Abstract
Background
Acute kidney injury (AKI) affects 45% of critically ill patients resulting in increased morbidity and mortality. The diagnostic standard, serum creatinine (SCr), is non-specific and may not increase until days after injury. There is significant need for a renal specific AKI biomarker detectable early enough that there would be a potential window for therapeutic intervention. In this study, we sought to identify a renal specific biomarker of AKI.
Methods
Gene expression data was analyzed from normal mouse tissues to identify kidney specific genes, one of which was Miox. Monoclonal antibodies were generated to recombinant myo-inositol oxygenase (MIOX), and an immunoassay was developed to quantify MIOX in plasma. The immunoassay was tested in animals and retrospectively in patients with and without AKI.
Results
Kidney tissue specificity of MIOX was supported by Western blot. Immunohistochemistry localized MIOX to the proximal renal tubule. Plasma MIOX, undetectable at baseline, increased 24 hours following AKI in mice. Plasma MIOX was increased in critically ill patients with AKI (12.4 ± 4.3 ng/mL, n=42) compared with patients without AKI (0.5 ± 0.3 ng/mL, n=17) and was highest in patients with oliguric AKI (20.2 ± 7.5 ng/mL, n=23). Plasma MIOX increased 54.3 ± 3.8 hours before the increase in SCr.
Conclusions
MIOX is a renal specific, proximal tubule protein that is increased in plasma of animals and critically ill patients with AKI. MIOX preceded the elevation in SCr by approximately two days in human patients. Large-scale studies are warranted to further investigate MIOX as an AKI biomarker.
Keywords: MIOX, acute kidney injury, biomarker, renal, myo-inositol oxygenase
Introduction
Acute kidney injury (AKI) is common amongst hospitalized and critically ill patients and its incidence is increasing (1-3). Approximately 45% of critically ill patients and 20% of hospitalized patients develop AKI (2, 4). This results in increased hospital stays, infectious complications and increased mortality at significant cost (5-8). Recent studies have linked AKI with future development of chronic kidney disease (9, 10). Multiple factors contribute to the development of AKI including sepsis, ischemia, drugs, intravenous contrast, and infection (11-16).
The current standard for identifying AKI, serum creatinine, is non-specific and insensitive (14, 17, 18). Serum creatinine may not increase until days after injury or 50% of renal function is lost. Serum creatinine is unable to accurately predict glomerular filtration rate (GFR) in the non-steady state of AKI, underestimating the renal function decline. Lastly, since serum creatinine depends on muscle mass and hepatic function, serum concentrations may differ depending on these factors (14, 17, 18).
There is a critical unmet need for a real-time, specific, and sensitive AKI biomarker. The American Society of Nephrology, Acute Dialysis Quality Initiative, and Acute Kidney Injury Network (AKIN) have prioritized the identification and validation of AKI biomarkers (3, 19). Early detection of AKI may allow for timely intervention and perhaps decrease its significant morbidity and mortality (20). Although multiple new drugs have been developed to treat AKI, they have not proven effective in the clinical setting (21-27). This has been attributed, in part, to the inability to detect AKI early. Multiple studies have investigated a variety of plasma and urine biomarkers for the diagnosis of AKI including IL-18, NGAL, KIM-1, among others (28-32). Although significant progress has been made, no specific, early biomarkers of AKI have translated into clinical practice.
Renal tubular proteins are ideal biomarkers of AKI as it is well established that tubular damage is an early event in renal ischemia. Although both proximal and distal tubules are injured, the S3 segment of the proximal tubule located in the outer medulla is particularly sensitive to decreased blood flow (7, 33). When tubular injury occurs, proximal tubular proteins may leak into the extratubular space and be excreted into urine or reabsorbed into plasma.
In the current study, we identified myo-inositol oxygenase (MIOX) as a kidney specific protein abundantly expressed in the proximal tubule. We developed a sensitive immunoassay to measure plasma MIOX and investigated the utility of plasma MIOX as an AKI biomarker in an animal model of AKI and retrospectively obtained samples from critically ill human patients.
Methods
Gene array analysis
Identification of kidney specific genes was performed according to methods described previously (35). Briefly, brain, liver, spleen, kidney, skeletal muscle, lung, pancreas, heart, and small intestine were dissected from three C57Bl/6 mice. Organs were snap frozen in liquid nitrogen and total RNA was isolated, converted into biotinylated cRNA, fragmented and applied to Mouse MU75A (Version 1) Genechip arrays (Affymetrix). The fluorescence intensity was scaled to 1500 and the mean difference values calculated using Affymetrix software by measuring the difference between the perfect match and mismatch oligonucleotides. These data were mined for genes with mean difference values >10,000 in the kidney, expressed at >10-fold amounts in the kidney relative to other tissues, and expressed in the proximal
Generation and purification of recombinant MIOX
The nucleotide sequence for human MIOX was inserted into a pGEX-4T1 vector (Life Technologies, Carlsbad, CA) for production in Escherichia coli. Recombinant MIOX was purified using immobilized glutathione (Pierce Chemical Company, #78250, Rockford, IL) according to manufacturer's instructions. Purified protein was analyzed using Lonza precast 10-20% SDS-PAGE (#58106, Walkersville, MD) with Novex pre-stained protein markers (#10748-010, Life Technologies) to confirm purity. Concentrations were estimated using absorbance at 280 nm with calculated extinction coefficients. MIOX and GST sequences were obtained from the Swiss-Prot website (http://www.expasy.org/sprout). Recombinant GST-MIOX was cleaved with thrombin to obtain recombinant MIOX (rMIOX) devoid of GST. This material was analyzed by denaturing gel electrophoresis and N-terminal Edman sequencing to confirm purity and identity.
