia scores in all monkeys and for each sex. To make sure that these correlations were not accounted for solely by the inclusion of monkeys without hair loss, these correlations were rerun with only the monkeys from the hair loss group.
Results
Three-facility comparison (female rhesus macaques).
Hair cortisol varied as a function of alopecia status (yes or no) and facility, and these findings were unaffected by the body weight or age of the monkeys. Overall, monkeys with hair loss showed higher levels of hair cortisol than did fully haired monkeys (F = 22.51, P < 0.001; 72.4 ± 6.1 pg/mg compared with 54.4 ± 2.4 pg/mg, respectively; yielding a moderate to large effect size (Cohen d = 0.72, r = 0.34)). Female monkeys at facility A showed higher hair cortisol levels overall compared with the monkeys at facilities B and C (F = 12.81, P < 0.01). However, these effects are best explained by the significant interaction of facility and alopecia status (F = 11.05, P < 0.01) such that the hair cortisol differential between alopecic and normally haired monkeys was highly significant at facility A (large effect size, Cohen d = 2.1, r = 0.72), significant at facility B (medium to large effect size, Cohen d = 0.82, r = 0.38), and not significant at facility C (Figure 2). In addition, there was a significant difference in the average amount of hair loss at each facility (F = 7.73; P < 0.01), with monkeys showing greater overall hair loss at facility A compared with facilities B and C. Hair cortisol levels were highly correlated with the amount of hair loss in female macaques overall (r = 0.82, P < 0.001). This effect could not be accounted for by the inclusion of the control monkeys, because a strong correlation also existed independently in the alopecic female monkeys (r = 0.84, P < 0.001). In a separate analysis, we examined the influence of housing on alopecia and hair cortisol. Hair loss was present in monkeys in all 3 housing situations (individual, paired, and grooming contact). However, hair cortisol was significantly higher in monkeys housed in grooming contact as compared with those housed either individually or in pairs (F = 5.27, P < 0.01; mean ± SEM: grooming contact, 88.30 ± 18.85 pg/mg; individual, 57.67 ± 2.82 pg/mg; paired, 66.74 ± 4.58 pg/mg). These differences were unrelated to alopecia status.
Figure 2.
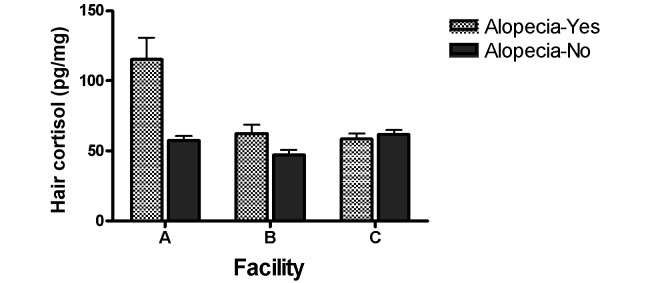
Hair cortisol in female rhesus monkeys as a function of facility.
Two-facility comparison (male and female macaques).
To examine the relationship between hair loss and hair cortisol in monkeys of both sexes, the analysis was restricted to facilities A and B. Once again, hair cortisol varied as a function of alopecia status and facility, and these findings were unaffected by the body weight or age of the monkeys. As reported in the previous section, monkeys with hair loss showed higher levels of hair cortisol than did their respective counterparts (F = 13.98, P < 0.01), and macaques at facility A showed higher cortisol levels than did those at facility B (F = 12.03, P < 0.01). Although the sex-associated effect did not reach statistical significance (P = 0.08), the difference was in the expected direction, with female macaques showing higher hair cortisol levels those of than male macaques. All of the 2-way interaction terms were significant. An interaction of facility and alopecia status (F = 9.97, P < 0.01) revealed that monkeys with alopecia at facility A had significantly elevated hair cortisol compared with all other groups. In addition, 2 significant interactions involving sex and facility (F = 6.39, P < 0.01) and sex and alopecia status (F = 11.23, P < 0.01) showed that this effect was primarily observed in female macaques at facility A (Figure 3). An examination of the relationship between hair cortisol and total amount of hair loss again revealed a high positive correlation across all monkeys (r = 0.71, P < 0.001). In addition, a positive correlation between hair cortisol and total amount of hair loss was noted for female (r = 0.84, P < 0.001) but not male (r = 0.31, P = 0.12) monkeys. The positive correlation between hair cortisol and amount of hair loss in female macaques was not the result of the inclusion of fully haired monkeys, because a positive correlation existed for alopecic female monkeys also (r = 0.83, P < 0.001). In fact, the exclusion of control males resulted in a positive correlation between hair loss and hair cortisol in male alopecic monkeys (r = 0.66, P = 0.02).
