Abstract
Do physiological changes occur shortly prior to psychotic relapse in schizophrenia outpatients? We addressed this question in a group of schizophrenia outpatients by measuring changes in symptoms and changes in activation of the sympathetic nervous system, as indexed by changes in skin conductance level (SCL), on a biweekly basis for between one and two years. All six outpatients exhibited heightened SCL within two weeks prior to relapse or exacerbation, compared to SCL proceeding continued remission. These results shed light on the psychotic relapse process and are consistent with neural diathesis-stress models of schizophrenia.
Keywords: diathesis-stress model, electrodermal activity, prodromal signs, relapse, schizophrenia, skin conductance
1. Introduction
The course of schizophrenia is often marked by periods of relative symptomatic remission punctuated by periods of psychotic symptoms. Phenomenological and behavioral changes are sometimes noticeable within a few days or weeks prior to the appearance of psychotic symptoms (the prodromal period) (Docherty et al., 1978; Herz & Melville, 1980; Subotnik & Nuechterlein, 1988). Common prodromal signs based on self-report include increases in anxiety, tension, somatic concerns, depression, sleep disturbance, social withdrawal, and cognitive inefficiency (see review by Norman & Malla, 1995). The low specificity of these self-reports, however, makes it desirable to search for biological markers of the prodromal period.
According to diathesis-stress models of schizophrenia, stressors that precede exacerbations of psychotic symptoms produce physiological changes that may serve as prodromal signs (Dawson et. al., 1983; Nuechterlein & Dawson, 1984; Walker & Diforio, 1997). One easily measured physiological sign of stress is increased electrodermal activity (EDA). EDA is controlled by the sympathetic nervous system (SNS) (Dawson et al., 2007), is known to increase in response to psychosocial stressors considered key in psychotic relapse in schizophrenia (Tarrier et al., 1979; Tarrier & Barrowclough, 1989), and has been found to increase during exacerbation of symptoms (Dawson et al., 1994).
Which appears first, the heightened EDA or the psychotic symptoms? In a small previous study, EDA arousal was found to be elevated in the weeks prior to a psychotic relapse or exacerbation in 4 out of 5 schizophrenic outpatients (Hazlett et. al., 1997). If this finding proves to be reliable, the hypothesized role of the stress response in triggering psychotic episodes would receive further support. Furthermore, monitoring such transient increases in arousal could potentially indicate when increased intervention (e.g., medication) could potentially prevent an imminent relapse. Therefore, we conducted an intensive prospective longitudinal examination of EDA and symptomatic state on a biweekly basis for up to two years in a group of relatively remitted schizophrenic outpatients. Thus, we were able to determine whether (1) psychotic relapses or exacerbations were preceded by heightened EDA arousal and whether (2) heightened EDA arousal was always followed by psychotic exacerbation or relapse.
2. Method
2.1 Participants
A total of 23 outpatients from the UCLA Aftercare Research Program with a diagnosis of schizophrenia or schizoaffective disorder, mainly schizophrenic, by Research Diagnostic Criteria (Spitzer, et. al., 1978) were recruited. Other inclusionary and exclusionary criteria for participation in the larger study are described by Nuechterlein et al. (1992). Data are reported here for a subset of six patients who were initially in psychotic remission and subsequently satisfied strict symptomatic criteria described in section 2.3, and for whom at least a year of data were obtained. The age, gender, diagnoses, and medication status of all six participants, and the total number of EDA tests completed, are displayed in Table 1.
Table 1.
Demographic, Diagnosis, Medication Information, and Number of EDA Tests
SZ = Schizophrenia diagnosis
SZAFF = Schizoaffective diagnosis
| Participant Number | Gender | Diagnosis | Age at Entry | Number of Tests Completed | Medication Pre-Remission | Medication Pre-Relapse |
|---|---|---|---|---|---|---|
| 1 | Male | SZ | 21.8 | 24 | risperidone 2.0 mg/day | risperidone 2.0 mg/day |
| 2 | Female | SZ | 25.4 | 49 | risperidone 4.5 mg/day | risperidone 4.5 mg/day |
| 3 | Male | SZ | 22.8 | 58 | risperidone 6.0 mg/day | risperidone 7.5 mg/day |
| 4 | Male | SZ | 38.0 | 72 | olanzapine 10.0 mg/day | olanzapine 10.0 mg/day |
| 5 | Male | SZ | 41.0 | 30 | fluphenazine 10.0 mg/day | fluphenazine 9.0 mg/day |
| 6 | Female | SZAFF | 41.3 | 43 | olanzapine 10.0 mg/day fluphenazine 6.25 mg/day | olanzapine 50.0 mg/day fluphenazine 10.0 mg/day |
2.2 Overall design
EDA testing was completed as close as possible to a biweekly schedule during the patients' normally scheduled clinic visits, at which time symptomatic states were also measured with the 24-item version of the Brief Psychiatric Rating Scale (BPRS; Lukoff, et. al., 1986; Overall & Gorham, 1962). EDA tests that were followed by psychotic relapse/exacerbation (“pre-relapse” test) and tests that were followed by continued symptomatic remission (“pre-remission” test) were then identified based on subsequent changes in the biweekly BPRS.