Anti-MIOX antibodies
Rabbit polyclonal antibodies were produced at Harlan Bioproducts for Science (Madison, WI) using recombinant GST-MIOX as an immunogen. Polyclonal antibodies were purified by first removing cross-reacting anti-GST with a GST column (#20205, Pierce) according to manufacturer's instructions. Antibodies were then affinity purified as described previously (34). Mouse monoclonal antibodies were produced at Maine Biotechnology Services, Inc (Portland, ME) using recombinant GST-MIOX as the immunogen. Monoclonal antibodies were purified using a protein A-agarose column, dialyzed against phosphate-buffered saline, pH 7.2 containing 0.05% NaN3 and quantified using absorbance at 280 nm. Monoclonal antibodies were typed using Isotyping cassettes (Pierce, #26179). Epitope mapping was performed using ABIMED spot peptide arrays as described previously (35). Briefly, peptide spot arrays composed of MIOX residues 1-10, 3-12, 5-14, and so on, were probed with the anti-MIOX antibodies. The epitope sequences were analyzed by BLAST using a non-redundant Homo sapiens database and default algorithm parameters (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Western blotting
Normal human kidney homogenate (#NLH-04) and pre-made tissue blots (TB-37-I) were purchased from G-Biosciences (Saint Louis, MO). Western blotting was performed as previously described (35). Briefly, blots were probed with 4 μg/mL of polyclonal or monoclonal antibody. Where indicated, both monoclonal antibodies were used simultaneously at the above concentrations. Anti-species GAR-AP (#111-055-047) and GAM-AP (#115-056-072, Jackson ImmunoResearch Laboratories, West Grove, PA) were used as detection antibodies at a 1/1,000 dilution.
Immunohistochemistry
Formalin-fixed paraffin embedded human kidney tissue was obtained from the Lauren V. Ackerman Laboratory of Surgical Pathology at Barnes Hospital. Samples were obtained from the uninvolved portions of kidneys resected for renal cell carcinoma. Single sections were stained following sodium citrate antigen retrieval using a Ventana autostainer as described previously (36). A final concentration of 5 μg/mL of the rabbit polyclonal anti-MIOX antibody was used.
MIOX Immunoassay
A sandwich immunoassay was developed for MIOX using monoclonal antibody 12H06 as capture antibody and biotinylated monoclonal antibody 01D10 as a capping antibody. This immunoassay was used to measure MIOX from human and mouse samples. Biotinylation of antibody 01D10 was performed using Sulfo-NHS-LC-Biotin (#21335, Pierce) according to manufacturer's instructions. The capture antibody was added to the plate at a concentration of 30 μg/mL. The capping antibody was used at a concentration of 0.33 μg/mL. Non-specific binding was blocked using Pierce Superblock (#37515), mouse immunoglobulin G (#SLM66, Equitech-Bio, Kerryville, TX) and 0.5 mg/mL Tween-20 (#P-1379, Sigma-Aldrich, Saint Louis, MO). Plasma samples, controls, and standards were diluted 1:8 in Superblock, mouse IgG, and Tween-20 prior to analysis. GST-MIOX was serially diluted for construction of a standard curve. The concentration of GST-MIOX was determined using amino acid analysis (AAA Services Lab, Inc., Damascus, OR). Streptavidin conjugated to ruthenium (#32AD, MesoScale Discovery, Rockville, MD) was added to samples and detected using a MesoScale Discovery Sector 2400 electrochemiluminescent plate reader. Spike-recovery was determined by adding recombinant MIOX to a final concentration of either 2 ng/mL or 10 ng/mL to control human heparin plasma. Dilutional linearity was evaluated by adding recombinant MIOX to a final concentration of 5 ng/mL to 1:8, 1:16, 1:32, and 1:64 dilutions of control human heparin plasma.
Animal Model
All animal studies were approved by the Animal Studies Committee of Washington University School of Medicine. C57Bl/6 mice (3 female and 4 male) ranging in age from 8-12 weeks were used. 1 week prior to surgery, serum was collected and frozen at −80°C. Animals were subjected to bilateral renal ischemia for 30 minutes. Briefly, animals were anesthetized with a mixture of ketamine and xylazine, their body temperature was maintained at 37°C on a heating pad and monitored with a rectal probe throughout surgery, and ischemia induced by bilateral clamping of renal vascular pedicles for 30 minutes. Two sham-operated animals (1 male and 1 female) underwent an identical procedure without vascular pedicle clamping. Twenty-four hours following surgery, the animals were sacrificed, serum was collected, and kidneys were perfused with 4% paraformaldehyde and placed in 4% paraformaldehyde (pH 7.4). Serum was stored at −80°C prior to immunoassay as described above for human plasma. Tissues were processed for routine hematoxylin and eosin (H&E) staining according to standard procedures.
