Abstract
Background
Biopharmaceutical development necessitates use of nonhuman primates in toxicology, leading to adoption of nontraditional methods including cognitive function assessment.
Methods
A two-object discrimination and reversal test in cynomolgus monkeys (Macaca fascicularis) was performed using a Wisconsin General Testing Apparatus (WGTA). Nonclinical study design and regulatory considerations dictate that infants are raised by their biological mothers until weaning at six months. Thirty four animals (six to 21 months of age) were trained to discriminate between two randomly selected stimulus objects to retrieve a reward. Following training, days to first reversal after interchanging the reward were measured.
Results
Both sexes acquired visual discrimination skills at similar rates. Trends in learning and reversals completed were uniform across age groups. Completing training early in some subjects had no impact on subsequent testing phases.
Conclusion
Weaned cynomolgus monkey infants can be successfully tested for cognitive abilities using the WGTA in a nonclinical laboratory setting.
Keywords: learning and memory, WGTA, enhanced pre- and postnatal study, cynomolgus monkey
Introduction
The use of the long-tailed cynomolgus monkey (Macaca fascicularis) as an experimental model in regulatory toxicology has become common because many of the biotherapeutics, especially monoclonal antibodies, are only pharmacologically active in nonhuman primates [4, 6]. Pre- and post-natal developmental (PPND) toxicology testing has evolved over the past few years to include in utero assessment. This type of study is referred to as enhanced pre- and postnatal development (ePPND [24, 27]) to distinguish it from the traditional Segment III PPND study. In our laboratory, ePPND study designs occasionally require additional specialized non-routine testing, specifically screens for learning and memory. In order to generate study data in regulatory studies investigating potential deficits in learning and memory as part of developmental neurotoxicity, we adopted and developed testing using stock cynomolgus monkey infants post-weaning using a Wisconsin General Testing Apparatus (WGTA [15]). Considering that reaching is a highly demanding motor skill for infants [20], hand preference was determined in a subset of infants to investigate a correlation between hand preference and two-object discrimination and reversal learning test performance.
In the present study, the experimental setup included training cynomolgus monkey infants naturally raised by their biological mothers up to weaning at six months of age. The raising of the infants by the biological mothers differs significantly from the common method of isolating the infants at birth and hand-raising them, a practice successfully in use in academic research institutions where testing using the WGTA is often performed [13, 14, 22]. The rearing environment in rhesus monkey infants has been associated with differences in cognitive performance [23]. This may also apply when infants are isolated from maternal animals immediately after birth and raised in a nursery. It has been suggested that nursery-reared primates do not experience psychological "maternal bonding" or immunological benefits of breast milk, so they may expected to be inferior to mother-raised monkeys in growth, health, survival, reproduction, and maternal abilities [21]. In experiments for object discrimination and reversal learning using the WGTA, rhesus monkey infants routinely have been separated from their mothers at birth and transferred to a nursery for rearing, but no abnormal behaviors associated with this early separation were discussed [11, 12, 13]. In addition, although nursery-reared rhesus monkey infants have been reported to exhibit reduced social contact, abnormal behavior and cognitive deficits, it was determined that once the criteria on cognitive testing was achieved, the nursery reared infants performed as well as the maternally reared animals, exhibiting no difference in the acquisition of simple object discrimination task [23]. A literature search indicated that there is a lack of testing data for cynomolgus monkey infants and juveniles, a commonly used animal model in nonclinical toxicology, which includes pre- and postnatal developmental studies [7, 18, 27]. In the present study, historical control data in naturally (maternally) reared infants and juveniles in a nonclinical laboratory are reported. Ultimately, the data reported forms an initial and essential contribution in on-going efforts to characterize an effective and practical monitoring model for learning and memory testing in nonhuman primate postnatal development and juvenile toxicology. The cynomolgus monkey appears to offer a good model for this testing paradigm.
Materials and Methods
Animals and Animal Care
Compliance with animal codes
All protocols and study procedures were approved prior to use by SNBL USA, Ltd. Institutional Animal Care and Use Committee.
Maternal Animals
Stock adult female cynomolgus monkeys (N = 34), in a reproductive colony pool colony bred for reproductive toxicology studies, delivered naturally and were allowed to breastfeed the infants until weaning at six months postnatal.
Infants/juveniles and WGTA Set-up
Table 1 is a list of animals and their unique animal identifiers used on this study. Animals were born and raised at the SNBL USA Everett Facility vivarium and housed in similar habitats throughout the experimental period. Postnatal age definitions for infants included animals six to 11 months of age, and juveniles 15 to 21 months of age. The experimental procedures used throughout the study did not involve pain or distress.
Table 1.
Unique identifying numbers and age of study animals.
| Male | Gestation Day at Birth |
Age (months) at Testing Start Date |
Females | Gestation Day at Birth |
Age (months) at Testing Start Date |
|---|---|---|---|---|---|
| 088044 | 167 | 8.5 | 077049 | 165 | 15.5 |
| 099066 | 180 | 7.2 | 077051 | 167 | 15.5 |
| 099047 | 171 | 6.6 | 088015 | 153 | 11.2 |
| 099052 | 161 | 6.6 | 099076 | 147 | 6.7 |
| 077042 | 159 | 21.2 | 099075 | 173 | 6.7 |
| 077056 | 164 | 19.7 | 088079 | 165 | 7.9 |
| 077050 | 168 | 21.1 | 102173* | 167 | 6.7 |
| 099106 | 157 | 7.4 | 102667* | 176 | 7.0 |
| 099110 | 167 | 7.4 | 102648* | 168 | 7.0 |
| 099115 | 167 | 7.3 | 099212 | 144 | 7.8 |
| 102162 | 148 | 6.8 | 100405 | 162 | 6.7 |
| 102165* | 155 | 6.8 | 100376 | 167 | 6.6 |
| 102170* | 171 | 7.7 | 101082 | 168 | 8.0 |
| 102163* | 171 | 6.8 | 101073 | 168 | 8.1 |
| 102653* | 167 | 6.8 | |||
| 099135 | 160 | 6.7 | |||
| 099150 | 171 | 7.2 | |||
| 100381 | 159 | 6.4 | |||
| 100382 | 171 | 6.5 | |||
| 100407 | 162 | 6.6 |
Tested for hand preference prior to start of WGTA testing
Following weaning at six months of age, infants were pair-housed in two run-through cages (each 76 × 58 × 71 cm) with temperature and light regulation, fed PMI's LabDiet® Laboratory Fiber-Plus® biscuits twice per day with water available ad libitum, and given enrichment items such as fresh fruit and vegetables up to three times per week. Feeding was withheld at least five hours before start of testing, but animals were fed a full meal as soon as testing was completed. The withholding of food prior to testing increases motivation for food reward-based assessments as animals are hungry in anticipation of mealtime. All animals were monitored on a daily basis for health and wellbeing.
