Abstract
Background
Short-duration studies show that salsalate improves glycemia in type 2 diabetes mellitus (T2DM).
Objective
To assess 1-year efficacy and safety of salsalate in T2DM.
Design
Placebo-controlled, parallel trial; computerized randomization and centralized allocation, with patients, providers, and researchers blinded to assignment. (ClinicalTrials.gov: NCT00799643)
Setting
3 private practices and 18 academic centers in the United States.
Patients
Persons aged 18 to 75 years with fasting glucose levels of 12.5 mmol/L or less (≤225 mg/dL) and hemoglobin A1c (HbA1c) levels of 7.0% to 9.5% who were treated for diabetes.
Intervention
286 participants were randomly assigned (between January 2009 and July 2011) to 48 weeks of placebo (n = 140) or salsalate, 3.5 g/d (n = 146), in addition to current therapies, and 283 participants were analyzed (placebo, n = 137; salsalate, n = 146).
Measurements
Change in hemoglobin A1c level (primary outcome) and safety and efficacy measures.
Results
The mean HbA1c level over 48 weeks was 0.37% lower in the salsalate group than in the placebo group (95% CI, −0.53% to −0.21%; P < 0.001). Glycemia improved despite more reductions in concomitant diabetes medications in salsalate recipients than in placebo recipients. Lower circulating leukocyte, neutrophil, and lymphocyte counts show the anti-inflammatory effects of salsalate. Adiponectin and hematocrit levels increased more and fasting glucose, uric acid, and triglyceride levels decreased with salsalate, but weight and low-density lipoprotein cholesterol levels also increased. Urinary albumin levels increased but reversed on discontinuation; estimated glomerular filtration rates were unchanged.
Limitation
Trial duration and number of patients studied were insufficient to determine long-term risk–benefit of salsalate in T2DM.
Conclusion
Salsalate improves glycemia in patients with T2DM and decreases inflammatory mediators. Continued evaluation of mixed cardiorenal signals is warranted.
Primary Funding Source
National Institutes of Health.
Salicylate is one of the oldest drugs in clinical practice, with documented use of relevant plant extracts for treating pain and inflammation dating back at least 3500 years (1). Nevertheless, its medicinal properties and mechanisms of action remain incompletely understood. Chemically pure forms were introduced during the 19th century (2, 3), but by the century’s end, salicylate had been acetylated by chemists to yield aspirin, which became the most used—and most marketed—drug in history (1, 4). The mechanism of aspirin is well-established; the acetyl group covalently modifies a serine at the active site of the cyclooxygenase (COX) enzymes (5), making it the prototypic nonsteroidal anti-inflammatory drug (NSAID). Salicylate lacks an acetyl group and, thus, must have a different mechanism of action. Neither salicylate nor prodrugs, including salsalate or trilisate, which are marketed for pain, have been tested for efficacy and safety under what regulatory agencies now consider to be current standard practice in clinical trials.
Interest in salicylate was renewed after suggestions that it lowers blood glucose in type 2 diabetes mellitus (T2DM) (6). Results from proof-of-principle studies using salsalate in patients with T2DM demonstrated reduced blood glucose, triglyceride, free fatty acid, and C-reactive protein concentrations; improved glucose utilization during euglycemic hyperinsulinemic clamp (defined as the glucose infusion rate required to maintain euglycemia at steady state during insulin infusion); and increased circulating insulin and adiponectin levels (7). The National Institutes of Health–sponsored TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) trials determine whether this generic and inexpensive drug is safe, tolerated, and efficacious in diabetes. Stage 1, a dose-ranging study, was reported (8); stage 2 of TINSAL-T2D is a larger study to assess the magnitude and durability of glycemic efficacy over 1 year, tolerability, and an array of safety variables relevant to patients with diabetes.
Methods
Design Overview
Stage 2 of TINSAL-T2D was a single-blind, placebo lead-in, randomized (1:1), placebo-controlled, parallel clinical trial to assess whether salsalate is superior to placebo in patients with T2DM and inadequate glycemic control. Participants were randomly assigned between January 2009 and July 2011, and the last participant visit occurred in September 2011.