2.3 Identification of clinical states
The BPRS was administered every two weeks and was used to determine the remitted, psychotic exacerbation and psychotic relapse states. Symptoms were rated on a scale from 1 (not present) to 7 (extremely severe), with ratings of 4 and higher being in the pathological range according to the BPRS anchor points (Ventura et al., 1993). A psychotic exacerbation was defined as an increase from the immediately preceding rating of at least two points in the sum of the three BPRS psychotic items (unusual thought content, conceptual disorganization, and hallucinations), and a rating above 3 on at least one of these items for at least two consecutive biweekly evaluations. A psychotic relapse was defined as an increase from the immediately preceding BPRS rating of at least two points in the sum of the psychotic items in which the score of at least one of the three psychotic items reached 6 or 7. The criteria for exacerbation are consistent with those for a “mild exacerbation” and the criteria for relapse are consistent with those for “relapse” recommended by Nuechterlein et al. (2006).
2.4 Final sample of participants
Of the 23 outpatients, 13 either left the program or did not continue bi-weekly attendance before data could be collected for the full one-year period. Of the remaining 10 participants, two had no remission periods satisfying criteria, one had no relapse or exacerbation, and one was found to have inaccurately reported symptoms. Thus, a total of six patients had both pre-remission and pre-relapse EDA tests.
2.5 Electrodermal tests and measures
EDA was recorded using standard methods (Dawson et al., 2007) during a 5-minute rest period. Two electrodermal measures were obtained: (1) the number of nonspecific skin conductance responses (NS-SCRs) and (2) the mean skin conductance level (SCL). NS-SCRs were defined as the number of responses of at least .05 μSiemens. SCL was defined as the mean of the skin conductance levels recorded at each minute during the rest period.
3. Results
The sums of the 3 BPRS psychotic items, the sums of the 24 BPRS items, the mean skin conductance level (SCL) and the mean rate of nonspecific skin conductance responses (NS-SCRs) were analyzed for each of the 6 participants. For each variable, difference scores were computed between the pre-remission and pre-relapse tests, and Sign Tests were performed on the difference scores.
The mean of the 24 BPRS items were at low levels during both the pre-remission and pre-relapse tests (Figure 1). The mean increased from the pre-relapse period to the relapse period in all patients, whereas there was no significant change from the pre-remission to the remission tests. Likewise, the sums of the 3 psychotic items were relatively low and did not differ significantly from each other during the pre-remission and pre-relapse tests. There was a significant increase in the psychotic ratings during the relapse period compared to the pre-relapse period (p = .016), but not during the remission period compared to the pre-remission period.
Figure 1.
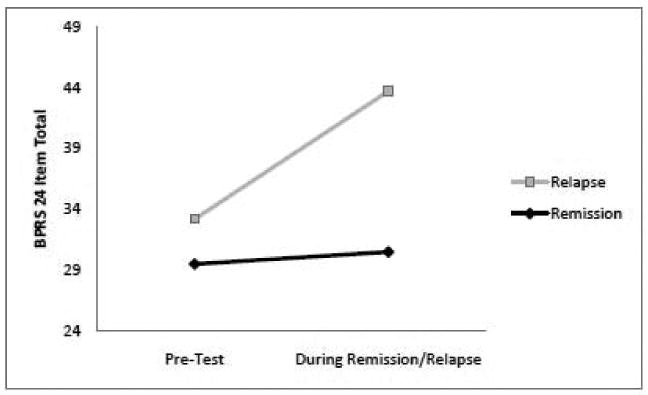
Mean total scores on the 24 item Brief Psychiatric Rating Scale (BPRS) during the pre-relapse and pre-remission test occasions and during the subsequent relapse and remission occasions. The change from pre-relapse to relapse is significant (p = .016); no other differences are significant. The minimum BPRS score is 24.
