Abstract
Victoria blight of Avena sativa (oat) is caused by the fungus Cochliobolus victoriae, which is pathogenic because of the production of the toxin victorin. The victorin-induced response in sensitive A. sativa has been characterized as a form of programmed cell death (PCD) and displays morphological and biochemical features similar to apoptosis, including chromatin condensation, DNA laddering, cell shrinkage, altered mitochondrial function, and ordered, substrate-specific proteolytic events. Victorin-induced proteolysis of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is shown to be prevented by caspase-specific and general protease inhibitors. Evidence is presented for a signaling cascade leading to Rubisco proteolysis that involves multiple proteases. Furthermore, two proteases that are apparently involved in the Rubisco proteolytic cascade were purified and characterized. These proteases exhibit caspase specificity and display amino acid sequences homologous to plant subtilisin-like Ser proteases. The proteases are constitutively present in an active form and are relocalized to the extracellular fluid after induction of PCD by either victorin or heat shock. The role of the enzymes as processive proteases involved in a signal cascade during the PCD response is discussed.
INTRODUCTION
Victoria blight of Avena sativa (oat) is caused by the necrotrophic fungus, Cochliobolus victoriae (Meehan and Murphy, 1946), which is pathogenic because of the production of the host-specific toxin, victorin (Meehan and Murphy, 1947). Isolates of C. victoriae that produce victorin are pathogenic on susceptible A. sativa (Meehan and Murphy, 1947), whereas mutants or outcrosses that do not produce the toxin are nonpathogenic. Host susceptibility and victorin sensitivity are conferred by a dominant allele at the Vb locus (Litzenberger, 1949). Homozygous recessive genotypes (vb/vb) are victorin insensitive and resistant to the fungus. The interaction between victorin and the Vb gene product induces a response in A. sativa that displays characteristics of programmed cell death (PCD) (Navarre and Wolpert, 1999; Tada et al., 2001; Yao et al., 2001; Curtis and Wolpert, 2002, 2004).
PCD is a genetically controlled, organized form of cellular suicide that functions in eliminating unnecessary or aged cells. It is essential for cellular maturation and morphogenesis and is required to maintain cellular homeostasis in multicellular organisms. In addition, improper regulation of PCD has been implicated in a wide variety of animal diseases (Polverini and Nör, 1999; Wang and Wang, 1999).
PCD also has been associated with several processes in plants, including senescence (Bleecker and Patterson, 1997; Miller et al., 1999; Schmid et al., 2001), stress (Katsuhara, 1997; Solomon et al., 1999), development (Runeberg-Roos and Saarma, 1998; Groover and Jones, 1999; Schmid et al., 1999), and the hypersensitive response (HR) to pathogens (Dangl et al., 1996; Mittler et al., 1997; Pontier et al., 1998; Mackey et al., 2002; Abramovitch et al., 2003). Currently, very little is known about the fundamental processes that control and regulate PCD in plants.
Apoptosis, the most characterized form of PCD, has been extensively studied in animal systems and can be distinguished by unique characteristics. Cells undergoing apoptosis display morphological changes, including cell shrinkage, chromatin condensation, and apoptotic body formation. Biochemically, apoptotic cells exhibit DNA fragmentation (also referred to as DNA laddering) and activation of a family of Cys proteases called caspases (cysteine aspartases) (reviewed in Vaux and Korsmeyer, 1999; Hengartner, 2000). The name caspase is derived from their active site residue (which is a Cys) and substrate specificity (caspases cleave only after aspartate residues, aspase).
The victorin-induced PCD response in sensitive A. sativa demonstrates similar morphological and biochemical traits to animal apoptosis, including cell shrinkage and collapse (Yao et al., 2001; Curtis and Wolpert, 2004), chromatin condensation (Yao et al., 2001), DNA laddering (Navarre and Wolpert, 1999; Tada et al., 2001), mitochondrial depolarization and permeability transition (Curtis and Wolpert, 2002, 2004), and ordered, substrate-specific proteolytic events (Navarre and Wolpert, 1999). Furthermore, victorin-induced PCD in A. sativa is easily initiated, proceeds in a rapid and synchronous manner, and appears to encompass at least all leaf mesophyll cells. Therefore, victorin treatment of A. sativa provides an appropriate system in which to study the mechanism and progression of plant PCD.
The proteolytic cleavage of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) has been identified as a specific PCD-induced event in victorin-treated A. sativa cells (Navarre and Wolpert, 1999). Rubisco cleavage occurs after the first 14 amino acids (apparently after glutamate-14) and is prevented by Cys protease inhibitors (Navarre and Wolpert, 1999). Rubisco degradation has been identified as a characteristic of senescence (Weidhase et al., 1987; Ferreira and Davies, 1989), also a form of PCD, and has been shown to occur in A. sativa chloroplast after oxidative stress (Casano and Trippi, 1992), a treatment shown to induce PCD (Amor et al., 1998; Solomon et al., 1999). Additionally, chloroplast-localized proteases have been reported that appear to recognize Rubisco as a substrate (Bushnell et al., 1993; Casano et al., 1994). These data indicate that a specific proteolytic process is required to degrade Rubisco and that this process is common to several types of PCD.
Proteolytic alteration of key cellular proteins is a fundamental characteristic of animal apoptosis and is executed by the highly specialized caspases. Multiple cellular targets exist for caspases, all of which are directly or indirectly involved in the ordered disassembly of the cell. Two types of caspases exist, initiator and effector caspases. Initiator caspases are activated by autoproteolysis, after which they are able to proteolytically activate effector caspases. Effector caspases target cellular proteins, such as poly(ADP-ribose) polymerase (PARP), which is proteolytically inactivated, resulting in a signature 89-kD fragment (reviewed in Solary et al., 1998). In addition, CAD, a caspase-activated DNase, is indirectly activated when an effector caspase cleaves ICAD (inhibitor of CAD), allowing CAD to disassociate and process DNA into nucleosomal fragments (reviewed in Nagata, 2000). Caspase proteolysis typifies the ordered processive nature of apoptosis because caspases function with exquisite control, cleaving at limited site(s) found within specifically targeted key proteins, differing from the degradative role of other proteolytic pathways required during apoptosis (e.g., the proteasome-ubiquitin pathway of protein degradation) (Solary et al., 1998).
Current research has demonstrated that caspase-specific inhibitors can prevent different forms of plant PCD, suggesting that caspase-like enzymes may be involved in these responses (reviewed in Woltering et al., 2002). For example, Tobacco mosaic virus–induced HR in Nicotiana tabacum leaves was prevented by treatment with short peptide inhibitors that preferentially inhibit caspase-1 and caspase-3 proteases (del Pozo and Lam, 1998). Other forms of PCD in N. tabacum, including those induced by bacteria (Richael et al., 2001) and treatment with a fungal elicitor (Elbaz et al., 2002), also were prevented by caspase inhibitors. Similar results were obtained with caspase inhibitors in preventing or reducing cell death after treatment with camptothecin (De Jong et al., 2000), staurosporine (Elbaz et al., 2002), and isopentenyladenosine (Mlejnek and Procházka, 2002). In addition, heat shock–induced PCD in N. tabacum suspension cells stimulated cleavage of PARP and activation of a caspase-3–like protease (Tian et al., 2000), whereas N. tabacum protoplast treated with menadione displayed PARP cleavage (Sun et al., 1999). However, to date, no protease that recognizes caspase substrates or is inhibited by caspase inhibitors has been identified in plants.
Studies have described proteases associated with several types of plant PCD, including senescence (Delorme et al., 2000; Schmid et al., 2001; Eason et al., 2002), oxidative stress (Solomon et al., 1999), seed development (Schmid et al., 1998, 1999; Wan et al., 2002), tracheary element development (Runeberg-Roos and Saarma, 1998; Groover and Jones, 1999), and the HR (Vera and Conejero, 1988; D'Silva et al., 1998; Krüger et al., 2002). However, characterization of these proteases reveals that they are degradative, not processive enzymes; thus, they are unlike caspases or other proteases involved in signaling pathways. A degradative nature suggests that their role in PCD may be associated with the terminal decomposition of the dying cell but not in the initiation or progression of the PCD signal.
This research describes the purification and characterization of two proteases that possess caspase-like specificity but contain an active-site Ser residue. Therefore, because of their aspartate specificity (aspase) and active-site Ser residue, they have been termed saspases. The saspases, purified from A. sativa, contain amino acid sequence similarities to subtilisin-like Ser proteases from plants and likely function in a PCD-induced signaling cascade involving other proteases that leads to the proteolytic processing of Rubisco. They also are constitutively present in the cell, not being transcriptionally or translationally activated during the response, but released into the extracellular fluid (ECF) upon induction of PCD. Heat shock–induced PCD also is further characterized and displays similar biochemical features as victorin-induced PCD, including DNA laddering, Rubisco proteolysis, and release of the saspases into the ECF. These results and the implications of numerous subtilisin-like Ser proteases involved in plant disease, development, and death are discussed.
RESULTS
Evidence for a Protease Cascade Leading to Rubisco Proteolysis
Rubisco proteolysis is a characteristic of victorin-induced PCD and has been shown to be inhibited by two general protease inhibitors, E-64 (a Cys protease inhibitor) and leupeptin (a Cys and Ser protease inhibitor) (Navarre and Wolpert, 1999). Two caspase and a granzyme B inhibitor were tested for their effects on Rubisco proteolysis. These and a large variety of other short peptide inhibitors (and corresponding substrates) were designed based on the caspase recognition sites in known substrates. These inhibitors are typically preferential for a given caspase but can often inhibit a wide variety of caspases (Kidd, 1998). One inhibitor initially evaluated, Ac-VAD-CMK, is considered a pan-caspase inhibitor because it can inhibit most caspases (Thornberry et al., 1997; Kidd, 1998; Ekert et al., 1999), whereas another inhibitor, Ac-DEVD-CMK, is more specific for caspase-3 (Thornberry et al., 1997; Ekert et al., 1999). In addition, we tested Z-AAD-CMK, which inhibits granzyme B (Ekert et al., 1999), a Ser protease that specifically cleaves peptide bonds after aspartate residues (the recognition sequence for the three inhibitors is in single-letter amino acid code). Preincubation of peeled A. sativa leaves with the caspase and granzyme B inhibitors prevented victorin-induced Rubisco proteolysis (Figure 1). The effectiveness of these inhibitors was similar to that of E-64 and leupeptin.
Figure 1.
Inhibition of Rubisco Proteolysis with Protease Inhibitors.
Leaf segments with epidermis removed were pretreated with 200 μM of the indicated inhibitor for 2 h then with 20 ng/mL of victorin for 4 h. Coomassie blue–stained 12% SDS-PAGE of total protein extract. Only the region containing Rubisco is shown.
The inhibition of Rubisco proteolysis by caspase-specific inhibitors suggested that a caspase-like protease(s) might be present in victorin-treated A. sativa tissue. Total soluble protein was extracted from 60 g of victorin-treated A. sativa leaves, and a low level of proteolytic activity that hydrolyzed the pan-caspase substrate Z-VAD-AFC was observed (data not shown). To concentrate the activity, the soluble protein extract was run through hydrophobic interaction chromatography (HIC) (see Methods). Two distinct proteolytic activities were separated during chromatography: activity A (Figure 2A, fractions 56 to 67), which hydrolyzed Z-DEVD-AFC (a caspase-3 substrate) and eluted at ∼0.45 M ammonium sulfate, and activity B (Figure 2A, fractions 76 to 100), which hydrolyzed Z-VAD-AFC and eluted at ∼0.3 M ammonium sulfate. The fractions containing each activity were individually combined for further analysis. Activity A hydrolyzed the substrate Z-DEVD-AFC but not Z-VAD-AFC or Z-AAD-AFC (a substrate for granzyme B) (Figure 2B), whereas activity B hydrolyzed Z-VAD-AFC and Z-AAD-AFC but not Z-DEVD-AFC (Figure 2C). Figure 2D illustrates the percentage of inhibition of activities A and B by the protease inhibitors used to prevented Rubisco proteolysis in Figure 1. Z-VAD-AFC hydrolysis by activity B was completely inhibited by the caspase inhibitors Ac-VAD-CMK and Z-AAD-CMK but was unaffected by Ac-DEVD-CMK, leupeptin, and E-64 (Figure 2D, open bars). However, activity A hydrolysis of Z-DEVD-AFC was only inhibited by Ac-DEVD-CMK (Figure 2D, closed bars). These data suggest that there are two distinct caspase-like protease activities involved in the proteolytic processing of Rubisco because granzyme B and both caspase-specific inhibitors all prevent Rubisco proteolysis in vivo, whereas two of the inhibitors inhibit only activity B, and the other inhibits only activity A. Furthermore, because neither caspase-like activity is inhibited by leupeptin or E-64, both of which prevent Rubisco proteolysis, a third non-caspase-like protease is also likely involved. Thus, a proteolytic cascade involving at least three proteases, two caspase-related and a non-caspase-like protease, is apparently activated during victorin-induced PCD, leading to the proteolytic processing of Rubisco.
