Abstract
Pancuronium is a long-duration neuromuscular blocking drug (NMBD) that has been used in anesthetized rabbits at 0.1 mg/kg. However, there are limited data regarding the time course for recovery from this dose either spontaneously or with pharmacologic reversal. Here we defined the potency, onset, and recovery characteristics for the intermediate-duration NMBD cisatracurium and CW002 (a novel cysteine-inactivated molecule) in the rabbit, and test the hypothesis that these drugs may be alternatives to 0.1 mg/kg pancuronium for survival procedures. New Zealand white rabbits anesthetized with isoflurane were studied in a cross-over design. Potencies of cisatracurium and CW002 were defined as the effective dose for 95% depression of evoked muscle twitch (ED95). Responses to 3×ED95 were used to define onset (time to maximal effect), recovery index (RI; time from 25% to 75% recovery of twitch), and duration (time to complete recovery). Responses to all drugs were determined with and without reversal by neostigmine–glycopyrrolate or l-cysteine. CW002 was 4-fold more potent than was cisatracurium, but their onset, RI, and duration were similar. Pancuronium had similar onset and RI but longer duration, compared with cisatracurium and CW002. Reversal shortened the recovery index and duration for all 3 drugs. At 3×ED95, cisatracurium and CW002 had the same onset as did standard-dose pancuronium, but durations were shorter and more predictable. In addition, CW002 can be reversed without the potential side effects of cholinergic manipulation. We conclude that cisatracurium and CW002 are viable alternatives to pancuronium for survival studies in rabbits.
Abbreviations: ED, effective dose; NMBD, neuromuscular blocking drug; RI, recovery index
Neuromuscular blocking drugs (NMBD) are frequently administered during surgery to provide profound muscle relaxation that cannot be achieved with general anesthesia alone. Although new NMBD have been introduced into human clinical practice over the last 20 y, surgical protocols involving many animal species continue to use older NMBD, largely because of tradition and a lack of knowledge concerning the potency and pharmacodynamics of new agents. Optimal pharmacologic management during and after experimental surgery in animal models is particularly important for both animal welfare and study outcomes.
Pancuronium bromide, a long-acting, nondepolarizing NMBD first synthesized in 1964,4 continues to be used in laboratory animals including rabbits.20,40,42 Beginning in the 1980s, a pancuronium dose of 0.1 mg/kg was reported for rabbits and has persisted over time.12,13,16,18,30,42 However, there are limited data defining the time course for complete recovery from this dose, either spontaneously or after pharmacologic reversal. In human medicine, intermediate-duration nondepolarizing neuromuscular blockers such as the benzylisoquinolinium compound cisatracurium besylate are now preferred, largely due to the potential for residual postoperative muscle weakness after pancuronium.7,37 To date, the relative potency, onset time, duration, and reversal characteristics for cisatracurium in rabbits remain unclear.
Over the last 10 y, a new class of neuromuscular blockers has been developed that is inactivated by interaction with the endogenous amino acid l-cysteine. The first in this series was gantacurium, an asymmetric benzylisoquinolinium α-chlorofumarate with an ultrashort duration due to very rapid molecular inactivation by endogenous l-cysteine.6,25,33 More recently, CW002, a nonhalogenated, symmetrical, benzylisoquinolinium fumarate, was developed to interact more slowly with l-cysteine,15,25 and provide an intermediate duration (approximately 40 min). Importantly, research has now shown that CW002 can be reversed at any time by injection of exogenous l-cysteine, a process that has been termed ‘facilitated molecular inactivation.’38 Functionally, cysteine binds to a portion of the CW002 molecule to produce a conformational change that dramatically decreases affinity for nicotinic receptors at the motor endplate.33 This modification is irreversible, and the CW002–cysteine adduction product is metabolized. From the clinical perspective, the ability to facilitate molecular inactivation of CW002 by injecting cysteine, an endogenously produced substance with no capacity for hypersensitivity reactions,15 at any time allows clinicians to tailor the duration of muscle relaxation to clinical need: a single dose can last 4 min or 40 min. In addition, unlike with most conventional NMBD, even complete paralysis due to CW002 can be reversed rapidly, and this process does not require the use of conventional anticholinesterase or antimuscarinic drugs, thus avoiding their potential side effects.24,25,33 To date, the potency and duration of CW002 in rabbits are unknown. We hypothesized that both cisatracurium and CW002 would have recovery profiles that are preferable to ‘standard-dose’ (0.1 mg/kg) pancuronium, rendering them viable alternatives for use in rabbits undergoing survival procedures. To test this hypothesis, we first defined the potencies of cisatracurium and CW002 in rabbits. Subsequently, the spontaneous and reversed recovery profiles for each drug given at clinically relevant doses were compared with those for the standard pancuronium dose.
Materials and Methods
Animals.