Setting and Participants
The study was conducted at 21 U.S. sites (3 private practice and 18 academic centers). Participants were recruited from practices or through advertising. Eligible adult patients were 75 years or younger; had hemoglobin A1c (HbA1c) levels of 7.0% to 9.5% at screening; and were treated by lifestyle modification or with metformin, insulin secretagogue, or dipeptidyl peptidase-4 inhibitor, alone or in combination. Participants using insulin, thiazolidinediones, glucagon-like peptide-1 agonists, NSAIDs, warfarin, or uricosuric agents were not eligible (Appendix 2, available at www.annals.org).
Randomization and Intervention
The protocol, approved by human subject institutional review boards, included 1-week screening; a 4-week, single-blind placebo run-in phase; pretreatment baseline evaluation; and 48 weeks of treatment. Salsalate was administered at 3.0 g daily for 2 weeks then escalated to 3.5 g daily, as tolerated, divided into 3 daily doses. Randomization was computer-generated in blocks of 4 with centralized allocation and codes secured at the data coordinating center. Participants were blinded during the run-in phase. Study participants, site investigators and staff, steering committee members, and data coordinating center staff responsible for clinical activities were blinded to treatment assignment. To assess study drug effect, we recommended that patients maintain stable dosages of diabetes, lipid-lowering, and hypertension medications for 24 weeks whenever possible. Dose reductions in diabetes medications were immediate for hypoglycemia. After 24 weeks of randomization, good clinical practice was recommended with planned “rescue therapy” for very poorly controlled diabetes (Appendix 2).
Follow-up and Outcomes
Clinic visits followed an overnight fast at screening; run-in; randomization; and 4, 8, 12, 16, 24, 36, and 48 weeks to assess safety, adherence, and treatment response. A visit after dosing occurred at 50 weeks. An additional visit at week 56 was added for patients with persistent tinnitus, elevated urinary albumin levels, or increased blood pressure. Adverse events were assessed by questionnaire at follow-up visits. Quality of life was assessed using the Short Form-36 Health Survey at baseline and weeks 24 and 48. Clinical laboratory evaluations were done at Quest Diagnostics (Chantilly, Virginia).
The primary outcome was change in HbA1c level. Key secondary outcomes included change in other variables to determine effects on glucose homeostasis and cardiometabolic risk (Appendix 2). Outcomes were assessed after the last patient visit. Hypoglycemia was classified as mild if symptoms were relieved by food or if documented blood glucose concentration was less than 3.3 mmol/L (<60 mg/dL) and severe if patients required assistance.
Statistical Analyses
We calculated a sample size of 286 to detect a 0.5% difference in HbA1c level between groups at week 48, based on 80% power, an α level of 0.05, an SD of 1.33, and a 20% withdrawal rate. We analyzed data following intention-to-treat principles, with persons in groups as assigned regardless of study drug adherence. Analyses included data from patients with baseline HbA1c measurements through the end of the trial or withdrawal.
Differences in baseline characteristics between groups used analysis of variance for normally distributed continuous traits and chi-square or Fisher exact test for categorical traits. For normally distributed continuous outcomes, we estimated mean group differences using linear-regression mixed models, including HbA1c measurements adjusted for baseline over the 48-week study. Natural log-transformations were used for variables with log-normal distributions. We assumed an autoregressive, moving-average covariance structure. Group was tested as a fixed effect and clinical center and study time were tested as random effects. For continuous outcomes with nonparametric distributions that could not be transformed to the normal distribution (alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase), we used the Wilcoxon test to compare change from baseline between groups at week 48. Differences between categorized outcomes were tested using chi-square analysis. For testing recurring events, such as hypoglycemia, a patient was categorized as ever or never having the event. All statistical tests report 2-sided P values; a P value less than 0.050 was considered significant. We used SAS, version 9.2 (SAS Institute, Cary, North Carolina) for analysis.
Role of the Funding Source
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, which participated in study design and data interpretation. Caraco Pharmaceutical Laboratories (Detroit, Michigan) provided salsalate and placebo, LifeScan (Milpitas, California) provided glucometers and test strips, and Mercodia (Uppsala, Sweden) provided insulin assay materials. No private company had roles in trial design, conduct, data analysis, or manuscript preparation.