Thirty-four animals were tested between the ages of six and 21 months (N = 29 infants [17 males, 12 females], six to 11 months of age; N = 5 juveniles [3 males, 2 females], 15 to 21 months of age) using a WGTA with real-time remote monitoring capabilities (see schematic presentation in Figure 1) to facilitate observation by third parties. The samples sizes were unequal concerning sex and age because these were stock animals born on different dates and available at the start of the testing period. Third party observers include quality assurance personnel who independently verify that procedures are in compliance with the study protocol, an essential requirement in nonclinical regulatory studies. Eight of the test infants (five male, three female) were evaluated for hand preference. As part of the testing, infants from ePPND studies placebo cohort are routinely evaluated for behavioral characteristics during the lactation and post-weaning phases.
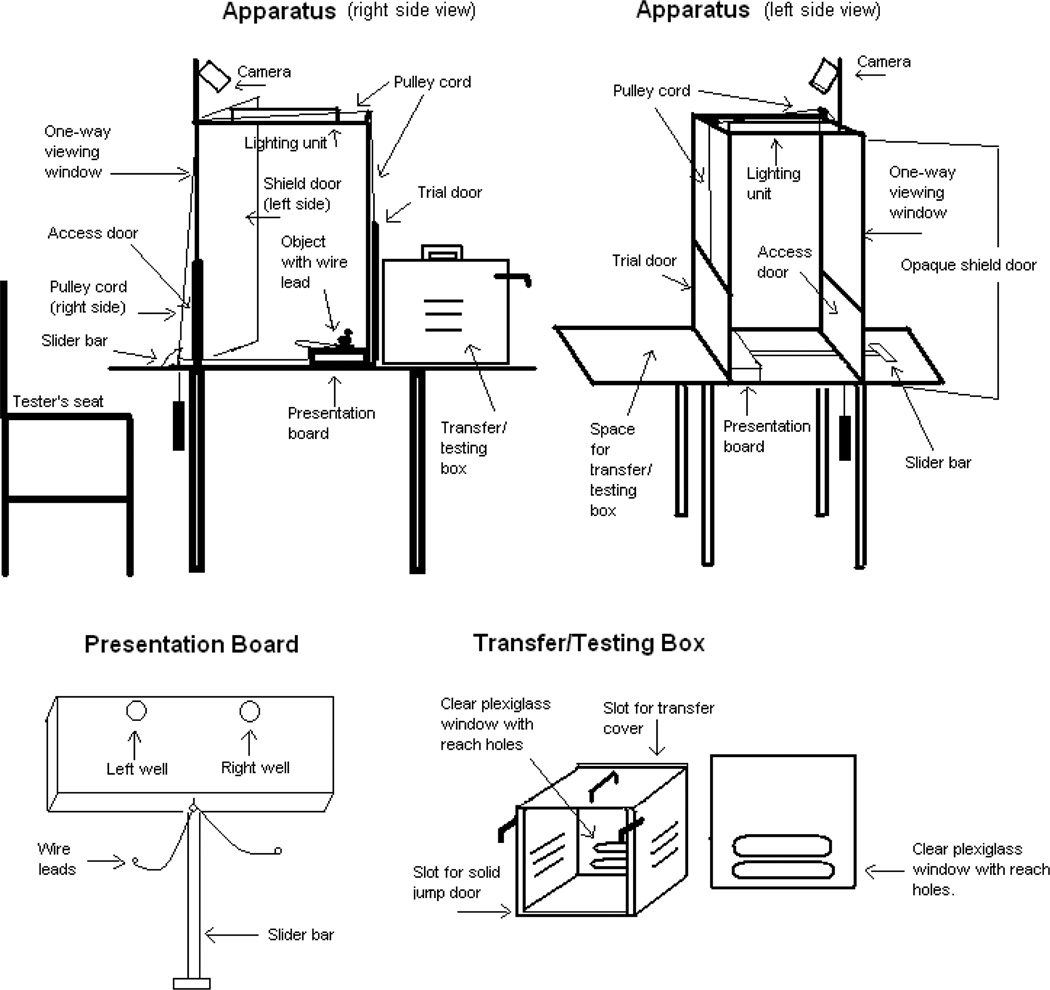
Figure 1.
A diagram of a WGTA apparatus set-up in a nonclinical toxicology testing laboratory (modified from [14]). A unique feature is the addition of a camera for remote monitoring for quality assurance activities.
Procedures
Discrimination and Reversal Learning
Refer to the WGTA diagram in Figure 1 for orientation of structures described in the following text. Dimensions of the main set-up are 79 × 48 × 56 cm.