Figure 2.
Characterization of Two Caspase-Like Proteolytic Activities.
(A) Activity profile of fractions from a HIC column. The protease in activity A was assayed by incubating 50 μL of each fraction with 50 μL of 20 mM Mops, pH 7, with 20 μM substrate for 2 h. Activity B was assayed as described in Methods. RFU, relative fluorescence units.
(B) Substrate profile of activity A. Activity A was assayed as described above for proteolytic activity with indicated substrates. RFU, relative fluorescence units.
(C) Substrate profile of activity B. RFU, relative fluorescence units.
(D) Inhibitor profile of activities A and B. Samples were pretreated with inhibitor (200 μM) for 2 h and then assayed for hydrolytic activity with Z-DEVD-AFC (activity A) or with Z-VAD-AFC (activity B). Expressed as percent inhibited of noninhibited control.
Reversible and Irreversible Inhibition by Caspase Inhibitors
The protease(s) constituting activity B from the HIC column, which hydrolyzed Z-VAD-AFC and Z-AAD-AFC, displayed the highest levels of activity; therefore, this activity was selected for further characterization. No further characterization of activity A, which hydrolyzed Z-DEVD-AFC, was performed in this study. Initial attempts to visualize the activity B protease with the biotinylated caspase inhibitor biotin-VAD-FMK on a biotin blot were unsuccessful. Biotin-VAD-FMK prevented Z-VAD-AFC hydrolysis but did not covalently label the protein (data not shown). Three types of inhibitors with the same tripeptide recognition motif, Ac-VAD-CMK, Z-VAD-FMK, and Ac-VAD-CHO, were tested for the nature of their binding to the protease (Figure 3A). Chloromethylketone (CMK)-based inhibitors irreversibly inhibit both Cys and Ser proteases, whereas FMK (fluoromethylketone)-based inhibitors irreversibly inhibit Cys proteases but reversibly inhibit Ser proteases. CHO (aldehyde)-based inhibitors reversibly inhibit both Cys and Ser proteases. All three of these inhibitors inhibited activity of the protease by at least 80% when incubated for 2 h (Figure 3A, initial column). However, after two washes, inhibition was lost from the FMK- and CHO-based inhibitors, indicating reversible inhibition (Figure 3A, 1st wash and 2nd wash columns). Only the CMK-based inhibitor irreversibly inhibited activity, indicating that the caspase-like protease is a Ser, not a Cys, protease. These results were later confirmed by amino acid sequencing (discussed below). Because the protease(s) with caspase-like hydrolytic activity in activity B is a Ser protease, we referred to this enzyme(s) as a saspase.
Figure 3.
The Caspase-Like Protease Is an 84-kD Ser Protease.
(A) Reversibility of inhibition of the protease in activity B. Three distinct inhibitors all containing the VAD recognition motif were incubated at a concentration of 200 μM with activity B for 2 h. Samples were washed twice in buffer then reconstituted to original volume. After initial incubation and after each wash, aliquots were removed and assayed for Z-VAD-AFC hydrolytic activity. Irreversible inhibition of the protease was achieved only with the CMK-based inhibitor.
(B) Biotin blot of the protease in activity B. Each lane contains a 30-μL sample of activity B from the HIC column. Samples were preincubated 2 h with a nonbiotinylated inhibitor (lanes 2 and 3) then incubated 2 h with biotin-YVAD-CMK (lanes 1 to 3). The caspase-like protease shows specific binding to biotin-YVAD-CMK because binding is competitive with Z-VAD-CMK, an inhibitor, but not Z-DEVD-CMK, which does not inhibit activity. Only the portion of the blot containing the 84-kD protein is shown.
The biotinylated inhibitor biotin-YVAD-CMK irreversibly binds and inhibits the activity of the saspase (activity B). However, because of the highly reactive nature of CMKs (Ekert et al., 1999), biotin-YVAD-CMK labeled numerous proteins in the fractions containing activity B from the HIC column. To determine if labeling of any of these proteins was specific, that is, if biotin-YVAD-CMK binds to the active site of one of these proteins, a nonbiotinylated inhibitor that also inhibits saspase activity (binds to the active site) was preincubated with the protein sample to identify proteins whose biotin-YVAD-CMK binding was outcompeted/blocked. Preaddition of Ac-VAD-CMK outcompeted binding of biotin-YVAD-CMK to an 84-kD protein (Figure 3B, lane 2) but did not affect labeling of any other proteins (data not shown). Conversely, preaddition of Ac-DEVD-CMK, which does not inhibit saspase (activity B) activity, did not block binding of biotin-YVAD-CMK to the 84-kD protein (Figure 3B, lane 3). Furthermore, other inhibitors that inhibit saspase activity (e.g., Z-AAD-CMK and Ac-IETD-CHO, discussed below) also were able to outcompete biotin-YVAD-CMK binding, whereas additional inhibitors that did not prevent activity (e.g., E-64 and leupeptin) did not outcompete binding (data not shown). The ligand-specific labeling of the 84-kD protein corresponds to the substrate and inhibitor specificity identified for the saspase, suggesting that the labeled 84-kD protein is the saspase. These results were later confirmed during purification of the saspase (see below).
Extracellular Localization of the Saspase after Victorin Treatment
Infiltration of victorin into unpeeled leaves caused the release of the saspase into the ECF. The use of leaves with the epidermis removed provides a very sensitive and rapid assay of victorin activity (Figure 1). However, because this assay cannot be used for the collection of the ECF, a different assay was developed. In this new assay, victorin was directly infiltrated into whole leaf segments, thus allowing the ECF to be removed by centrifugation. However, this leaf infiltration assay is less sensitive. Infiltration of victorin at 1 μg/mL resulted in the first observation of Rubisco proteolysis and DNA laddering (discussed below) at 8 h after infiltration. Observation of levels of Rubisco proteolysis and DNA laddering comparable to that seen after 4-h incubation of peeled A. sativa leaf segments in 20 ng/mL of victorin were not seen until 15 h after infiltration (data not shown). Thus, the relative rates of Rubisco proteolysis and DNA laddering and, presumably, release of the saspases into the ECF are different in the two assays.
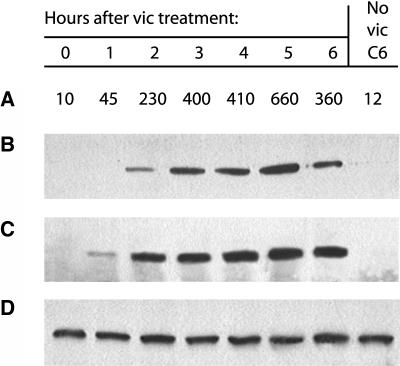
ECF was collected from victorin-infiltrated leaves at 0 to 6 h after infiltration with victorin, and then saspase activity was measured by Z-VAD-AFC hydrolysis (Figure 4A). Protease activity was first detected at 1 h and was maximal at 5 h. The same ECF samples were incubated with 200 μM biotin-YVAD-CMK for 2 h, separated by SDS-PAGE, and blotted for biotin visualization. The appearance of the 84-kD saspase band in the ECF (Figure 4B) coincided with hydrolytic activity. Four-centimeter leaf sections were used for extraction of the ECF, and typically, eight leaf sections were used per treatment/experiment. For the first 5 h (the time when maximum saspase activity was recovered), the quantity of ECF recovered in untreated leaves ranged from 6.5 to 8 μL/leaf segment and averaged 7.5 μL (n = 56), whereas toxin-treated leaf segments ranged from 6.5 to 10.75 μL/leaf segment and averaged 9 μL (n = 52). Slightly more ECF was consistently recovered from toxin-treated leaf segments at all time points likely because of the fact that toxin causes substantial leakage of electrolytes (e.g., Wheeler and Black, 1962; Sammadar and Scheffer, 1968), which would increase the water potential of treated cells.
Figure 4.
Release of the Saspase into the ECF after Victorin Treatment.
(A) and (B) Leaves were infiltrated with 1 μg/mL of victorin (vic) or water (no victorin control), and the ECF was removed at the indicated time.
(A) Z-VAD-AFC hydrolytic activity in the ECF. Activity expressed as relative fluorescence units.
(B) Biotin blot of the ECF. Twenty microliters of each sample was incubated with 200 μM biotin-YVAD-CMK for 2 h before electrophoresis and blotting.
(C) and (D) Leaves were preinfiltrated with 400 μM biotin-YVAD-CMK, preincubated 2 h, then infiltrated with 1 μg/mL of victorin mixed with 100 μM biotin-YVAD-CMK.
(C) Biotin blot of the ECF.
(D) Biotin blot of total protein extract. Note that the saspase is uniformly labeled in all samples including the water only/no victorin control (last lane).
Biotin-YVAD-CMK prevented Rubisco proteolysis with the same effectiveness as the other caspase inhibitors (data not shown), and furthermore, infiltration of leaves with biotin-YVAD-CMK provided in vivo labeling of the saspase. In Figure 4C, the ECF was collected from leaves that were preinfiltrated with 400 μM biotin-YVAD-CMK for 2 h and then infiltrated with 1 μg/mL of victorin mixed with 100 μM biotin-YVAD-CMK. Accumulation of the saspase in the ECF (Figure 4C) followed approximately the same pattern as shown in Figure 4B (activity could not be measured because of inhibition by biotin-YVAD-CMK). Analysis of total cellular protein prelabeled with biotin-YVAD-CMK (Figure 4D) indicates a constant level of saspase labeling throughout all time points, including the 6 h no victorin control (Figure 4D, last lane). These results suggest that transcriptional or translational activation of the saspase is apparently not required. In addition, treatment of leaves with cyclohexamide before victorin treatment had no effect on accumulation of the saspase in the ECF (data not shown).
Heat Shock–Induced PCD Is Similar to Victorin-Induced PCD
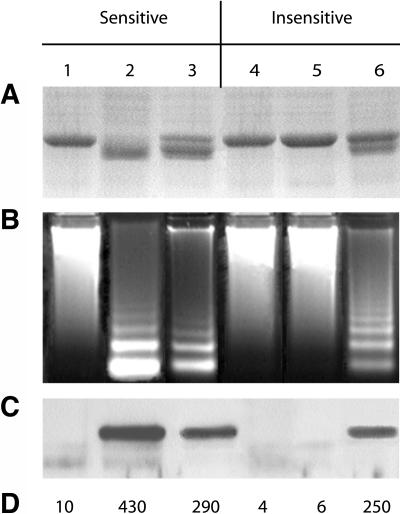
Heat shock has been successfully used to induce PCD in Cucumis sativus (cucumber) (Balk et al., 1999) and N. tabacum (Tian et al., 2000). We wanted to ascertain weather or not heat shock could induce characteristics similar to victorin-induced PCD in A. sativa. Victorin-sensitive and -insensitive leaves were infiltrated with water (Figures 5A to 5D, lanes 1 and 4), 1000 ng/mL of victorin (Figures 5A to 5D, lanes 2 and 5), or heat shock (45°C for 1 h) (Figures 5A to 5D, lanes 3 and 6). The ECF was collected 4 h after start of the treatment, and protein and DNA samples were collected after 15 h. Heat shock induces the typical characteristics of victorin-induced PCD in victorin-sensitive and -insensitive A. sativa, including Rubisco proteolysis (Figure 5A, lanes 3 and 6), DNA laddering (Figure 5B, lanes 3 and 6), and release of the 84-kD saspase into the ECF (Figures 5C and 5D, lanes 3 and 6). The water controls for both sensitive (Figure 5, lane 1) and insensitive (Figure 5, lane 4) leaves did not display any of the characteristics associated with PCD, nor did victorin-treated insensitive leaves (Figure 5, lane 5). Heat shock–induced Rubisco proteolysis, like that induced by victorin, was prevented by the caspase inhibitors Ac-VAD-CMK and Ac-DEVD-CMK, the granzyme B inhibitor Ac-AAD-CMK, and the general Cys protease inhibitors E-64 and leupeptin (Figure 6).