Figure 3.
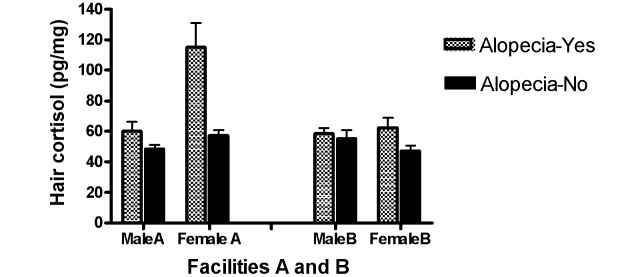
Hair cortisol in male and female rhesus monkeys housed at facilities A and B.
Patterns of hair loss.
Hair loss was relatively equally distributed across the left side, right side, and back, yielding high correlations (that is, greater than 90%) for all 3 positions (left compared with right, r = 0.96, P < 0.001; left compared with prone, r = 0.97, P < 0.001; right compared with prone, r = 0.95, P < 0.001). In addition some monkeys showed unusual patterns of hair loss. The particular monkey shown in Figure 1 had generalized hair loss along the back, with 2 unique patterns, one on each arm. The left upper arm contained a square pattern with clearly defined edges and hair in the center, and the right upper arm contained a W-shaped pattern with clear edges. In fact, these patterns conformed exactly to the size of the cage mesh against which this animal typically rested and may represent very mild, friction-induced hair loss. We subsequently examined all the monkeys in this study for the presence of clearly defined straight lines, edges, and squares. Of the 99 monkeys examined, 18 showed clear evidence of cage mesh patterns. The presence of cage mesh patterns was noted in all 3 facilities and in both sexes (10 male, 8 female). Of these 18 monkeys, 11 showed no other examples of hair loss and previously had been classified as having no alopecia (less than 5% hair loss). The remaining 7 monkeys, like the monkey in Figure 1, showed both friction-induced and presumably other patterns of hair loss.
Discussion
This study provides strong—but not universal—evidence of an association between the HPA axis and hair loss in rhesus monkeys without an apparent hair-plucking disorder. Rhesus macaques with considerable hair loss showed chronically elevated concentrations of cortisol as measured in hair. This relationship was unrelated to the month in which the sample was collected, the age of the monkey, or its body weight. However, this pattern was not observed in all facilities examined. In facility C, hair loss was not associated with increased elevations of hair cortisol. This differential finding reinforces the notion that alopecia is a multietiologic phenomenon and cautions against widespread generalizations.
The reason for this facility effect remains elusive. The largest effect was observed at facility A, where individual monkeys had the highest levels of total body hair loss. But this effect was also present in the monkeys at facility B, where hair loss was more intermediate and similar to that of the monkeys maintained at facility C. Potential contributing factors include differences in the genetic backgrounds in the monkeys housed at these facilities, rearing and housing procedures, the type of research being conducted, and total length of time spent in the facility. Because animals transfer in and out of facilities and in and out of research projects, these variables can be challenging to track systematically in relation to hair loss.
Although our data provide clear evidence of a relationship between HPA axis activity and hair loss in some monkeys, the demonstration of an association is not proof of a causal relationship—herein lies the cautionary tale. Some persons might suppose that elevated levels of hair cortisol occurred first, perhaps in response to environmental stress and led to the hair loss; however, it is equally plausible that hair loss occurred first and then led to elevations in hair cortisol, perhaps as a result of alterations in thermoregulation.5 Another relevant issue concerns the source of the cortisol measured in the hair samples. Although diffusion from the circulation is thought to be the major route of incorporation of cortisol into hair,22 local synthesis within the hair follicle has been demonstrated.12 Moreover, the skin is responsive to both local and psychogenic stressors,8 thus complicating the interpretation of the observed relationship between alopecia and hair cortisol levels. Finally, both type 1 and type 2 11β-hydroxysteroid dehydrogenases—enzymes that interconvert cortisol and the inactive metabolite cortisone—have been detected in several types of skin cells.27,31 Accordingly, hair cortisol concentrations may be regulated, in part, by the local activity of these enzymes.