SPF male New Zealand White rabbits (n = 10; weight, 3.0 to 4.0 kg; Crlc:KBL(NZW) Charles River Laboratories, Saint-Constant, Quebec) were used. Rabbits were maintained in accordance with the Guide for the Care and Use of Laboratory Animals17 in an AAALAC-accredited facility. All procedures in the study were approved by the Weill Cornell Medical College IACUC. Rabbits were housed individually in stainless steel mobile 6-cage units (4.17 ft2 in each cage; Lock Solutions, Laurence Harbor, NJ) containing removable plastic (GE Noryl, Selkirk, NY) sleeves with a perforated floor and removable excreta pans. Pans were lined with cage board (Techboard, Shepherd Specialty Products, Somerville, NJ) and were changed 5 times each week; cage sleeves and the rack were changed every 2 wk. The rabbits received a commercial high-fiber pelleted laboratory diet (Purina Hi-Fiber Rabbit Diet, Purina Mills, St Louis, MO) ad libitum and were maintained on municipal water in glass bottles with rubber stoppers and stainless-steel sippers. Rabbits were housed at 68 ± 2 °F (20 ± 1 °C) at 30% to 70% relative humidity on a 12:12-h light:dark cycle (lights on, 0600 to 1800). Environmental enrichment included Western timothy hay (Oxbow Animal Health, Murdock, NE) daily, weekly toy rotations, and food enrichment 3 times each week.
Drugs and solutions.
CW002 (Cedarburg Hauser Pharmaceuticals, Grafton, WI) was diluted with sterile saline (0.9% Sodium Chloride Injection, Baxter Healthcare, Deerfield, IL). l-Cysteine hydrochloride (Sigma–Aldrich, St Louis, MO) was prepared by dissolving the drug in sterile saline to a concentration of approximately 100 mg/mL and buffering with sodium hydroxide to pH 4.5 to 5.5. CW002 and l-cysteine hydrochloride were administered by using 0.2-μm syringe filters (Pall, Port Washington, NY). Anesthetics used were ketamine HCl (Ketathesia, Butler Animal Health Supply, Dublin, OH), xylazine (AnaSed, Lloyd Laboratories, Shenandoah, IA), and isoflurane (Isothesia, Butler Schein Animal Health). Other NMBD used included cisatracurium besylate (Nimbex Injection, Abbott Laboratories, North Chicago, IL) and pancuronium bromide (Pavulon, Teva Pharmaceuticals, Sellersville, PA). Neostigmine methylsulfate injection (APP Pharmaceuticals, Schaumburg, IL) with glycopyrrolate injection solution (Baxter Healthcare) were used to reverse cisatracurium and pancuronium.
Anesthesia and monitoring.
All rabbits were anesthetized by using ketamine (35 mg/kg IM) and xylazine (5 mg/kg IM), and catheters were inserted in an auricular vein and artery. The trachea was intubated and the lungs mechanically ventilated (Ohmeda, Madison, WI) with a mixture of approximately 30% oxygen, approximately 70% nitrogen, and 1.8% to 2.0% isoflurane. Inhaled and exhaled gas concentrations were quantified by infrared analysis (Datex Ultima, Helsinki, Finland), and side-stream spirometry was used to monitor inspiratory pressures, volumes, and flow rates. Systemic arterial oxygen saturation was monitored continuously, and end-tidal carbon dioxide was maintained at approximately 32 to 35 mm Hg. Fluid deficits and maintenance needs (7 to 10 mL/kg IV hourly) were provided by using normal 0.9% saline solution throughout the procedure. Body temperature was measured with a rectal probe and maintained at 37° to 38°C with an underbody air-circulating heating blanket (Bair hugger, Arizant Healthcare, Eden Prairie, MN). The electrocardiogram, heart rate, and direct arterial blood pressure were recorded continuously to data acquisition software (Iox2, EMKA Technologies, Falls Church, VA).
Neuromuscular monitoring.
Neuromuscular function was measured by hindlimb mechanomyography. A fine-needle electrode was inserted through the skin over the common peroneal nerve, which was electrically stimulated (Innervator 252 peripheral nerve stimulator, Fisher and Paykel Electronics, Auckland, NZ) to elicit twitches of the left cranial tibial muscle. The left hindlimb was stabilized securely, and a superficial segment of the cranial tibial muscle tendon palpated during nerve stimulation to produce movement. The tendon then was encircled with a 2-0 silk suture (Ethicon, San Lorenzo, PR) passed percutaneously and attached to an isometric force transducer (FT10 C, Grass Instruments, Quincy, MA). Tension on the suture was increased until maximal force in response to nerve stimulation was achieved. This preload then was maintained and measured continuously as the resting tension between stimuli. A train-of-4 stimulation pattern of 4 supramaximal stimuli (0.15 Hz; duration, 0.2 msec) delivered every 500 msec at 12-s intervals was applied. This pattern provides a sensitive index of neuromuscular blockade and residual weakness,2,3 with repetitive nerve stimulation (4 twitches, twitch 1 [T1] through twitch 4 [T4]) causing a progressive decrease in the amount of acetylcholine released from the motor nerve endings.1 Consequently, this decrease in acetylcholine indicates a progressive decrease in the size of the endplate potential, which is reflected in the ratio of the amplitude of the fourth (T4) response to that of the first (T1).1 Before the procedures were initiated, a 15- to 20-min stabilization period occurred.
Determination of potency.