All animals were tested in a modified WGTA located in a quiet, dark room with additional sound masking provided by a white-noise generator. Adaptation to stimuli, presentation board, food rewards, transfer/testing box and testing room began on postnatal day 120 for the infants tested between six and 11 months of age (N = 29) while co-housed with maternal animals. Adaptation for the older age juveniles (N = 5, tested between 15 and 21 months of age) was conducted individually and began 4 weeks prior to the initiation of the training phase. The procedure for adaptation training was performed at least twice weekly until just prior to the WGTA testing procedures. Adaption was conducted while animals were in their home cages and included hanging the presentation board onto the front of the home cage, placing a reward at random (e.g. piece of grape) and allowing at least 5 minutes for retrieval, followed by placing the stimulus object to be used for the WGTA testing (only one object used per session). Animal adaptation to the transfer box was performed at least once every other week for infant/dam pairs, and three to five times per week for weaned infants and juveniles. The transfer/testing box was hung onto the front of the animal’s cage or held up to the front of the cage, opened and the animal allowed access into the box. WGTA testing room adaptation involved transferring the animal using the transfer/testing box, placing the box on the apparatus, and removing the transfer cover on the box, so that the animal could reach out once the trial door was opened. A food treat was placed on the presentation board, and animals were encouraged to reach for the reward. At minimum, each animal was adapted to the testing room in two separate occasions before the start of the actual WGTA testing procedures.
Details of the WGTA methodology are extensively documented [1, 15]. The WGTA set-up included ensuring that the manual guillotine door (trial door) attached to the pulley cord was operable and in the lowered position and all equipment to be used by the tester were not visible to the animal. As shown in the diagram in Figure 1, the WGTA has a guillotine door that when lowered blocks the animal from reaching through the testing cage reach holes or viewing through the clear Plexiglas window. Raising the door allows the animal access to the presentation board that contains two wells for reward placement. Stimuli are attached to a presentation board using wire leads and clasps (see also Figure 2). An animal was moved to the testing room in a familiar small transfer/testing box. The WGTA transfer/testing box is centered between the two pre-measured marks on the back of the WGTA. After closing the testing room door, the transfer cover that shields the clear Plexiglas of the transfer/testing box is removed. The tester sits or stands at the opposite end of the WGTA facing the testing box and animal (tester must maintain the selected position throughout testing). The slider bar is used to pull back the presentation board. The access door is opened to set up the presentation board with stimuli and reward. The access door is then closed and the slider bar used to push the presentation board against the trial door, assuring that the board is within arm’s reach of the animal. The pulley cord is used to lift the guillotine trial door in a quick motion until the mark on the door reaches the pre-measured mark on the frame of the WGTA. The tester times and monitors the animal through the one-way Plexiglas window of the WGTA. Upon making a response, as defined by displacing the stimulus just enough so that the animal can retrieve the reward or see inside the well, the trial ends and the trial door is immediately lowered by the tester. The presentation board is set with stimuli and reward display for the next trial. The trial time, captured by stopwatches, is defined as the time period between the tester raising the door and the animal touching the stimuli. Parameters recorded by the tester include the animal’s response and the elapsed time to respond.
Figure 2.
Photograph of a WGTA testing station. The transfer cage is labeled T and presentation board as PB, with a blue and white toy as the stimulus objects covering the wells for reward placement.
To allow third party remote monitoring, a necessary requirement in nonclinical laboratories for maintaining regulatory compliance, a video-monitoring camera is mounted (refer to diagram in Figure 1). The camera is connected to a computer screen away from the testing rooms. This set-up allows real time observations as the testing proceeds.
In order to minimize variability, each animal was assigned to the same tester throughout the experimental period. The tester was not allowed to perform any other procedures on the infants (e.g. blood sample collection). Use of the same make and color of personal protective gear was required. The small transfer box and WGTA station remained the same for each animal until the testing was completed.
In the current study, two food wells on the presentation board were covered by the stimulus objects. The animals were trained to reach out through the test cage and displace objects on the presentation board in order to retrieve a food reward from a well. On testing days, no food or enrichment was provided until the completion of testing. The visual discrimination reversal task used colored toys (blue and white, see Figure 2) as the stimulus objects. During the training phase, animals were trained to displace each stimulus object to retrieve a food reward. An animal had to deliberately touch or take the food reward during each trial for a correct response. Moving the stimulus object without taking or touching the reward was considered a balk (no response). Each animal progressed through subsequent stages after correct responses were made for five consecutive trials at each stage. There are six stages of the training phase (all animals begin training on Stage 2) as shown in Table 2.
Table 2.
Definitions for the testing stages of the training phase.
| Stage | Description |
|---|---|
| 1* | Reward offered by hand centered between the two wells |
| 2 | Reward placed uncovered in front of the right or left well |
| 3 | Reward placed in the right or left well, not covered by stimulus object |
| 4 | Reward placed in the right or left well, stimulus object placed behind the well containing the reward |
| 5 | Reward placed in the right or left well with a stimulus object covering half the well with the reward |
| 6 | Reward placed in the right or left well with stimulus object fully covering the baited well |
This stage is used only if an animal balks at Stage 2 for one week (5 consecutive training sessions).
Stimulus object: appropriate-sized object, a toy in this case, used to cover a well containing a reward
An animal that balked for five consecutive trials during stages three to six progressively dropped back one stage at a time (holding at Stage 2). The animal had to complete the standard criterion (five correct consecutive trials or five consecutive balks) to once again move forward or back a stage. An animal was marked as meeting the training criteria to move on to the next testing phase (Object Discrimination and Reversal) after completing at least 18 of 20 correct trials at Stage 6 during a single training session.