Figure 5.
Heat Shock–Induced PCD in Victorin-Sensitive and -Insensitive A. sativa.
Victorin-sensitive and -insensitive A. sativa leaves were infiltrated with water (lanes 1, 3, 4, and 6) or 1 μg/mL of victorin (lanes 2 and 5). Water-infiltrated leaves in lanes 3 and 6 were heat shocked for 60 min at 45°C and then incubated at 25°C for indicated times below.
(A) Coomassie blue–stained SDS-polyacrylamide gel of total protein extracted 15 h after treatment. Rubisco cleavage is seen in sensitive leaves treated with victorin and sensitive and insensitive leaves that were heat shocked. Only the region containing Rubisco is shown.
(B) 1.5% agarose gel of DNA extracted 15 h after treatment.
(C) Biotin blot of ECF collected 4 h after treatment. Samples were incubated with 200 μM biotin-YVAD-CMK for 2 h before electrophoresis.
(D) Z-VAD-AFC hydrolytic activity in the ECF. Activity expressed as relative fluorescence units.
Figure 6.
Inhibition of Heat Shock–Induced Rubisco Proteolysis.
A. sativa leaves were preincubated with 400 μM of indicated inhibitor for 2 h and then heat shocked for 60 min at 45°C (sample in lane 1 did not undergo heat shock). Proteins were isolated 15 h after heat shock. Only the region containing Rubisco is shown.
Purification of Saspase-1 and Saspase-2
The purification protocol from 200 g of victorin-treated (100 ng/mL) A. sativa leaves is summarized in Table 1. The proteolytic activity in the crude extract and the 50% ammonium sulfate fraction was either too dilute to obtain an accurate measurement of activity or contained an inhibitory compound because total activity (Table 1, column 3) more than doubled after the HIC column. Anion exchange chromatography separated the saspase activity into two closely eluting peaks (Figure 7): the first was termed Saspase-1 (SAS-1) and the second Saspase-2 (SAS-2). SAS-1 and SAS-2 were purified to homogeneity by size exclusion chromatography and both migrated in an SDS-PAGE with an apparent mass of 84 kD, as detected by silver staining (Figure 8).
Table 1.
Purification of SAS-1 and SAS-2 from 200 g of Victorin-Treated Leaves
| Purification Step | Total Protein (mg) | Total Activity (pmol/min)a | Specific Activity (pmol/min/mg)a | Purification Fold | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 812 | 1577.6 | 1.9 | 1 | 100 |
| (NH4)2SO4 pellet | 380 | 1520.0 | 4.0 | 2.1 | 96 |
| HIC column | 45.46 | 3957.4 | 87.1 | 44.8 | 250 |
| Heparin affinity | 12 | 2502.4 | 208.5 | 107.4 | 158 |
| Anion exchange | |||||
| SAS-1 | 0.082 | 844.3 | 10296.3 | 5307.4 | 53.5 |
| SAS-2 | 0.106 | 1087.7 | 10261.3 | 5289.3 | 68.9 |
| Size exclusion | |||||
| SAS-1 | 0.042 | 573.9 | 13664.3 | 7043.5 | 36.4 |
| SAS-2 | 0.054 | 739.4 | 13692.6 | 7058.1 | 46.9 |
1 pmol of substrate cleavage = 6.024 fluorescence units.
Figure 7.
Anion Exchange Chromatography Separated Two Distinct Peaks of Saspase Activity.
Silver stained gel of active fractions (20 μL loaded/lane) eluted off the anion exchange column. Z-VAD-AFC hydrolytic activity of each sample is indicated below the gel, expressed as relative fluorescence units. Fractions 42 to 52 (SAS-1) and 54 to 64 (SAS-2) were pooled for individual purification of SAS-1 and SAS-2 by size exclusion chromatography.
Figure 8.
Electrophoresis of SAS-1 and SAS-2 after Purification by Size Exclusion Chromatography.
Silver stained gel of purified SAS-1 and SAS-2; 15 μL of active fraction was loaded per lane.
SAS-1 and SAS-2 Are Subtilisin-Like Ser Proteases
NH2-terminal amino acid sequencing was performed on gel-purified SAS-1 and SAS-2 after anion exchange chromatography. SAS-1 and SAS-2 possessed nearly identical NH2-terminal sequences, with the only difference being the lack of the first Thr residue at the NH2 terminus of SAS-1 (Table 2). Consistent with the inhibitor studies implicating a Ser protease, the protein sequence of the NH2 terminus of both SAS-1 and SAS-2 were homologous to the mature NH2 terminus of several plant subtilisin-like Ser proteases (Table 2). Additional sequence information was obtained from three internal peptides produced by a partial tryptic digest of SAS-2 (no internal sequence data was obtained from SAS-1). These three peptides also exhibited homology to plant subtilisin-like Ser proteases (Table 2). A putative subtilisin-like Ser protease from rice (Oryza sativa) was the most similar protein identified (77.4%; 48 out of 62 amino acids identical).
Table 2.
Comparison of Partial Amino Acid Sequence of SAS-1 and SAS-2 with Other Known Subtilisin-Like Ser Proteases
| Protein | Amino Terminus | Internal Peptide 1 |
|---|---|---|
| SAS-1 | -THTPEFLGLSAAGG-LWEAS-EYG | - - - - - - - - - - - |
| SAS-2 | TTHTPEFLGLSAAGG-LWEAS-EYG | VHPDWSPAAVR |
| Rice subtilisin | 109-TTHTPEFLGVSGAGG-LWETA-SYG-133 | 545-VHPEWSPAAIR-556 |
| P69A | 115-TTHTSSFLGLQQNMG-VWKDS-NYG-137 | 548-THPDWSPAAIK-558 |
| P69B | 115-TTHTPSFLGLQQNMG-VWKDS-NYG-137 | 547-THPDWSPAVIK-557 |
| P69C | 115-TTHTPSFLGLQQNMG-LWKDS-NYG-137 | 547-SHPDWSPAVIK-557 |
| ARA12 | 107-TTRTPLFLGLDEHTADLFPEAGSYS-131 | 558-VHPEWSPAAIR-568 |
| Cucumisin | 111-TTRSWDFLGFPLTVP- - - RRS-QVE-131 | 541-YNPTWSPAAIK-551 |
| Protein | Internal Peptide 2 | Internal Peptide 3 |
| SAS-1 | - - - - - - - - - - - - - - - | - - - - - - - - - - - - - |
| SAS-2 | EVTNVGDGPASYTAK | SPIVATTASSTPF |
| Rice subtilisin | 674-VVTNVGAGAASYRAK-688 | 748-SPIVATTLSSTRL-760 |
| P69A | 667-TVTNVGDAKSSYKVE-681 | 737-SPI-ALLLIQ- - - - 745 |
| P69B | 666-TVTNVGDATSSYKVE-680 | 738-SPI-AVV-SA- - - - 745 |
| P69C | 667-TVTNVGDAKSSYTVQ-681 | 739-SPI-AVEFALATK-750 |
| ARA12 | 666-NVDGVGAYKYTRTVT-679 | 750-SP-VAISWT- - - - - 757 |
| Cucumisin | 655-TLTSVAPQASTYRAM-670 | 723-SPITITSLV- - - - - 731 |
Rice subtilisin, putative subtilisin-like protease (accession number BAB89803). P69A, P69B, and P69C, subtilisin-like proteases from L. esculentum (accession numbers CAA76724, CAA76725, and T06577, respectively). ARA12, subtilisin-like protease from Arabidopsis (accession number NP_569048). Cucumisin, subtilisin-like protease form Cucumis melo (accession number A55800). Identical amino acids are in bold.
Characterization and Comparison of SAS-1 and SAS-2
Matrix-assisted laser-desorption ionization time of flight (MALDI-TOF) mass spectrometry performed on purified SAS-1 and SAS-2 indicated that both masses were ∼74,500 D ± 1900 D (Figure 9). Because of the size of the protein analyzed and the sensitivity of the instrument, the peak width was too large to obtain a more accurate measurement of mass. Nevertheless, these data indicate that the actual molecular mass of both SAS-1 and SAS-2 is ∼10,000 D smaller than that indicated by their migration in SDS-PAGE (cf. Figures 7 and 8 with Figure 9).
Figure 9.
Molecular Mass of both SAS-1 and SAS-2 Is ∼74.5 kD as Indicated by Mass Spectrometry.
Spectrograph of purified SAS-1 (top spectrograph) and SAS-2 (bottom spectrograph) were individually analyzed by MOLDI-TOF mass spectrometry. First peak is the doubly charged ion, and the second peak is the singly charged ion.
Peptide maps were created from MALDI-TOF mass spectrometry analysis of a partial tryptic digest of both SAS-1 and SAS-2 (Figure 10). A comparison of the two peptide maps indicate that SAS-1 and SAS-2 have in common all major peaks in the profile, and furthermore, the mass of the peaks are nearly identical. Two major peaks with mass differences are indicated with arrows in Figure 10. In both cases, the mass of the peak is larger in SAS-1, with peak A having a mass difference of 16.1 D and peak B of 14.21 D. This size variation may indicate a single amino acid substitution or a difference in posttranslational modifications.
Figure 10.
Peptide Maps of SAS-1 and SAS-2.
Partial tryptic digest of SAS-1 and SAS-2 analyzed by MALDI-TOF mass spectrometry. Major peptide peaks with molecular mass differences between SAS-1 and SAS-2 are indicated by an arrow. m/z, mass-to-charge ratio.
The effect of pH on hydrolytic activity was assayed for SAS-1 and SAS-2 with Z-VAD-AFC as a substrate. Figure 11 illustrates that maximal activity for both enzymes is observed at pH 6.5, and SAS-1 and SAS-2 also share similar patterns of activity as the pH is increased or decreased.
Figure 11.
Effects of pH on Hydrolytic Activity of SAS-1 and SAS-2.
Partially purified SAS-1 and SAS-2 (post-anion exchange chromatography) were assayed for hydrolytic activity at different pHs with the substrate Z-VAD-AFC. RFU, relative fluorescence units.
Salts generally enhanced saspase activity. The effects of different salts on Z-VAD-AFC hydrolysis by SAS-1 and SAS-2 are presented in Table 3. Monovalent salts (i.e., NaCl and KCl) were less efficient at stimulating hydrolysis than divalent salts (i.e., MgCl2 and CaCl2). The heavy metal salt ZnCl2 inhibited activity with or without the addition of NaCl.
Table 3.
Effect of Different Salts on Hydrolytic Activity of SAS-1 and SAS-2
| Activity
|
|||
|---|---|---|---|
| Salt | Concentration | SAS-1 | SAS-2 |
| NaCl | 10 mM | 15 ± 1.6 | 11 ± 3.1 |
| 100 mM | 48 ± 7.1 | 41 ± 0.8 | |
| 500 mM | 95 ± 2.7 | 100 ± 0.4 | |
| KCl | 10 mM | 13 ± 3.3 | 6 ± 5.0 |
| 100 mM | 42 ± 0.8 | 51 ± 3.4 | |
| 500 mM | 108 ± 6.1 | 128 ± 10.7 | |
| CaCl2 | 10 mM | 53 ± 3.1 | 65 ± 9.8 |
| 100 mM | 102 ± 4.3 | 118 ± 3.9 | |
| MgCl2 | 10 mM | 50 ± 1.9 | 65 ± 2.1 |
| 100 mM | 90 ± 2.2 | 113 ± 3.7 | |
| ZnCl2 | 10 mM | 16 ± 1.5 | 28 ± 2.8 |
| 100 mM | 20 ± 4.2 | 18 ± 5.6 | |
| ZnCl2 (with 500 mM NaCl) | 10 mM | 25 ± 6.1 | 32 ± 2.7 |
| 100 mM | 1 ± 0.5 | 1 ± 1.0 | |
Partially purified enzymes assayed 1 h in indicated salt with the substrate Z-VAD-AFC. Activity is expressed as relative fluorescence units.