Each rabbit was studied 4 times (once each week), with the drug order varied. Potency of CW002 and cisatracurium for producing neuromuscular blockade was assessed by bolus administration of multiple intravenous doses starting at 0.005 and 0.015 mg/kg, respectively (Figure 1). After full recovery of twitch amplitude and verification of normal train-of-4 stimulation (approximately 40 min), an additional rest period of at least 45 min (4 or more half-lives for both drugs26,33) occurred before injection of the next dose. The response to 3 doses yielding between 5% and 99% neuromuscular blockade, along with the low and high doses that produced 0% or 100% block, respectively, were used to quantify the effective dose for producing 95% blockade (ED95) from a 4-compartment dose response model (SigmaPlot, Systat Software, San Jose, CA). The mean of individual ED95 values for cisatracurium and CW002 then was used as a relative potency index. Due to its long duration and the availability of historical data, the potency of pancuronium was not determined.
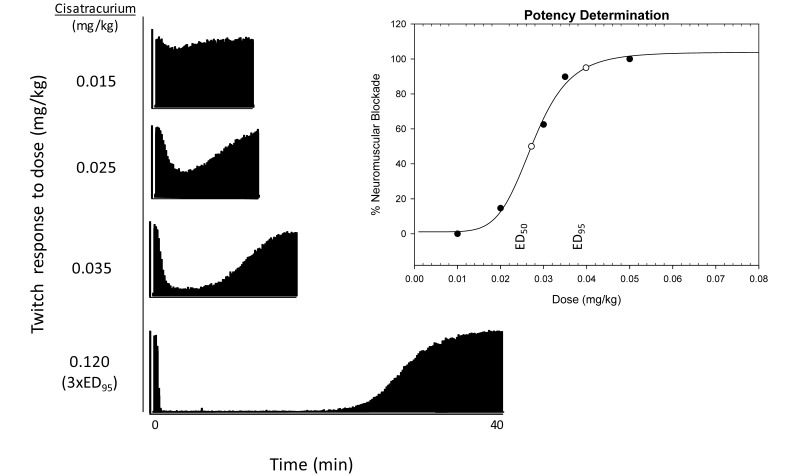
Figure 1.
Representative mechanomyographic tracings showing the cisatracurium dose–response data used to derive an ED95 of 0.037 ± 0.002 mg/kg. The bottom panel depicts the response to a 3×ED95 dose. Potency then was determined by using this dose–response model, from which 100% was estimated from twice the dose response and 0% is estimated from 1/5 of the dose response. The closed circles indicate the different doses administered to determine potency; open circles represent the doses estimated by the dose–response model; and ED50 and ED95 represent the effective doses of NMBD required to reduce twitch amplitude by 50% and 95%, respectively.
Determination of recovery.
After potency determination, in a subsequent experiment, rabbits received a dose of 3×ED95, a common clinical dose previously used in pharmacodynamic studies of CW002 and cisatracurium.8,15,32 The onset (time to complete loss of T1), percentage of T1 recovery (Figure 2, recovery profile of the first twitch in train-of-4 stimulation), recovery index (RI; time between 25% and 75% T1 recovery), and duration (time to T4 amplitude ≥ 90% of T1 amplitude) were all noted. These parameters were defined for the ‘standard’ pancuronium dose (0.1 mg/kg).
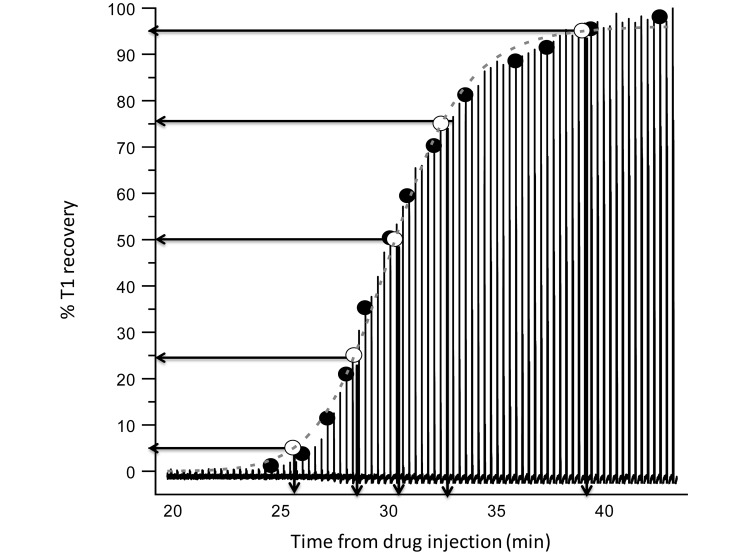
Figure 2.
Representative analysis of twitch recovery profile. Amplitude of T1 was measured directly at regular intervals (closed circles), and resulting data were used to generate a best-fit equation (dashed line) from which 5%, 25%, 50%, 75%, and 95% recovery points (open circles) were derived.
Evaluation of reversal characteristics.
During separate experiments, the same 3×ED95 dose of cisatracurium or 0.1 mg/kg of pancuronium was administered; when there was a 5% recovery of T1 amplitude (some degree of spontaneous recovery is required prior to effective reversal), neuromuscular blockade was reversed by using previously reported doses of neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg).11,22,36 In the case of CW002, a 3×ED95 dose was administered and then reversed with 30 mg/kg l-cysteine (projected clinical dose33) 5 min after CW002 injection; unlike conventional NMBD, CW002 can be reversed at any time, therefore a 5% T1 recovery threshold was not used.33,38 For each drug, the RI after full reversal was determined for comparison to that measured during spontaneous recovery. After full return of neuromuscular function (10 to 15 min after all 4 twitches returned to equal height), the rabbits were awakened, the trachea extubated, and activity monitored for clinical signs of recurrent muscle weakness over the next 3 h.