Given the lack of directional information between alopecia and hair cortisol, perhaps an experimental procedure could be used to establish the exact nature of this relationship in monkeys. Indeed, in rats and mice, hair loss has been studied by applying experimental stressors by using a depilated model. However, this approach is problematic inasmuch as the rapid removal of hair might be stressful to monkeys in ways unrelated to various environmental stressors (for example, the response of other monkeys to it, changes in skin sensitivity). Furthermore, rapid depilation does not mirror the typical time course of hair loss in monkeys. For example, we documented hair loss in an adult female macaque that was separated physically but not visually from her adult female partner after a severe aggressive bout. The female macaques had been paired for 8 y. During the next 8 wk of physical separation, the monkeys recovered, and modifications were made to their pen environment to enable more rapid separation. At the end of the second week, one female macaque (aggressor) showed mild bilateral hair loss on both upper and lower legs that became worse as the weeks progressed. During the fourth week, the hair loss extended to the area of her lower lumbar spine and gradually rose to the lower thoracic spine during the seventh week. Upon successful reunion with her partner, no further hair loss was observed, and she regained all her hair by 6 wk later. This anecdote demonstrates that hair loss, possibly stress-related, can occur rapidly, but not within a very short period of time (for example, a day). This experience also draws attention to the fact that hair loss frequently is episodic, waxing and waning over time and exposure to events. Therefore, more specific questions can be raised such as: 1) do hair cortisol concentrations decline as hair grows back? and 2) can specific events be identified retrospectively that precede the onset of hair loss and the elevation in hair cortisol?
The results of the current study also show that hair loss is not synonymous with hair-plucking behavior. We exerted extensive efforts to eliminate hair-pluckers in advance of study initiation. Hair pluckers, identified through a part of each facility's behavioral assessment program, were excluded as potential subjects (this population was quite small, see reference 19). Videotapes of the subjects selected for this study were examined for hair plucking behavior during the Human Intruder Test. No additional animal was excluded from this study on the basis of this screening. Finally, our photographs showed a very high correlation of hair loss in individual monkeys across all 3 orientations. Although monkeys might show bilateral hair-plucking of arms and legs, it is difficult to imagine that monkeys would also pull the same amount of hair from various parts of the back, some areas of which would be very difficult to reach. These steps do not absolutely guarantee that hair plucking was absent in the population studied, but they make it unlikely.
In this study, we uncovered unique patterns of hair loss that are best explained as created by the pressure associated with resting against surfaces (for example, cage mesh). Such friction-induced hair loss has been observed in humans wearing orthodontic head gear18 and in nurses wearing caps pinned to their hair.9 In the monkeys showing these precise patterns of hair loss, the patterns contributed little to the sum of the total body hair loss and generally were restricted to one side of the outer arms or legs. Whether simple friction is also responsible for more amorphous patches of hair loss is difficult to determine.
Our data indicate a relationship between alopecia and glucocorticoid concentrations in hair in some rhesus macaques. This study did not identify the basis for this relationship. Delineating the mechanisms that underlie this relationship is an important future aim that will depend on extensive retrospective analyses of husbandry practices and of colony and health records across 3 facilities.
Acknowledgments
This research is supported by grant nos. R24OD01180-15 (to MN), P51OD011133 to Texas Biomedical Research Institute (SNPRC), 8P51OD011092-53 to the Oregon National Primate Research Center (ONPRC), P51OD010425 to the Washington National Primate Research Center (WaNPRC), and P51OD011103 to the New England Primate Research Center (NEPRC). We thank the technicians who assisted in data collection and analysis: Kim Linsenbardt (SNPRC), Nicola D Robertson (ONPRC), and Grace Lee and Rose Kroeker (WaNPRC). We thank Dr David Arnold (UMass) for assistance with the statistical analysis.
