Abstract
During the second half of 2013, a total of 26 deaths involving para-methyl-4-methylaminorex (4,4’-DMAR) were reported to the European Monitoring Centre for Drugs and Drug Addiction. While aminorex and 4-methylaminorex (4-MAR) are known psychostimulants, nothing is known about the comparatively new para-methyl analogue. Analytical characterization of two independent samples obtained from online vendors confirmed the presence of the (±)-cis isomer that also appeared to be involved in at least 18 of the 26 deaths. Extensive characterizations included crystal structure analysis, single, tandem and high-resolution mass spectrometry, liquid and gas chromatography and nuclear magnetic resonance spectroscopy. For the work described here, both the (±)-cis and (±)-trans racemates were also synthesized, confirming that the differentiation between these two forms was straight-forward. Monoamine transporter activity was studied using rat brain synaptosomes. (±)-cis-4,4'-DMAR was a potent, efficacious substrate-type releaser at transporters for dopamine, norepinephrine and serotonin with EC50 values of 8.6 ± 1.1 nM (DAT), 26.9 ± 5.9 nM (NET) and 18.5 ± 2.8 nM (SERT), respectively. A comparison with d-amphetamine, aminorex and (±)-cis-4-MAR revealed that activity at SERT varied more than 100-fold across the four drugs, with (±)-cis-4,4’-DMAR exhibiting the highest potency for releasing 5-HT. The potent releasing activity of (±)-cis-4,4’-DMAR at all three monoamine transporters predicts a potential for serious side-effects such as psychotic symptoms, agitation, hyperthermia and cardiovascular stimulation, especially after high-dose exposure or following combination with other psychostimulants.
Keywords: Aminorex, 4-methylaminorex, para-methyl-4-methylaminorex, new psychoactive substances, psychostimulants, internet, monoamine transporters, synaptosomes
Introduction
The global emergence of novel psychoactive drugs of abuse has triggered the need for close monitoring around the world.[1–3] From a European perspective, most of these substances, including so-called “designer drugs,” are classified as "new psychoactive substances" and, following the definition set out by the European Council Decision 2005/387/JHA, are not under international control.[4] Many of the new psychoactive substances appearing in the recreational drug marketplace are not really new, but are analogs of older chemical structures that were identified during the medication discovery process. For example, the development of centrally-active appetite suppressants has been a lucrative area of drug discovery for several decades. Many of the anorexigenic substances identified exhibit psychostimulant properties and had to be removed from clinical use due to adverse effects that included a range of cardiovascular toxicities.[5]
One class of anorexigenic compounds studied in the early 1960s was based on the 5-phenyl-4,5-dihydrooxazol-2-amine (2-amino-5-phenyl oxazoline, aminorex) template structure (Figure 1A). Numerous analogs of aminorex that showed reduced beef broth consumption in rats were prepared and evaluated.[6] The majority of these analogs published by Poos et al.[6,7] retained the primary amine function and a number of modifications of the phenyl ring were also introduced. While some modifications led to significant loss of activity, others were able to retain stimulant-like character when compared to aminorex. Aminorex and its 4-methyl analog 4-methylaminorex (4-MAR) showed anorexigenic effects in rats comparable to d-amphetamine and methamphetamine, but effects of aminorex were longer-lasting.[8] Although aminorex was briefly available in some European countries, case reports of the occurrence of primary pulmonary hypertension led to its removal from the market in the early 1970s.[9,10] Importantly, serotonergic mechanisms were implicated in the development of aminorex-induced primary pulmonary hypertension.[11,12]
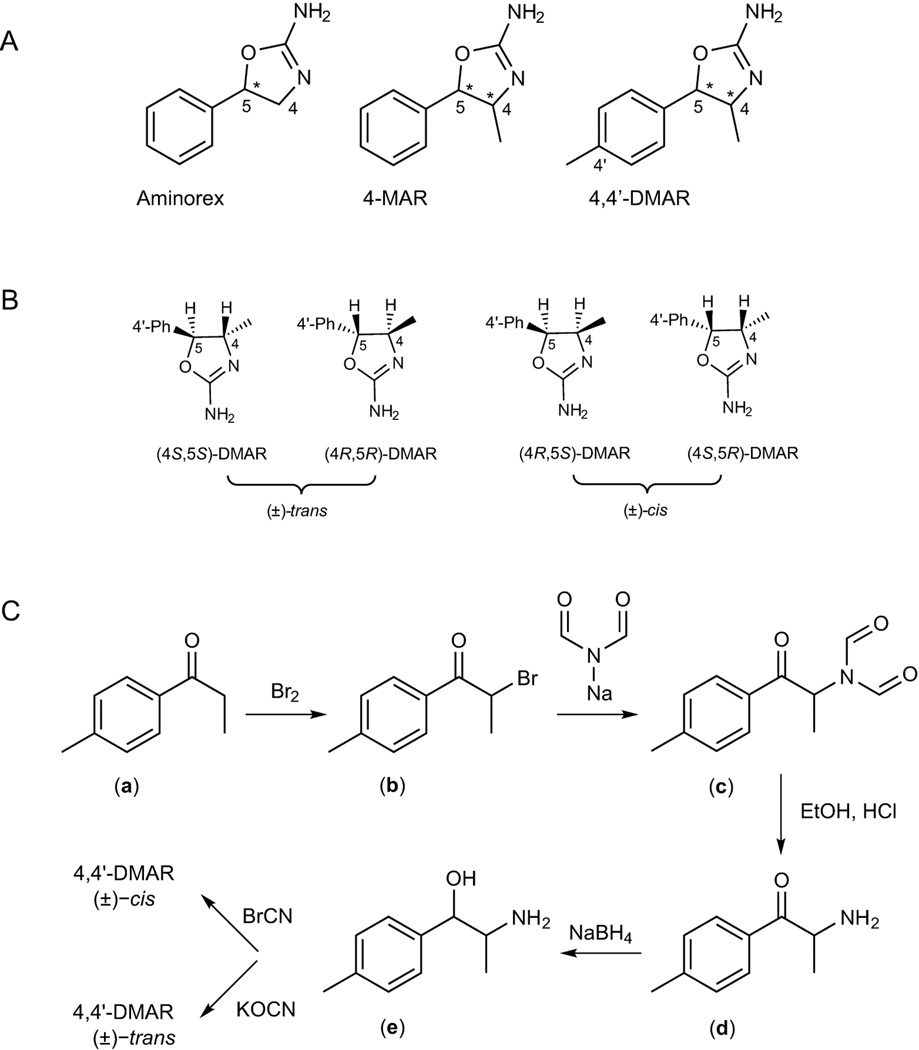
Figure 1.
A: Chemical structures of the three psychostimulants aminorex, 4-methylaminorex (4-MAR) and para-methyl-4-methylaminorex (4,4'-DMAR). B: structural representations of all four existing 4,4'-DMAR enantiomers; 4'-Ph represents the para-substituted phenyl ring. C: synthetic route to both (±)-cis- and (±)-trans-4,4'-DMAR. Both isomers were prepared from the same 4'-methylnorephedrine precursor (e) using cyanogen bromide or potassium cyanate to yield the (±)-cis- and (±)-trans product, respectively.
214×246mm (300 × 300 DPI)
Although aminorex itself has not been frequently reported as a street drug,[13] recent interest in this particular substance has re-emerged following the observation that it is formed in vivo in horses following levamisole administration[14] and in human cocaine users,[15,16] since levamisole is frequently used as a cocaine adulterant.[17] Reports about the detection and characterization of 4-MAR, on the other hand, began to appear following a case report about a fatality in the late 1980s.[18] The presence of two chiral carbons in 4-MAR gives rise to two diastereomeric cis- and trans racemates that have been previously studied.[19–23] The preparation and characterization of the four cis- and trans 3,4-dimethylaminorex enantiomers, i.e. no substituent on the phenyl ring, have also been described.[24]
para-Methyl-4-methylaminorex (4,4'-DMAR) appears to be absent from the currently available scientific literature with the exception of the trans-(4S,5S)-enantiomer (Figure 1B) that was featured in a Japanese patent application on oxa(thia)zolidines.[25] Europol and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) have recently issued a public early-warning notification about the association between 4,4'-DMAR and eighteen deaths in the United Kingdom and eight deaths in Hungary in 2013.[26] The European early-warning system received notifications from Denmark, Finland, Hungary, Sweden and the United Kingdom following its first detection in 2012 in the Netherlands.[26,27] The identification of (±)-cis-4,4'-DMAR and its involvement in the fatalities reported in Northern Ireland were carried out in the authors' laboratories. (±)-cis-4,4'-DMAR was also detected in tablets obtained from a police seizure (‘Speckled Cherry’ and ‘Speckled Cross’ motifs).[28]
An analytical characterization of (±)-cis-4,4'-DMAR HCl obtained from an internet vendor was underway when it was realized that this substance was implicated in the recent fatalities encountered in Northern Ireland. It was then decided to extend the investigation to organic synthesis of both (±)-cis- and (±)-trans racemates in order to establish the potential for unambiguous differentiation under routine analytical conditions (Figure 1C). A very recent test purchase from an alternative online vendor also confirmed that (±)-cis-4,4’-DMAR is available for purchase as a research chemical.