Human Patients
All human studies were approved by the internal review board for human studies at Washington University School of Medicine. Over a period of two years, laboratory data from adult patients in the surgical or medical intensive care units was screened for increases in serum creatinine occurring over a 24-72 hour time period. Laboratory data was also screened from age matched patients in the surgical or medical intensive care units or on hospital floors for stable serum creatinine over 72 hours. Patients with chronic kidney disease or who did not have Foley catheters were not included. Remnant heparin plasma samples were obtained from the Clinical Chemistry Laboratory of Barnes Hospital. Plasma samples were obtained before the increase in serum creatinine (designated time 0) and at the time of the serum creatinine increase (designated time 54). For patients with stable serum creatinine, one representative sample was retrieved from the Clinical Chemistry Laboratory. 500 μL aliquots of plasma were prepared and frozen at −80°C prior to analysis. Patient medical records were reviewed for demographic information, urine output, and diagnosis. Oliguria was defined as a urine output <0.5 mL/kg/h for at least 6 hours.
Statistics
Quantitative data are presented as mean ± SEM unless otherwise indicated. All statistical analyses were performed using GraphPad Prism software. Comparison of mulitple groups of patients was evaluated using the Kruskal-Wallis test with Dunn's correction. Standard curves were generated using a four parameter logistic curve fit with 1/y weighting. Significance was defined as p <0.05.
Results
Identification of myo-inositol oxygenase as a renal specific biomarker
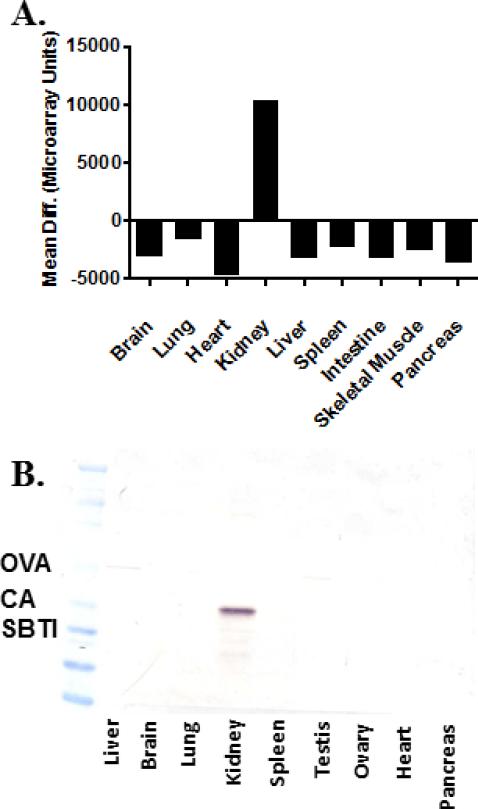
We sought to identify mouse genes expressed in the kidney by a factor of at least 10-fold compared with other tissues. Using this strategy, we identified Miox as a renal specific, abundant gene (Figure 1A). The Miox gene encodes the protein myo-inositol oxygenase (Miox). In order to confirm the tissue specific nature of the human homolog of MIOX, human tissue homogenates from liver, brain, lung, kidney, spleen, testis, ovary, heart, and pancreas were probed using an anti-MIOX rabbit polyclonal antibody. Consistent with the mouse gene profiling data, MIOX protein was only detected in human kidney homogenate (Figure 1B).
Figure 1.
A. Miox mRNA expression in mouse tissues; analyzed as previously described (35) B. MIOX expression in human tissue homogenates (50 μg protein/lane) using a rabbit polyclonal anti-MIOX antibody. The observed protein migrates between carbonic anhydrase (CA, 29 kDa) and ovalbumin (OVA, 45 kDa). SBTI – soybean trypsin inhibitor (20 kDa). No staining was observed in the absence of primary antibody.
Characterization of anti-MIOX antibodies
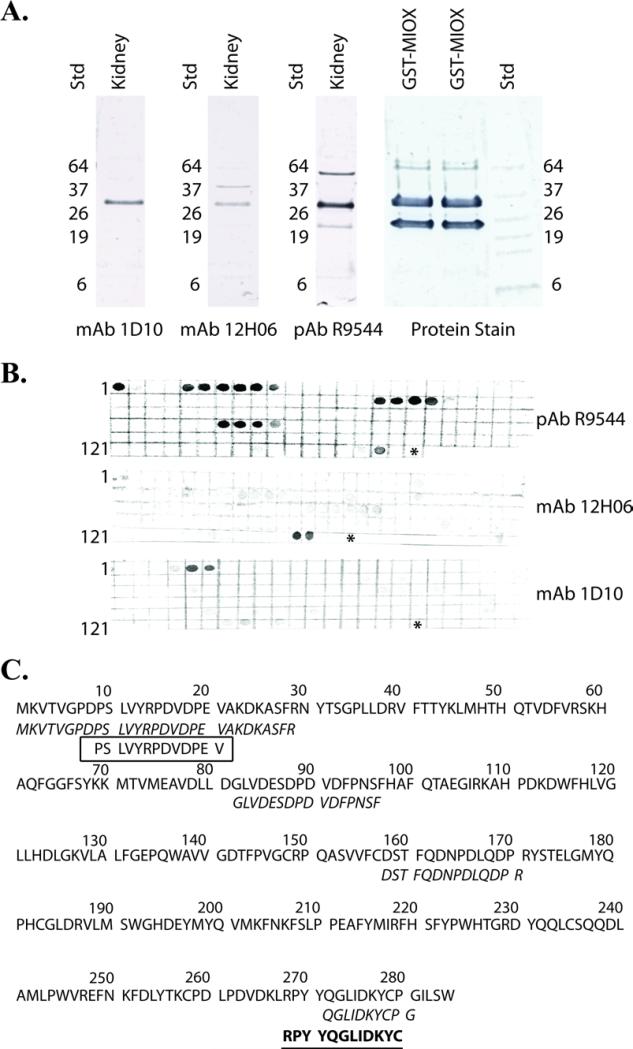
Rabbit polyclonal and mouse monoclonal anti-MIOX antibodies were generated using recombinant GST-MIOX as antigen. In order to confirm the identity of recombinant GST-MIOX, the protein was first cleaved into GST and MIOX using thrombin. The cleaved proteins were separated using SDS-PAGE and subjected to Edman sequencing (Figure 2A). The protein migrating between 26kDa and 37kDa gave a sequence of GSPEFKVTVG corresponding to the N-terminus of MIOX. The protein near 26kDa gave a sequence of MSPILGYWKI corresponding to the N-terminus of S. japonicum GST. Both monoclonal antibodies were isotyped and found to be IgG2bκ. Western blots were used to confirm the antibodies’ ability to recognize recombinant MIOX and endogenous MIOX present in normal human kidney homogenate (Figure 2A). The polyclonal and monoclonal antibodies recognized a protein with molecular mass between 26kDa and 37kDa that co-migrated with the recombinant MIOX protein (Figure 2A). These results are consistent with the expected molecular weight of MIOX, 33kDa.