Most important, SCL was higher during the pre-relapse test than during the pre-remission test for each of the six patients (p = .016). As shown in Figure 2, there is considerable between-patient variability in the absolute values of SCL, but the SCL for each patient was higher during the pre-relapse test than during the pre-remission test. In contrast, the rates of NS-SCRs did not discriminate the two states.
Figure 2.
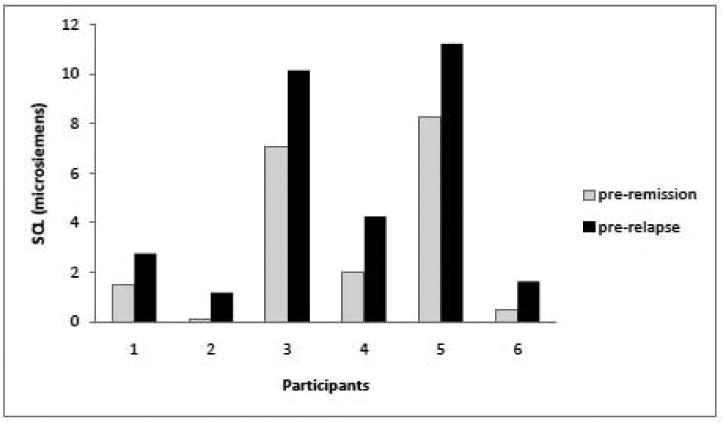
Mean skin conductance level for each of six patients during the pre-relapse test and the pre-remission test. Patients were in similar remitted states during both the pre-relapse and pre-remission tests but exhibited higher SCL during the pre-relapse tests.
In addition to these tests, three of the six patients had one or more additional psychotic exacerbations less severe than those described above. Of the total of 10 relapses/exacerbations seen across all patients, 8 (80%) were preceded by SCL increases. Thus, SCL showed good sensitivity in prediction of schizophrenic relapse/exacerbation.
On the other hand, specificity was not as high as sensitivity in that several occasions of heightened SCL were not followed by relapses or exacerbations. Four of six patients had other EDA tests besides the pre-relapse tests where SCL was equally high or higher, but without a return of psychotic symptoms. Thus, although relapses or exacerbations were commonly preceded by increases in SCL, such increases were not always followed by relapses or exacerbations.
4. Discussion
Increases in sympathetic nervous system activity, indexed by heightened SCL, occurred reliably in the weeks preceding psychotic relapse and exacerbation, thus replicating and extending findings reported in an independent sample of patients (Hazlett et al., 1997). The present results shed light on the nature of the psychotic relapse process and are consistent with the neural diathesis-stress model of Walker and Diforio (1997) in which the HPA axis activation associated with the stress response, which would be expected to accompany SNS activation, induces dopamine release through the effect of heightened cortisol on dopamine. The results are also consistent with the noradrenalin hypothesis of Yamamoto and Hornykiewcz (2004).
One limitation of this study concerns the relatively small sample. However, the results are statistically significant, and are even more so when combined with those reported previously in an independent sample. Another limitation is that the patients were initially in stable remission, making it possible to define a discrete point of the onset of symptomatic relapse or exacerbation. Defining this time point would be more difficult among patients with persisting symptoms of various degrees (R. Zarate, personal communication, 2009). Research with larger and more heterogeneous samples is needed to test the generality of these results.
An interesting direction for future research is to measure the prodromal signs of sympathetic activation within the ultra high-risk paradigm that identifies distal prodromal signs (Cannon et al., 2007). Such distal signs may identify who is likely to show psychotic symptoms within 1 to 2 years, whereas the proximal signs may identify when psychotic symptoms are likely to occur within a few weeks.
Acknowledgments
We thank William C. Williams and Bill Troyer for providing computer scoring programs. We gratefully acknowledge the following individuals for the administration and scoring of the Brief Psychiatric Rating Scale (BPRS): Sally Friedlob, Debbie Gioia-Hasick, Kim Field and Rosemary Collier. Training in the use of the BPRS was provided by the Diagnosis and Pathology Unit of the UCLA Clinical research Center for the Study of Schizophrenia. We thank the treating psychiatrists at the UCLA Aftercare Research Program: Michael Gitlin and Ben Siegal. Finally, we thank the patients who patiently gave of their time that made this research possible.