At present, information about the biological mechanism of action of (±)-cis-4,4’-DMAR is not available. Drugs with a similar amphetamine-like structure, such as aminorex, are known to act as substrates for monoamine transporter proteins, thereby releasing monoamine neurotransmitters - dopamine, norepinephrine, and serotonin - in the central nervous system.[11,29] It was therefore deemed important to assess the monoamine transporter activity of (±)-cis-4,4’-DMAR and its closely related analogue (±)-cis-4-MAR. For comparison, d-amphetamine and aminorex, which are substances with known pharmacology, were also included as reference compounds.
Experimental
Materials
All reagents and dry solvents used in the syntheses were obtained from Sigma Aldrich Ltd. (Arklow, Ireland). LC-MS grade solvents were obtained from Fisher Scientific (Dublin, Ireland). d-Amphetamine (amphetamine), aminorex, and (±)-cis-4-MAR were obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program (Rockville, MD, USA). (±)-cis-4,4'-DMAR hydrochloride was obtained via a donation from an Internet vendor (July 2013) and purified by recrystallization. A test purchase from an alternative vendor was also carried out in March 2014.
Synthesis procedures
2-Amino-1-(4-methylphenyl)propan-1-ol (4-methylnorephedrine)
Bromine (5.16 ml, 100 mmol) in dichloromethane (50 ml) was added dropwise to a solution of 4-methylpropiophenone (a) (14.82 g, 100 mmol) in dichloromethane (100 ml). The mixture was then stirred for 1 h at room temperature and dried (anhydrous magnesium sulphate). Following removal of the solvent, α-bromo-4-methylpropiophenone (b) (21.49 g, 94.6 mmol, 95%) remained as yellow crystals. The alpha bromo ketone (20.98 g, 92.4 mmol) was dissolved in acetonitrile (100 ml) and sodium diformylamide (11.01 g, 116 mmol) was added. The mixture was refluxed for 4 h. Following removal of the solvent, the residue was partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulphate) and the solvent was removed to give the N,N– diformylamide derivative (c) (14.26 g, 65.0 mmol, 56%) as a yellow oil. This was dissolved in 5% ethanolic hydrochloric acid (200 ml) and stirred overnight at room temperature. The solution was evaporated to dryness, acetone was added, and the precipitate was collected by filtration. The primary amine intermediate (d) was then used directly without the need for further purification. This was dissolved in methanol (500 ml), cooled in an ice bath and sodium borohydride (26.86 g, 0.71 mol) was added over a 1.5 h period. Removal of the solvent, partitioning between dichloromethane/water, drying (anhydrous magnesium sulphate) and evaporation of the dichloromethane yielded 2-amino-1-(4-methylphenyl)propan-1-ol (e) (6.42 g, 38.9 mmol, 39% from 4-methylpropiophenone) as a colorless solid: m.p. 103–105 °C. 1H NMR (CDCl3) δ 7.23 (d, J = 8.0 Hz, 2 H, Ar H), 7.16 (d, J = 8.0 Hz, 2 H, Ar H), 4.53 (d, J = 4.3 Hz, 1 H, CH(OH)), 3.16–3.18 (m, 1 H, CH(CH3)), 2.35 (s, 3 H, Ar-CH3) and 0.99 (d, J = 6.5 Hz, 3 H, CH(CH3)); 13C NMR (CDCl3) δ 138.19 (Ar C), 136.09 (Ar C), 128.71 (Ar CH), 126.30 (Ar CH), 77.17 (CH(OH)), 51.86 (CH(CH3)), 20.94 (Ar-CH3) and 17.84 (CH(CH3)); HR-ESIMS found 166.1222 (theor. for [M+H]+, C11H16NO, 166.1226).
(±)-cis-4-Methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine ((±)-cis-4,4'-DMAR)
A solution of cyanogen bromide (0.963 g, 9.1 mmol) in methanol (3 ml) was added to a mixture of 2-amino-1-(4-methylphenyl)propan-1-ol (e) (1.36 g, 8.2 mmol) and anhydrous sodium acetate (2.04 g, 24.9 mmol) in methanol (20 ml) with cooling in an ice bath. The mixture was stirred for 3.5 h, the volatiles were removed and saturated sodium carbonate was added to the residue. The mixture was shaken until a white precipitate formed. This was filtered to afford a colorless solid (1.52 g). Purification of 700 mg product was carried out by flash chromatography (dichloromethane:methanol, 8:2) which gave a colorless solid (144 mg, 20% based on (e)): m.p. 136–138 °C. 1H NMR (CDCl3) δ 7.20 (d, J = 7.8 Hz, 2 H, Ar H), 7.12 (d, J = 7.8 Hz, 2 H, Ar H), 5.74 (d, J = 8.7 Hz, H-5), 4.41 (dq, J = 8.7, 6.8 Hz, H-4), 2.38 (s, 3 H, Ar-CH3) and 0.84 (d, J = 6.8 Hz, 3 H, CH3); 13C NMR (CDCl3) δ 160.90 (C-2), 138.30 (Ar C), 131.71 (Ar C), 129.04 (Ar CH), 125.85 (Ar CH), 85.59 (C-5), 59.50 (C-4), 21.07 (Ar-CH3) and 17.59 (CH3); HR-ESIMS found 191.1175 (theor. for [M+H]+, C10H15N2O, 191.1179).
(±)-trans-4-Methyl-5-(4-methylphenyl)-4,5-dihydrooxazol-2-amine ((±)-trans-4,4'-DMAR)
A mixture 2-amino-1-(4-methylphenyl)propan-1-ol hydrochloride (e) (prepared from 830 mg (5 mmol) of the free base and ethereal hydrogen chloride) and potassium cyanate (434 mg, 5.4 mmol) in water (5 ml) was refluxed for 3 h. Aqueous hydrochloric acid (2 M, 5 ml) was then added slowly followed by heating at reflux for an additional 2 h. The mixture was allowed to cool to room temperature and saturated aqueous sodium carbonate (50 ml) was added until a precipitate formed. The solid was collected by filtration to afford a pink powder (746 mg). Purification by flash chromatography (dichloromethane:methanol, 8:2) afforded a colorless solid (119 mg, 0.63 mmol, 13%): m.p. 101–103 °C. 1H NMR (CDCl3) δ 7.23 (m, 4 H, Ar H), 5.08 (d, J = 7.7 Hz, 1 H, H-5), 4.05 (dq, J = 7.7, 6.2 Hz, 1 H, H-4), 2.38 (s, 3 H, Ar-CH3) and 1.40 (d, J = 6.2 Hz, 3 H, CH3); 13C NMR (CDCl3) δ 160.49 (C-2), 139.34 (Ar C), 133.84 (Ar C), 129.76 (Ar CH), 126.31 (Ar CH), 90.25 (C-5), 63.71 (C-4), 21.03 (Ar-CH3) and 20.08 (CH3); HR-ESIMS found 191.1176 (theor. for [M+H+], C10H15N2O, 191.1179).
Instrumentation
Gas chromatography ion trap mass spectrometry
GC ion trap MS data for (±)-cis-4,4'-DMAR (1 mg/mL in methanol) were obtained in electron (EI) and chemical ionization (CI) mode (scan range m/z 41–m/z 500) using a Varian 450-GC gas chromatograph coupled to a Varian 220-MS ion trap mass spectrometer. A Varian 8400 autosampler was employed with a CP-1177 injector (275 °C) in split mode (1:50). Data acquisition was performed with the MS Data Review function of the Workstation software, version 6.91. Transfer line, manifold and ion trap temperatures were set at 310, 80 and 220 °C, respectively. The liquid CI reagent was HPLC grade methanol. CI ionization parameters (0.4 s/scan): CI storage level 19.0 m/z; ejection amplitude 15.0 m/z; background mass 55 m/z; maximum ionization time 2000 µs; maximum reaction time 40 ms; target TIC 5000 counts. A 30 m × 0.25 mm (0.25 µm film thickness) Agilent J&W VF-5ms GC column (Strathaven, UK) was employed for separation. The temperature profile was as follows: The starting temperature was set at 50 °C and held for 1 min. The temperature then increased at 20 °C/min to 300 °C and held constant for 5.00 minutes to give a total run time of 18.50 min.
Gas chromatography quadrupole mass spectrometry
Samples were analyzed on an Agilent 7890A GC coupled to a 5975C Mass Selective Detector. A HP-5MS column (30 m × 0.25 mm × 0.25 µm) was used with helium carrier gas at a constant flow of 1 mL/min and a split ratio of 5:1. The injector was set at 225 °C and the transfer line at 250 °C. The initial oven temperature was 80 °C, held for 4 minutes, then ramped at 40 °C/min. to 290 °C with a hold time of 10 minutes to give a total run time of 19.25 minutes.