Figure 2.
A. Western blot of 10 μg of normal human kidney homogenate (kidney) using the rabbit anti-MIOX polyclonal antibody and the mouse monoclonal antibodies 12H06 and 1D10. 10 μg of recombinant GST-MIOX (rMIOX) was cut with thrombin, run on the same gel, transferred to PVDF and protein stained (Protein Stain). The protein stained bands were subjected to Edman sequencing and confirmed to represent MIOX and GST as indicated. The remaining lanes were cut and stained with the rabbit polyclonal anti-MIOX antibody (pAb R9544), mouse monoclonal anti-MIOX antibody 12H06 (mAb 12H06) and mouse monoclonal antibody 1D10 (mAb 1D10). Non-specific bands were identified using pAb R9544 (~64 kDa and ~22 kDa) and the mAb 12H06 (~40 kDa). The only band that reacted with all three antibodies corresponded to recombinant MIOX (~33 kDa). B. Identification of anti-MIOX antibody epitopes. Spot-peptide membrane array immunostaining for rabbit polyclonal antibody R9544, mouse monoclonal antibody spot was comprised by a 10-mer synthetic peptide with spot 1 = residues 1-10, spot 2 = residues 3-12, spot 3 = 5-14 and so on until the entire sequence was covered. The numbers to the left of the blots correspond to the spot number at the beginning of the row. An asterisk marks the end of the sequence. C. Representation of anti-MIOX antibody epitopes. The amino acid sequence of MIOX is shown. The epitope map of the rabbit polyclonal anti-MIOX antibody is italicized, mouse monoclonal antibody 12H06 is bold and underlined, and the mouse monoclonal antibody 1D10 is boxed. The mAb 1D10 antibody recognizes an epitope near the N-terminus and the mAb 12H06 antibody recognizes an epitope near the C-terminus. The polyclonal antibody reacts with N- and C-terminal epitopes and two internal epitopes.
The linear epitopes of the anti-MIOX antibodies were determined using spot-peptide membrane arrays containing the full-length amino acid sequence of MIOX. The two mouse monoclonal antibodies mapped to opposite ends of the MIOX sequence (Figure 2B). The antibody designated 01D10 mapped to the N-terminal sequence of MIOX (Figure 2C). The mouse monoclonal antibody designated 12H06 mapped to the C-terminal region of MIOX (Figure 2C). Human MIOX showed 89.8% homology to mouse Miox. The peptide epitope recognized by the 12H06 mouse monoclonal antibody was 100% identical to the corresponding mouse Miox peptide sequence. The epitope recognized by the 01D10 mouse monoclonal antibody differed by one amino acid. The valine at amino acid position 21 is replaced by a methionine in mouse Miox. The rabbit anti-MIOX polyclonal antibody R9544 demonstrated 5 regions of intense staining, a finding consistent with the polyclonal nature of this antibody (Figure 2C). Each monoclonal epitope sequence was validated for their uniqueness in a non-redundant Homo sapiens database using BLAST. A critical output parameter from BLAST is the expected value (E value). The E value is a measure of the chance that a random alignment from the probed database would produce the same normalized score. Based on the E value, there is a less than 1 in >1,000,000 chance that the epitope sequences could be randomly found in the database (data not shown). The lowest E value of a non-MIOX protein was 2.3, compared to a MIOX E value of 1×10−6, indicating the highly specific nature of the antibody epitopes. Individual peptides corresponding to the mapped epitopes for both monoclonal antibodies were produced. Pre-incubation using the N-terminal peptide epitope, but not the C-terminal peptide epitope, inhibited binding of the 01D10 mouse monoclonal anti-MIOX antibody to a MIOX spot peptide array. Similarly, pre-incubation using the C-terminal peptide epitope, but not the N-terminal peptide epitope, inhibited binding of the 12H06 mouse monoclonal anti-MIOX antibody to a MIOX spot peptide array (data not shown).
Immunohistochemical analysis of MIOX in human kidney
Human kidney tissues were obtained from uninvolved portions of partial nephrectomy specimens from patients with renal cell carcinoma. Single formal-infixed paraffin embedded tissue sections were stained using the rabbit polyclonal anti-MIOX antibody. Consistent with previous studies, the MIOX protein showed strong cytoplasmic immunoreactivity in cells morphologically consistent with proximal tubules (Figure 3) (37).
Figure 3.