Blood pressure and heart rate analysis.
Mean arterial blood pressure and heart rate were recorded continuously in both analog and digital formats. Data were plotted at 2-s intervals, and changes in mean arterial blood pressure and heart rate over the first 5 min after drug administration were quantified by AUC analysis to incorporate both increases and decreases.18,34
Statistics and data analysis.
Potency data between cisatracurium and CW002 was compared by using t tests. Neuromuscular function data were compared between groups by ANOVA (Newman–Keuls test) and within groups (reversal data) by paired t tests. Statistical comparison of changes in heart rate and mean arterial blood pressure produced by individual drugs was based on AUC analysis using ANOVA and the Newman–Keuls test. Data are presented as mean ± SEM, with a P value of 0.05 or less considered significant. For drug duration data, CV were calculated (SD / mean) and used as indexes of variability.
Results
Determination of potency.
On average, CW002 (ED95, 0.01 ± 0.001 mg/kg) was more potent (P < 0.001) than was cisatracurium (ED95, 0.04 ± 0.003 mg/kg; Table 1).
Table 1.
Potency and pharmacodynamics of study drugs administered at 3xED95
| ED95 (mg/kg) | ED50 (mg/kg) | Onset (min) | T1 5% (min) | Recovery index (min) | Duration (min) | |
| Pancuronium | not applicable | not applicable | 1.4 ± 0.3 | 33.4 ± 4.8a | 7.7 ± 0.7 | 55.8 ± 6.4a |
| Cisatracurium | 0.04 ± 0.002 | 0.02 ± 0.002 | 1.5 ± 0.2 | 20.5 ± 1.6 | 5.6 ± 0.8 | 38.4 ± 2.7 |
| CW002 | 0.01 ± 0.001b | 0.006 ± 0.0004b | 1.5 ± 0.1 | 21.0 ± 2.7 | 6.5 ± 0.5 | 39.9 ± 2.5 |
Value for pancuronium significantly (P < 0.05) different from those of cisatracurium and CW002.
Value for CW002 significantly (P < 0.05) different from that for cisatracurium.
Determination of onset and spontaneous recovery.
The pharmacodynamics of clinically relevant doses of cisatracurium and CW002 in comparison to the standard pancuronium dose of 0.1 mg/kg are summarized in Table 1. All 3 drugs had a similar onset time of approximately 1.5 min (P = 0.93), but cisatracurium and CW002 had a faster return of T1% to 5% of the maximal amplitude than did the standard pancuronium dose (P = 0.03). Figure 3 A depicts interval T1 recovery (5%, 25%, 50%, 75%, 95%) after all 3 drugs and shows that the faster return of neuromuscular function after cisatracurium and CW002 relative to the standard dose of pancuronium was maintained at all intervals (all P < 0.03). However, once recovery had begun, RI did not differ (P = 0.12) among the 3 drugs (Table 1). Ultimately, cisatracurium and CW002 both had shorter durations (mean, 38.4 ± 2.7 min; range, 32 to 45 min, CV, 16% and mean, 39.9 ± 2.5 min; range, 34 to 46 min; CV, 14%, respectively) than did the standard pancuronium dose (mean, 55.8 ± 6.4 min; range, 42 to 70 min; CV, 26%; P = 0.02; Table 1).
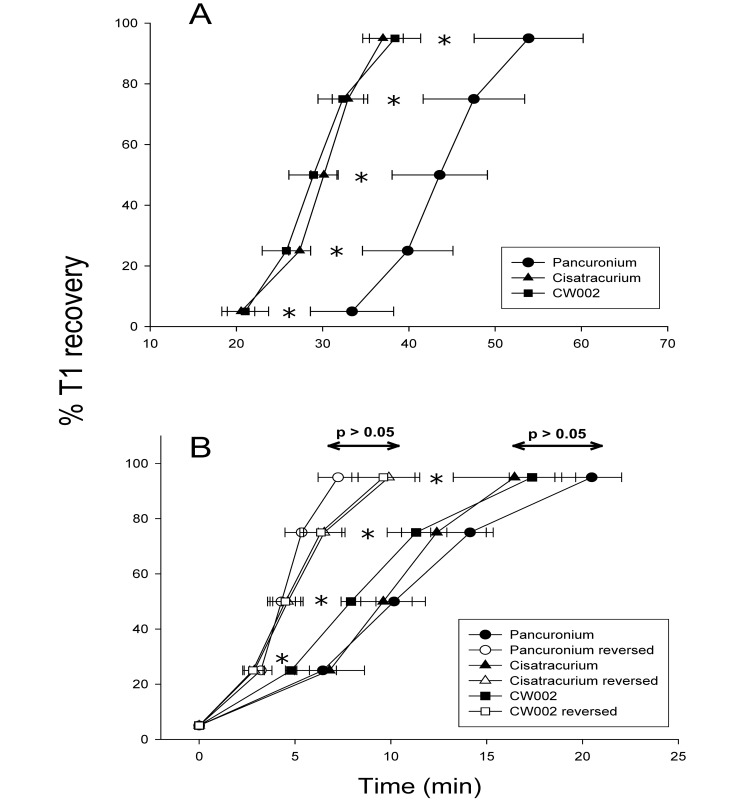
Figure 3.