Nuclear magnetic resonance spectroscopy
Both synthesized (±)-cis- and (±)-trans-4,4'-DMAR isomers were prepared as free bases and dissolved in CDCl3. 1H (600 MHz) and 13C (150 MHz) NMR spectra were recorded on a Bruker AV600 NMR spectrometer using a 5 mm TCI cryoprobe. 1H NMR spectra were referenced to an external TMS reference at δ = 0 ppm. The analyses of the donated and purchased (±)-cis-4,4'-DMAR hydrochloride salts were carried out in CD3OD on a Bruker Avance 300 system (1H 300 MHz; DEPTQ 75 MHz).
Liquid chromatography electrospray triple quadrupole mass spectrometry
LC-MS/MS experiments were carried out on a Waters (Micromass) Quattro Premier with an ESI source and interfaced to a Waters Alliance 2695 HPLC system (Waters Ltd., Hertfordshire, UK) operated under Masslynx v.4.1 software. The chromatographic method included a Synergi Max RP column (4 µm; 150 mm × 4.6 mm) which was from Phenomenex (Cheshire, UK). The column oven was maintained at 25 °C. Solvent A was 10 mM aqueous ammonium formate, 0.1% formic acid and Solvent B was methanol with 10 mM aqueous ammonium formate, 0.1% formic acid. The gradient elution (flow rate 0.8 mL/min) was programmed as follows: 0–1 min 90% A and 10% B, 1–20 min gradient to 10% A and 90% B, 20–22 min returned to 90% A and 10% B, and equilibrated for 8 min. The eluent was split between a PDA 996 detector/Quattro MS/MS (80/20). The injection volume was 10 µL and MS/MS data were collected in positive ion mode by multiple reaction monitoring (MRM). The optimized source conditions were as follows: capillary 3.12 kV, cone 15 V, rf lens 0.1 V, source temperature 100 °C, desolvation temperature 400 °C, cone gas flow 50 L/h, desolvation gas flow 500 L/h. Quadrupole 1 (Q1) parameters: LM resolution 14.0, HM resolution 14.0, ion energy 1.0. Collision Cell (Q2) Entrance -1, collision voltage 20 V, Exit 1. Quadrupole 3 (Q2) parameters were LM resolution 14.0, HM resolution 14.0, ion energy 1.0. Multiplier voltage was 650 V and the collision gas was Argon (0.3 mL/min flow). The protonated precursor ion was at m/z 191 and four product ions were collected, i.e. at m/z 148, m/z 131, m/z 91and m/z 56, respectively, Dwell time for each channel was 0.05 s. Interchannel delay was 0.02 s.
The 4,4'-DMAR compounds were also injected by direct infusion (10 µL/min) in order to obtain the corresponding product ions. The standard LC-MS/MS settings were applied however, masses were collected over the range between m/z 45 and m/z 200; collision voltage 23 eV; desolvation temperature 200°C; desolvation gas flow 200 L/h.
Liquid chromatography electrospray single quadrupole mass spectrometry
LC-MS analyses were performed on an Agilent 1100 LC system. Separation was obtained on an Allure PFP Propyl column (5 µm, 50 mm × 2.1 mm) from Restek (Bellefonte, PA, USA) and the aqueous mobile phase A consisted of 0.05% formic acid in water whereas mobile phase B was prepared from 0.05% formic acid in acetonitrile, respectively. The Agilent LC-MSD settings were as follows: positive electrospray mode, capillary voltage 3000 V, drying gas (N2) 12 L/min at 350 °C, nebulizer gas (N2) pressure 60 psi, EIC m/z 191 and 148, fragmentor voltage 70 V. Samples for LC-MS analysis (1 µL injection volume) were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) at a concentration of 5 µg/ml. The following gradient elution program was used: 0–4 min 2% A, then increase to 30% over 30 min using a linear gradient. The flow rate was 1 mL/min and the column temperature was 30 °C.
High-resolution electrospray mass spectrometry
HR-ESI mass spectra for the synthesized (±)-cis- and (±)-trans-4,4'-DMAR isomers were recorded by direct injection into a LTQ Orbitrap Discovery (Thermo Fisher, UK). Samples were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) and infused at a rate of 5 µL/min. Full accurate high-resolution (30000) mass scans were performed in positive electrospray mode. Measured accurate masses were within ± 5ppm of the theoretical masses. The following conditions were used: drying gas (N2) 10 L/min, capillary temperature 310 °C, spray voltage 4 V, capillary voltage 22 V and tube lens 77 V.
Analysis of the donated (±)-cis-4,4'-DMAR HCl was carried out by UHPLC-Q-TOF-MS analysis. UHPLC separation employed a mobile phases consisting of 100% acetonitrile with 1% formic acid and an aqueous solution of 1% formic acid. The column was maintained at 40 °C with a 0.6 mL/min flow rate and 5.5 min acquisition time. The elution was a 5–70% acetonitrile gradient ramp over 3.5 min, then up to 95% acetonitrile in 1 min and held for 0.5 min before returning to 5% acetonitrile in 0.5 min. Q-TOF-MS data were acquired in positive mode scanning from m/z 100–1000 with and without auto MS/MS fragmentation. Ionization was achieved with an Agilent JetStream electrospray source and infused internal reference masses. Agilent 6540 Q-TOF-MS parameters: gas temperature 325 °C, drying gas 10 L/min and sheath gas temperature 400 °C. Internal reference masses of m/z 121.05087 Da and m/z 922.00979 were used.
X-ray crystallography
Data collection was carried out on a Bruker APEX 2000 CCD Diffractometer using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). Data were corrected for Lorentz and polarization effects and empirical absorption correction applied. Structure solution by direct methods and structure refinement on F2 employed SHELXTL version 6.10(1).[30] Hydrogen atoms were included in calculated positions (C-H = 0.95 – 1.00 Å) riding on the bonded atom with isotropic displacement parameters set to 1.5Ueq(C) for methyl H atoms and 1.2Ueq(C) for all other H atoms. All non-H atoms were refined with anisotropic displacement parameters.
Monoamine transporter release
Male Sprague-Dawley rats (250–300 g, Charles River Laboratories, Wilmington, MA, USA) were housed 2 per cage and maintained on a 12-hour light-dark cycle. Food and water were provided ad libitum. Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Care and Use Committee of the Intramural Research Program of NIDA (Baltimore, MD, USA).
Rats were euthanized by CO2 narcosis and brains were processed to yield synaptosomes as previously described.[31,32] For release assays, 9 nM [3H]-1-methyl-4-phenylpyridinium ([3H]MPP+) was used as the radiolabeled substrate for dopamine transporters (DAT) and norepinephrine transporters (NET), while 5 nM [3H]5-HT was used as the radiolabeled substrate for 5-HT transporters (SERT). All buffers used in the release assay methods contained 1 µM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for a single transporter by including unlabeled blockers to prevent the uptake of [3H]MPP+ or [3H]5-HT by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h (steady state). Release assays were initiated by adding 850 µl of preloaded synaptosomes to 150 µl of test drug. Release was terminated by vacuum filtration and retained radioactivity was quantified by scintillation counting.
Results and discussion
The full analytical characterization and identification of (±)-cis-4,4'-DMAR (4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine or 4-methyl-5-(p-tolyl)-4,5-dihydrooxazol-2-amine) began with a product donated by an online vendor following a recognition that it was involved in eight fatal intoxications in Hungary.[26,33] Soon afterward, (±)-cis-4,4'-DMAR was associated with 18 fatal intoxications in Northern Ireland.[28] This prompted the preparation of both racemic cis and trans isomers and the investigation of the monoamine transporter activity of (±)-cis-4,4'-DMAR. In addition, a recent test purchase of a 4,4'-DMAR product from an alternative online vendor confirmed the presence of the (±)-cis isomer and showed that this substance is commercially available as a research chemical.
Compared to many other new psychoactive substances (NPS) that have been identified in many countries, little is known in the scientific literature about (±)-cis-4,4'-DMAR. One of the names that appears to be associated with this substance as a product sold by online vendors, is "Serotoni" but others may include 4,4'-dimethyl-aminorex, p4-DMAR, 4,4'-DMAR, 4-methyl-euphoria and 4-methyl-U4Euh.[33] The "euphoria" or "U4Euh" component of this name refers to 4-methylaminorex (4-MAX, 4-MAR) (Figure 1A), a psychostimulant that appeared in the 1980s as a street drug.[18–20] However, as discussed in the Introduction, the presence of a number of aminorex analogs can be traced back to the early 1960s, when they were prepared and evaluated as potential anorexigenic substances with central nervous system activity.