A. Formalin fixed paraffin embedded human kidney tissue was stained using the rabbit polyclonal anti-MIOX antibody. Intense staining of the renal cortex is seen (40×). B. On high power, cells consistent with proximal tubules (*) showed strong staining. Adjacent distal tubules (arrow), glomeruli (G), and blood vessels showed no significant immunoreactivity (400×).
MIOX immunoassay
The mouse monoclonal antibodies were optimized for use in a sandwich immunoassay. The monoclonal antibody 12H06 was used as a capture antibody and biotinylated monoclonal antibody 01D10 was used as a capping antibody. The immunoassay performance characteristics are summarized in Supplemental Table 1. The limit of detection, defined as a signal to noise ratio of 2 relative to background, was 115 ± 55 pg/mL (n=13). Human plasma samples stored at 4°C or −80°C for ten days showed no significant difference in MIOX concentration (absolute mean % difference = 7.2 ± 3.6%, n=4). The interassay coefficient of variation (CV) was 15.2 ± 3.4% (n=9, mean ± SD). All samples underwent two freeze thaw cycles. The intraassay CV was 7.9 ± 5.2% (n=19; mean ± SD). GST-MIOX spiked into human heparinzed plasma demonstrated 94 ± 19% (mean ± SD) recovery of the expected signal (n=8). The MIOX immunoassay demonstrated dilutional linearity recovery of 109 ± 12% (n=9; mean ± SD; Supplemental Figure 1).
Mouse AKI model
Miox was not detected in the serum of normal mice or sham operated mice 24 hours post-operatively (Figure 4A). In contrast, Miox was markedly increased in the serum of mice 24 hours following 30 minutes of bilateral ischemia reperfusion injury (Figure 4A). Routine histologic examination revealed significant tubular necrosis in the kidneys 24 hours after injury (Figure 4B). Sham-operated animals showed no evidence of tubular necrosis in the kidneys 24 hours after injury (Figure 4B). Sham-operated animals showed no evidence of tubular necrosis.
Figure 4.
Mouse AKI model. A. Miox was measured in mouse serum at baseline and 24 hours following AKI (n=5). No Miox was detected at baseline. Serum Miox was elevated 24 hours post-injury (2.8 ± 0.7 ng/mL; mean ± SEM). *p<0.02 (paired t-B. Representative section of renal cortex 24 hours post-injury. Extensive tubular necrosis is evident (arrows, H&E, 400×). No tubular necrosis was observed sham-operated animals.
Analysis of plasma from AKI
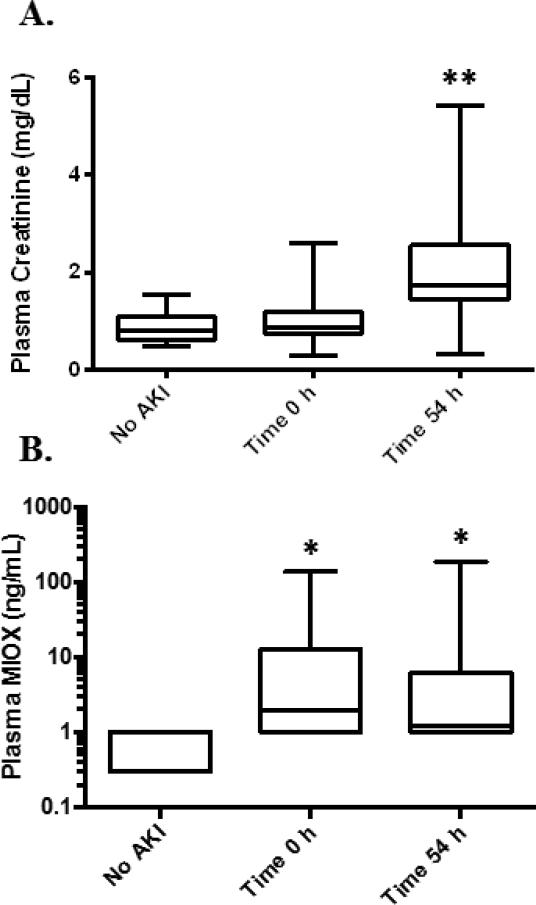
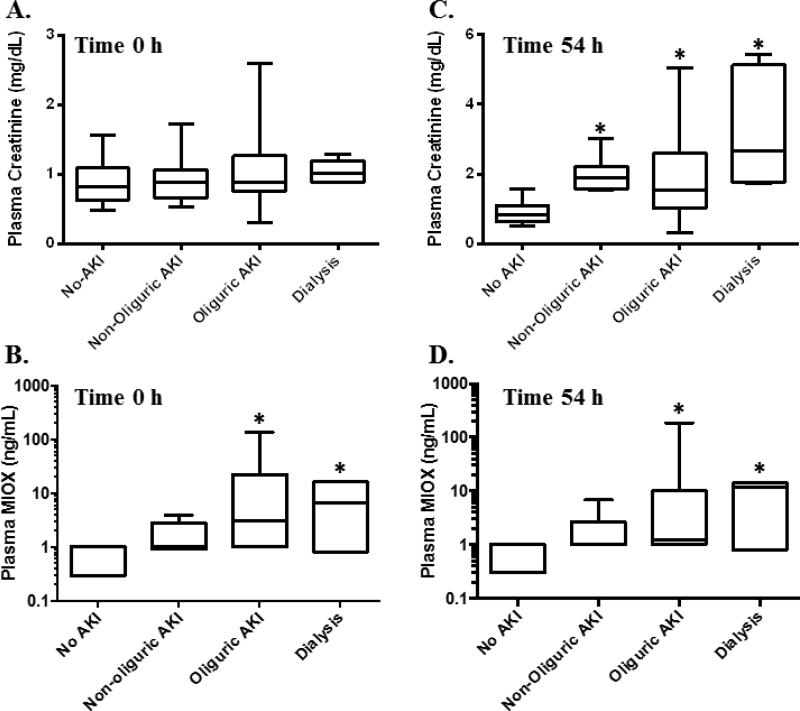
Patients in an intensive care unit (ICU) were screened for AKI defined according to the AKIN criteria (3). Patients were screened for serum creatinine increases of at least 1.5 times baseline or an absolute increase of 0.3 mg/dL over a 1-3 day time period. Once a patient was identified who had an increase in serum creatinine, remnant heparin plasma samples were collected from the Barnes Hospital Clinical Chemistry Laboratory at the time of the serum creatinine increase and 1-3 days before the serum creatinine increase (time 0). Serum creatinine increased an average of 54 ± 3.8 hours (n=33; time 54) after the time 0 sample. The patient characteristics are summarized in Table 1. Oliguria was defined according to the AKIN criteria as <0.5 mL/kg/h for at least 6 hours (3). A total of 42 patients fulfilled criteria for at least stage I AKI defined as a relative increase in serum creatinine of 50% or an absolute increase in serum creatinine of 0.3 mg/dL, and/or a decrease in urine output to <0.5 mL/kg/h for at least 6 hours (3). Of the 42 patients with AKI, 33 had increases in serum creatinine of at least 1.5 times baseline. Nine patients had a urine output of <0.5 ml/kg/h for at least 6 hours without a change in serum creatinine. 