References
- 1.Aoki E, Shibasaki T, Kawana S. 2003. Intermittent foot-shock stress prolongs the telogen stage in the hair cycle of mice. Exp Dermatol 12:371–377 [DOI] [PubMed] [Google Scholar]
- 2.Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry 63:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport MD, Tiefenbacher ST, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261 [DOI] [PubMed] [Google Scholar]
- 4.Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. 2010. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology 35:1404–1409 [DOI] [PubMed] [Google Scholar]
- 5.Djordjević J, Cvijić G, Davidović V. 2003. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol Res 52:67–72 [PubMed] [Google Scholar]
- 6.Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. 2011. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology 36:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-de-la-Vara Mdel R, Valdez RA, Lemus-Ramirez V, Vázquez-Chagoyán JC, Villa-Godoy A, Romano MC. 2011. Effects of adrenocorticotropic hormone challenge and age on hair cortisol concentrations in dairy cattle. Can J Vet Res 75:216–221 [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. 2012. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis. Dermatol Res Pract 2012:403908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SM, Lee WS, Choi EH, Lee SH, Ahn SK. 1999. Nurse's cap alopecia. Int J Dermatol 38:187–191 [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 11.Isbell LA. 1995. Seasonal and social correlates of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya. Am J Primatol 36:61–70 [DOI] [PubMed] [Google Scholar]
- 12.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. 2005. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal axis and synthesize cortisol. FASEB J 19:1332–1334 [DOI] [PubMed] [Google Scholar]
- 13.Katayama M, Aoki E, Suzuki H, Kawana S. 2007. Foot-shock stress prolongs the telogen stage of the spontaneous hair cycle in a nondepilated mouse model. Exp Dermatol 16:553–560 [DOI] [PubMed] [Google Scholar]
- 14.Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K. 2010. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. J Med Primatol 39:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroeker R, Bellanca RU, Lee GH, Thom JP, Worlein JM. 2014. Alopecia in 3 macaque species housed in a laboratory environment. Am J Primatol 76:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lair S, Crawshaw GJ, Mehren KG, Perrone MA. 1999. Diagnosis of hypothyroidism in a Western lowland gorilla (Gorilla gorilla gorilla) using human thyroid-stimulating hormone assay. J Zoo Wildl Med 30:537–540 [PubMed] [Google Scholar]
- 17.Laudenslager ML, Natvig C, Corcoran CA, Blevins MW, Pierre PJ, Bennett AJ. 2013. The influences of perinatal challenge persist into the adolescent period in socially housed bonnet macaques (Macaca radiata). Dev Psychobiol 55:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonardi R, Lombardo C, Loreto C, Caltabiano R. 2008. Pressure alopecia from orthodontic headgear. Am J Orthod Dentofacial Orthop 134:456–458 [DOI] [PubMed] [Google Scholar]
- 19.Lutz CK, Coleman K, Worlein J, Novak MA. 2013. Hair loss and hair pulling in rhesus macaques. J Am Assoc Lab Anim Sci 52:454–457 [PMC free article] [PubMed] [Google Scholar]
- 20.Malinow MR, Bardana EJ, Pirofsky B, Craig S, McLaughlin P. 1982. Systemic lupus erythematosus-like syndrome in monkeys fed alfalfa sprouts: role of a nonprotein amino acid. Science 216:415–417 [DOI] [PubMed] [Google Scholar]
- 21.Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. 2011. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab 96:E1862–E1865 [DOI] [PubMed] [Google Scholar]
- 22.Meyer JS, Novak MA. 2012. Minireview: hair cortisol: a novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 153:4120–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak MA, Kelly BJ, Bayne K, Meyer JS. 2012. Behavioral pathologies, p 177–196 In: Abee CR, Mansfield K, Tardif SD, Morris T. Nonhuman primates in biomedical disease. New York (NY): Elsevier [Google Scholar]
- 24.Novak MA, Meyer JM. 2009. Alopecia: possible causes and treatments with an emphasis on captive nonhuman primates. Comp Med 59:18–26 [PMC free article] [PubMed] [Google Scholar]
- 25.Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. 2011. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress 14:73–81 [DOI] [PubMed] [Google Scholar]
- 26.Sawyer LS, Moon-Fanelli AA, Dodman NH. 1999. Psychogenic alopecia in cats: 11 cases (1993–1996). J Am Vet Med Assoc 214:71–74 [PubMed] [Google Scholar]
- 27.Smith RE, Maguire JA, Stein-Oakley AN, Sasano H, Takahashi K-I, Fukushima K, Krozowski ZS. 1996. Localization of 11β-hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab 81:3244–3248 [DOI] [PubMed] [Google Scholar]
- 28.Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. 2010. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol 85:357–360 [DOI] [PubMed] [Google Scholar]
- 29.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup FJ. 2006. Coat condition, housing condition and measurement of faecal cortisol metabolites—a noninvasive study about alopecia in captive rhesus macaques (Macaca mulatta). J Med Primatol 35:3–11 [DOI] [PubMed] [Google Scholar]
- 30.Swenerton H, Hurley LS. 1980. Zinc deficiency in rhesus and bonnet monkeys, including effects on reproduction. J Nutr 110:575–583 [DOI] [PubMed] [Google Scholar]
- 31.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. 2011. Localization, age- and site-dependent expression, and regulation of 11β-hydroxysteroid dehydrogenase type I in skin. J Invest Dermatol 131:30–36 [DOI] [PubMed] [Google Scholar]
- 32.Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. 2008. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–488 [DOI] [PubMed] [Google Scholar]
- 33.Wikramanayake TC, Alvarez-Connelly E, Simon J, Mauro LM, Guzman J, Elgart G, Schachner LA, Chen J, Plano LR, Jimenez JJ. 2010. Heat treatment increases the incidence of alopecia aureate in the C3H/HeJ mouse model. Cell Stress Chaperones 15:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. 2007. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology 92:42–49 [DOI] [PubMed] [Google Scholar]