SAS-1 and SAS-2 were tested for hydrolytic activity against a variety of caspase and granzyme B–specific peptide substrates (Table 4). The two enzymes displayed indistinguishable substrate recognition specificity, whereas SAS-2 possessed slightly higher hydrolysis rates when standardized against Z-VAD-AFC hydrolysis. Furthermore, neither saspase hydrolyzed any non-caspase substrate tested (with the exception of the granzyme B substrate), including two subtilisin A substrates and the general protease substrate casein (Table 5), nor did they cleave purified Rubisco or BSA (data not shown). These data indicate that both SAS-1 and SAS-2, like caspases, specifically cleave after Asp residues and, also similar to caspases, possess specificity beyond recognition of the P1 residue because not all substrates with Asp in the P1 position are hydrolyzed by SAS-1 or SAS-2.
Table 4.
Hydrolytic Activity of SAS-1 and SAS-2 toward Caspase and Granzyme B Substrates
| Percentage of Z-VAD-AFC
|
|||
|---|---|---|---|
| Substrate | Protease with Optimal Specificity | SAS-1 | SAS-2 |
| Z-VAD-AFCa | Pan-caspase | 100% ± 1.2% | 100% ± 1.9% |
| Z-YVAD-AFCa | Caspase-1 | 19% ± 2.1% | 28% ± 2.5% |
| Z-VDVAD-AFCa | Caspase-2 | 0% ± 0% | 0% ± 0% |
| Z-DEVD-AFCa | Caspase-3 | 0% ± 0% | 0% ± 0% |
| Ac-LEVD-AFCa | Caspase-4 | 39% ± 4.4% | 51% ± 2.6% |
| Z-WEHD-AFCa | Caspase-5 | 0% ± 0% | 0% ± 0% |
| Ac-VEHD-AFCa | Caspase-6 | 311% ± 21.5% | 366% ± 15.6% |
| Ac-VEID-AFCa | Caspase-6 | 0% ± 0% | 0% ± 0% |
| Ac-VKMD-AFCa | Caspase-6 | 623% ± 32.5% | 654% ± 28.8% |
| Ac-VNLD-AFCa | Caspase-6 | 384% ± 18.2% | 521% ± 21.8% |
| Ac-IETD-AFCa | Caspase-8 | 130% ± 10.1% | 172% ± 14.7% |
| Ac-LEHD-AFCa | Caspase-9 | 105% ± 9.6% | 140% ± 10.2% |
| Z-AAD-AFCa | Granzyme B | 144% ± 13.9% | 183% ± 20.1% |
Z-VAD-AFC was used as the 100% control.
Z, benzyloxycarbonyl; Ac, acetyl; AFC, 7-amido-4-(trifluoromethyl)coumarin.
Recognition sequence in single-letter amino acid code.
Table 5.
Hydrolytic Activity of SAS-1 and SAS-2 toward Various Protease Substrates
| Percentage of Z-VAD-AFC
|
|||
|---|---|---|---|
| Substrate | Protease with Optimal Specificity | SAS-1 | SAS-2 |
| Z-VAD-AFCa | Pan-caspase | 100% ± 2.8% | 100% ± 2.1% |
| Casein | General protease | 0% ± 0% | 0% ± 0% |
| Boc-GGL-pNAa | Subtilisin A | 0% ± 0% | 0% ± 0% |
| A-AAL-pNAa | Subtilisin A | 0% ± 0% | 0% ± 0% |
| SY-AFCa | Dipeptydylpeptidase I | 0% ± 0% | 0% ± 0% |
| GF-AFCa | Dipeptydylpeptidase I | 0% ± 0% | 0% ± 0% |
| PR-AFCa | Dipeptydylpeptidase I | 0% ± 0% | 0% ± 0% |
| GP-AFCa | Dipeptydylpeptidase IV | 0% ± 0% | 0% ± 0% |
| Z-RR-AMCa | Cathepsin B | 0% ± 0% | 0% ± 0% |
| Suc-AAPF-AMCa | Chymotrypsin | 0% ± 0% | 0% ± 0% |
| Z-AKR-AMCa | ICRM Ser protease I | 0% ± 0% | 0% ± 0% |
| H-L-AMCa | Aminopeptidase | 0% ± 0% | 0% ± 0% |
Z-VAD-AFC was used as the 100% control.
Z, benzyloxycarbonyl; Boc, tert-butyloxycarbonyl; Suc, N-succinyl; AFC, 7-amido-4-(trifluoromethyl)coumarin; AMC, 7-amido-4-methylcoumarin.
Recognition sequence in single-letter amino acid code.
The effects of various caspase and the granzyme B inhibitors on SAS-1 and SAS-2 Z-VAD-AFC hydrolytic activity are summarized in Table 6. Similar to their substrate recognition profiles, SAS-1 and SAS-2 also share nearly identical inhibitor profiles. Several non-caspase-specific protease inhibitors were tested for their ability to prevent Z-VAD-AFC hydrolysis (Table 7). Only the subtilisin-specific inhibitor Z-GF-NHO-Bz had a strong inhibitory effect on both SAS-1 and SAS-2, whereas all other inhibitors, including another subtilisin-specific inhibitor, Boc-APF-NHO-Bz, had only an intermediate or weak inhibitory effect on hydrolytic activity of both enzymes.
Table 6.
Effects of Caspase and Granzyme B Inhibitors on SAS-1 and SAS-2 Hydrolytic Activity
| Percent Activity of Noninhibited
|
|||
|---|---|---|---|
| Inhibitor | Preferred Protease Target | SAS-1 | SAS-2 |
| No inhibitor | - | 100% ± 3.0% | 100% ± 2.6% |
| Ac-VAD-CMKa | Pan-Caspase | 1% ± 0.6% | 1% ± 0.1% |
| B-YVAD-CMKa | Caspase-1 | 1% ± 0.1% | 1% ± 0.0% |
| Ac-WEHD-CHOa | Caspase-1 | 1% ± 0.5% | 1% ± 1.2% |
| Ac-LDESD-CHOa | Caspase-2 | 39% ± 2.4% | 49% ± 4.4% |
| Ac-VDVAD-CHOa | Caspase-2 | 1% ± 0.9% | 2% ± 0.8% |
| Ac-DEVD-CMKa | Caspase-3 | 90% ± 4.9% | 91% ± 5.6% |
| Ac-DMQD-CHOa | Caspase-3 | 1% ± 0.7% | 1% ± 0.4% |
| Ac-LEVD-CHOa | Caspase-4 | 1% ± 0.2% | 1% ± 0.9% |
| Ac-VEID-CHOa | Caspase-6 | 1% ± 0.2% | 1% ± 0.8% |
| Ac-IETD-CHOa | Caspase-8 | 1% ± 0.4% | 1% ± 1.5% |
| Ac-LEHD-CMKa | Caspase-9 | 47% ± 1.9% | 58% ± 5.2% |
| Ac-LEED-CHOa | Caspase-13 | 68% ± 3.2% | 67% ± 4.7% |
| Z-AAD-CMKa | Granzyme B | 1% ± 0.6% | 1% ± 1.0% |
Enzymes were preincubated with 200 μM inhibitor for 2 h and then assayed for Z-VAD-AFC hydrolytic activity for 1 h.
Z, benzyloxycarbonyl; Ac, acetyl; B, biotin.
Recognition sequence in single-letter amino acid code.
Table 7.
Effects of Various Protease Inhibitors on SAS-1 and SAS-2 Hydrolytic Activity
| Percent Activity of Noninhibited
|
|||
|---|---|---|---|
| Inhibitor | Preferred Protease Target | SAS-1 | SAS-2 |
| No inhibitor | - | 100% ± 2.2% | 100% ± 3.0% |
| Z-GF-NHO-Bza | Subtilisin | 1% ± 0.5% | 1% ± 2.2% |
| Boc-APF-NHO-Bza | Subtilisin | 93% ± 4.2% | 92% ± 3.8% |
| Aprotinin | Ser | 73% ± 8.1% | 77% ± 2.8% |
| AEBSFb | Ser | 64% ± 5.4% | 73% ± 4.2% |
| PMSFc | Ser | 60% ± 5.9% | 70% ± 3.4% |
| Leupeptin | Ser and Cys | 88% ± 2.5% | 81% ± 2.5% |
| Antipain | Ser and Cys | 100% ± 3.9% | 100% ± 2.7% |
| E-64d | Cys | 79% ± 5.1% | 81% ± 3.2% |
| PCMBe | Thiol | 89% ± 4.0% | 100% ± 1.9% |
| Pepstatin A | Aspartate | 91% ± 2.5% | 89% ± 4.1% |
Purified SAS-1 and SAS-2 were preincubated with 200 μM inhibitor for 2 h, except PMSF (1 mM) and aprotinin (1 μg/mL), and then assayed for Z-VAD-AFC hydrolytic activity for 1 h.
Recognition sequence in single-letter amino acid code. NHO, nitrosyl; Bz, benzoyl.
4-(2-aminoethyl)-benzenesulfonyl-fluoride.
Phenylmethylsulfonyl fluoride.
Trans-epoxysuccinyl-l-leucylamido-(4-guanidino)-butane.
p-Chloromercuribenzoic acid.
Autoproteolysis of SAS-1 and SAS-2
Gel-purified (post-anion exchange chromatogaphy) SAS-1 and SAS-2 were used to produce polyclonal antibodies in rabbits. The antisera produced from SAS-1 and SAS-2 were cross-reactive with each other (data not shown). Because SAS-2 antiserum had a higher titer of polyclonal antibodies, it was used for protein gel blot analysis.
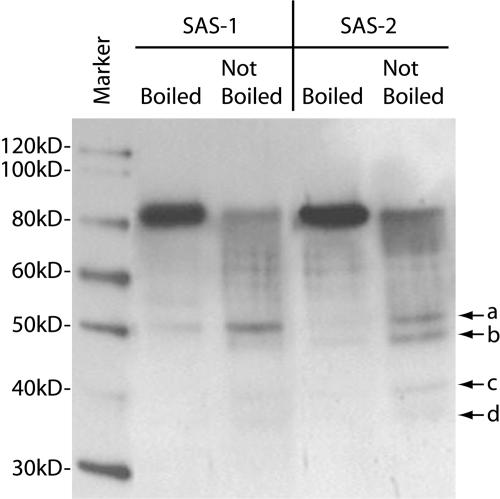
A protein gel blot of purified SAS-1 and SAS-2 probed with SAS-2 antiserum is shown in Figure 12. After addition of SDS-loading buffer to each sample (∼200 ng purified enzyme), the samples were either boiled 5 min (Figure 12, lanes 2 and 4) or not boiled (Figure 12, lanes 3 and 5) before loading on the gel. Nonboiled samples of both enzymes underwent autoproteolysis and accumulated lower molecular mass bands. The SAS-2 sample contained four distinct proteolytic bands—a, b, c, and d— whereas the SAS-1 sample only contained three bands—a, c, and d. Bands c and d are more visible when overexposed (data not shown).
Figure 12.
Autoproteolysis of SAS-1 and SAS-2.
Protein gel blot of purified SAS-1 and SAS-2 (200 ng each lane) boiled and not boiled before electrophoresis. Nonboiled enzymes underwent autoproteolysis with the appearance of lower molecular weight bands (a, b, c, and d).
DISCUSSION
PCD and a Protease Cascade
The PCD response in plants is currently under intense characterization by many research laboratories. Apoptosis is the most well-characterized form of PCD. Because caspases can play such a critical role in the process and because numerous experiments have shown that caspase-specific inhibitors prevent some forms of PCD in plants (del Pozo and Lam, 1998; Sun et al., 1999; Tian et al., 2000; Richael et al., 2001; Elbaz et al., 2002; Mlejnek and Procházka, 2002), it has been speculated that proteases similar to caspases are involved in plant PCD. We have purified and characterized two subtilisin-like Ser proteases that possess specificity for caspase-specific substrates and inhibitors and apparently participate in a signaling cascade involving multiple proteases, leading to the proteolysis of Rubisco. The proteases have been referred to as saspases.