17 hospitalized or critically ill patients who did not meet criteria for AKI served as controls. There was no significant difference in age between the patient groups. There were a greater proportion of male patients within the AKI group. Plasma MIOX was measured in critically ill patients with AKI at time 0 h (n=42) and time 54 h (n=37), the average time serum creatinine increased (Figure 5). Samples were not available at the time of serum creatinine increase for 5 patients. Plasma MIOX was significantly elevated in patients with AKI at time 0 (12.4 ± 4.3 ng/mL) and at time 54 (10.1 ± 5.3 ng/mL) relative to controls (0.5 ± 0.3 ng/mL; p=0.002). Patients with oliguric AKI had significantly higher plasma MIOX values at time 0 (20.2 ± 7.5 ng/mL, n=23) and time 54 (17.1 ± 11.0 ng/mL, n=17) compared with controls (Figure 6; p<0.05). Significantly higher plasma MIOX concentrations were observed in patients with dialysis requiring AKI at time 0 (8.2 ± 3.5 ng/mL, n=5) and time 54 (8.2 ± 3.1 ng/mL, n=5) compared with controls (Figure 6; p<0.05). All plasma MIOX concentrations were determined prior to initiation of dialysis. The increase in plasma MIOX preceded the increase in serum creatinine by a mean of 54.3 ± 3.8 hours (n=33).
Tables Patient characteristics. Values are reported as mean ± SEM. Patients designated as No AKI were either hospitalized or critically ill and no significant increases in serum creatinine or decreases in urine output.
| No AKI (n=17) | AKI (n=42) | Non-oliguric AKI (n=14) | Oliguric AKI (n=23) | Dialysis-requiring AKI (n=5) | |
|---|---|---|---|---|---|
| Age (years) | 58 ± 4 | 58 ± 2 | 63 ± 4 | 56 ± 2 | 57 ± 8 |
| M:F | 8:9 | 24:18 | 7:7 | 14:9 | 3:2 |
| Race | |||||
| Caucasian | 14/17 (82%) | 31/42 (74%) | 11/14 (79%) | 16/23 (70%) | 4/5 (80%) |
| African American | 2/17 (12%) | 10/42 (24%) | 2/14 (14%) | 7/23 (30%) | 1/5 (20%) |
| Hispanic | 0 | 1/42 (2%) | 1/14 (7%) | 0 | 0 |
| Unknown | 1/17 (6%) | 0 | 0 | 0 | 0 |
| Peak SCr (mg/dL) | 0.88 ± 0.08 | 2.03 ± 0.17a | 1.95 ± 0.12a | 1.81 ± 0.19a | 3.28 ± 0.78a |
| Peak BUN (g/dL) | 16 ± 2 | 36 ± 3a | 38 ± 4a | 31 ± 2a | 57 ± 20a |
p<0.005 when compared with the control group (Kruskal-Wallis). AKI - acute kidney injury; SCr - serum creatinine; BUN - blood urea nitrogen.
Figure 5.
MIOX in critically ill and hospitalized patients. A. Serum creatinine (SCr) peaked 54.3±3.8 hours (Time 54 h) relative to the preceding SCr measurement (Time 0 h), and was elevated at Time 54 in patients with AKI (**p<0.005). B. Patients with AKI showed higher plasma MIOX concentrations at Time 0 h and Time 54 h compared to patients without AKI (*p=0.002).
Figure 6.
A. Serum creatinine is similar amongst all groups at Time 0h. B. Plasma MIOX increased at Time 0h in patients with oliguric and dialysis-requiring AKI. C. Serum creatinine increased at Time 54h in patients with AKI. D. Plasma MIOX was increased at Time 54h in patients with oliguric and dialysis requiring AKI relative to patients without AKI. *p<0.05.