Mass spectrometry and chromatography
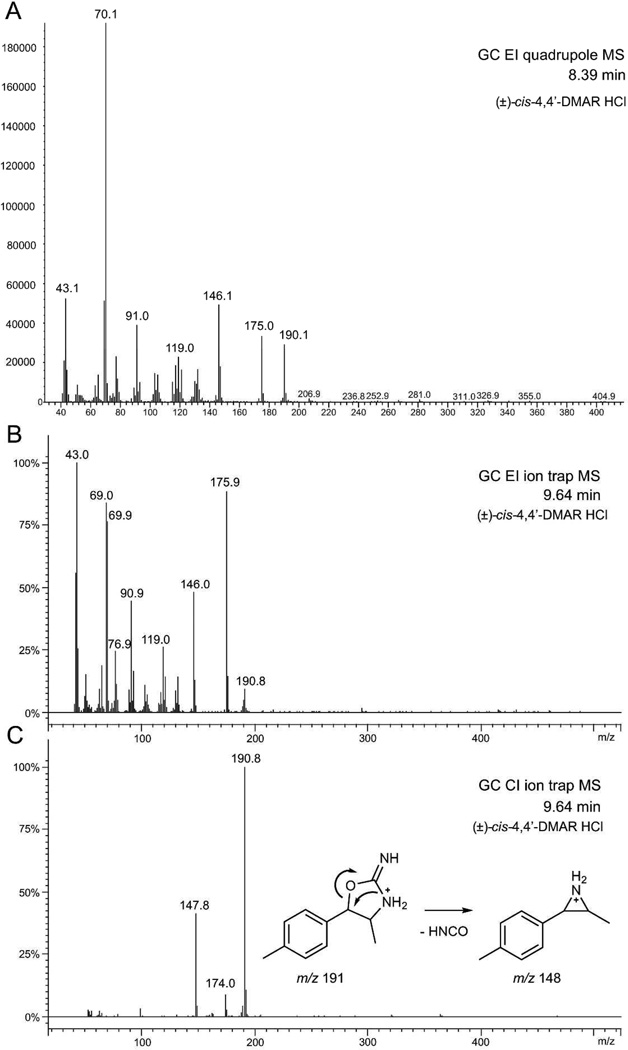
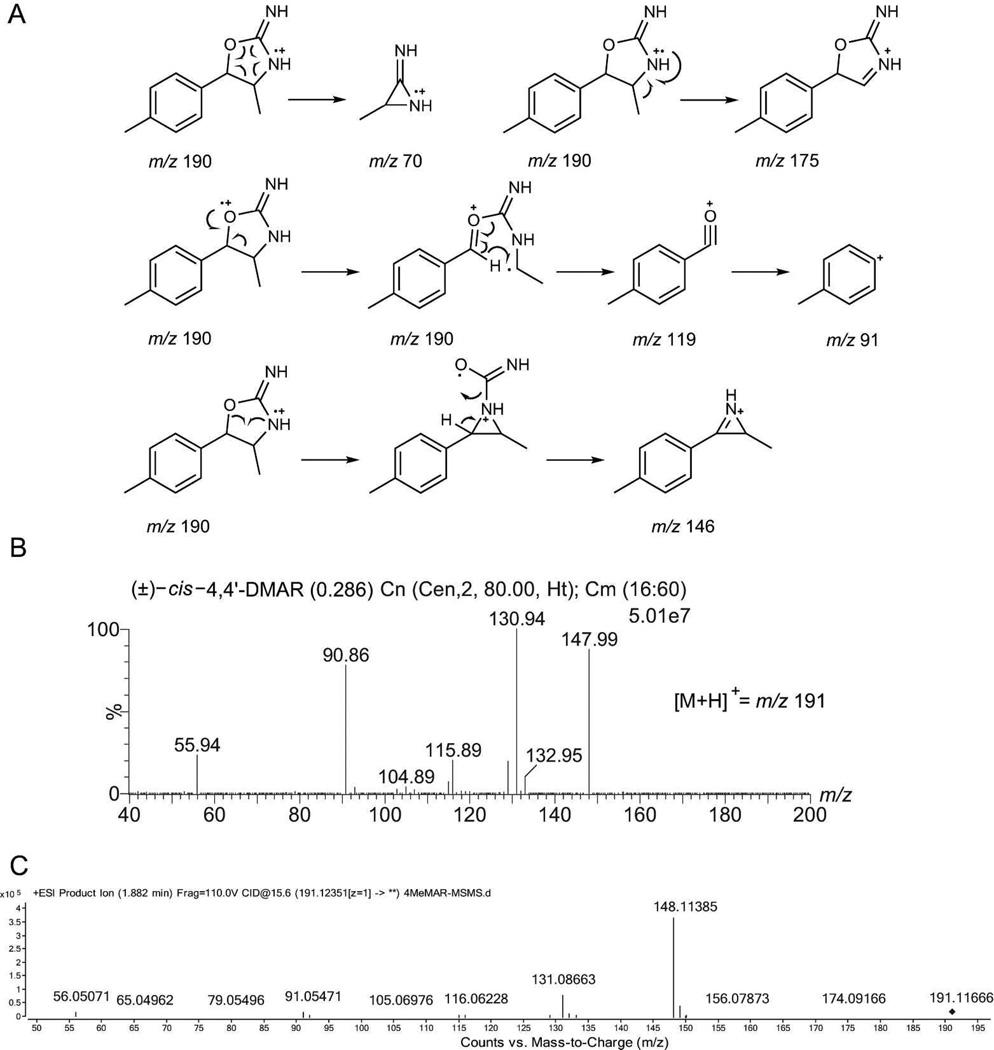
Figure 2 shows the electron ionization (EI) and chemical ionization (CI) mass spectra (MS) obtained for the donated (±)-cis-4,4'-DMAR following sample introduction by gas chromatography (GC). A comparison between quadrupole and ion trap EI-MS (Figure 2A and 2B) revealed a difference in relative abundance for a number of fragments. Among the most notable differences was the formation of the base peak at m/z 70 when using the quadrupole mass analyzer. Ion trap MS displayed a base peak at m/z 43 and an intense species at m/z 176 that was absent under quadrupole MS conditions where a fragment at m/z 175 was observed instead. The EI spectra of both synthesized cis and trans racemates were identical as expected and may be found as supplementary data. The m/z 70 has previously been observed in the EI-MS of 4-MAR[18–20,22] which indicated that the presence of the additional 4'-CH3 group in 4,4'-DMAR did not form part of base peak formation which represented fragmentation of the substituted oxalole-2-amine ring. A proposed fragmentation pattern for base peak formation at m/z 70 following neutral loss of 4-methylbenzaldehyde is shown in Figure 3A, thus, giving rise to a radical cation. The suggested species, which may be considered as a 3-methylaziridin-2-imine fragment, would give rise to a composition of C3H6N2 which was consistent with high-resolution EI-MS data reported for 4-MAR.[20] The base peak reported for aminorex, i.e. lacking the 4-CH3 substituent present in 4-MAR, showed the expected mass difference of 14 Da which led to m/z 56.[13] The impact of the 4'-CH3 group could however be noticed when considering the appearance of species at m/z 119, m/z 146 and m/z 175 (Figure 2A and 2B) that corresponded to their 4-MAR counterparts at m/z 105, m/z 132 and m/z 161, respectively, thus, also displaying a shift of 14 Da.[18–20,22] A possible equivalent of m/z 146 which was observed with 4.4'-DMAR (Figures 2A/2B and 3A) may have been the m/z 118 reported in the EI-MS of aminorex that lacks both methyl groups at positions 4 and 4' but in both cases a loss of CONH2 may have accounted for their appearance.[13] Proposed fragmentation pathways under EI-MS conditions are shown in Figure 3A. The CI-MS spectrum revealed the presence of a fragment of minor abundance at m/z 174, possibly loss of NH3, and m/z 148 (Figure 2C). A suggested mechanism for the formation of m/z 148 under CI-MS conditions is shown in Figure 2C.
Figure 2.
Mass spectra of (±)-cis-4,4'-DMAR HCl obtained from an Internet vendor using gas chromatography for sample introduction. A: electron ionization (EI) quadrupole MS. B: EI ion trap MS which varied from quadrupole MS in terms of relative abundance values. C: CI ion trap MS and a suggested loss from of NH3 and HNCO from the protonated molecule.
246×411mm (300 × 300 DPI)
Figure 3.
A: Proposed EI-MS fragmentation pattern for (±)-cis-4,4'-DMAR HCl. The following mass spectra were obtained from the donated (±)-cis-4,4'-DMAR HCl. Both isomers yielded identical mass spectra, see supplemental information for comparison. B: ESI-triple quadrupole MS/MS. C: High-resolution UHPLC-Q-TOF-MS/MS.
221×233mm (300 × 300 DPI)
Figure 3B shows the product ion spectrum obtained from direct infusion of the donated (±)-cis-4,4'-DMAR hydrochloride salt which indicated that the four most prominent product ions useful for multiple reaction monitoring purposes were detected at m/z 148, 131, 91 and 56, respectively. The synthesized cis and trans racemates yielded identical mass spectra (supplementary information). A quadrupole time-of-flight (Q-TOF) MS/MS analysis of the donated compound confirmed the associated mass of the protonated molecule (theory C11H15N2O+ 191.1179, observed 191.1167). A similar key product ion, also observed after GC-CI-MS analysis, was detected under both MS/MS conditions (m/z 148). High resolution QTOF-MS/MS data indicated the loss of 43 Da (HNCO) resulting in a species at m/z 148 (theory C10H14N+ 148.1121, observed 148.1139) (Figure 2C for suggested structure). The loss of 43 Da was described previously following thermospray triple-quadrupole MS/MS analyses of 4-MAR and closely related metabolites and analogs during metabolism studies in rats. However, since 4-MAR lacks the methyl substituent on the phenyl ring, the resulting ion was detected at m/z 134 instead of m/z 148 ion observed in the present study with (±)-cis-4,4'-DMAR.[34] The m/z 148 species was also observed following increased cone voltage settings (in-source CID) under single stage LC-MS conditions.