Object discrimination and reversal testing were divided into two phases, learning phase and reversal phase. In the learning phase, the aim was to determine the number of days needed for each animal to learn to discriminate between two stimulus objects. The animal was given a choice of displacing one of two different stimulus objects placed over the right and left wells (see Figure 2). One object (right or left side initially randomly selected) was considered the correct choice and baited with a food reward for every trial. A trial ended when the animal had made a response or after 30 to 35 seconds had elapsed (correct choice: when the correct stimulus object covering the food reward was displaced; incorrect choice: when the incorrect stimulus object not covering the food reward was displaced or when both objects were simultaneously displaced). Twenty trials were conducted per testing session. The criterion for completing the learning phase was 23 correct responses in 25 consecutive trials across two consecutive testing days. In the reversal phase, the aim was to determine the number of days needed for each animal to inhibit a learned response in order to learn a new response. This phase was conducted in the same manner as the learning phase, except the correct and incorrect objects from the learning phase were reversed. The incorrect object from the learning phase now contained the reward and was thus considered the correct object. Criterion for completing a reversal was the same as in the learning phase, 23 correct responses in 25 consecutive trials across two consecutive testing days. Once criterion was reached for the first reversal, the correct and incorrect objects were once again switched. Testing sessions continued until the animal had completed a total of 30 sessions, which could include learning and reversal sessions.
Hand preference
The WGTA environment and pre-testing paradigm were used to assess initial hand use preference in eight infants (five males and three females, available at the time of initiation of hand preference testing). Adaptation and training for testing followed the same procedure described in the “Discrimination and Reversal-Learning” section. Testing for hand preference was performed during the adaptation phase, once daily for 10 days, divided into two, five-consecutive-day sessions in a two-week period. Twenty trials were conducted per session. For each trial, the hand that reached out for a stimulus object was marked as the preferred hand. Right or left hand preference was designated for an animal if a hand was used to move the stimulus object in 70% or more of the trials. No hand preference was designated if an animal used both hands in less than 70% of the trials. The designation of percentage was arbitrary but a hand use rate of 70% or greater was considered adequate to assign hand preference with confidence at this age of postnatal development.
Statistical Analysis
Descriptive statistics (mean and standard deviation) were conducted for each parameter. The primary aim of this study was to establish the WGTA testing paradigm and to train staff involved in the conduct of nonclinical studies. Even though sample size per sex and age was relatively small and unequal, analysis of these end-points was made and presented graphically. Sample size for gestation days at birth included all the 34 subjects.
Continuous data (testing phase days to reach criterion) from each end-point (training and learning days to criterion, and 1st reversal) were compared by one-way analysis of variance (ANOVA) using general linear modeling (GLM [SAS Institute, Carey, NC, USA]) in JMP10 (SAS Institute) for the three end-points: effects of gestation days at birth, age, and sex. The GLM version allowed inclusion of age and gestation age as non-categorical variables. Power calculations for the three end-points were performed using group sizes of 10, 12 and 20 to further characterize the sensitivity of the test under the specified criteria for the three phases.
Results
The summary statistics data are graphically presented in Figures 3 to 6. Statistical analysis showed no effects of age, sex, gestational age at birth on the 3 endpoints, with the exception of an effect of age at testing on the training days to criterion (p < 0.05) but this was probably due to the pre-weaning adaptation. Power calculations showed that with group sizes of 10, 12 and 20 it would only be possible to detect an effect on training sessions with a power of 0.8. Another approach looked at the number of animals that passed training and learning criteria, and completed learning and first reversal in the 30 sessions. This measure had high power for N = 12 per group, which is more reflective of the number of infants that are available by postnatal day 180 (start of testing) in a standard ePPND study with a starting group size of 20 (for example, SNBL USA background data: mean N = 13, range 10 to 16, with 67% with N ≥ 12).
Figure 3.
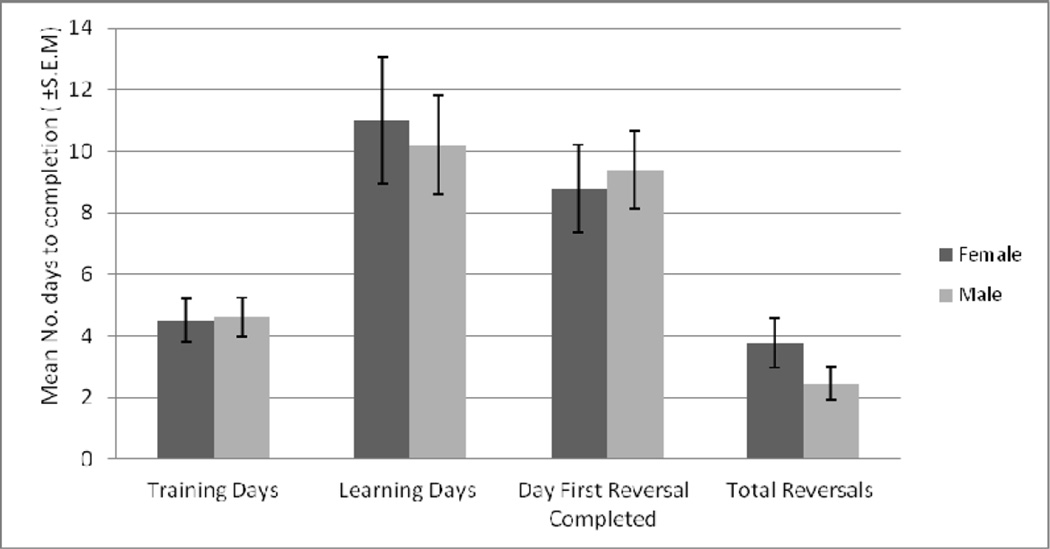
Sex comparisons for task completion. Mean number of days WGTA testing phases for males (training = 5 [N=20], learning = 10 [N=19], first reversal = 9 [N=13]) and females (training = 5 [N=14], learning = 11 [N=13], first reversal = 9 [N=8]). No differences in the number of days to completion were noted between females and males in any of the phases. Vertical bars indicate standard error of the mean.
Figure 6.