The saspase proteases were originally implicated based on the effects of inhibitors that prevented victorin and heat shock–induced Rubisco proteolysis, a characteristic of PCD in A. sativa. Other inhibitors that prevent this response (Ac-DEVD-CMK, E-64, and leupeptin) do not inhibit the activity of either saspase, indicating the involvement of additional proteases. The identification of another protease with specificity for DEVD derived substrates and inhibitors, but not E-64 or leupeptin, suggested that at least two other proteases function within a protease cascade, with either or both of the saspase enzymes, leading to the proteolysis of Rubisco.
This evidence suggests that the saspases are components of a PCD-induced protease cascade that ultimately leads to the activation of a protease that is targeted to the chloroplast to cleave Rubisco. Although cleavage of Rubisco is inhibited by Ac-VAD-CMK and Z-AAD-CMK, both of which are recognized by the saspases, it is not likely that either saspase directly cleaves Rubisco in vivo. Four lines of evidence suggest that an alternative protease cleaves Rubisco: (1) E-64, leupeptin, and Ac-DEVD-CMK prevent Rubisco cleavage but do not affect SAS-1 or SAS-2 activity or their release into the ECF; (2) the cleavage site in Rubisco (Navarre and Wolpert, 1999) does not conform to the substrate specificity for the saspases; (3) purified SAS-1 and SAS-2 do not cleave Rubisco in vitro (data not shown); and (4) the saspases appear to function extracellularly.
A Gene Family
The caspase-like activity was purified into two distinct proteases, SAS-1 and SAS-2. The two proteases are nearly indistinguishable, in that their substrate and inhibitor profiles are identical, they have the same pH and salt requirements, they are predicted to have equivalent molecular masses determined by either electrophoretic migration or mass spectrometry, they possess similar peptide maps, they have an identical NH2-terminal sequence (except the first amino acid of SAS-1), and they both bind heparin and are autoproteolytic. Although they recognize the same substrates and inhibitors, SAS-2 does have a higher hydrolysis rate for several caspase substrates and is slightly more sensitive to some caspase inhibitors. Furthermore, the difference in mass between two of the several major peaks in their peptide maps suggest a differential posttranslational modification (such as a methyl group) or a single amino acid substitution. The comparisons between SAS-1 and SAS-2 indicate that they are different yet highly similar proteases, most likely from two separate genes. But the possibility exists that they are differentiated by unique posttranslational modifications to the same gene product.
Gene families of subtilisin-like Ser proteases have been identified in several plant species. Lycopersicon esculentum (tomato) has had 10 genes for subtilisin-like Ser proteases thus far cloned and characterized (Vera and Conejero, 1988; Tornero et al., 1997; Jordá et al., 1999, 2000; Meichtry et al., 1999; Janzik et al., 2000; Riggs et al., 2001). Whereas Arabidopsis (Arabidopsis thaliana) has had only four genes characterized (Neuteboom et al., 1999; Berger and Altmann, 2000; Tanaka et al., 2001; Hamilton et al., 2003), at least 10 more genes have been annotated as being subtilisin-like Ser proteases. Rice has had only one subtilisin-like Ser protease gene characterized (Yamagata et al., 2000), but seven have been annotated from the genome, two of which have high sequence similarity to SAS-1 and SAS-2. Thus, it is likely that two or more subtilisin-like Ser proteases are present in A. sativa, and quite possibly a more divergent protease with DEVD specificity functions along with SAS-1 and SAS-2 within the protease cascade leading to Rubisco proteolysis. Because of the structural and immunological similarities between the saspases and the difficulty in purifying large amounts from the ECF, we have not been able to distinguish which saspase, if not both, is released into the ECF. However, initial studies have indicated that after induction of PCD and removal of the ECF from victorin-treated leaves, total saspase protein remaining in the whole leaf is dramatically reduced, suggesting that both SAS-1 and SAS-2 are released into the ECF. Further experiments need to be performed to confirm these observations.
Processive Protease and Family Relations
The substrate specificity of SAS-1 and SAS-2 is distinct from all other known subtilisin-like Ser proteases. Among the substrates tested, only certain caspase substrates and the granzyme B substrate were hydrolyzed and to varying degrees, indicating that although an aspartate residue is required in the P1 position, specificity extends beyond this one residue. No other identified protease from plants has been reported to be specific for caspase substrates or to cleave exclusively after an aspartate residue. Plant subtilisin-like Ser proteases differ widely in their substrate specificity. Cucumisin (Yamagata et al., 1994), macuralisin (Rudenskaya et al., 1995), taraxalism (Rudenskaya et al., 1998), hordolisin (Terp et al., 2000), and Ser endopeptidase-1 (Fontanini and Jones, 2002) have been reported as having a broad substrate range and are thought to be degradative proteases, whereas Auxin Induced in Root3 (Neuteboom et al., 1999), Abnormal Leaf Shape1 (Tanaka et al., 2001), Stomatal Density and Distribution1 (SDD1) (Berger and Altmann, 2000), Tomato subtilase1 (LeSBT1) (Janzik et al., 2000), 50-kD Systemin Binding Protein (Schaller and Ryan, 1994), and Ara12 (Hamilton et al., 2003) are described as possessing greater substrate specificity and are thought to function as processive proteases. Until more is known about the biochemical and physiological properties of plant subtilisin-like Ser proteases, the biological significance of the difference between a degradative or processive function may prove challenging to determine. Because SAS-1 and SAS-2 do not generally degrade proteins or non-caspase-specific substrates (e.g., casein), require an aspartate residue in the P1 position of the substrate, and are likely involved in a signaling cascade, it appears evident that they function as processive proteases.
Plant subtilisin-like Ser proteases are members of the Pyrolysin family of subtilisin-like Ser proteases (Siezen and Leunissen, 1997) and are predicted, based on DNA sequence of the cloned proteases, to be expressed as pre-pro-protein precursors. Another family of subtilisin-like Ser proteases, the Kexin family, also is expressed as pre-pro-protein precursors. Kex2 from Saccharomyces cerevisiae, the prototype member of this family, has been well-characterized (Mizuno et al., 1988). In Kex2, the pre-domain is a signal peptide that directs the enzyme into the secretory system of the endoplasmic reticulum (ER) and is cleaved during import (Van de Ven et al., 1991). The pro-domain is autocatalytically cleaved while in the ER, which results in the formation of the active, mature form of the protease (Gluschankof and Fuller, 1994). The pre- and pro-domains of plant subtilisin-like Ser proteases are thought to each function in a similar manner to those of the Kex2 enzyme because several plant subtilisin-like Ser proteases have been localized extracellularly (Vera and Conejero, 1988; Yamagata et al., 1994; Taylor et al., 1997), and the NH2 terminus of mature, active enzymes corresponds to the predicted mature NH2 terminus absent the pre- and pro-domains (Terp et al., 2000; Popovič et al., 2002).
The saspases from A. sativa are extracellularly localized during the PCD response, and their NH2-terminal sequence is similar to the mature, processed NH2 terminus of most plant subtilisin-like Ser proteases, characteristics which correspond in function to the pre-pro-protein model of subtilisin-like Ser proteases. Furthermore, because pro-domains are autocatalytically processed and the pro-domain cleavage site indicates, to some degree, the cleavage specificity for the enzyme, it might be expected that in the saspases, the amino acid preceeding the cleavage site would be an aspartate. Interestingly, the protein with the most similarity to the sequenced saspase peptides, the putative protease annotated from the rice genome, does have an aspartate residue in the P1 position of the predicted pro-domain cleavage site, suggesting that the putative rice protease may possess similar cleavage specificity to the saspases from A. sativa.
Heparin and Autoproteolysis
In the early stages of characterization and before purification and partial sequence analysis, it was clear that the saspases were high molecular mass, extracellular Ser proteases apparently involved in a protease cascade. That depiction strongly resembled a Drosophila melanogaster protease called gastrulation defective (GD), which is a 72-kD extracellular Ser protease involved in a protease cascade leading to dorsal-ventral signaling (Konrad et al., 1998; Han et al., 2000; DeLotto, 2001; Dissing et al., 2001; LeMosy et al., 2001). After purification and partial sequencing, it became clear that GD and SAS-1 and SAS-2 are structurally unrelated, although they may share some similar biological characteristics. Two other characteristics were later identified as being in common between the saspases and GD, namely heparin binding and autoproteolysis.
Heparin, a sulfated glycosaminoglycan, is involved in several biological activities in animals, including binding antithrombin III. Such binding accelerates the rate of antithrombin III–mediated inhibition of Ser proteases involved in the blood coagulation cascade (reviewed in Petitou et al., 1988; Capila and Linhardt, 2002). Heparan sulfate, a heparin derivative, is ubiquitously distributed on the surfaces of animal cells and in the extracellular matrix where it mediates various physiological and pathological processes (reviewed in Capila and Linhardt, 2002). Though the biological significance of GD binding heparin is not known, Dissing et al. (2001) suggest that the pipe gene product, a heparan sulfate 2-O-sulfotransferase, might regulate activity of GD through binding of a yet unknown sulfated proteoglycan. Possibly, the binding of SAS-1 and SAS-2 to a heparin-like molecule in plants may have a regulatory role, similar to Ser proteases involved in the blood coagulation cascade or the putative role of heparin-like molecules in regulating GD.
Autoproteolysis by GD, SAS-1, and SAS-2 is an intriguing yet confounding characteristic. GD has been shown to undergo autoproteolysis only in the presence of the Snake protease and into two distinct bands of 44 and 46 kD (Dissing et al., 2001). Neither proteolytic activity nor a biological function has been identified for the products of proteolysis, though it was suggested that the unprocessed form of the protease was the zymogen and the proteolytic products might function as the active component of the cascade (Dissing et al., 2001). SAS-1 and SAS-2 autoproteolysis has only been observed in gel where it is likely dependent on concentration and the state of denaturation. Both proteases are stable at room temperature in biological buffers for several days (data not shown). Autoproteolysis by SAS-1 and SAS-2 is not unique among plant subtilisin-like Ser proteases because two other proteases have been shown to undergo a form of self-catalyzed proteolysis. LeSBT1, a 73-kD protease from L. esculentum, is autoprocessed into a 68-kD protease at pH 5.0 and 6, whereas it completely autodegrades at pH 4.0 (Janzik et al., 2000), and hordolisin from Hordeum vulgare (barley) was autoprocessed from 74 to 62 kD during storage at −20°C (Terp et al., 2000).
Regulation and Localization
A unique characteristic of the saspases is that they appear to be constitutively present in A. sativa cells, and release of active saspase into the ECF but not de novo synthesis of the enzyme is correlated with the induction of PCD. This is in contrast with other proteases associated with plant PCD (Delorme et al., 2000; Eason et al., 2002; Wan et al., 2002) or the HR (Vera and Conejero, 1988; Krüger et al., 2002), which are typically regulated at the level of expression. P-69B and P-69C, members of a family of L. esculentum subtilisin-like Ser proteases, are two pathogenesis-related (PR) proteins that show increased expression levels during HR (Jordá et al., 1999). The pathogen-induced P-69 enzymes also are secreted into the extracellular space (Tornero et al., 1996a). However, unlike saspases, these proteins are not present in the cell before induction of the HR (Tornero et al., 1997).
Release of the saspase(s) into the ECF occurs before the onset of other markers of PCD, indicating that saspase release could be causal to but is not a consequence of PCD. The saspase(s) starts to appear in the ECF at 1 h after victorin treatment, at least 7 h before other signs of PCD, such as Rubisco proteolysis and DNA laddering, start to occur. This indicates that saspase appearance in the ECF is not because of cell lysis but is a regulated event. This conclusion is further supported by time-course studies (Curtis and Wolpert, 2004) that have established that the plasma membrane and tonoplast of victorin-treated A. sativa cells remain structurally intact after the point at which Rubisco proteolysis, DNA laddering, and even cell collapse occur.
The question arises as to where the saspases are localized before release into the ECF. They could be retained in the ER where they await processing and secretion. The Kexin family of subtilisin-like Ser proteases is known to autocatalytically remove their pro-domain in the ER before continuing in the secretory pathway (Gluschankof and Fuller, 1994; Hill et al., 1995). A mutation in the Kex2 protease that inhibits removal of the pro-domain stimulates accumulation of proKex2 in the ER (Gluschankof and Fuller, 1994). The pro-domain is thought to contain an ER retention sequence. If the saspases are stored in the ER while awaiting processing and secretion, then an inactive pro-saspase form should be identifiable. However, analysis of proteins labeled in vivo with biotin-YVAD-CMK and extracted under denaturing conditions (phenol extracted) from victorin-treated and untreated tissue revealed similar concentrations of only the mature processed proteases (Figure 4D). Soluble protein extracts from victorin-treated and untreated tissue also contained comparable amounts of saspase activity (data not shown). These data suggest that the saspases are likely sequestered as active proteases.