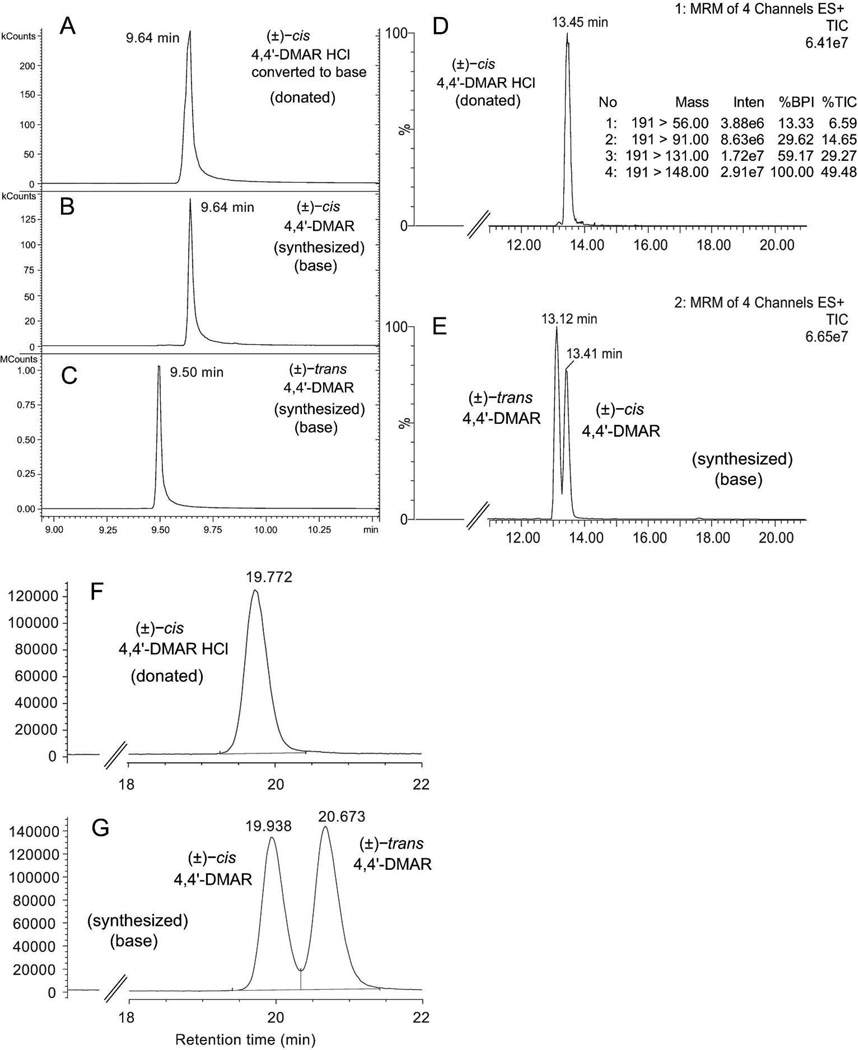
GC-MS analysis of the (±)-cis-4,4'-DMAR HCl salt using several instruments available in the authors' laboratories revealed that peak shapes and signal responses were occasionally quite variable. This was attributed to differences of GC conditions including liners, temperature and columns and a more dramatic example is shown in the supplementary information where degradation into two peaks occurred. However, conversion of the donated (±)-cis-4,4'-DMAR hydrochloride salt into the free base improved detectability significantly (Figure 4A) which allowed for GC comparison with (±)-cis and (±)-trans-4,4'-DMAR standards synthesized as free bases (Figure 4A–C). Analysis by liquid chromatography electrospray triple quadrupole mass spectrometry (LC-ESI-MS/MS) also confirmed the ability to separate both isomers and the corresponding multiple reaction monitoring traces are shown in Figures 4D and 4E, respectively. A further improvement in separation was obtained when employing an alternative method of separation involving single stage LC-ESI-MS detection (Figure 4F and 4G). All three chromatographic methods were able to achieve separation between the two isomers.
Figure 4.
Chromatographic analysis of donated and synthesized 4,4'-DMAR. A–C: GC-MS analysis confirmed that the donated 4,4'-DMAR HCl was consistent with the synthesized (±)-cis-4,4'-DMAR standard. Conversion of the hydrochloride salt to the free base (trace B) improved detectability. D and E: LC-ESI-quadrupole-MS/MS analysis in MRM mode to obtain separation. F and G: LC-single quadrupole-MS analysis of the donated (±)-cis-4,4'-DMAR HCl and improved separation of cis and trans racemates.
247×302mm (300 × 300 DPI)
Synthesis and nuclear magnetic resonance spectroscopy
The synthesis employed for the preparation of the racemic isomers (Figure 1C) included the traditional approach via α-bromination[35] of the 4-methylpropiophenone precursor (a) followed by reaction of the brominated intermediate (b) with sodium diformylamide to give (c).[36] Hydrolysis under acidic conditions provided access to the primary 4-methylcathinone (normephedrone) (d) which, following its reduction to the alcohol (e), was either converted to (±)-cis- (via cyanogen bromide) or (±)-trans (via potassium cyanate) 4,4'-DMAR, respectively, which was adopted from previously published work by Poos et al.[6] and Fodor et al.[37] Forensic work carried out by the Drug Enforcement Administration indicated that application of the potassium cyanate route to norephedrine gave (±)-trans 4-MAR, whereas cyanogen bromide would have been expected to give the (±)-cis product.[23] Previously, norpseudoephedrine was used to yield the trans-(4R,5R)-isomer while the use of norephedrine, both reacted with cyanogen bromide, gave cis-(4S,5R)-4-MAR.[20]
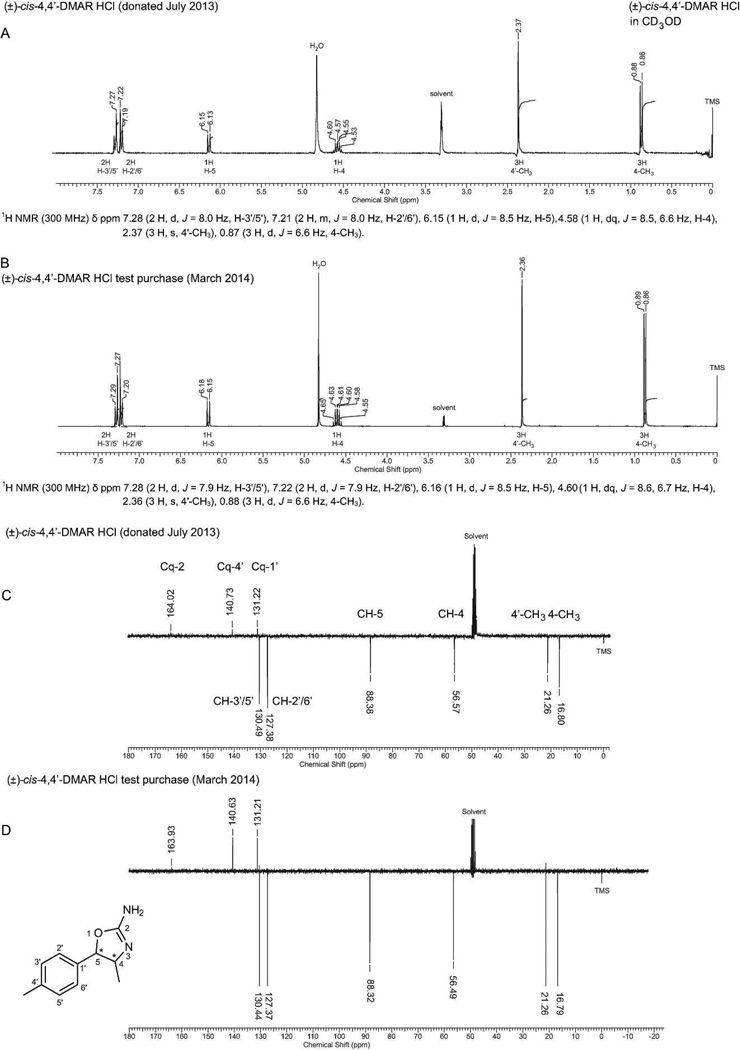
The NMR spectra associated with both racemic 4,4'-DMAR isomers shared some key features that were consistent with those reported previously with cis- and trans-4-MAR which facilitated differentiation between these isomers. In the proton NMR, for example, changes in the chemical shifts were observed for the 4-CH3, H-4 and H-5 resonances. The free base of (±)-cis-4,4'-DMAR studied here, showed that the H-4 and H-5 signals were recorded at 4.41 ppm and 5.74 ppm, respectively, whereas an upfield shift to 4.05 and 5.08 ppm was observed in (±)-trans-4,4'-DMAR. In contrast, a downfield shift was observed for the 4-CH3 doublet which shifted from 0.84 ppm (cis) to 1.40 ppm (trans). The same tendency was also reported for 4-MAR[19–21] and 3,4-dimethylaminorex.[24] On the other hand, the carbon NMR revealed that both C-4 and C-5 in the two 4,4'-DMAR isomers experienced a downfield shift, that is, from 59.50 ppm and 85.59 ppm (cis) to 63.71 ppm and 90.25 ppm (trans), respectively. Similarly, this trend was consistent with carbon NMR data reported for 4-MAR.[19,20] For completeness, Figure 5 provides the 1H and 13C NMR data obtained from the donated (±)-cis-4,4'-DMAR HCl salt. A comparison with data acquired from a test purchase from an alternative Internet vendor confirmed the presence of the same cis-isomer.