Results
Baseline Characteristics
Of the 638 patients screened, 326 entered the 4-week placebo run-in phase and 286 were randomly assigned (140 to placebo and 146 to salsalate) (Figure 1). Groups were similar for multiple baseline characteristics (Table 1). Most participants (88.1%) used metformin, 40.9% took a single diabetes drug, 49% used dual therapy, and 5.6% were already using triple therapy. Only 4.5% of participants were treated with lifestyle modifications alone. Although HbA1c levels were 7.0% to 9.5% at screening (mean, 7.85% [95% CI, 7.77% to 7.93%]), 5 weeks later at randomization after the single-blind placebo run-in phase, the mean HbA1c level decreased by 0.15% (CI, −0.21% to −0.09%; P <0.001, 1-sample t-test). Results were reported for the 137 placebo recipients and 146 salsalate recipients with a baseline HbA1c measurement. For the primary outcome of change in HbA1c level, the placebo group was missing 16% of measurements and the salsalate group was missing 17% due to participant withdrawal and laboratory specimen problems (Figure 2, A). When possible, missing laboratory results were redrawn at interim visits or at the next scheduled visit.
Figure 1. Study flow diagram.
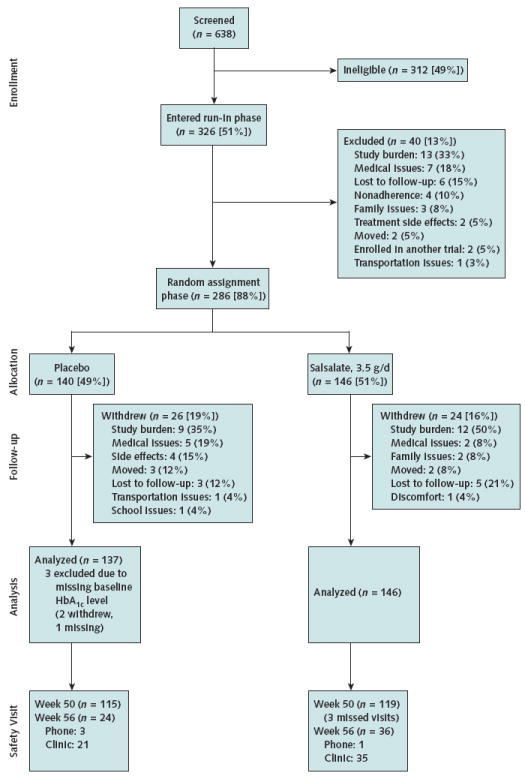
All data were used through trial completion or point of withdrawal for patients with a baseline HbA1c measurement. Two participants withdrew after randomization but before the blood draw; 1 additional participant did not have baseline HbA1c measurement from the laboratory. Percentages may not sum to 100 due to rounding. HbA1c = hemoglobin A1c.
Table 1.
Baseline Characteristics, by Treatment Group*
| Characteristic | Total (n = 286) | Placebo (n = 140) | Salsalate (n = 146) |
|---|---|---|---|
|
| |||
| Mean age (SD), y | 55.8 (9.6) | 55.8 (10.0) | 55.8 (9.2) |
|
| |||
| Male sex | 156 (54.5) | 74 (52.9) | 82 (56.2) |
| Race/ethnicity† | |||
|
| |||
| White | 151 (52.8) | 75 (53.6) | 76 (52.1) |
|
| |||
| Black | 95 (33.2) | 47 (33.6) | 48 (32.9) |
|
| |||
| Other | 40 (14.0) | 18 (12.9) | 22 (15.1) |
|
| |||
| BMI (SD), kg/m2 | 33.3 (6.7) | 33.2 (6.8) | 33.3 (6.7) |
|
| |||
| Median time since diabetes diagnosis (min, max), y | 4.9 (0.1, 38.3) | 4.9 (0.2, 35.0) | 5.3 (0.1, 38.3) |
|
| |||
| Medical history | |||
| Established CVD‡ | 32 (11.2) | 14 (10.0) | 18 (12.3) |
|
| |||
| Hypertension§ | 208 (72.7) | 101 (72.1) | 107 (73.3) |
|
| |||
| Dyslipidemia∥ | 199 (69.6) | 97 (69.3) | 102 (69.9) |
|
| |||
| Family history¶ of T1DM | 13 (4.5) | 6 (4.3) | 7 (4.8) |
|
| |||