Another possible saspase-retention area is the cell exterior, where it could associate with the extracellular matrix or a membrane protein. Heparin or heparin-like molecules are typically extracellular, either membrane bound or soluble (Petitou et al., 1988; Stringer et al., 2002), and because the saspases are ultimately extracellular, it is possible that a heparin-like molecule plays a physiologically significant role, either before release of the saspases or sometime during their biological function. Whatever their storage location, the fact that the saspases appear to be constitutively present in an active form suggests that they can be rapidly released and that a quick response is important for PCD.
PCD and Saspase Function
Though no physiological substrate(s) of the saspases has been identified, data suggest that they activate, either directly or indirectly, other proteases. Because the saspases recognize two of the inhibitors (and their complementary substrates) that prevent Rubisco proteolysis and because victorin treatment affects localization of the saspases, it appears likely that they are involved in the signal cascade leading to Rubisco proteolysis. The saspases also likely function early in the signal cascade because their release into the ECF is not prevented by any of the protease inhibitors that prevent Rubisco proteolysis, indicating that these inhibitors function either downstream or after release of the saspases. Furthermore, because multiple proteases appear to be involved in this cascade, one or both of the saspases are likely involved in activation of these other proteases. Are these proteases the direct substrate, or do the saspases interact with an extracellular protein, possibly membrane associated, which facilitates the activation of intracellular proteases? Several reports have implicated the interaction of extracellular proteases with Leu-rich repeat (LRR) proteins (LRP) during the HR and developmental processes. The pathogen-induced, subtilisin-like Ser protease P-69 from L. esculentum is able to proteolytically process an extracellular LRP (Tornero et al., 1996b). The LRP, predicted to be soluble in the extracellular matrix, also has increased expression levels during pathogen attack, but the function of the processed or unprocessed form is yet to be determined. Krüger et al. (2002) describe an extracellular Cys protease, Required for Cladosporium fulvum Resistance3 (Rcr-3), that is required for Cf-2–dependent HR. Cf-2 is a resistance gene in L. esculentum that encodes a transmembrane LRP with an extracellular LRR domain. Not only is Rcr-3 required for Cf-2–dependent HR, it also is necessary for suppression of Cf-2–dependent autonecrosis. Two other extracellular proteases, SDD1, a subtilisin-like Ser protease (Berger and Altmann, 2000), and Brassinosteroid-Insensitive1, a Ser carboxypeptidase (Li et al., 2001), have been described as necessary for stomata development and brassinosteroid signaling, respectively. Both proteases function upstream of LRR receptor–like proteins that are required for continuation of their respective signaling pathways (Li et al., 2001; Serna and Fenoll, 2002). Whether or not the saspases interact with another protease, an LRR receptor–like protein, or a ligand for a membrane receptor is currently not known. Identification of the physiological substrate for the saspases will be an important step in furthering the understanding of the processes of PCD in A. sativa.
Recently, Woltering et al. (2002) asked the question, “Do plant caspases exist?” This research addresses that question by providing insight into enzymes that possess caspase-like activity, though not structurally related to caspases. Numerous studies have indicated the involvement of caspase-like proteases in the regulation of plant PCD. Caspase-specific inhibitors prevent PCD in response to bacteria (del Pozo and Lam, 1998; Richael et al., 2001), fungal elicitors (Elbaz et al., 2002), and various forms of stress (Sun et al., 1999; Tian et al., 2000; Elbaz et al., 2002; Mlejnek and Procházka, 2002; Woltering et al., 2002). Although these reports indicate that there are proteases in plants that are inhibited by caspase-specific inhibitors and implicated in PCD, no enzyme had yet been identified that pertains to these observations. Saspases, plant enzymes possessing caspase-like specificity and associated with the PCD response, are subtilisin-like Ser proteases, and partial amino acid sequence analysis revealed homology to all plant subtilisin-like Ser proteases. The unusual requirement for aspartate residues in the P1 position of the substrate, the specificity of the enzyme among aspartate-containing substrates, the lack of general proteolytic activity, and the possible involvement in a signal cascade suggest that the saspases function as processive proteases. Release of one or both of the saspases into the ECF occurs during victorin and heat shock–induced PCD, two treatments that share other characteristics of PCD, namely Rubisco proteolysis and DNA laddering. The saspases appear to be involved in a PCD-induced signaling cascade based on inhibitor studies and relocalization during the PCD response, but unfortunately no direct evidence of their involvement has been identified. Initial experiments to induce PCD in A. sativa leaves by infiltration of purified saspase have provided interesting but inconsistent results. The ability to purify large amounts of enzyme (needed for these types of experiments) without first cloning the gene has been the largest impediment. The identification of the putative protease from rice, which possesses the highest amino acid sequence similarity, should facilitate cloning of the saspase genes from A. sativa. It also will be of interest to see if this putative protease from rice has caspase-like activity and if it is involved in rice PCD responses.
METHODS
Experimental Materials
A. sativa seedlings of the line X469 (victorin sensitive) and X424 (victorin insensitive) were grown in a growth chamber for 6 to 7 d under a 16-h photoperiod at 24°C. All protease substrates and caspase inhibitors were purchased from Calbiochem (La Jolla, CA). The remaining chemicals and protease inhibitors were purchased from Sigma (St. Louis, MO).
Treatment of A. sativa Tissue
A. sativa leaves were treated with victorin by one of three methods. A. sativa leaves, with their epidermis removed, were floated on 20 mM 3-(N-morpholino)-propanesulfonic acid (Mops), pH 6.5, supplemented with 20 ng/mL of victorin. For leaf infiltration assays, intact A. sativa leaves were infiltrated with 1 μg/mL of victorin in water and then cut into 4-cm leaf segments. The third method, which was used to purify large quantities of protein, treated leaf slices (2- to 3-mm cross-sectional pieces) with 100 ng/mL of victorin in water.
For heat shock treatments, 4-cm water-infiltrated leaf segments were incubated for 60 min at 45°C. Leaves were water infiltrated to facilitate collection of ECF. After heat shock, leaves were incubated at 25°C for the times indicated before collection of the ECF, total protein, and DNA.
Total protein and DNA were extracted from leaf samples by homogenizing 1 cm of leaf tissue in 200 μL of phenol combined with 200 μL of Tes buffer (50 mM Tris-HCl, pH 7.6, 5 mM EDTA, and 2% [w/v] SDS). After homogenization, the phases were thoroughly mixed and separated by centrifugation for 10 min. The protein (phenol) phase was removed, and the proteins were precipitated by mixing with 5× volume 0.1 M ammonium acetate and 2.5% (v/v) β-mercaptoethanol in cold methanol and incubating the mixture a minimum of 4 h at 4°C. Samples were then centrifuged for 10 min to pellet the proteins, washed in cold methanol, and centrifuged again. Precipitated samples were dried and resuspended in 100 μL of SDS-loading buffer (62.5 mM Tris-HCl, pH 6.8, 2.3% [w/v] SDS, 5% [v/v] β-mercaptoethanol, and bromophenol blue [trace amount]). For SDS-PAGE analysis of Rubisco proteolysis, 8 μL of sample was loaded on the gel. For biotin blots of total cellular protein, 30 μL of sample was loaded on the gel.
The DNA (aqueous) phase was precipitated by mixing with 2.5× volume cold ethanol and 0.1× volume 3 M Na acetate and incubating overnight at −20°C. DNA samples were centrifuged for 10 min, washed in 70% (v/v) cold ethanol, and centrifuged again. Precipitated DNA samples were dried and resuspended in 20 μL of TE buffer (10 mM Tris-HCl, pH 7.5, and 1 mM EDTA) supplemented with 40 μg/mL of RNase. The entire sample was loaded on a 1.5% (w/v) agarose gel.
ECF was collected by cutting the 4-cm leaf segments into two, 2-cm segments and placing them vertically in a 1.5-mL microcentrifuge tube with a pinhole in the bottom. The tubes were placed inside a collection tube and centrifuged for 10 min at 1000g in a swinging bucket rotor. To assay for proteolytic activity, 10 μL of ECF was used per reaction. For biotin blots, 30 μL of biotin-YVAD-CMK–labeled ECF was loaded per lane for each gel.
Protease Activity Assays
Substrate hydrolysis was measured, unless otherwise indicated, by adding 10 μL of an enzyme sample to 90 μL of 20 mM Mops, pH 6.5, and 0.5 M NaCl supplemented with 20 μM of the indicated substrate for a final volume of 100 μL. Samples were incubated at 25°C for 60 min in 96-well microtiter plates. Fluorescence produced by hydrolysis of the substrates was read with a SpectroMax Gemini fluorometer (Molecular Devices; Sunnyvale, CA). All assays were performed in triplicate.
Reversible and Irreversible Binding Experiments
Activity B from the HIC column was assayed for inhibition with three inhibitors: Z-VAD-CHO, Z-VAD-FMK, and Ac-VAD-CMK. The inhibitors (200 μM) were each added to 200 μL of sample equilibrated in 20 mM Mops, pH 6.5, 1 mM DTT, and 100 mM NaCl, then incubated for 2 h. Ten-microliter aliquots from each treatment were assayed for VAD-AFC hydrolysis (see above). Samples were washed with 1 mL of 20 mM Mops, pH 6.5, 1 mM DTT, and 100 mM NaCl and concentrated back to the original volume with a 2-mL Centricon-30 (30,000 molecular weight cutoff [MWCO]) (Amicon, Beverly, MA). Three, 10-μL aliquots were assayed after each wash step.
Purification of SAS-1 and SAS-2
Soluble Protein Preparation and Ammonium Sulfate Precipitation
A. sativa leaves (200 g) were sliced into 2- to 3-mm pieces and incubated 4 h with 100 ng/mL of victorin in water. The slices were then homogenized with a mortar and pestle in cold 20 mM Mops, pH 7.0, with 1 mM DTT. The homogenate was filtered through four layers of cheesecloth and centrifuged for 10 min at 10,000g. The resultant supernatant was removed and centrifuged for 1 h at 100,000g. The soluble protein solution was mixed with 50% (w/v) ammonium sulfate for 30 min at 4°C, centrifuged for 30 min at 10,000g, and the pellet was solubilized in 20 mM Mops, pH 7, containing 1 mM DTT and 1 M ammonium sulfate. The solubilized pellet was mixed for 30 min at 4°C, centrifuged for 10 min at 10,000g to remove insoluble material, and filtered through a 0.22-μm filter.
HIC
Before the establishment of a purification protocol, initial characterization of protease activity was performed on soluble protein extracted from 60 g of A. sativa tissue (prepared as above but without ammonium sulfate precipitation) after separation and concentration by HIC. HIC was performed in a 15-mm × 40-cm column packed with Phenol Sepharose high-performance resin (Amersham Bioscience; Piscataway, NJ) equilibrated in 20 mM Mops, pH 7, 1 mM DTT, and 1 M ammonium sulfate. The sample was adjusted to contain 20 mM Mops, pH 7, 1 mM DTT, and 1 M ammonium sulfate and loaded directly onto the column. The column was then washed in the same buffer and eluted with a 200-min, linear gradient of 1.0 to 0 M ammonium sulfate with a flow rate of 4 mL/min. For initial characterization of protease activity, all fractions were assayed with the substrates Z-VAD-AFC and Z-DEVD-AFC. Two proteolytic activities were identified: activity A eluting at ∼0.45 M ammonium sulfate and activity B eluting at ∼0.3 M. For the purification protocol, the sample obtained from the ammonium sulfate precipitation step was loaded and ran on the HIC column as described above. Throughout the purification protocol, fractions were assayed for Z-VAD-AFC hydrolytic activity.
Heparin Affinity Chromatography
Active fractions from the HIC column were pooled, and the buffer was exchanged with 20 mM Mops, pH 7, 1 mM DTT, and 50 mM NaCl by filtering through an Amicon Ultra-15, 30,000 MWCO centrifugal filter device (Millipore, Bedford, MA). After buffer exchange, the sample was loaded onto a 5-mL Heparin HP affinity column (Amersham Bioscience), washed with 20 mM Mops, pH 7, 1 mM DTT, and 50 mM NaCl, and eluted in 20 mM Mops, pH 7, 1 mM DTT, and 2 M NaCl.