Figure 5.
1H NMR and DEPTQ comparison of (±)-cis-4,4'-DMAR HCl obtained from a donation (July 2013) and a test purchase from an alternative Internet vendor (March 2014). A spectral comparison revealed that both samples were spectroscopically identical. Note the difference in chemical shifts observed with the synthesized (±)-cis free base (e.g. H-4 and H-5, see Experimental section) as a reflection of salt formation and use of different deuterated solvents (CDCl3 base vs. CD3OD HCl salt). A: 1H NMR of donated product. B: 1H NMR of test purchase. This was then followed by 13C NMR analysis. C: DEPTQ of donated product. D: DEPTQ of test purchase.
289×410mm (300 × 300 DPI)
X-Ray crystallography
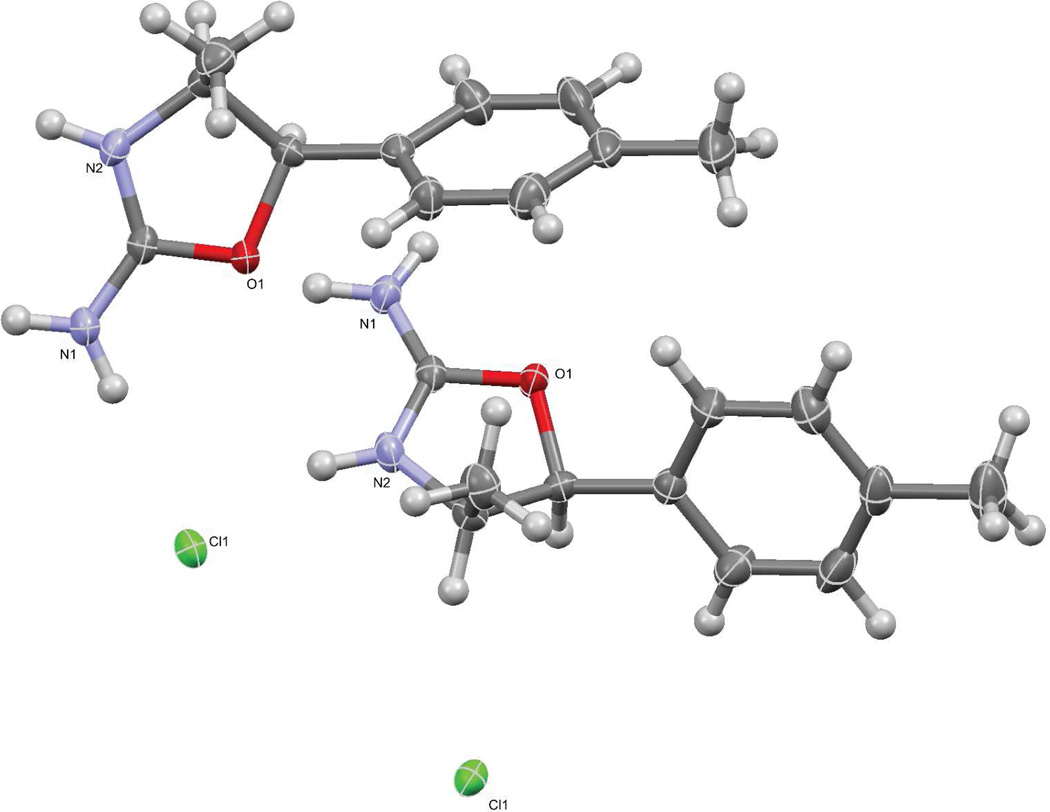
Single crystal X-ray data obtained from the originally donated sample provided unequivocal proof that the sample was the racemic (±)-cis-4,4'-DMAR HCl (dihedral angle 30°). Both the 4R,5S, and 4S,5R- enantiomers were identified in the unit cell (Figure 6). The bond lengths of C-N bonds were practically the same length N(1)-C(1) 1.293(2) Å and N(2)-C(1) 1.307(2) Å, respectively, which indicated that the nitrogen incorporated into the heterocycle was protonated. Interestingly, both nitrogen atoms were bonding to the same Cl− ion albeit from different molecules in the same unit cell which suggested that the positive charge may have been distributed between both nitrogen atoms.
Figure 6.
Crystal structure of (±)-cis-4,4'-DMAR HCl.
586×469mm (300 × 300 DPI)
Although it was not possible to compare the reactivity or conformation of a substance in solution to that of a crystalline substance, it was interesting to see that the ionic charge may have been distributed over the two nitrogen atoms. Interestingly, early work on the reaction of methylaminorex analogs with methyl iodide showed methylation of the ring nitrogen (N2) rather than the less hindered nitrogen (N1) belonging to the primary amine function.[37] Further studies may be needed to explore whether this may be accounted for by charge distribution over both nitrogen atoms.
Pharmacology
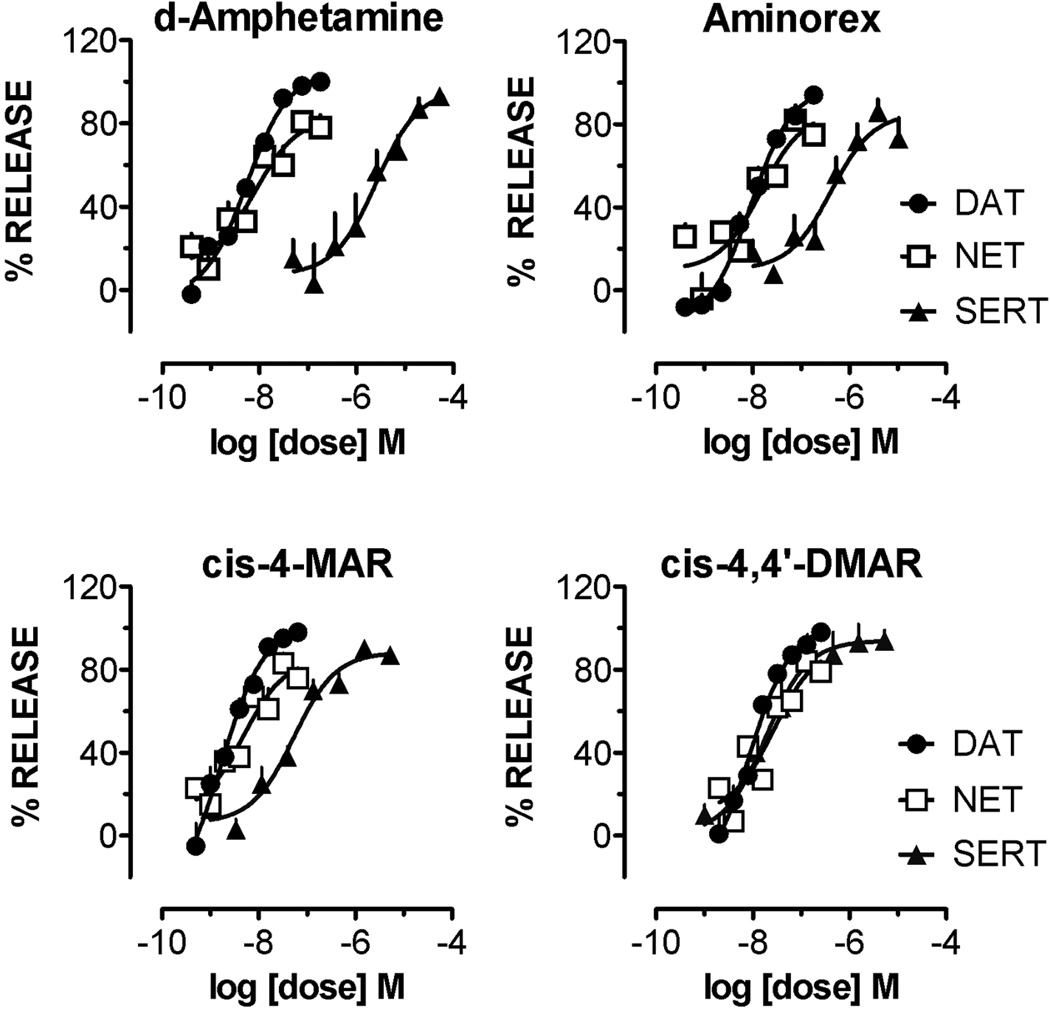
Figure 7 shows the dose-response effects of d-amphetamine, aminorex, (±)-cis-4-MAR and (±)-cis-4,4’-DMAR to evoke release at the three monoamine transporters DAT, NET and SERT. Table 1 summarizes potency values (EC50 concentration) for the test drugs based on data depicted in Figure 7. All of the drugs displayed potent releasing activity at DAT, with EC50 values ranging from 1.7 ± 0.2 nM for (±)-cis-4-MAR to 9.1 ± 0.9 nM for aminorex. The drugs were also quite potent at NET, with EC50 values ranging from 4.8 ± 0.9 nM for (±)-cis- 4-MAR to 26.9 ± 5.9 nM for (±)-cis-4,4’-DMAR. Activity at SERT varied more than 100-fold across the four drugs, with (±)-cis-4,4’-DMAR exhibiting the highest potency at releasing 5-HT (EC50=18.5 ± 2.8 nM). All test drugs were fully efficacious in their ability to evoke release at DAT, NET and SERT, i.e., drug effects achieved 100% of maximal release. When considering the overall transporter selectivity of the test compounds, d-amphetamine was the most selective for DAT/NET over SERT, with a DAT/SERT ratio of 473, whereas (±)-cis-4,4’-DMAR was essentially non-selective with a DAT/SERT ratio of 2.