Role of Funding Source: This research was supported by USPHS grants MH-37705 (Keith Nuechterlein, P.I.), and MH46433 (Michael Dawson, P.I.) from the National Institute of Mental Health. The NIMH had no further role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
All authors declare they have no conflicts of interest.
Contributors
MD and AS conceptualized the study design, analyzed the data and prepared the original manuscript. AR collected, organized and helped interpret the data. JV oversaw the quality of the BPRS data collection. KN and KS had broad oversight of the large study in which these patients were participants and they also helped interpret the results and edit the manuscript. All authors commented on the manuscript at various stages of preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cannon TD, Cornblatt B, McGorry P. Editor's introduction: The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull. 2007;33:661–664. doi: 10.1093/schbul/sbm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Nuechterlein KH, Liberman RP. Relapse in schizophrenic disorders: Possible contributing factors and implications for behavior therapy. In: Rosenbaum M, Franks CM, Jaffe Y, editors. Perspectives on behavior therapy in the eighties. Springer Verlag; New York: 1983. pp. 265–286. [Google Scholar]
- Dawson ME, Nuechterlein KH, Schell AM, Gitlin M, Ventura J. Autonomic abnormalities in schizophrenia: State or trait indicators? Arch Gen Psychiatry. 1994;51:813–824. doi: 10.1001/archpsyc.1994.03950100061006. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd. Cambridge University Press; New York: 2007. pp. 159–181. [Google Scholar]
- Docherty JP, Van Kammen DP, Siris SG, Marder SR. Stages of onset of schizophrenic psychosis. Am J Psychiatry. 1978;135:420–426. doi: 10.1176/ajp.135.4.420. [DOI] [PubMed] [Google Scholar]
- Hazlett H, Dawson ME, Schell AM, Nuechterlein KH. Electrodermal activity as a prodromal sign in schizophrenia. Biol Psychiatry. 1997;41:111–113. doi: 10.1016/s0006-3223(96)00351-4. [DOI] [PubMed] [Google Scholar]
- Herz MI, Melville C. Relapse in schizophrenia. Am J Psychiatry. 1980;137:801–805. doi: 10.1176/ajp.137.7.801. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for expanded Brief Psychiatric Rating Scale (BPRS) Schizophr Bull. 1986;12:594–602. [Google Scholar]
- Marder SR, Mintz J, Van Putten T, Lebell M, McKenzie J. Prodromal symptoms as predictors of schizophrenic relapse. In: Lieberman JA, Kane JM, editors. Predictors of Relapse in Schizophrenia. American Psychiatric Press; Washington, D.C.: 1986. pp. 48–57. [Google Scholar]
- Norman RMG, Malla AK. Prodromal symptoms of relapse in schizophrenia: A review. Schizophr Bull. 1995;21:527–539. doi: 10.1093/schbul/21.4.527. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull. 1984;10:300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, et al. Developmental Processes in Schizophrenic Disorders: Longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–426. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Miklowitz DJ, Ventura J, Gitlin MJ, Stoddard M, Lukoff D. Classifying episodes in schizophrenia and bipolar disorder: Criteria for relapse and remission applied to recent-onset samples. Psychiatry Res. 2006;144:153–166. doi: 10.1016/j.psychres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: Rational and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Subotnik KL, Nuechterlein KH. Prodromal signs and symptoms of schizophrenic relapse. J Abn Psychol. 1988;97:405–412. doi: 10.1037//0021-843x.97.4.405. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Vaughn C, Lader MH, Leff JP. Bodily reactions to people and events in schizophrenics. Arch Gen Psychiatry. 1979;36:311–315. doi: 10.1001/archpsyc.1979.01780030077007. [DOI] [PubMed] [Google Scholar]
- Tarrier N, Barrowclough C. Electrodermal activity as a predictor of schizophrenic relapse. Psychopathology. 1989;22:320–324. doi: 10.1159/000284614. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RL, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS), Expanded Version (4.0): Scales, anchor points, and administration manual. International J Method in Psychiatric Research. 1993;3:227–243. [Google Scholar]
- Walker EE, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hornykiewicz O. Proposal for a noradrenalin hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:913–922. doi: 10.1016/j.pnpbp.2004.05.033. [DOI] [PubMed] [Google Scholar]