Anion Exchange Chromatography
Active fractions from the heparin column were prepared for anion exchange chromatography by replacing the buffer with 50 mM Tris-HCl, pH 8.5, 2 mM EDTA, and 1 mM DTT (by concentration and washing in an Amicon Ultra-15). The resulting sample was loaded onto a Mono Q fast-protein liquid chromatography 5/5 column (Amersham Bioscience) and eluted with a 200-min linear gradient of 0 to 0.3 M NaCl with a flow rate of 0.5 mL/min. Two peaks of proteolytic activity eluted from the Mono Q column and were combined into two separate fractions called SAS-1 and SAS-2 (see Results).
Size Exclusion Chromatography
The two active fractions from the Mono Q column were individually concentrated with an Amicon Ultra-15 into 4 mL with the buffer consisting of 20 mM Mops, pH 6.5, 1 mM DTT, and 150 mM NaCl. Each 4-mL sample was loaded onto a Sephacryl S-200 (16/60) column (Amersham Bioscience) and run at 0.5 mL/min. Fractions containing proteolytic activity (Z-VAD-AFC) were checked for purity by silver staining of an SDS-PAGE.
Substrate, Inhibitor, pH, and Salt Profiles
Samples of SAS-1 and SAS-2 from the Mono Q column were used for the substrate, inhibitor, pH, and salt profile assays. Conditions were as described above for substrate and inhibitor assays and as described below for the pH and salt assays. All activity assays were performed in triplicate.
The optimal pH for hydrolytic activity against the substrate Z-VAD-AFC was measured over the pH range of 5.5 to 7.5. A 20-mM Mes buffer containing 0.5 M NaCl was used for the assays at pH 5.5, 6.0, and 6.5, and 20 mM Mops and 0.5 M NaCl were used for assays at pH 6.5, 7.0, and 7.5.
Five salts, NaCl, KCl, MgCl2, CaCl2, and ZnCl2, were tested at 10 mM and 100 mM in 20 mM Mops, pH 6.5, or at 10, 100, and 500 mM in 20 mM Mops, pH 6.5, for their affect on hydrolytic activity against Z-VAD-AFC.
Polyclonal Antibody Production
Polyclonal antibodies against SAS-1 and SAS-2 were produced at the Laboratory of Animal Research, Oregon State University by subcutaneous injection in rabbits. After anion exchange chromatography, SAS-1 and SAS-2 (∼1 μg) were gel purified and mixed with the adjuvant TiterMax Gold (Sigma) before injection. Booster injections (∼1 ug of SAS-1 and SAS-2 mixed in TiterMax Gold) were administered at weeks 4 and 8, and final serums were collected at week 11.
Silver Staining and Protein Blotting
All enzyme samples were analyzed on an 8% SDS-PAGE. Silver staining was performed following the protocol developed by Blum et al. (1987). Electroblotting was done in a Trans-Blot sd semi-dry transfer cell (Bio-Rad, Hercules, CA) with transfer buffer consisting of 25 mM Tris, 192 mM glycine, and 0.02% SDS in 20% methanol. Proteins were blotted onto a Protran nitrocellulose membrane (Keene, NH) with a 0.45-μm pore size (Schleicher and Schuell).
Membranes were blocked with 1% BSA in TBST (100 mM Tris-HCl, pH 7.6, 0.9% NaCl, and 0.1% Tween 20) for 20 min. For protein gel blots, SAS-2 polyclonal antibody was added at a 1:2000 dilution in TBST for 1 h and washed three times for 15 min each in 1× TBST, and then 2° antibody (goat anti-rabbit conjugated to horseradish peroxidase) (Santa Cruz Biotechnology, Santa Cruz, CA) was added at a 1:100,000 dilution for 1 h. Blots were washed four times for 15 min each in TBST before development with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). Biotin blots were incubated with NeutrAvidin conjugated to horseradish peroxidase (Pierce) at a 1:50,000 dilution for 1 h, washed four times for 15 min each, and developed the same as protein gel blots.
Amino Acid Sequencing and Mass Spectrometry
SAS-1 and SAS-2 samples from the Mono Q column were run in an SDS-PAGE, blotted onto a polyvinylidene difluoride membrane, and stained with 0.1% Coomassie Brilliant Blue R 250 in 1% acetic acid and 40% methanol. The 84-kD bands were excised and destained in 50% methanol. NH3-terminal sequencing was conducted by Edmonds degradation at The Institute of Molecular Biology, University of Oregon (Eugene, OR).
Purified SAS-1 and SAS-2 was analyzed by MALDI-TOF mass spectrometry at the Mass Spectrometry Laboratory, Oregon State University (Corvallis, OR).
The peptide maps of SAS-1 and SAS-2 were produced at the Stanford PAN Biotechnology Facility, Stanford University (Stanford, CA). Samples were excised from an SDS-PAGE, partially digested in-gel by trypsin, separated by high-performance liquid chromatography, and analyzed by MALDI-TOF mass spectrometry. Three internal peptides of SAS-2 produced by the in-gel tryptic digest were NH2-terminally sequenced by Edmonds degradation at the Stanford PAN Biotechnology Facility.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers BAB89803, CAA76724, CAA76725, T06577, NP_569048, and A55800.
Acknowledgments
We thank Lilo Barofsky for her help with the mass spectrometry analysis and Donald Buhler for the use of the fluorometer. We are also grateful to Jennifer Lorang, Teresa Sweat, and Marc Curtis for their scientific discussion and technical assistance. This work was supported in part by grants from the USDA National Research Initiative Competitive Grants Program (1999-02404 and 2001-35319-10896) and the National Science Foundation (IBN-9631442).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Thomas J. Wolpert (wolpertt@science.oregonstate.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017947.
References
- Abramovitch, R.B., Kim, Y.J., Chen, S., Dickman, M.B., and Martin, G.B. (2003). Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 22, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor, Y., Babiychuk, E., Inzé, D., and Levine, A. (1998). The involvement of poly(ADP-ribose) polymerase in the oxidative stress responses in plants. FEBS Lett. 440, 1–7. [DOI] [PubMed] [Google Scholar]
- Balk, J., Leaver, C.J., and McCabe, P.F. (1999). Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett. 463, 151–154. [DOI] [PubMed] [Google Scholar]
- Berger, D., and Altmann, T. (2000). A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14, 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Bleecker, A.B., and Patterson, S.E. (1997). Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 9, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, H., Beier, H., and Gross, H.J. (1987). Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8, 93–99. [Google Scholar]
- Bushnell, T.P., Bushnell, D., and Jagendorf, A.T. (1993). A purified zinc protease of pea chloroplasts, EP1, degrades the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 103, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capila, I., and Linhardt, R.J. (2002). Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 41, 391–412. [DOI] [PubMed] [Google Scholar]
- Casano, L.M., Lascano, H.R., and Trippi, V.S. (1994). Hydroxyl radicals and a thylakoid-bound endopeptidase are involved in light- and oxygen-induced proteolysis in oat chloroplast. Plant Cell Physiol. 35, 145–152. [Google Scholar]
- Casano, L.M., and Trippi, V.S. (1992). The effect of oxygen radicals on proteolysis in isolated oat chloroplasts. Plant Cell Physiol. 33, 329–332. [Google Scholar]
- Curtis, M.J., and Wolpert, T.J. (2002). The oat mitochondrial permeability transition and its implication in victorin binding and induced cell death. Plant J. 29, 295–312. [DOI] [PubMed] [Google Scholar]
- Curtis, M.J., and Wolpert, T.J. (2004). The victorin induced mitochondrial permeability transition precedes cell shrinkage and biochemical markers of cell death and shrinkage occurs without loss of membrane integrity. Plant J., in press. [DOI] [PubMed]
- Dangl, J.L., Dietrich, R.A., and Richberg, M.H. (1996). Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 8, 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong, A.J., Hoeberichts, F.A., Yakimova, E.T., Maximova, E., and Woltering, E.J. (2000). Chemical-induced apoptotic cell death in tomato cells: Involvement of caspase-like proteases. Planta 211, 656–662. [DOI] [PubMed] [Google Scholar]
- Delorme, V.G.R., McCabe, P.F., Kim, D., and Leaver, C.J. (2000). A matrix metalloproteinase gene is expressed at the boundary of senescence and programmed cell death in cucumber. Plant Physiol. 123, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLotto, R. (2001). Gastrulation defective, a complement factor C2/B-like protease, interprets a ventral prepattern in Drosophila. EMBO Rep. 21, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo, O., and Lam, E. (1998). Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr. Biol. 8, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Dissing, M., Giordano, H., and DeLotto, R. (2001). Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 20, 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva, I., Poirier, G.G., and Heath, M.C. (1998). Activation of cysteine proteases in cowpea plants during the hypersensitive response — A form of programmed cell death. Exp. Cell Res. 245, 389–399. [DOI] [PubMed] [Google Scholar]
- Eason, J.R., Ryan, D.J., Pinkney, T.T., and O'Donoghue, E.M. (2002). Programmed cell death during flower senescence: Isolation and characterization of cysteine proteinases from Sandersonia aurantiaca. Funct. Plant Biol. 29, 1055–1064. [DOI] [PubMed] [Google Scholar]
- Ekert, P.G., Silke, J., and Vaux, D.L. (1999). Caspase inhibitors. Cell Death Differ. 6, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Elbaz, M., Avni, A., and Weil, M. (2002). Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ. 9, 726–733. [DOI] [PubMed] [Google Scholar]
- Ferreira, R.B., and Davies, D.D. (1989). Conversion of ribulose-1,5-bisphosphate carboxylase to an acidic and catalytically inactive form by extracts of osmotically stressed Lemna minor fronds. Planta 179, 448–455. [DOI] [PubMed] [Google Scholar]
- Fontanini, D., and Jones, B.L. (2002). SEP-1 — A subtilisin-like serine endopeptidase from germinated seeds of Hordeum vulgare L. cv. Morex. Planta 215, 885–893. [DOI] [PubMed] [Google Scholar]
- Gluschankof, P., and Fuller, R.S. (1994). A C-terminal domain conserved in precursor processing proteases is required for intramolecular N-terminal maturation of pro-Kex2 protease. EMBO J. 13, 2280–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover, A., and Jones, A.M. (1999). Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 119, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, J.M.U., Simpson, D.J., Hyman, S.C., Nidimda, B.K., and Slabas, A.R. (2003). Ara12 subtilisin-like protease from Arabisopsis thaliana: Purification, substrate specificity and tissue localization. Biochem. J. 370, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J.-H., Lee, S.H., Tan, Y.-Q., LeMosy, E.K., and Hashimoto, C. (2000). Gastrulation defective is a serine protease involved in activating the receptor Toll to polarize the Drosophila embryo. Proc. Natl. Acad. Sci. USA 97, 9093–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner, M.O. (2000). The biochemistry of apoptosis. Nature 407, 770–776. [DOI] [PubMed] [Google Scholar]
- Hill, R.M., Ledgerwood, E.C., Brennan, S.O., Pu, L.-P., Loh, Y.P., Christie, D.L., and Birch, N.P. (1995). Comparison of the molecular forms of the Kex2/subtilisin-like serine proteases SPC2, SPC3, and Furin in neuroendocrine secretory vesicles reveals differences in carboxyl-terminus truncation and membrane association. J. Neurochem. 65, 2318–2326. [DOI] [PubMed] [Google Scholar]
- Janzik, I., Macheroux, P., Amrhein, N., and Schaller, A. (2000). LeSBT1, a subtilase from tomato plants. J. Biol. Chem. 275, 5193–5199. [DOI] [PubMed] [Google Scholar]
- Jordá, L., Coego, A., Conejero, V., and Vera, P. (1999). A genomic cluster containing four differentially regulated subtilisin-like processing protease genes is in tomato plants. J. Biol. Chem. 274, 2360–2365. [DOI] [PubMed] [Google Scholar]