| Family history¶ of T2DM | 190 (66.4) | 93 (66.4) | 97 (66.4) |
|
| |||
| Family history¶ of CVD | 163 (57.0) | 82 (58.6) | 81 (55.5) |
|
| |||
| Taking diabetes medications Metformin | 252 (88.1) | 124 (88.6) | 128 (87.7) |
|
| |||
| Insulin secretagogue | 149 (52.1) | 65 (46.4) | 84 (57.5) |
|
| |||
| α-Glucosidase inhibitor | 1 (0.3) | 0 (0.0) | 1 (0.7) |
|
| |||
| DPP-4 inhibitor | 43 (15.0) | 20 (14.3) | 23 (15.8) |
|
| |||
| Lifestyle only (no diabetes drugs) | 13 (4.5) | 7 (5.0) | 6 (4.1) |
|
| |||
| Taking 1 diabetes medication | 117 (40.9) | 62 (44.3) | 55 (37.7) |
|
| |||
| Taking 2 diabetes medications | 140 (49.0) | 66 (47.1) | 74 (50.7) |
|
| |||
| Taking 3 diabetes medications | 16 (5.6) | 5 (3.6) | 11 (7.5) |
|
| |||
| Taking lipid medications** | 180 (62.9) | 85 (60.7) | 95 (65.1) |
|
| |||
| Statin | 171 (59.8) | 80 (57.1) | 91 (62.3) |
|
| |||
| Other lipid medications | 28 (9.8) | 13 (9.3) | 15 (10.3) |
|
| |||
| Taking antihypertensive medications†† | 185 (64.7) | 91 (65.0) | 94 (64.4) |
|
| |||
| ACE inhibitor or ARB | 159 (55.6) | 75 (53.6) | 84 (57.5) |
|
| |||
| Other antihypertensive medications | 111 (38.8) | 62 (44.3) | 49 (33.6) |
|
| |||
| Taking low-dose aspirin‡‡ | 116 (40.6) | 57 (40.7) | 59 (40.4) |
ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; BMI = body mass index; CVD = cardiovascular disease; DPP-4 = dipeptidyl peptidase-4; max = maximum; min = minimum; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
All values are numbers (percentages) unless otherwise indicated. Percentages may not sum to 100 due to rounding.
Patients appearing in >1 category are grouped with “other.”
A history of stroke, angina, coronary artery bypass graft surgery, or percutaneous transluminal coronary angioplasty.
Systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or taking antihypertensive drugs, including loop, thiazide, or potassium-sparing diuretics, potassium supplements, ACE inhibitors, ARBs, calcium-channel blockers, peripheral α-blockers, central α-adrenergic agonists, β-blockers, vasodilators, or reserpine.
Low-density lipoprotein cholesterol level >3.89 mmol/L (>150 mg/dL) or taking cholesterol-lowering drugs, including bile acid sequestrants, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), fibrates, cholesterol absorption inhibitors, niacin, and nicotinic acid.
First-degree relatives.
Some participants take statins and “other” lipid medications.
Some participants take ACE inhibitors or ARBs and “other” antihypertensive agents.
81–325 mg/d.
Figure 2. Glycemic effects of salsalate.