(A) Comparative recovery profiles for pancuronium (0.1 mg/kg), cisatracurium (0.12 mg/kg), and CW002 (0.03 mg/kg). Cisatracurium and CW002 have faster spontaneous recovery profiles than does pancuronium. (B) Comparison of spontaneous and reversed recovery profiles for pancuronium (0.1 mg/kg), cisatracurium (0.12 mg/kg), and CW002 (0.03 mg/kg) with data normalized to the point of T1 return. For pancuronium and cisatracurium, neostigmine–glycopyrrolate markedly accelerated recovery. l-Cysteine markedly accelerated recovery after CW002. *, Significant (P < 0.05) difference between spontaneous and pharmacologic reversal of the same agent at the same percentage of T1 recovery.
Evaluation of reversal characteristics.
Figure 3 B contrasts spontaneous and reversed recovery profiles for pancuronium, cisatracurium, and CW002. To facilitate comparison, data were normalized to the point of 5% T1 return for spontaneous recovery (designated as 0 on time scale), reflecting the point at which neostigmine–glycopyrrolate or l-cysteine was given. Reversal of pancuronium and cisatracurium with neostigmine–glycopyrrolate markedly accelerated the recovery of T1 amplitude (P ≤ 0.03 for all comparisons). Similarly, reversal of CW002 with l-cysteine also accelerated recovery of T1 amplitude relative to spontaneous recovery (P ≤ 0.01 for all comparisons). With reversal, there were no differences in RI among the study drugs (pancuronium, 2.1 ± 0.4 min; cistaracurium, 2.6 ± 0.7 min; CW002, 3.7 ± 0.6 min; P = 0.19).
Blood pressure and heart rate analysis.
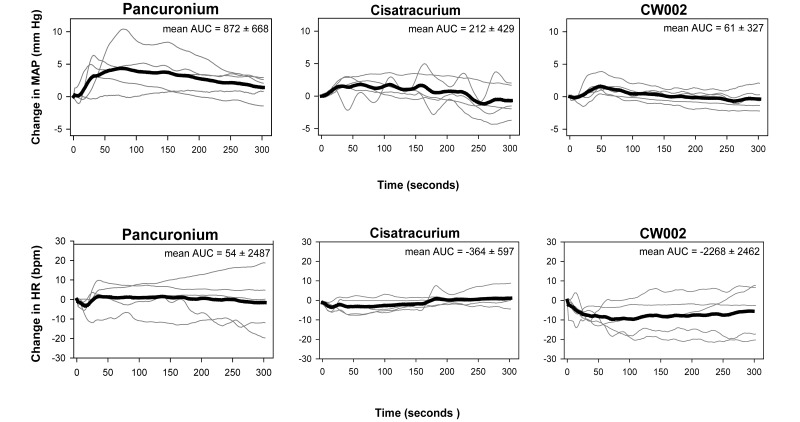
Heart rate and mean arterial pressure were plotted to illustrate both individual and mean responses over the first 5 min after drug injection (Figure 4). This approach indicated considerable variability in the response among individual rabbits, but there were no differences overall in the AUC for either heart rate or mean arterial pressure.
Figure 4.
Heart rate (HR) and mean arterial blood pressure (MAP) analysis for pancuronium (0.1 mg/kg), cisatracurium (0.12 mg/kg), and CW002 (0.03 mg/kg). No significant differences occurred between agents.
Discussion
Results of the study indicate that the conventional drug cisatracurium and the novel compound CW002 are potent NMBD in rabbits, with the same onset as that of a standard pancuronium dose but with shorter durations. Cisatracurium and CW002 both reached the clinical time point for reversal (return of T1 amplitude to 5% of the maximum) significantly faster than did pancuronium. However after pharmacologic reversal, the recovery characteristics for all 3 drugs were accelerated similarly. None of the drugs studied produced significant hemodynamic effects at the doses used.
Although the existing literature suggests that 0.1 mg/kg pancuronium will have a long duration in rabbits, this dose historically has been used for even short procedures without pharmacologic reversal.13,23,29 To our knowledge, the origin of the 0.1 mg/kg dose is unclear but may have been extrapolated from human medicine.12,16 For the current study, we chose to compare newer NMBD with this reported dose because the long duration of action for pancuronium complicates specific determination of potency, and there are ample historical data. Our results confirm that 0.1 mg/kg pancuronium produces relatively long neuromuscular blockade in rabbits but with considerable variability (range, 42 to 70 min; CV, 26%). In contrast, the duration ranges for cisatracurium (32 to 45 min) and CW002 (34 to 46 min) combined with their CV (approximately 15%) at the common clinical dose of 3×ED95 suggest more predictable responses than that to pancuronium.