Figure 7.
Dose-response effects of d-amphetamine, aminorex, (±)-cis-4,MAR and (±)-cis-4,4'-DMAR to evoke release from monoamine transporters in rat brain synaptosomes. DAT: dopamine transporter; NET: norepinephrine transporter; SERT: serotonin transporter. (±)-cis-4,4'-DMAR elicited potent release of all three monoamines including serotonin.
151×146mm (300 × 300 DPI)
Table 1.
Stimulation of release in rat brain synaptosomes
| Drug | Release at DAT EC50 (nM)a |
Release at NET EC50 (nM)a |
Release at SERT EC50 (nM)a |
DAT/SERT ratiob |
|---|---|---|---|---|
| d-Amphetamine | 5.5 ± 0.5 | 8.2 ± 1.6 | 2602 ± 494 | 473 |
| Aminorex | 9.1 ± 0.9 | 15.1 ± 3.5 | 414 ± 78 | 45 |
| (±)-cis-4-MAR | 1.7 ± 0.2 | 4.8 ± 0.9 | 53.2 ± 6.8 | 31 |
| (±)-cis-4,4'-DMAR | 8.6 ± 1.1 | 26.9 ± 5.9 | 18.5 ± 2.8 | 2 |
Data are expressed as mean ± SD for N = 3–4 experiments performed in triplicate.
DAT/SERT ratio calculated by (EC50 at DAT)−1/(EC50 at SERT)−1; higher value indicates greater DAT selectivity.
These results indicated that (±)-cis-4,4’-DMAR is a potent efficacious releaser at DAT, NET and SERT in rat brain tissue. The potency of (±)-cis-4,4’-DMAR at DAT and NET rivals that of other psychomotor stimulant drugs like d-amphetamine and aminorex. However, (±)-cis-4,4’-DMAR had much more potent actions at SERT when compared to the other drugs examined in the present study. Because (±)-cis-4,4’-DMAR was shown to be a potent substrate-type releaser at DAT, NET, and SERT, it is possible that this drug generates exceptionally high levels of extracellular neurotransmitters that may be responsible for the range of serious side-effects after high dose exposure and/or following combination with other substances that may interact with monoamine transporters. Indeed, the early-warning notification issued by EMCDDA-Europol included warnings about adverse effects associated with 4,4’-DMAR such as agitation, hyperthermia, foaming at the mouth, breathing problems and cardiac arrest.[26]
Due to the absence of clinical studies, little is known about active dosage levels for 4,4'-DMAR in humans. A range between 30 and 300 mg has been suggested, although it is unclear whether this referred to a particular isomer.[33] In general, aminorex analogs have not been explored extensively as so-called designer-drugs, even though the stimulant properties of a number of analogs, including 4-MAR, have been known since the 1960s when they were investigated and formulated as potential anorexigens.[6,7,38–40] The possibility of encountering these analogs as psychoactive substances on the street has been discussed,[41] although it appears that the only analogue that received some attention during the 1980s and early 1990s was (±)-cis-4-MAR,[18–20] which was shown to have amphetaminelike abuse liability..[42–50] Interestingly, it was reported that (±)-cis-4-MAR lacked adverse central nervous system stimulation in a test subject when orally administered at a 0.25 mg/kg level as a nasal decongestant.[51]
From a chemical perspective, the synthetic route used to prepare both (±)-cis- and (±)-trans aminorex analogs shares a number of steps that are also employed for the synthesis of cathinone analogs (Figure 1C). Where secondary (or tertiary) cathinones are used as starting materials, N-mono or disubstituted analogs may arise which might open the possibility to re-cycle cathinones for their conversion to aminorex analogs. For example, 4-methyl-N-methcathinone (mephedrone) may be converted to the N-methyl analogue of 4,4'-DMAR; this may have to be taken into account from a forensic viewpoint. It is currently unknown whether this or similar analogs have psychoactive properties, although it has been demonstrated that the N-methyl and N,N-dimethylated analogs of 4-MAR maintained anorectic properties in rats.[6] Another issue of forensic importance is the ability to differentiate between positional isomers (e.g. "Serotoni 2.0"),[33] and it remains to be seen when and/or whether these are encountered on the market.
In summary, psychotic symptoms, agitation and hyperthermia following 4,4'-DMAR ingestion may be due to overstimulation of central dopamine and serotonin systems, whereas dangerous cardiovascular effects may be associated with excessive norepinephrine release in the periphery. An additional reason for concern is the fact that some of the deaths reported in Northern Ireland were also associated with 4,4’-DMAR-containing tablets (‘Speckled Cherry’ and ‘Speckled Cross’ motifs)[28] which raises the possibility that these may be circulating as either MDMA or ecstasy-type products. Unintended combinations between (±)-cis-4,4’-DMAR and other amphetamine-type substances may potentiate the risk of adverse and possibly fatal outcomes as recently observed in a number of cases reported in Northern Ireland.
Conclusion
The in-depth chemical and analytical investigation of (±)-cis-4,4’-dimethylaminorex (4,4'-DMAR) reported here was prompted by the dramatic occurrence of 26 fatal intoxications in Hungary and Northern Ireland. The synthesis of 4,4'-DMAR and the differentiation between the (±)-cis- and (±)-trans isomers was shown to be straight-forward. (±)-cis-4,4’-DMAR, d-amphetamine, aminorex and (±)-cis-4-methylaminorex (4-MAR) were fully efficacious substrate-type releasers of dopamine, norepinephrine and serotonin in rat brain synaptosomes. Most importantly, (±)-cis-4,4’-DMAR was a far more potent serotonin releaser than d-amphetamine, aminorex or 4MAR. It might be predicted that high dosages and/or combinations of (±)-cis-4,4’-DMAR with other amphetamine-type substances may potentiate the risk of adverse and possibly fatal outcomes. A recent test purchase from the internet also confirmed the presence of (±)-cis-4,4’-DMAR, proving that this substance is freely available.