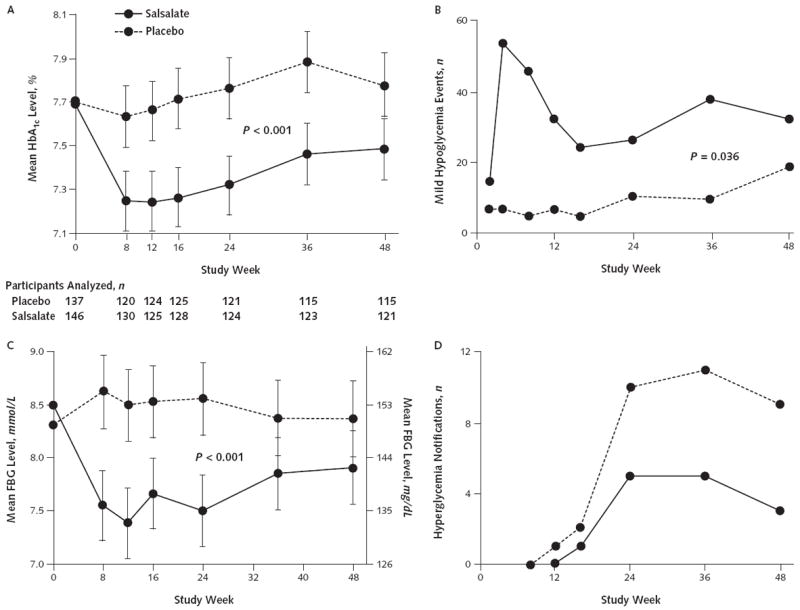
HbA1c (A) and FBG (C) levels are graphed as unadjusted means and 95% CIs. In panel A, the numbers of participants analyzed for the primary end point of HbA1c levels at each time point are displayed below study week. Mild hypoglycemia events (B) and notifications for hyperglycemia (D) sent to the primary caregivers are based on HbA1c levels >10.5% before week 24 and >9.5% after week 24. Participants could have >1 mild hypoglycemic event or exceed the hyperglycemic threshold >1 time during the trial. FBG = fasting blood glucose; HbA1c = hemoglobin A1c.
Study Adherence
Mean medication adherence rates were 91% for placebo and 92% for salsalate; 7.7% of participants had mean adherence less than 80%. Expected visits were 98%completed. Total and time to withdrawal did not differ between salsalate and placebo groups. No participants were unblinded during the trial.
Hemoglobin A1c Level and Glycemic Control
The mean difference in HbA1c levels over 48 weeks between the salsalate and placebo groups was −0.37% (CI, −0.53% to −0.21%; P < 0.001). The mean HbA1c level in the salsalate group was 0.33% lower than at baseline after 48 weeks of treatment (CI, −0.44% to −0.22%; P < 0.001) and essentially unchanged (increase of 0.04%) over 48 weeks in the placebo group (CI, −0.08% to 0.15%; P = 0.51). Significant differences between groups were seen at every time point (Figure 2, A).
There was an interaction between baseline HbA1c levels and group (P < 0.001). Baseline HbA1c levels were not associated with change in HbA1c levels in the placebo group (P = 0.93). For salsalate, baseline glycemia affected the magnitude of glycemic decreases, because participants with greater baseline HbA1c levels had the greatest magnitudes of change. For every 1% increase in baseline HbA1c level, the mean (±SE) decrease in HbA1c levels over 48 weeks was 0.43% ± 0.081% greater (P < 0.001). At 48 weeks, more patients receiving salsalate (41% salsalate v. 23% placebo) achieved reductions of 0.5% or greater in HbA1c levels (P = 0.005).
Consistent with HbA1c levels decreasing, the mean change in fasting glucose level was −0.83 mmol/L (−15 mg/dL) greater for salsalate than placebo over 48 weeks (CI, −1.14 to −0.53 mmol/L [−20.5 to −9.6 mg/dL]; P < 0.001) (Figure 2, C).
Also related to glycemic control, mild hypoglycemic events occurred more frequently with salsalate than placebo. Forty-one participants receiving salsalate and 23 receiving placebo had mild hypoglycemic events (P = 0.036) (Figure 2, B), with a 6-fold increased relative risk for mild hypoglycemia when salsalate was added to sulfonylurea (P < 0.001). Fewer safety alerts for crossing hyperglycemic thresholds occurred for salsalate than placebo (Figure 2, D). Glycemia improved despite adjustments in concomitant diabetes medications in 76 participants (27%). Whereas numbers of adjustments were similar for salsalate (n = 39) and placebo (n = 37), dose reductions and discontinuations were more frequent for salsalate (62%) than placebo (13%). Conversely, concomitant diabetes medications were increased and new therapies instituted more frequently for patients receiving placebo (87%) than those receiving salsalate (38%) (P < 0.001) (Appendix Tables 1 and 2, available at www.annals.org).