In human medicine, pancuronium use has become relatively uncommon, having been supplanted by modern intermediate-duration NMBD. In rabbits, the use of cisatracurium35 and vecuronium39 have been described, but to our knowledge, no publications describe the pharmacodynamics of these drugs. In contrast, one group studied the potency of the intermediate NMBD rocuronmium in rabbits.21 These investigators reported an ED50 for rocuronium of 0.056 mg/kg. In keeping with many previous publications,5,14,15,41 the current study used ED95 as a potency index. However, analysis of the data indicates an ED50 for cistatracurium of 0.024 mg/kg, which suggests that the drug is more potent in rabbits than is rocuronium. Although these data need to be interpreted in the context of different background anesthetics (isoflurane in the current study, propofol infusion in the previously cited study21), a previous report indicated only modest anesthetic-induced differences in rocuronium potency.27 Regardless of potency, when these drugs are administered in doses that produce complete neuromuscular blockade, data from the current study suggest that the rate of spontaneous recovery in the rabbit for both cisatracurium (RI, 5.6 ± 0.8 min) and CW002 (RI, 6.5 ± 0.5 min) may be faster than that previously reported for rocuronium (RI, 9.1 ± 2.9 min).21
Results of our study highlight importance of the monitoring and reversal of neuromuscular blockade. Appropriate neuromuscular monitoring is critical for the prevention of postoperative residual weakness and its associated complications.10 Pancuronium, cisatracurium, and CW002 all showed spontaneous recovery profiles that, in the absence of neuromuscular function monitoring, might lead clinicians to under-appreciate residual weakness when only functional indices such as respiratory effort are used. For example, during spontaneous recovery from pancuronium, the average time for 25% to 75% recovery of T1 measured at the hindlimb was nearly 8 min; without neuromuscular function monitoring, diaphragmatic movement during this interval could give the impression of sufficient recovery for spontaneous ventilation and discontinuation of inhaled anesthetic, when in fact the rabbit remains too weak for adequate respiratory function. The resulting hypoventilation then can lead to hypercarbia, acidosis, and potentiation of residual neuromuscular blockade.28 Study data confirm that pharmacologic reversal of residual neuromuscular blockade in rabbits markedly accelerates recovery of full muscle function. Although the initial recovery was, on average, faster for cisatracurium than for pancuronium (time to return of T1 was, on average, 13 min faster), once neostigmine–glycopyrrolate was injected, the rate of recovery was accelerated by 70% or greater for pancuronium and by 50% or greater for cisatracurium.
Although the results of our study appear consistent with the concept that the risk of complications resulting from persistent weakness can be reduced by pharmacologic reversal, in order for neostigmine reversal of conventional NMBD like pancuronium and cisatracurium to be fully effective, some degree of spontaneous recovery has to be present first.22,24,31 As a means to negate this limitation, recent research has focused on alternative methods that will allow for NMBD reversal at any time, even in the setting of complete paralysis. Toward this end, the drug sugammadex has been introduced for reversal of rocuronium.9 A cyclodextrin with multiple binding sites for rocuronium, sugammadex essentially chelates the molecule from the blood, thus rapidly reducing rocuronium concentration at the motor end plate.9 Although the drug is commercially available in Europe, widespread use of sugammadex has been hampered by high cost; sugammadex is not yet approved for sale in the United States. As an alternative to rocuronium–sugammadex, the current study focused on CW002, which is a novel, intermediate-duration NMBD currently in human clinical trials that can be rapidly and inexpensively reversed at any time by using the amino acid cysteine.38 The unique pharmacology of CW002 makes it possible for clinicians to tailor the duration of muscle relaxation to specific clinical needs. The current study confirms that, as with other species, CW002 can be easily reversed by using l-cysteine in rabbits, although their response may be slightly slower than those in other species.33,38 Although study results show the efficacy of l-cysteine reversal in the absence of any spontaneous muscle recovery, the acceleration of RI was not different from that produced by neostigmine for pancuronium and cisatracurium.
The results of our current study need to be interpreted in the context of several limitations. First, unlike those of the other drugs studied, the dose of pancuronium was not based specifically on potency. Accordingly, the pharmacodynamic parameters described for pancuronium need to be considered in this light. However, because the dose used was that widely published for rabbits and because the onset time was the same as that determined for the 3×ED95 doses of the other study drugs, we believe the comparisons are relevant. Second, we cannot say with certainty that the potency and recovery characteristics of the study drugs will be the same in the contexts of different anesthetic techniques. In addition, due to the lack of pharmacokinetic studies in rabbits, the dosing interval used for estimating potency was based on human half-life data for cisatracurium and CW002. Although neuromuscular function had returned to normal for an extended period between doses, there is the possibility that residual—but not pharmacologically active—drug concentrations led to overestimation of potency.
In summary, at 3×ED95, cisatracurium and CW002 have the same onset and RI as does standard-dose pancuronium but shorter and more predictable durations. CW002 is 4-fold more potent than cisatracurium and can be reversed at any time without the potential side-effects of cholinergic manipulation. In addition, when pharmacologically reversed, RI and duration were significantly shortened for all 3 drugs. We conclude that cisatracurium and CW002 are viable alternatives to pancuronium for survival studies in rabbits.