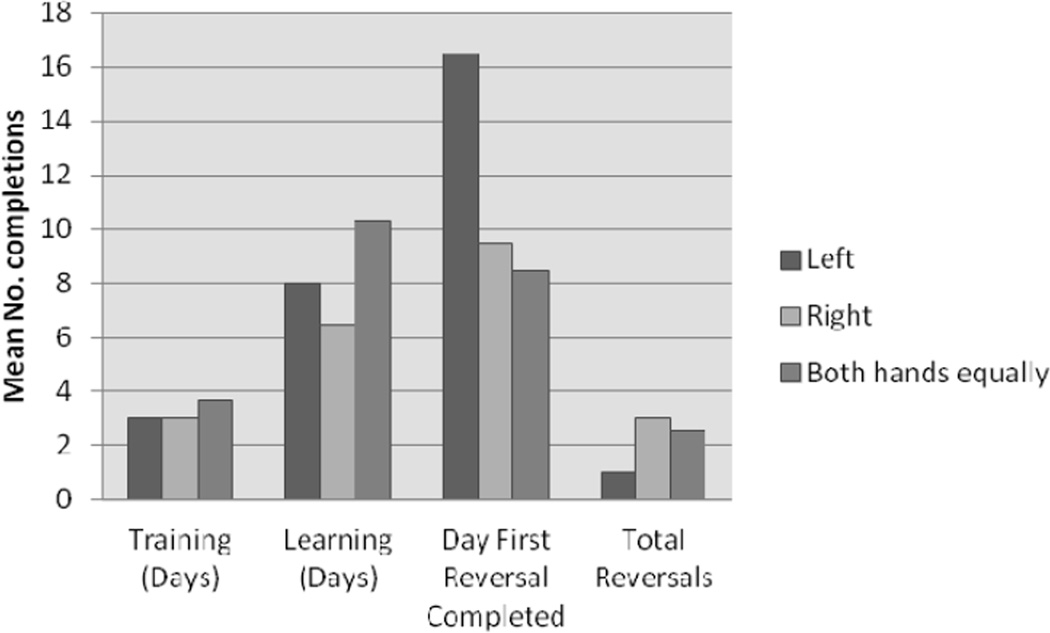
Hand preference in relation to WGTA task completion. Days to 1st reversal was relatively long in infants with left hand preference (N=3) when compared to right (N=2) or both (N=3) hands preference infants.
Object Discrimination and Reversal Learning
Animals that were excluded or that did not meet the criteria to move to the next testing phase are listed in Table 3. This included one female and one male that failed to achieve the criterion in the learning phase and therefore did not undergo testing in the reversal phase. In addition, six males and five females failed to learn that the reward well and stimuli had been reversed and therefore failed to reach criterion for reversal.
Table 3.
Animals that did not meet criteria at learning or training testing phases.
| Animal No. (Sex) |
Training Phase Completed (Days) |
Learning Phase Completed (Days) |
First Reversal Completed (Days) |
|---|---|---|---|
| 077051 (F) | 9 | 24 | FC |
| 088015 (F) | 4 | 20 | FC |
| 088079 (F) | 2 | 17 | FC |
| 100376 (F) | 11 | 4 | FC |
| 102667 (F) | 5 | 20 | FC |
| 101073 (F) | 5 | FC | NA |
| 099066 (M) | 6 | 18 | FC |
| 099106 (M) | 9 | 23 | FC |
| 099110 (M) | 3 | 26 | FC |
| 099115 (M) | 3 | FC | NA |
| 102165 (M) | 2 | 12 | FC |
| 099135 (M) | 6 | 14 | FC |
| 100382 (M) | 8 | 13 | FC |
FC: Failed to reach criterion; NA: Not applicable because animals did not achieve criterion in the Learning Phase to warrant further testing in the reversal phase
The number of days required to complete the different phases of the WGTA task was similar between males and females as shown in Figure 3. On average, the reversal phase took the least number of days, while the learning phase took the most number of days. In both male and female animals, the failure rate in subsequent phases of testing (Table 4) was low in the training-to-learning phases, and relatively high but comparable between training-to-first reversal and learning-to-first reversal.
Table 4.
Failure rate of testing in subsequent phases between males and females.
| % Failure rate | |||
|---|---|---|---|
| Sex | Training- to- learning |
Training-to- 1st reversal |
Learning- to-1st reversal |
| Male | 5.0 | 35.0 | 31.6 |
| Female | 7.1 | 42.9 | 38.5 |
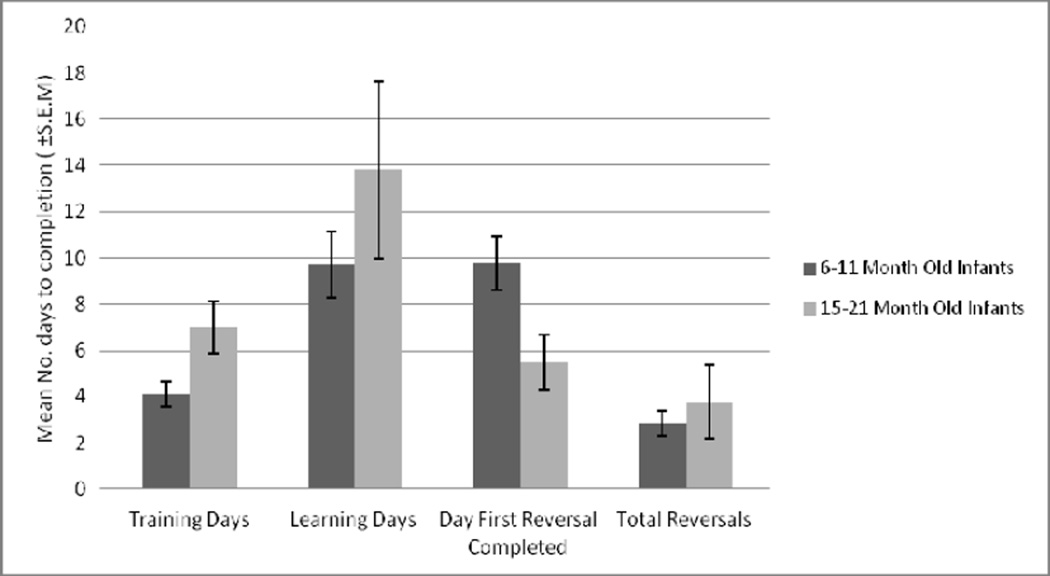
On average, animals six to 11 months of age took fewer days to complete training and learning compared to animals 15 to 21 months of age (Figure 4). It took more days to first reversal learning in younger animals compared to the older age infants, but the total numbers of reversals were similar. Therefore the tendency of completing training and learning relatively early did not seem to have an overall impact on total reversals completed. In the two different age categories, the failure rate in subsequent phases of testing (Table 5) was low in the training-to-learning phases, and relatively high in the training-to-first reversal and learning-to-first reversal. The 15 to 21 month age category had a lower failure rate compared to the six to 11 month age category in all phases.