Discussion
An ideal AKI biomarker should be kidney specific, rapidly detectable following injury, correlate with the degree of damage, and be easily measured. A variety of approaches have been used to identify such a biomarker. In the current study, we first sought to identify kidney specific genes. Since the proximal tubule is the major site of ischemic damage, genes specific to the proximal tubule were targeted. Using differential gene expression profiling, MIOX was identified as a renal specific proximal tubule gene. Tissue specificity of MIOX was confirmed using Western blot. MIOX localized to the renal proximal tubule using immunohistochemistry. Mouse monoclonal anti-MIOX antibodies were developed and characterized. These monoclonal antibodies were optimized for use in a sandwich immunoassay to quantify MIOX in plasma. This assay was used to investigate MIOX as a biomarker of AKI. Mice subjected to bilateral renal ischemiareperfusion showed elevated serum MIOX 24 hours post-injury. Critically ill patients with AKI showed elevated plasma MIOX compared to control patients without AKI. Plasma MIOX was highest in patients with oliguric and dialysis-requiring AKI. The elevation in plasma MIOX occurred approximately 2 days prior to the elevation in serum creatinine.
MIOX is a renal specific enzyme that catalyzes the first committed step in myo-inositol metabolism (38). The MIOX mRNA transcript is reportedly downregulated in a rat model of ischemic AKI (37). In this study, the authors hypothesized MIOX mRNA loss was directly related to the degree of necrosis following renal ischemia. In the current study, demonstration of increased plasma MIOX following AKI may also be related to necrosis of the proximal tubule, although secretion cannot be ruled out.
It is noteworthy that MIOX was not detected in plasma of all patients with AKI defined using the AKIN criteria (3). Since the timing of renal injury was unknown in this patient population, it is possible that an elevation in plasma MIOX may have occurred prior to or after samples were obtained. Since serum creatinine is a non-specific biomarker of renal injury, it is possible that a subset of patients developed elevated serum creatinine in the absence of proximal tubule cell damage. Alternatively, AKI may have occurred in the absence of proximal tubule injury.
Other proteins have been investigated as potential kidney injury biomarkers. Previous studies demonstrated that neutrophil gelatinase associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), α-glutathione-S-transferase (α-GST), liver type fatty acid-binding protein 1 (FABP1) and N-acetyl-β-D-glucosaminidase (NAG) are detectable in urine following renal injury (32, 39, 40). However, none of these has yet translated into clinical use. In contrast to these biomarkers, MIOX is a kidney specific protein. MIOX is also an endogenous kidney protein. Therefore, it may be released more rapidly than the inducible biomarkers KIM-1, NGAL, and FABP1. Measuring these analytes together may provide more detailed information regarding the timing of renal injury. Interestingly, MIOX did not appear to signal non-oliguric kidney injury in this retrospective study. It will be important to confirm this result in a prospective study. Nonetheless, it is most likely that a panel of biomarkers will be necessary to accurately detect AKI, determine severity, and localize the portion of the nephron injured in a given clinical scenario.
There are several limitations to this study. The human samples were collected in a retrospective manner from critically ill and hospitalized patients. The study population was screened for patients with clinical evidence of AKI and is therefore not an accurate representation of all critically ill patients. Since these samples were obtained retrospectively, they were not immediately frozen following collection. Rather, the samples were stored at 4°C for 1-7 days prior to aliquoting and freezing. Although the MIOX immonoassay does not appear to be significantly affected by storing plasma for up to ten days at 4°C, it is important to note that the samples were not all handled identically. This may have introduced artifact into the analysis. Previous studies confirmed the kidney specific nature of MIOX. However, it is unknown whether MIOX expression patterns may change in other tissues following AKI or multi-organ failure. Additional studies are necessary to investigate the tissue expression profile of MIOX in these settings to explore the renal specific nature of its origin.
In conclusion, we developed an immunoassay to quantify the kidney specific protein MIOX in human plasma. We demonstrated that plasma MIOX was elevated in animals and critically ill patients with AKI. In critically ill patients, MIOX increased approximately 2 days prior to the increase in serum creatinine, potentially opening a therapeutic window. Additional studies are warranted to further investigate the potential of MIOX as an early, kidney specific biomarker of AKI.
Supplementary Material
Acknowledgements
We thank Amanda Knoten for technical assistance with animal studies. Studies were supported in part by a Washington University O'Brien Center for Kidney Disease Research Pilot and Feasibility Grant (P30 DK07933-05) to J.P.G. and by R01 DK081644 to S.J.