References
- 1.Ali HH, Utting JE, Gray C. 1970. Stimulus frequency in the detection of neuromuscular block in humans. Br J Anaesth 42:967–978 [DOI] [PubMed] [Google Scholar]
- 2.Ali HH, Utting JE, Gray TC. 1971. Quantitative assessment of residual antidepolarizing block. I. Br J Anaesth 43:473–477 [DOI] [PubMed] [Google Scholar]
- 3.Ali HH, Utting JE, Gray TC. 1971. Quantitative assessment of residual antidepolarizing block. II. Br J Anaesth 43:478–485 [DOI] [PubMed] [Google Scholar]
- 4.Baird WL, Reid AM. 1967. The neuromuscular blocking properties of a new steroid compound, pancuronium bromide. A pilot study in man. Br J Anaesth 39:775–780 [DOI] [PubMed] [Google Scholar]
- 5.Belmont MR, Lien CA, Quessy S, Abou-Donia MM, Abalos A, Eppich L, Savarese JJ. 1995. The clinical neuromuscular pharmacology of 51W89 in patients receiving nitrous oxide/opioid/barbiturate anesthesia. Anesthesiology 82:1139–1145 [DOI] [PubMed] [Google Scholar]
- 6.Belmont MR, Lien CA, Tjan J, Bradley E, Stein B, Patel SS, Savarese JJ. 2004. Clinical pharmacology of GW280430A in humans. Anesthesiology 100:768–773 [DOI] [PubMed] [Google Scholar]
- 7.Bevan DR, Smith CE, Donati F. 1988. Postoperative neuromuscular blockade: a comparison between atracurium, vecuronium, and pancuronium. Anesthesiology 69:272–276 [PubMed] [Google Scholar]
- 8.Bluestein LS, Stinson LW, Jr, Lennon RL, Quessy SN, Wilson RM. 1996. Evaluation of cisatracurium, a new neuromuscular blocking agent, for tracheal intubation. Can J Anaesth 43:925–931 [DOI] [PubMed] [Google Scholar]
- 9.Bom A, Bradley M, Cameron K, Clark JK, van Egmond J, Feilden H, MacLean EJ, Muir AW, Palin R, Rees DC, Zhang M-Q. 2002. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl 41:266–270 [DOI] [PubMed] [Google Scholar]
- 10.Brull SJ, Murphy GS. 2010. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg 111:129–140 [DOI] [PubMed] [Google Scholar]
- 11.Cammu G, de Baerdemaeker L, den Blauwen N, de Mey JC, Struys M, Mortier E. 2002. Postoperative residual curarization with cisatracurium and rocuronium infusions. Eur J Anaesthesiol 19:129–134 [DOI] [PubMed] [Google Scholar]
- 12.Flecknell PA. 1996. Special techniques. Laboratory animal anaesthesia: a practical introduction for research workers and technicians. San Diego (CA): Academic Press [Google Scholar]
- 13.Fok TF, al-Essa M, Monkman S, Dolovich M, Girard L, Coates G, Kirpalani H. 1997. Delivery of metered-dose inhaler aerosols to paralyzed and nonparalyzed rabbits. Crit Care Med 25:140–144 [DOI] [PubMed] [Google Scholar]
- 14.Heerdt PM, Kang R, The A, Hashim M, Mook RJ, Jr, Savarese JJ. 2004. Cardiopulmonary effects of the novel neuromuscular blocking drug GW280430A (AV430A) in dogs. Anesthesiology 100:846–851 [DOI] [PubMed] [Google Scholar]
- 15.Heerdt PM, Malhotra JK, Pan BY, Sunaga H, Savarese JJ. 2010. Pharmacodynamics and cardiopulmonary side effects of CW002, a cysteine-reversible neuromuscular blocking drug in dogs. Anesthesiology 112:910–916 [DOI] [PubMed] [Google Scholar]
- 16.Hindman BJ, Funatsu N, Cheng DC, Bolles R, Todd MM, Tinker JH. 1990. Differential effect of oncotic pressure on cerebral and extracerebral water content during cardiopulmonary bypass in rabbits. Anesthesiology 73:951–957 [DOI] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 18.Ivey ES, Morrisey JK. 2000. Therapeutics for rabbits. Vet Clin North Am Exot Anim Pract 3:183–220 [vii.] [DOI] [PubMed] [Google Scholar]
- 19.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. 1981. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34:362–366 [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Bhargava V, Lal HA, Mittal RK. 2011. Variability in the muscle composition of rat esophagus and neural pathway of lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 301:G1014–G1019 [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Shim JC, Jun JH, Lee KH, Chung CW. 1999. Rabbits treated with chronic isepamicin are resistant to mivacurium and rocuronium. Anesth Analg 88:654–658. [DOI] [PubMed] [Google Scholar]
- 22.Kopman AF, Zank LM, Ng J, Neuman GG. 2004. Antagonism of cisatracurium and rocuronium block at a tactile train-of-4 count-of-2: should quantitative assessment of neuromuscular function be mandatory? Anesth Analg 98:102–106 [DOI] [PubMed] [Google Scholar]