Figure 4.
Age comparisons for task completion. Mean number of days WGTA testing phases for 2 different age groups, 6–11 months of age (training = 4 [N=29], learning = 10 [N=27], to 1st reversal = 10 [N=17]), 15–21 months of age (training = 7 [N=5], learning = 14 [N=4], to 1st reversal = 6 [N=4]).
Table 5.
Failure rate of testing in subsequent phases between the two different age categories.
| % Failure rate | |||
|---|---|---|---|
| Age (months) |
Training-to- learning |
Training-to- 1st reversal |
Learning-to- 1st reversal |
| 6–11 | 7 | 38 | 37 |
| 15–21 | 0 | 20 | 20 |
Balks and incorrect responses
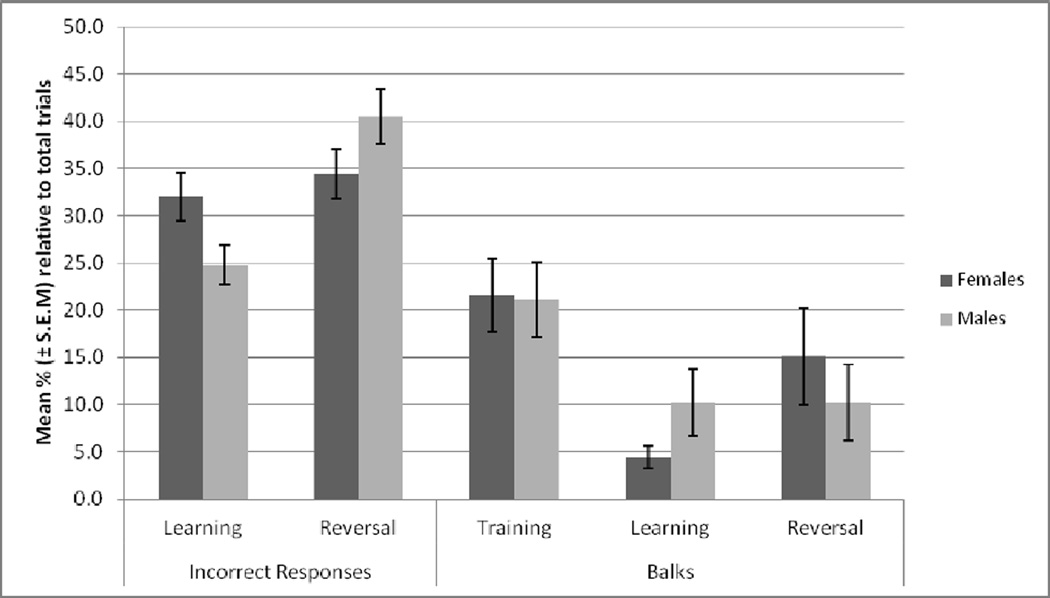
As shown in Figure 5, the incidence of the number of incorrect responses (when an animal displaces an object under which there is no reward or displaces both objects at the same time) was not significantly different between the learning and reversal phases, and between males and females. The incidence of balks (when an animal does not deliberately touch or take the food reward before the end of the trial) during the learning phase tended to be higher in males during the learning phase, while no significant differences were seen during the training and reversal phases. Balks during the training phase were slightly higher than the learning and reversal phases.
Figure 5.
Incorrect responses during the learning (N=13 females; N=19 males) and to 1st reversal (N=8 females; N=13 males) phases. The ratio of the incorrect responses was overall higher compared to the balks ratio (training, N=14 females and N=20 males; learning N=13 females and N=19 males; to 1st reversal, N=8 females and N=13 males). No differences between females and males were noted in the incorrect responses or balks categories.
Hand preference
Left, right or no hand preference was exhibited by three of eight, two of eight and three of eight animals, respectively (see Figure 6 and Table 6). There was no apparent relationship between performance in the different phases of the two-object discrimination and reversal test and handed preference assigned prior to start of testing. One female with no hand preference took a long time (20 days) to meet criteria in the learning phase. Out of 1000 total counts for the five animals that showed either right or left hand preference (two animals had a preference for the right hand, and three animals had a preference for the left hand), agreement for frequency of use of the right and left hands was 85% and 78%, respectively (mean = 82%). It must be emphasized that this was a one-time measure, and therefore there was no establishment of stable preferences for the use of one hand over the other. The design was simply to investigate whether initial hand preference correlated with outcomes in any testing phases of the two-object discrimination and reversal test.
Table 6.
Individual data for hand preference trials. Number of animals was similar between left, right or no hand preference.
| Animal ID |
Number right hand |
% of total* trials |
Number left hand |
% of total trials |
Number both hands |
% of total trials |
Number by mouth |
% of total trials |
Balked | % of total trials |
Hand Assigned |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 102162 | 56 | 28 | 142 | 71 | 1 | 1 | 1 | 1 | 0 | 0 | L |
| 102165 | 26 | 13 | 170 | 85 | 4 | 2 | 0 | 0 | 0 | 0 | L |
| 102170 | 150 | 75 | 47 | 24 | 3 | 2 | 0 | 0 | 0 | 0 | R |
| 102163 | 43 | 22 | 156 | 78 | 1 | 1 | 0 | 0 | 0 | 0 | L |
| 102653 | 73 | 37 | 122 | 61 | 5 | 3 | 0 | 0 | 0 | 0 | A |
| 102173 | 191 | 96 | 9 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | R |
| 102667 | 83 | 42 | 93 | 47 | 0 | 0 | 0 | 0 | 24 | 12 | A |
| 102648 | 105 | 53 | 75 | 38 | 19 | 10 | 1 | 1 | 0 | 0 | A |
L, left; R, Right; A, no preference.