Abbreviations
- GFR
glomerular filtration rate
- MIOX
myo-inositol oxygenase
- rMIOX
recombinant myo-inositol oxygenase
- AKI
acute kidney injury
- AKIN
Acute Kidney Injury Network
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- PVDF
polyvinylidene difluoride
- BLAST
basic local alignment search text, amino acids are abbreviated using standard one letter codes
- Human genes
myo-inositol oxygenase (MIOX)
- Mouse genes
myo-inositol oxygenase (Miox)
References
- 1.Cruz DN, Ricci Z, Ronco C. Clinical review: Rifle and akin-time for reappraisal. Crit Care. 2009;13:211. doi: 10.1186/cc7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li PK, Burdmann EA, Mehta RL. Acute kidney injury: Global health alert. Kidney Int. 2013;83:372–6. doi: 10.1038/ki.2012.427. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–66. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb J, Ikizler TA. Acute kidney injury: Changing lexicography, definitions, and epidemiology. Kidney Int. 2007;71:971–6. doi: 10.1038/sj.ki.5002224. [DOI] [PubMed] [Google Scholar]
- 6.Palevsky PM. Renal support in acute kidney injury-how much is enough? N Engl J Med. 2009;361:1699–701. doi: 10.1056/NEJMe0907831. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singbartl K, Kellum JA. Aki in the icu: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–25. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 9.Bydash JR, Ishani A. Acute kidney injury and chronic kidney disease: A work in progress. Clin J Am Soc Nephrol. 2011;6:2555–7. doi: 10.2215/CJN.09560911. [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81:442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. Us renal data system 2010 annual data report. Am J Kidney Dis. 2011;57:A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012;81:1172–8. doi: 10.1038/ki.2010.475. [DOI] [PubMed] [Google Scholar]
- 13.Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122:2451–5. doi: 10.1161/CIRCULATIONAHA.110.953851. [DOI] [PubMed] [Google Scholar]
- 14.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 15.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 16.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 17.Bolignano D. Serum creatinine and the search for new biomarkers of acute kidney injury (aki): The story continues. Clin Chem Lab Med. 2012;50:1495–9. doi: 10.1515/cclm-2012-0099. [DOI] [PubMed] [Google Scholar]
- 18.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–5. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 19.Kellum JA, Mehta RL, Levin A, Molitoris BA, Warnock DG, Shah SV, et al. Development of a clinical research agenda for acute kidney injury using an international, interdisciplinary, three-step modified delphi process. Clin J Am Soc Nephrol. 2008;3:887–94. doi: 10.2215/CJN.04891107. [DOI] [PubMed] [Google Scholar]
- 20.Schrier RW. Need to intervene in established acute renal failure. J Am Soc Nephrol. 2004;15:2756–8. doi: 10.1097/01.ASN.0000141324.49873.11. [DOI] [PubMed] [Google Scholar]
- 21.Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, et al. Anaritide in acute tubular necrosis. Auriculin anaritide acute renal failure study group. N Engl J Med. 1997;336:828–34. doi: 10.1056/NEJM199703203361203. [DOI] [PubMed] [Google Scholar]
- 22.Conger JD, Falk SA, Yuan BH, Schrier RW. Atrial natriuretic peptide and dopamine in a rat model of ischemic acute renal failure. Kidney Int. 1989;35:1126–32. doi: 10.1038/ki.1989.100. [DOI] [PubMed] [Google Scholar]
- 23.Denton MD, Chertow GM, Brady HR. “Renal-dose” dopamine for the treatment of acute renal failure: Scientific rationale, experimental studies and clinical trials. Kidney Int. 1996;50:4–14. doi: 10.1038/ki.1996.280. [DOI] [PubMed] [Google Scholar]
- 24.Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, et al. Multicenter clinical trial of recombinant human insulin-like growth factor i in patients with acute renal failure. Kidney Int. 1999;55:2423–32. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi S, Kashihara Y, Ikegami Y, Morimoto K, Miyamoto M, Nakao K. Insulin-like growth factor-i ameliorates transient ischemia-induced acute renal failure in rats. J Pharmacol Exp Ther. 1993;267:919–26. [PubMed] [Google Scholar]
- 26.Tumlin JA, Finkel KW, Murray PT, Samuels J, Cotsonis G, Shaw AD. Fenoldopam mesylate in early acute tubular necrosis: A randomized, doubleblind, placebo-controlled clinical trial. Am J Kidney Dis. 2005;46:26–34. doi: 10.1053/j.ajkd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP. A trial of thyroxine in acute renal failure. Kidney Int. 2000;57:293–8. doi: 10.1046/j.1523-1755.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- 28.Devarajan P. Review: Neutrophil gelatinase-associated lipocalin: A troponinlike biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 29.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, et al. Renal biomarker qualification submission: A dialog between the fda-emea and predictive safety testing consortium. Nat Biotechnol. 2010;28:455–62. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 30.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Nephron Clin Pract. 2008;109:c192–7. doi: 10.1159/000142928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810–20. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 33.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crimmins DL, Brada NA, Lockwood CM, Griest TA, Waldemer RJ, Cervinski MA, et al. Etrap (efficient trapping and purification) of target protein polyclonal antibodies from gst-protein immune sera. Biotechnol Appl Biochem. 2010;57:127–38. doi: 10.1042/BA20100279. [DOI] [PubMed] [Google Scholar]
- 35.Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, Ladenson JH. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–21. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 36.Gaut JP, Crimmins DL, Lockwood CM, McQuillan JJ, Ladenson JH. Expression of the na(+)/k(+)-transporting atpase gamma subunit fxyd2 in renal tumors. Mod Pathol. 2012 doi: 10.1038/modpathol.2012.202. [DOI] [PubMed] [Google Scholar]
- 37.Hu E, Chen Z, Fredrickson T, Gellai M, Jugus M, Contino L, et al. Identification of a novel kidney-specific gene downregulated in acute ischemic renal failure. Am J Physiol Renal Physiol. 2000;279:F426–39. doi: 10.1152/ajprenal.2000.279.3.F426. [DOI] [PubMed] [Google Scholar]
- 38.Thorsell AG, Persson C, Voevodskaya N, Busam RD, Hammarstrom M, Graslund S, et al. Structural and biophysical characterization of human myoinositol oxygenase. J Biol Chem. 2008;283:15209–16. doi: 10.1074/jbc.M800348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T, et al. Urinary fatty acid-binding protein 1: An early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296:F669–79. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543–51. doi: 10.1093/ndt/18.3.543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.