- 23.Kraft SA, Larson CP, Jr, Shuer LM, Steinberg GK, Benson GV, Pearl RG. 1990. Effect of hyperglycemia on neuronal changes in a rabbit model of focal cerebral ischemia. Stroke 21:447–450 [DOI] [PubMed] [Google Scholar]
- 24.Lien CA. 2011. Development and potential clinical impairment of ultrashort-acting neuromuscular blocking agents. Br J Anaesth 107 Suppl 1:i60–i71 [DOI] [PubMed] [Google Scholar]
- 25.Lien CA, Savard P, Belmont M, Sunaga H, Savarese JJ. 2009. Fumarates: unique nondepolarizing neuromuscular blocking agents that are antagonized by cysteine. J Crit Care 24:50–57 [DOI] [PubMed] [Google Scholar]
- 26.Lien CA, Schmith VD, Belmont MR, Abalos A, Kisor DF, Savarese JJ. 1996. Pharmacokinetics of cisatracurium in patients receiving nitrous oxide–opioid–barbiturate anesthesia. Anesthesiology 84:300–308 [DOI] [PubMed] [Google Scholar]
- 27.Lowry DW, Mirakhur RK, McCarthy GJ, Carroll MT, McCourt KC. 1998. Neuromuscular effects of rocuronium during sevoflurane, isoflurane, and intravenous anesthesia. Anesth Analg 87:936–940 [DOI] [PubMed] [Google Scholar]
- 28.Miller RD, Roderick LL. 1978. Acid–base balance and neostigmine antagonism of pancuronium neuromuscular blockade. Br J Anaesth 50:317–324 [DOI] [PubMed] [Google Scholar]
- 29.Mills P, Sessler DI, Moseley M, Chew W, Pereira B, James TL, Litt L. 1987. An in vivo 19F nuclear magnetic resonance study of isoflurane elimination from the rabbit brain. Anesthesiology 67:169–173 [DOI] [PubMed] [Google Scholar]
- 30.Muzzin S, Trippenbach T, Baconnier P, Benchetrit G. 1989. Entrainment of the respiratory rhythm by periodic lung inflation during vagal cooling. Respir Physiol 75:157–172 [DOI] [PubMed] [Google Scholar]
- 31.Nielsen HK, May O. 1994. The optimal administration time for neostigmine following atracurium blockade. Kinetics of antagonists. Anaesthesist 43:528–533 [DOI] [PubMed] [Google Scholar]
- 32.Savarese JJ, Lien CA, Belmont MR, Wastila WB. 1997. The clinical pharmacology of new benzylisoquinoline-diester compounds, with special consideration of cisatracurium and mivacurium. Anaesthesist 46:840–849 [DOI] [PubMed] [Google Scholar]
- 33.Savarese JJ, McGilvra JD, Sunaga H, Belmont MR, Van Ornum SG, Savard PM, Heerdt PM. 2010. Rapid chemical antagonism of neuromuscular blockade by l-cysteine adduction to and inactivation of the olefinic (double-bonded) isoquinolinium diester compounds gantacurium (AV430A), CW 002, and CW 011. Anesthesiology 113:58–73 [DOI] [PubMed] [Google Scholar]
- 34.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr. 2003. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88:553–558 [DOI] [PubMed] [Google Scholar]
- 35.Scheufler KM, Thees C, Nadstawek J, Zentner J. 2003. S(+)-ketamine attenuates myogenic motor-evoked potentials at or distal to the spinal α-motoneuron. Anesth Analg 96:238–244 [DOI] [PubMed] [Google Scholar]
- 36.Shorten GD, Ali HH, Goudsouzian NG. 1993. Neostigmine and edrophonium antagonism of moderate neuromuscular block induced by pancuronium or tubocurarine. Br J Anaesth 70:160–162 [DOI] [PubMed] [Google Scholar]
- 37.Sparr HJ, Beaufort TM, Fuchs-Buder T. 2001. Newer neuromuscular blocking agents: how do they compare with established agents? Drugs 61:919–942 [DOI] [PubMed] [Google Scholar]
- 38.Sunaga H, Malhotra JK, Yoon E, Savarese JJ, Heerdt PM. 2010. Cysteine reversal of the novel neuromuscular blocking drug CW002 in dogs: pharmacodynamics, acute cardiovascular effects, and preliminary toxicology. Anesthesiology 112:900–909 [DOI] [PubMed] [Google Scholar]
- 39.Terakawa Y, Ichinohe T, Kaneko Y. 2010. Rocuronium and vecuronium do not affect mandibular bone marrow and masseter muscular blood flow in rabbits. J Oral Maxillofac Surg 68:15–20 [DOI] [PubMed] [Google Scholar]
- 40.Veres-Nyeki KO, Rieben R, Spadavecchia C, Bergadano A. 2012. Pancuronium dose refinement in experimental pigs used in cardiovascular research. Vet Anaesth Analg 39:529–532 [DOI] [PubMed] [Google Scholar]
- 41.Wastila WB, Maehr RB, Turner GL, Hill DA, Phil M, Savarese JJ. 1996. Comparative pharmacology of cisatracurium (51W89), atracurium, and 5 isomers in cats. Anesthesiology 85:169–177 [DOI] [PubMed] [Google Scholar]
- 42.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. 2013. The comparison of spontaneous breathing and muscle paralysis in 2 different severities of experimental lung injury. Crit Care Med 41:536–545 [DOI] [PubMed] [Google Scholar]