- Jordá, L., Coego, A., Conejero, V., and Vera, P. (2000). Characterization of P69E and P69F, two differentially regulated genes encoding new members of the subtilisin-like proteinase family from tomato plants. Plant Physiol. 122, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd, V.J. (1998). Proteilytic activities that mediate apoptosis. Annu. Rev. Physiol. 60, 533–573. [DOI] [PubMed] [Google Scholar]
- Katsuhara, M. (1997). Apoptosis-like cell death in barley roots under salt stress. Plant Cell Physiol. 38, 1091–1093. [Google Scholar]
- Konrad, K.D., Goralski, T.J., Mahowald, A.P., and March, J.L. (1998). The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc. Natl. Acad. Sci. USA 95, 6819–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger, J., Thomas, C.M., Golstein, C., Dixon, M.S., Smoker, M., Tang, S., Mulder, L., and Jones, J.D.G. (2002). A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296, 744–747. [DOI] [PubMed] [Google Scholar]
- Li, J., Lease, K.A., Tax, F.E., and Walker, J.C. (2001). BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98, 5916–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMosy, E.K., Tan, Y.-Q., and Hashimoto, C. (2001). Activation of a protease cascade involved in patterning the Drosophila embryo. Proc. Natl. Acad. Sci. USA 98, 5055–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenberger, C.S. (1949). Nature of susceptibility to Helminthosporium victoriae and resistance to Puccinia coronata in Victoria oats. Phytopathology 39, 300–318. [Google Scholar]
- Mackey, D., Holt III, B.F., Wiig, A., and Dangl, J.L. (2002). RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108, 743–754. [DOI] [PubMed] [Google Scholar]
- Meehan, F., and Murphy, H.C. (1946). A new Helminthosporium blight of oats. Science 104, 413–414. [DOI] [PubMed] [Google Scholar]
- Meehan, F., and Murphy, H.C. (1947). Differential phytotoxicity of metabolic by-products of Helminthosporium victoriae. Science 106, 270–272. [DOI] [PubMed] [Google Scholar]
- Meichtry, J., Amrhein, N., and Schaller, A. (1999). Characterization of the subtilase gene family in tomato (Lycopersicon esculentum Mill.). Plant Mol. Biol. 39, 749–760. [DOI] [PubMed] [Google Scholar]
- Miller, J.D., Arteca, R.N., and Pell, E.J. (1999). Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol. 120, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R., Del Pozo, O., Meisel, K., and Lam, E. (1997). Pathogen-induced programmed cell death in plants, a possible defense mechanism. Dev. Genet. 21, 279–289. [DOI] [PubMed] [Google Scholar]
- Mizuno, K., Nakamura, T., Ohshima, T., Tanaka, S., and Matsuo, H. (1988). Yeast Kex2 gene encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem. Biophys. Res. Commun. 156, 246–254. [DOI] [PubMed] [Google Scholar]
- Mlejnek, P., and Procházka, S. (2002). Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215, 158–166. [DOI] [PubMed] [Google Scholar]
- Nagata, S. (2000). Apoptotic DNA fragmentation. Exp. Cell Res. 256, 12–18. [DOI] [PubMed] [Google Scholar]
- Navarre, D.A., and Wolpert, T.J. (1999). Victorin induction of an apoptotic, senescence-like response in oats. Plant Cell 11, 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuteboom, L.W., Ng, J.M.Y., Kuyper, M., Clijdesdale, O.R., Hooykaas, P.J.J., and van der Zaal, B.J. (1999). Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol. Biol. 39, 273–287. [DOI] [PubMed] [Google Scholar]
- Petitou, M., Lormeau, J.-C., and Choay, J. (1988). Interaction of heparin and antithrombin III. Eur. J. Biochem. 176, 637–640. [DOI] [PubMed] [Google Scholar]
- Polverini, P.J., and Nör, J.E. (1999). Apoptosis and predisposition to oral cancer. Crit. Rev. Oral Biol. Med. 10, 139–152. [DOI] [PubMed] [Google Scholar]
- Pontier, D., Tronchet, M., Rogowsky, P., Lam, E., and Roby, D. (1998). Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol. Plant Microbe Interact. 11, 544–554. [DOI] [PubMed] [Google Scholar]
- Popovič, T., Puizdar, V., and Brzin, J. (2002). A novel subtilase from common bean leaves. FEBS Lett. 530, 163–168. [DOI] [PubMed] [Google Scholar]
- Richael, C., Lincoln, J.E., Bostock, R.M., and Gilchrist, D.G. (2001). Caspase inhibitors reduce symptom development and limit bacterial proliferation in susceptible plant tissues. Physiol. Mol. Plant Pathol. 59, 213–221. [Google Scholar]
- Riggs, C.D., Zeman, K., DeGuzman, R., Rzepczyk, A., and Taylor, A.A. (2001). Antisense inhibition of a tomato meiotic proteinase suggests functional redundancy of proteinases during microsporogenesis. Genome 44, 644–650. [PubMed] [Google Scholar]
- Rudenskaya, G.N., Bogacheva, A.M., Preusser, A., Kuznetsova, A.V., Dunaevsky, Y.E., Golovkin, B.N., and Stepanov, V.M. (1998). Taraxalisin — A serine proteinase from dandelion Taraxacum officinale Webb s.l. FEBS Lett. 437, 237–240. [DOI] [PubMed] [Google Scholar]
- Rudenskaya, G.N., Bogdanova, E.A., Revina, L.P., Golovkin, B.N., and Stepanov, V.M. (1995). Macluralisin — A serine proteinase from fruits of Maclura pomifera (Raf.) Schneid. Planta 196, 174–179. [DOI] [PubMed] [Google Scholar]
- Runeberg-Roos, P., and Saarma, M. (1998). Phytepsin, a barley vacuolar aspartic proteinase, is highly expressed during autolysis of developing tracheary elements and sieve cells. Plant J. 15, 139–145. [DOI] [PubMed] [Google Scholar]
- Sammadar, K.R., and Scheffer, R.P. (1968). Effect of the specific toxin in Helminthosporium victoriae on host cell membranes. Plant Physiol. 43, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, A., and Ryan, C.A. (1994). Identification of a 50-kDa systemin-binding protein in tomato plasma membranes having Kex2p-like properties. Proc. Natl. Acad. Sci. USA 91, 11802–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M., Simpson, D., and Gietl, C. (1999). Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc. Natl. Acad. Sci. USA 96, 14159–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M., Simpson, D., Kalousek, F., and Gietl, C. (1998). A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta 206, 466–475. [DOI] [PubMed] [Google Scholar]
- Schmid, M., Simpson, D., Sarioglu, H., Lottspeich, F., and Gietl, C. (2001). The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 98, 5353–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (2002). Reinforcing the idea of signalling in the stomatal pathway. Trends Genet. 18, 597–600. [DOI] [PubMed] [Google Scholar]
- Siezen, R.J., and Leunissen, J.A.M. (1997). Subtilases: The superfamily of subtilisin-like serine proteases. Protein Sci. 6, 501–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solary, E., Eymin, B., Droin, N., and Haugg, M. (1998). Proteases, proteolysis, and apoptosis. Cell Biol. Toxicol. 14, 121–132. [DOI] [PubMed] [Google Scholar]
- Solomon, M., Belenghi, B., Delledonne, M., Menachem, E., and Levine, A. (1999). The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer, S.E., Forster, M.J., Mulloy, B., Bishop, C.R., Graham, G.J., and Gallagher, J.T. (2002). Characterization of the binding site on heparan sulfate for macrophage inflammatory protein 1α. Blood 100, 1543–1550. [PubMed] [Google Scholar]
- Sun, Y.-L., Zhao, Y., Hong, X., and Zhai, Z.-H. (1999). Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Lett. 462, 317–321. [DOI] [PubMed] [Google Scholar]
- Tada, Y., Hata, S., Takata, Y., Nakayashiki, H., Tosa, Y., and Mayama, S. (2001). Induction and signaling of an apoptotic response typified by DNA laddering in the defense response of oats to infection and elicitors. Mol. Plant Microbe Interact. 14, 477–486. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Onouchi, H., Kondo, M., Hara-Nishimura, I., Nishimura, M., Machida, C., and Machida, Y. (2001). A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128, 4681–4689. [DOI] [PubMed] [Google Scholar]
- Taylor, A.A., Horch, A., Rzepczyk, A., Hasenkampf, C.A., and Riggs, C.D. (1997). Maturation and secretion of a serine proteinase is associated with events of late microsporogenesis. Plant J. 12, 1261–1271. [DOI] [PubMed] [Google Scholar]
- Terp, N., Thomsen, K.K., Svendsen, I., Davy, A., and Simpson, D.J. (2000). Purification and characterization of hordolisin, a subtilisin-like serine endoprotease from barley. J. Plant Physiol. 156, 468–476. [Google Scholar]
- Thornberry, N.A., Rano, T.A., Peterson, E.P., Rasper, D.M., Timkey, T., Garcia-Calvo, M., Houtzager, V.M., Nordstrom, P.A., Roy, S., Vaillancourt, J.P., Chapman, K.T., and Nicholson, D.W. (1997). A combinatorial approach defines specificities of members of the caspase family and granzyme B. J. Biol. Chem. 272, 17907–17911. [DOI] [PubMed] [Google Scholar]
- Tian, R.-H., Zhang, G.-Y., Yan, C.-H., and Dai, Y.-R. (2000). Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Lett. 474, 11–15. [DOI] [PubMed] [Google Scholar]
- Tornero, P., Conejero, V., and Vera, P. (1996. a). Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: Similarity of functional domains to subtilisin-like endoproteases. Proc. Natl. Acad. Sci. USA 93, 6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P., Conejero, V., and Vera, P. (1997). Identification of a new pathogen-induced member of the subtilisin-like processing protease family from plants. J. Biol. Chem. 272, 14412–14419. [DOI] [PubMed] [Google Scholar]
- Tornero, P., Mayda, E., Gómez, M.D., Canas, L., Conejero, V., and Vera, P. (1996. b). Characterization of LRP, a leucine-rich repeat (LRR) protein from tomato plants that is processed during pathogenesis. Plant J. 10, 315–330. [DOI] [PubMed] [Google Scholar]
- Van de Ven, W.J.M., Creemers, J.W.M., and Roebroek, A.J.M. (1991). Furin: The prototype mammalian subtilisin-like proprotein-processing enzyme. Enzyme 45, 257–270. [DOI] [PubMed] [Google Scholar]
- Vaux, D.L., and Korsmeyer, S.J. (1999). Cell death in development. Cell 96, 245–254. [DOI] [PubMed] [Google Scholar]
- Vera, P., and Conejero, V. (1988). Pathogenesis-related proteins of tomato. Plant Physiol. 87, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, L., Xia, Q., Qiu, X., and Selvaraj, G. (2002). Early stages of seed development in Brassica napus: A seed coat-specific cysteine proteinase associated with programmed cell death of the inner integument. Plant J. 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Wang, T.H., and Wang, H.S. (1999). Apoptosis: (2) Characteristics of apoptosis. J. Formos. Med. Assoc. 98, 531–542. [PubMed] [Google Scholar]
- Weidhase, R.A., Lehmann, J., Kramell, H., Sembdner, G., and Parthier, B. (1987). Degradation of ribulose-1,5-bisphosphate carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methyl ester, and counteraction by cytokinin. Physiol. Plant 69, 161–166. [Google Scholar]
- Wheeler, H., and Black, H.S. (1962). Change in permeability induced by victorin. Science 137, 983–984. [DOI] [PubMed] [Google Scholar]
- Woltering, E.J., van der Bent, A., and Hoeberichts, F.A. (2002). Do plant caspases exist? Plant Physiol. 130, 1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata, H., Masuzawa, T., Nagaoka, Y., Ohnishi, T., and Iwasaki, T. (1994). Cucumisin, a serine protease from melon fruits, shares structural homolgy with subtilisin and is generated from a large precursor. J. Biol. Chem. 269, 32725–32731. [PubMed] [Google Scholar]
- Yamagata, H., Uesugi, M., Saka, K., Iwasake, T., and Aizono, Y. (2000). Molecular cloning and characterization of a cDNA and a gene for subtilisin-like serine proteases from rice (Oryza sativa L.) and Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 64, 1947–1957. [DOI] [PubMed] [Google Scholar]
- Yao, N., Tada, Y., Park, P., Nakayashiki, H., Tosa, Y., and Mayama, S. (2001). Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J. 28, 13–26. [DOI] [PubMed] [Google Scholar]