A total of 200 each animal for number of trials.
Discussion
The primary aim of this study was to adopt and apply the two-object discrimination and subsequent reversal learning for cognitive function testing using cynomolgus monkeys in a nonclinical laboratory setting performing studies under Good Laboratory Practices regulations [26]. In this test, infant and juvenile cynomolgus monkeys learned to associate a blue or white toy (stimulus features) with location of a hidden reward [16]. It was ultimately determined whether and at what rate an animal was able to comprehend a reversal in the stimulus-reward association that had previously been formed. The secondary aim was to facilitate comparisons between control cohorts and test article treatment groups in future testing in our laboratory. The two-object discrimination and reversal task with other modifications has been extensively used in research settings for cognitive tests in nonhuman primates [10, 11, 13, 14, 17, 22]. To meet both scientific and regulatory expectations in our laboratory, stock infant and juvenile cynomolgus monkeys were selected and used in this study which allowed quality assurance activities to be part of the subsequent nonclinical studies. The data reported herein serve as a contribution to background data in future experiments run under similar conditions, and affirm the application of the test post-weaning of infants following maternal rearing.
Animals six to 11 months of age appeared to take fewer days to complete training compared to animals 15 to 21 months of age. This had no impact on the subsequent learning and reversal phase testing. The difference in completing training between the two age groups was attributed to adaptation training starting at four to five months of age for the younger animals. However, this does not rule out the possibility that the difference could be significant because early training could potentially trigger early neurodevelopmental cues that normally could occur at a later age if no such training is offered. No differences in test results were seen between male and female animals. Significant sex differences have been reported, with three-month-old rhesus females being superior to males [2, 3], 75-day-old males being superior to age-matched females [9], and 4.8-month-old pig-tailed macaque males performing better than age-matched females [17]. The gender difference was absent in six-month-old monkeys and in adults [3]. In the present study, animals were six months or older at the start of testing and no gender difference in testing outcome was observed. Therefore, it is possible that age may play a role in the sex differences in task performance in the published literature.
Another aspect investigated in the current study was hand preference. Adult monkey hand preference is primarily shaped by experience, with no evidence to support right or left preference as a characteristic of the species [8]. In the present study, our primary focus was to relate initial hand preference to learning and reversal in juvenile cynomolgus monkeys. There was no hand preference correlation in the two-object discrimination and reversal learning test, but it appeared that the right or left-handed juveniles achieved testing criteria at a faster rate than infants with no initial hand preference. The significance of this is not apparent, but it may be argued that the juveniles with either left or right hand preference may be slightly ahead in the development of the brain regions associated with learning and reversal. The hand preference test involved the animal’s ability to reach for an object covering a reward and moving it to obtain the reward presented in a restricted or standardized reaching on a board in front of the testing cage. Our experimental set-up and conduct was not conducive to hand preference bias because the food reward could only be obtained after moving an object placed over the reward on either the right or left side.
All infants and juveniles in this study were reared naturally until weaning at six months of age. The approach commonly used when testing infants is to isolate them immediately after delivery and raise them by hand rearing in a nursery setting [10, 11, 13, 14, 17, 22]. In the present study, no behavioral abnormalities were identified in any infant at the initiation of the training phase. Even though it is not widely reported that isolating infants can lead to abnormalities, nursery rearing is a complex experimental condition and can result in behavioral abnormalities [23] that may be attributable to lack of mother and social contact [25] or loss of controllability over the environment [19]. The maternal-infant interaction allowed in our experimental model minimizes or eliminates potential for confounding behavioral abnormalities.
The power analysis measure that had a high power for N = 12 per group is more reflective of the number of infants that are available by postnatal day 180 (start of testing in a nonclinical laboratory setting) in a standard ePPND study with a starting group size of 20 (for example, SNBL USA background data: mean N = 13, range 10 to 16 [67% of these N ≥ 12]. It is highly likely that if a developmental neurotoxicant has an effect on cognitive function, the number of animals required to show an effect could be as low as eight (this represents the average number of animals per group used in chronic standard toxicology studies [6]). Generally in nonhuman primates, there can be wide inter- or intra-animal variations, which may sometimes give confounding statistical findings that may have no biological significance. In other cases of data analysis in nonhuman primates, there may be no statistical significance, but an effect clearly can be demonstrated, especially when baseline data are used to compare to experimental data of the same animal. In a recent study [5], it was argued that using the WGTA for cognitive testing in infants might not be applicable in nonhuman primates. However, this argument was based strictly on statistical analysis of control groups and comparison with rodents (which have minimal inter- or intra-animal variability), thus not providing a complete picture on ability to detect cognitive and other types of end-points using WGTA testing [2, 10, 11, 13, 14, 17, 22, 23].
In conclusion, the two-object discrimination and reversal learning test used in this study provide a basis for developing this type of testing in a nonclinical laboratory setting for use in regulated studies where maternal exposure occurs during pregnancy, for example in ePPND studies. Infant and juvenile performance, as shown by the data reported here, is in general agreement with what would be expected based on two-object discrimination and reversal data by WGTA reported in the literature. It can further be argued that this test can be applied in older animals in nonclinical studies when it is suspected that a test compound may have a neurotoxic effect that may affect cognitive function.
Acknowledgments
We thank Dr. Mari Golub for the statistical analysis and SNBL USA technical staff for dedication to their work and animal welfare.