The paradoxical increase in fasting insulin and decrease in C-peptide concentrations for salsalate compared with placebo were seen, as in previous reports (Table 2) (7, 8).
Table 2.
Baseline Clinical and Biochemical Status and Change From Baseline During Study, by Treatment Group
| Variable | Placebo
|
Salsalate
|
Difference in Change (95% CI)* | P Value† | ||
|---|---|---|---|---|---|---|
| Mean Baseline Value (SD) | Mean Change From Baseline (95% CI) | Mean Baseline Value (SD) | Mean Change From Baseline (95% CI) | |||
| Vital signs | ||||||
|
| ||||||
| Weight, kg | 95.4 (22.3) | −0.40 (−0.76 to −0.04) | 97.0 (22.7) | 0.94 (0.59 to 1.30) | 1.34 (0.85 to 1.84) | <0.001 |
|
| ||||||
| Heart rate, beats/min | 72 (10) | 1.1 (0.2 to 2.0) | 74 (10) | −0.7 (−1.6 to 0.2) | −1.8 (−3.0 to 0.5) | 0.007 |
|
| ||||||
| Systolic BP, mm Hg | 126.4 (13.9) | 0.6 (−0.6 to 1.8) | 125.9 (12.9) | 2.1 (1.0 to 3.3) | 1.6 (−0.1 to 3.2) | 0.063 |
|
| ||||||
| Diastolic BP, mm Hg | 76.9 (8.0) | 0.5 (−0.3 to 1.2) | 76.0 (8.8) | 0.8 (0.1 to 1.6) | 0.4 (−0.7 to 1.4) | 0.49 |
| Endocrine | ||||||
|
| ||||||
| HbA1c level, % | 7.7 (0.7) | 0.04 (−0.07 to 0.15) | 7.7 (0.7) | −0.33 (−0.44 to −0.22) | −0.37 (−0.53 to −0.21) | <0.001 |
|
| ||||||
| Fasting glucose level | ||||||
| mmol/L | 8.30 (2.09) | 0.11 (−0.11 to 0.33) | 8.49 (2.14) | −0.72 (−0.94 to −0.51) | −0.83 (−1.14 to −0.53) | <0.001 |
| mg/dL | 150 (38) | 2.0 (−1.9 to 5.9) | 153 (39) | −13.1 (−16.9 to −9.2) | −15.0 (−20.5 to −9.6) | <0.001 |
|
| ||||||
| Insulin level, pmol/L | 87.4 (56.1 to 141.2)‡ | 2.9 (−4.0 to 10.4)§ | 96.1 (69.5 to 152.4)‡ | 15.2 (6.9 to 24.0)§ | 10.5 (0.3 to 21.8) | 0.042§ |
|
| ||||||
| C-peptide level | ||||||
| nmol/L | 0.92 (0.42) | 0.01 (−0.03 to 0.05) | 1.00 (0.43) | −0.07 (−0.11 to −0.03) | −0.08 (−0.14 to −0.02) | 0.009 |
| ng/mL | 2.76 (1.25) | 0.03 (−0.10 to 0.16) | 2.94 (1.28) | −0.21 (−0.34 to −0.08) | −0.24 (−0.42 to −0.06) | 0.009 |
| Lipids | ||||||
|
| ||||||
| Total cholesterol level | ||||||
| mmol/L | 4.29 (1.04) | 0.00 (−0.08 to 0.09) | 4.27 (1.09) | 0.22 (0.14 to 0.31) | 0.22 (0.10 to 0.34) | <0.001 |
| mg/dL | 166 (40) | 0.0 (−3.3 to 3.3) | 165 (42) | 8.6 (5.4 to 11.9) | 8.6 (4.0 to 13.2) | <0.001 |
|
| ||||||
| HDL cholesterol level | ||||||
| mmol/L | 1.26 (0.33) | 0.03 (0.01 to 0.05) | 1.20 (0.32) | 0.02 (0.00 to 0.04) | −0.01 (−0.04 to 0.02) | 0.50 |
| mg/dL | 48.65 (12.74) | 1.16 (0.39 to 1.93) | 46.33 (12.36) | 0.77 (0.00 to 1.54) | −0.39 (−1.54 to 0.77) | 0.50 |