Supplementary Material
References
- 1.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Action on new drugs. [11 March 2014]; Available at: http://www.emcdda.europa.eu/activities/action-on-new-drugs. [Google Scholar]
- 2.EMCDDA-Europol. EU drug markets report: a strategic analysis. Lisbon: EMCDDA; 2013. Jan, [11 March 2014]. Avilable at: http://www.emcdda.europa.eu/publications/joint-publications/drug-markets. [Google Scholar]
- 3.United Nations Office on Drugs and Crime (UNODC) A Report from the Global SMART Programme March 2013. Vienna: United Nations Publication; 2013. [11 March 2014]. The challenge of new psychoactive substances. Available at: www.unodc.org/documents/scientific/NPS_2013_SMART.pdf. [Google Scholar]
- 4.Council Decision 2005/387/JHA of 20 May 2005 on the information exchange, risk-assessment and control of new psychoactive substances. Official J. EU. 2005 L 127/32. [Google Scholar]
- 5.Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277. doi: 10.2165/00002018-200629040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Poos GI, Carson JR, Rosenau JD, Roszkowski AP, Kelley NM, McGowin J. 2-Amino-5-aryl-2-oxazolines. Potent new anorectic agents. J. Med. Chem. 1963;6:266. doi: 10.1021/jm00339a011. [DOI] [PubMed] [Google Scholar]
- 7.Poos GI. 2-Amino-5-acryloxazoline products. US3161650. Patent. 1964 Dec 15;
- 8.Roszkowski AP, Kelley NM. A rapid method for assessing drug inhibition of feeding behavior. J. Pharmacol. Exp. Ther. 1963;140:367. [PubMed] [Google Scholar]
- 9.Gurtner HP. Aminorex and pulmonary hypertension. A review. Cor Vasa. 1985;27:160. [PubMed] [Google Scholar]
- 10.Gurtner HP. Pulmonary hypertension,"plexogenic pulmonary arteriopathy" and the appetite depressant drug aminorex: post or propter? Bull. Eur. Physiopathol. Respir. 1979;15:897. [PubMed] [Google Scholar]
- 11.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation. 1999;100:869. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- 12.Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br. J. Pharmacol. 2008;155:455. doi: 10.1038/bjp.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster ME, Davis FT. Appearance of aminorex as a designer analog of methylaminorex. J. Forensic Sci. 1991;36:587. [Google Scholar]
- 14.Barker SA. The formation of aminorex in racehorses following levamisole administration. A quantitative and chiral analysis following synthetic aminorex or levamisole administration vs. aminorex-positive samples from the field: a preliminary report. J. Vet. Pharmacol. Ther. 2009;32:160. doi: 10.1111/j.1365-2885.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 15.Hess C, Ritke N, Broecker S, Madea B, Musshoff F. Metabolism of levamisole and kinetics of levamisole and aminorex in urine by means of LC-QTOF-HRMS and LC-QqQ-MS. Anal. Bioanal. Chem. 2013;405:4077. doi: 10.1007/s00216-013-6829-x. [DOI] [PubMed] [Google Scholar]
- 16.Hess C, Ritke N, Sydow K, Mehling L-M, Ruehs H, Madea B, Musshoff F. Determination of levamisole, aminorex, and pemoline in plasma by means of liquid chromatography-mass spectrometry and application to a pharmacokinetic study of levamisole. Drug Test. Anal. 2014 doi: 10.1002/dta.1619. in press. [DOI] [PubMed] [Google Scholar]
- 17.Karch SB, Mari F, Bartolini V, Bertol E. Aminorex poisoning in cocaine abusers. Int. J. Cardiol. 2012;158:344. doi: 10.1016/j.ijcard.2011.06.105. [DOI] [PubMed] [Google Scholar]
- 18.Davis FT, Brewster ME. A fatality involving U4Euh, a cyclic derivative of phenylpropanolamine. J. Forensic Sci. 1988;33:549. [PubMed] [Google Scholar]
- 19.By AW, Dawson BA, Lodge BA, Sy WW. Spectral distinction between cis- and trans-4-methylaminorex. Forensic Sci. Int. 1989;43:83. [Google Scholar]
- 20.Klein RFX, Sperling AR, Cooper DA, Kram TC. The stereoisomers of 4-methylaminorex. J. Forensic Sci. 1989;34:962. [Google Scholar]
- 21.Caldwell GW, Rothchild R, Valentin I. Proton 1H NMR spectral simplification with chiral solvating agents and with achiral and chiral lanthanide shift reagents. Cis-4,5-Dihydro-4-methyl-5-phenyl-2-oxazolamine, "cis-4-methylaminorex," a potent stimulant and anorectic. Spectrosc. Lett. 1990;23:589. [Google Scholar]
- 22.Kankaanpää A, Meririnne E, Ellermaa S, Ariniemi K, Seppälä T. Detection and assay of cis- and trans-isomers of 4-methylaminorex in urine, plasma and tissue samples. Forensic Sci. Int. 2001;121:57. doi: 10.1016/s0379-0738(01)00453-4. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez WR, Allred RA. Synthesis of trans-4-methylaminorex from norephedrine and potassium cyanate. Microgram J. 2005;3:154. [Google Scholar]
- 24.Noggle FT, Jr, Clark CR, DeRuiter J. Liquid chromatographic and spectral analysis of the stereoisomers of dimethylaminorex. J. AOAC Int. 1992;75:423. [Google Scholar]
- 25.Takagi M, Ishimitsu K, Nishibe T. Preparation of oxa(thia)zolidine derivative as anti-inflammatory agents. WO2001072723. PCT Int. Appl. 2001
- 26.EMCDDA-Europol. Dangerous synthetic drugs hit the EU market. [05 March 2014]; Available at: http://www.emcdda.europa.eu/news/2014/europol-emcdda1. [Google Scholar]
- 27.EMCDDA-Europol. EMCDDA-Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA. Lisbon: EMCDDA; 2013. May, [10 March 2014]. New drugs in Europe, 2012. Available at: http://www.emcdda.europa.eu/publications/implementation-reports/2012. [Google Scholar]
- 28.Cosbey S, Kirk S, McNaul M, Peters L, Prentice B, Quinn A, Elliott SP, Brandt SD, Archer RP. Multiple fatalities involving a new designer drug – para-methyl-4-methylaminorex. J. Anal. Toxicol. 2014 doi: 10.1093/jat/bku031. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 29.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 30.Sheldrick GM. SHELXTL Version 6.10. Madison, Wisconsin, USA: Bruker AXS, Inc.; [Google Scholar]
- 31.Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307:138. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 32.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serotoni. [06 April 2014]; Available at: http://serotoni.info. [Google Scholar]
- 34.Henderson GL, Harkey MR, Chueh YT. Metabolism of 4-methylaminorex ("EU4EA") in the rat. J. Anal. Toxicol. 1995;19:563. doi: 10.1093/jat/19.7.563. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt C. Einwirkung von Phtalimidkalium auf einige sauerstoffhaltige Halogenverbindungen. Ber. Dtsch. Chem. Ges. 1889;22:3249. [Google Scholar]
- 36.Han YL, Hu HW. A convenient synthesis of aminomethyl ketones (α-amino ketones) Synthesis. 1990:615. [Google Scholar]
- 37.Fodor G, Koczka K. 155. The stereochemical course of the conversion of 2-ureidoalcohols into oxazolidines. Part I. J. Chem. Soc. 1952:850. [Google Scholar]
- 38.McNeill Laboratories, Inc. Procédé de production de nouvelles 2-aminooxazolines et leurs sels d'addition avec les acides, et produits obtenus. BE628803. Patent. 1963 Jun 17;
- 39.McNeill Laboratories, Inc. Compose de la classe des 2-amino-5-aryl-oxazolines et compositions pharmaceutiques le contenant. FR2448M. Patent. 1964 Apr 6;
- 40.2-Amino-5-aryloxazoline compositions and methods of using same. US3278382A. Patent. 1966 Oct 11;
- 41.Cooper DA. Future synthetic drugs of abuse. In: Castonguay RT, editor. Proceedings of the international symposium on the forensic aspects of controlled substances: March 28 – April 1. Washington, D.C.: Laboratory Division, Federal Bureau of Investigation, U.S. Dept. of Justice; 1988. p. 79. [Google Scholar]
- 42.Bunker CF, Johnson M, Gibb JW, Bush LG, Hanson GR. Neurochemical effects of an acute treatment with 4-methylaminorex: a new stimulant of abuse. Eur. J. Pharmacol. 1990;180:103. doi: 10.1016/0014-2999(90)90597-y. [DOI] [PubMed] [Google Scholar]
- 43.Glennon RA, Misenheimer B. Stimulus properties of a new designer drug: 4-methylaminorex ("U4Euh") Pharmacol. Biochem. Behav. 1990;35:517. doi: 10.1016/0091-3057(90)90282-m. [DOI] [PubMed] [Google Scholar]
- 44.Mansbach RS, Sannerud CA, Griffiths RR, Balster RL, Harris LS. Intravenous self-administration of 4-methylaminorex in primates. Drug Alcohol Depend. 1990;26:137. doi: 10.1016/0376-8716(90)90120-4. [DOI] [PubMed] [Google Scholar]
- 45.Hanson GR, Bunker CF, Johnson M, Bush L, Gibb JW. Response of monoaminergic and neuropeptide systems to 4-methylaminorex: a new stimulant of abuse. Eur. J. Pharmacol. 1992;218:287. doi: 10.1016/0014-2999(92)90181-3. [DOI] [PubMed] [Google Scholar]
- 46.Young R, Glennon RA. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacol. Biochem. Behav. 1993;45:229. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]
- 47.Batsche K, Ashby CR, Jr, Lee C, Schwartz J, Wang RY. The behavioral effects of the stereoisomers of 4-methylaminorex, a psychostimulant, in the rat. J. Pharmacol. Exp. Ther. 1994;269:1029. [PubMed] [Google Scholar]
- 48.Ashby CR, Jr, Pan H, Minabe Y, Toor A, Fishkin L, Wang RY. Comparison of the action of the stereoisomers of the psychostimulant 4-methylaminorex (4-MAX) on midbrain dopamine cells in the rat: an extracellular single unit study. Synapse. 1995;20:351. doi: 10.1002/syn.890200408. [DOI] [PubMed] [Google Scholar]
- 49.Russell BR, Beresford RA, Schmierer DM, McNaughton N, Clark CR. Stimulus properties of some analogs of 4-methylaminorex. Pharmacol. Biochem. Behav. 1995;51:375. doi: 10.1016/0091-3057(94)00407-a. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Russell B, Schmierer D, Laverty R. The effects of aminorex and related compounds on brain monoamines and metabolites in CBA mice. J. Pharm. Pharmacol. 1997;49:89. doi: 10.1111/j.2042-7158.1997.tb06758.x. [DOI] [PubMed] [Google Scholar]
- 51.Goodman RM. Methods of decongesting the nose without adverse stimulant effects. US4980364A. Patent. 1990 Dec 25;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.