References
- 1.Bachevalier J, Mishkin M. An early and a late developing system for learning and retention in infant monkeys. Behav Neurosci. 1984;98:770–778. doi: 10.1037//0735-7044.98.5.770. [DOI] [PubMed] [Google Scholar]
- 2.Bachevalier J, Hagger C, Bercu B. Gender differences in visual habit formation in 3-month-old rhesus monkeys. Dev Psychobiol. 1989;22:585–599. doi: 10.1002/dev.420220605. [DOI] [PubMed] [Google Scholar]
- 3.Bachevalier Jocelyne, Brickson Mimi, Hagger Corinne, Mishkin Mortimer. Age and sex differences in the effects of selective temporal lobe lesion on the formation of visual discrimination habits in rhesus monkeysMacaca mulatta . Behav Neurosci. 1990;104:885–899. doi: 10.1037//0735-7044.104.6.885. [DOI] [PubMed] [Google Scholar]
- 4.Buckley LA, Chapman K, Burns-Naas LA, Todd MD, Martin PL, Lansita JA. Considerations regarding nonhuman primate use in safety assessment of biopharmaceuticals. Int J Toxicol. 2011;30:583–590. doi: 10.1177/1091581811415875. [DOI] [PubMed] [Google Scholar]
- 5.Cappon GD, Bowman CJ, Hurtt ME, Grantham LE., 2nd Object Discrimination Reversal As a Method to Assess Cognitive Impairment in Nonhuman Primate Enhanced Pre- and Postnatal Developmental (ePPND) Studies: Statistical Power Analysis. Birth Defects Res B Dev Reprod Toxicol. 2012;95:354–362. doi: 10.1002/bdrb.21025. [DOI] [PubMed] [Google Scholar]
- 6.Chapman KL, Andrews L, Bajramovic JJ, Baldrick P, Black LE, Bowman CJ, Buckley LA, Coney LA, Couch J, Maggie Dempster A, de Haan L, Jones K, Pullen N, de Boer AS, Sims J, Ian Ragan C. The design of chronic toxicology studies of monoclonal antibodies: implications for the reduction in use of non-human primates. Regul Toxicol Pharmacol. 2012;62:347–354. doi: 10.1016/j.yrtph.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Chellman GJ, Bussiere JL, Makori N, Martin PL, Ooshima Y, Weinbauer GF. Developmental and reproductive toxicology studies in nonhuman primates. Birth Defects Res B Dev Reprod Toxicol. 2009;86:446–462. doi: 10.1002/bdrb.20216. [DOI] [PubMed] [Google Scholar]
- 8.Deuel RK, Dunlop NL. Implications for the Study of Cerebral Dominance. Arch Neurol. 1980;37:217–221. doi: 10.1001/archneur.1980.00500530055008. [DOI] [PubMed] [Google Scholar]
- 9.Goldman PS, Crawford HT, Stokes LP, Galkin TW, Rosvold HE. Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkey. Science. 1974;186:540–542. doi: 10.1126/science.186.4163.540. [DOI] [PubMed] [Google Scholar]
- 10.Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004;26:799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Golub MS, Hogrefe CE, Tarantal AF, Germann SL, Beard JL, Georgieff MK, Calatroni A, Lozoff B. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83:647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- 14.Golub MS, Slotkin TA, Tarantal AF, Pinkerton KE. Visual recognition memory and auditory brainstem response in infant rhesus monkeys exposed perinatally to environmental tobacco smoke. Brain Res. 2007;1151:102–106. doi: 10.1016/j.brainres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Harlow HF, Bromer JA. A test apparatus for monkeys. Psychol Rec. 1938;2:434–436. [Google Scholar]
- 16.Harlow HF. The development of learning in the Rhesus monkey. Am Sci. 1959;47:459–479. [Google Scholar]
- 17.Mandell DJ, Sackett GP. Comparability of developmental cognitive assessments between standard and computer testing methods. Dev Psychobiol. 2009;51:1–13. doi: 10.1002/dev.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin PL, Sachs C, Imai N, Tsusaki H, Oneda S, Jiao Q, Treacy G. Development in the cynomolgus macaque following administration of ustekinumab, a human anti-IL-12/23p40 monoclonal antibody, during pregnancy and lactation. Birth Defects Res B Dev Reprod Toxicol. 2010;89:351–363. doi: 10.1002/bdrb.20250. [DOI] [PubMed] [Google Scholar]
- 19.Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu Rev Psychol. 1985;36:495–429. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- 20.Nelson EL, Konidaris GD, Berthier NE, Braun MC, Novak MF, Suomi SJ, Novak MA. Kinematics of reaching and implications for handedness in rhesus monkey infants. Dev Psychobiol. 2012;54:460–467. doi: 10.1002/dev.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys (Macaca nemestrina) Am J Primatol. 2002;56:165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- 22.Sackett G, Ruppenthal G, Hewitson L, Simerly C, Schatten G. Neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies. Dev Psychobiol. 2006;48:243–265. doi: 10.1002/dev.20132. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 24.Stewart J. Developmental toxicity testing of monoclonal antibodies: An enhanced pre- and postnatal study design option. Reprod Toxicol. 2009;28:220–225. doi: 10.1016/j.reprotox.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Suomi SJ, Harlow HF, Domek CJ. Effect of repetitive infant-infant separation of young monkeys. J Abnorm Psychol. 1970;76:161–172. doi: 10.1037/h0029809. [DOI] [PubMed] [Google Scholar]
- 26.Good Laboratory Practice for Nonclinical Laboratory Studies. US FDA 21 CFR Part 58. [Google Scholar]
- 27.Weinbauer GF, Fuchs A, Niehaus M, Luetjens CM. The enhanced pre- and postnatal study for nonhuman primates: update and perspectives. Birth Defects Res C Embryo Today. 2011;93:324–333. doi: 10.1002/bdrc.20220. [DOI] [PubMed] [Google Scholar]