|
| ||||||
| LDL cholesterol level | ||||||
| mmol/L | 2.64 (0.82) | −0.02 (−0.10 to 0.05) | 2.64 (0.89) | 0.27 (0.20 to 0.34) | 0.29 (0.19 to 0.40) | <0.001 |
| mg/dL | 102 (32) | −0.8 (−3.7 to 2.1) | 102 (35) | 10.4 (7.6 to 13.3) | 11.2 (7.2 to 15.3) | <0.001) |
|
| ||||||
| Triglyceride level | ||||||
| mmol/L | 1.51 (1.07 to 2.19)‡ | −0.05 (−0.11 to 0.02)§ | 1.56 (1.12 to 2.20)‡ | −0.18 (−0.24 to −0.12)§ | −0.14 (−0.22 to −0.05)§ | 0.002§ |
| mg/dL | 134 (95 to 194)‡ | −4.4 (−9.7 to −1.8)§ | 138 (99 to 195)‡ | −15.9 (−21.2 to −10.6)§ | −12.4 (−19.5 to −4.4)§ | 0.002§ |
|
| ||||||
| FFA level, mmol/L | 0.48 (0.23) | −0.02 (−0.04 to 0.01) | 0.50 (0.22) | −0.00 (−0.03 to 0.02) | 0.02 (−0.02 to 0.05) | 0.41 |
|
| ||||||
| Total–HDL cholesterol ratio | 3.58 (1.07) | −0.10 (−0.19 to −0.01) | 3.75 (1.16) | 0.21 (0.12 to 0.30) | 0.31 (0.19 to 0.44) | <0.001 |
| Renal | ||||||
|
| ||||||
| Creatinine level | ||||||
| μmol/L | 73.5 (14.9) | −0.5 (−1.7 to 0.7) | 72.4 (15.0) | 1.8 (0.6 to 3.0) | 2.3 (0.6 to 4.0) | 0.007 |
| mg/dL | 0.83 (0.17) | −0.01 (−0.02 to 0.01) | 0.82 (0.17) | 0.02 (0.01 to 0.03) | 0.03 (0.01 to 0.05) | 0.007 |
|
| ||||||
| MDRD eGFR, mL/min per 1.73 m2 | 99.0 (19.9) | 1.2 (−0.9 to 3.3) | 101.6 (21.7) | −0.8 (−2.8 to 1.3) | −2.0 (−4.9 to 0.9) | 0.178 |
|
| ||||||
| Cystatin C level, mg/L | 71.7 (15.1) | −1.2 (−2.6 to 0.2) | 72.2 (14.6) | −1.9 (−3.2 to −0.5) | −0.7 (−2.6 to 1.3) | 0.50 |
|
| ||||||
| Cystatin C–based eGFR, mL/min per 1.73 m2 | 22.7 (4.4) | 0.4 (−0.05 to 0.8) | 22.4 (4.2) | 0.71 (0.3 to 1.1) | 0.3 (−0.3 to 0.9) | 0.28 |
|
| ||||||
| Uric acid level, μmol/L | 349 (90) | −10.7 (−21.6 to 0.28) | 371 (87) | −71.3 (−82.1 to −60.5) | −60.7 (−76.0 to −45.3) | <0.001 |
|
| ||||||
| ACR, μg/mg | 8.0 (5.0 to 13.0)‡ | 1.1 (1.0 to 1.2) | 7.0 (5.0 to 13.0)‡ | 2.6 (2.3 to 2.8) | 2.3 (2.0 to 2.7) | <0.001§ |
| Hepatic | ||||||
|
| ||||||
| ALT level, U/L | 0.35 (0.27 to 0.52)‡ | 0.00 (−0.10 to 0.05)‡ | 0.33 (0.25 to 0.52)‡ | −0.03 (−0.10 to 0.03)‡ | 0.068∥ | |
|
| ||||||
| AST level, U/L | 0.32 (0.25 to 0.42)‡ | 0.00 (−0.05 to 0.05)‡ | 0.32 (0.25 to 0.40)‡ | 0.00 (−0.05 to 0.03)‡ | 0.81∥ | |
|
| ||||||
| GGT level, U/L | 0.45 (0.32 to 0.72)‡ | 0.00 (−0.12 to 0.05)‡ | 0.43 (0.32 to 0.60)‡ | −0.03 (−0.12 to 0.03)‡ | <0.001∥ | |
|
| ||||||
| Albumin level, g/L | 44.0 (2.9) | −0.03 (−0.3 to 0.2) | 43.7 (2.7) | −1.9 (−2.2 to −1.7) | −1.9 (−2.3 to −1.5) | <0.001 |
| Other | ||||||
|
| ||||||
| Hematocrit, % | 41.2 (4.1) | −0.19 (−0.48 to 0.10) | 41.3 (4.3) | 0.60 (0.31 to 0.88) | 0.79 (0.39 to 1.19) | <0.001 |
|
| ||||||
| Leukocyte count, × 109 cells/L | 6.60 (1.93) | 0.00 (−0.14 to 0.15) | 7.00 (2.09) | −0.63 (−0.77 to −0.48) | −0.63 (−0.83 to −0.43) | <0.001 |
|
| ||||||
| Neutrophil count, × 109 cells/L | 2.12 (0.72) | −0.02 (−0.07 to 0.04) | 2.16 (0.67) | −0.27 (−0.32 to 0.21) | −0.25 (−0.32 to −0.18) | 0.001 |
|
| ||||||
| Lymphocyte count, × 109 cells/L | 3.89 (1.44) | −0.4 (−0.17 to 0.10) | 4.31 (1.70) | −0.36 (−0.50 to −0.23) | −0.32 (−0.52 to −013) | <0.001 |
|
| ||||||
| Adiponectin level, μg/mL | 3.98 (3.02 to 6.00)‡ | 0.25 (0.08 to 0.43)§ | 4.17 (3.06 to 5.86)‡ | 1.51 (1.29 to 1.74)§ | 1.16 (0.88 to 1.47)§ | <0.001§ |
|
| ||||||
| hs-CRP level, nmol/L | 33.1 (16.3 to 58.8)‡ | −0.68 (−3.51 to 2.45)§ | 32.5 (14.7 to 67.7)‡ | −2.45 (−5.06 to 0.43)§ | −1.80 (−5.51 to 2.46)§ | 0.39§ |
|
| ||||||
| TNF-α level, pg/mL | 1.69 (0.79) | 0.00 (−0.07 to 0.07)§ | 1.83 (2.31) | 0.05 (−0.02 to 0.11)§ | 0.05 (−0.05 to 0.15)§ | 0.32§ |
|
| ||||||
| TNF–receptor 1 level, ng/mL | 3.06 (0.72) | −0.04 (−0.11 to 0.03)§ | 3.04 (0.83) | −0.06 (−0.13 to 0.01)§ | −0.00 (−0.13 to 0.08)§ | 0.62§ |
|
| ||||||
| TNF–receptor 2 level, ng/mL | 8.21 (1.95) | −0.21 (−0.41 to −0.01)§ | 8.14 (2.20) | −0.05 (−0.25 to 0.15)§ | 0.16 (−0.13 to 0.46)§ | 0.28§ |
ACR = albumin–creatinine ratio; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BP = blood pressure; eGFR = estimated glomerular filtration rate; FFA = free fatty acid; GGT= γ-glutamyltransferase; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; MDRD = Modification of Diet in Renal Disease; TNF = tumor necrosis factor.
Salsalate minus placebo.
Unless otherwise noted, P values are mixed-model tests of the overall treatment effect after adjustment for clinical and follow-up time over the 48-wk study.
Median (25th–75th percentiles).
Test based on natural log-transformation, results are back-transformed, and the change ratio is multiplied by the group mean.
Wilcoxon 2-sample test; 2-sided P > |Z|. The probability test statistic was calculated using the Z-score from a standard normal table; there are no estimates of a difference in change when rank testing was used.
Other Measures of Efficacy and Safety
Anti-inflammatory effects of salsalate were evidenced by changes in circulating leukocyte and differential counts. Mean differences in change over 48 weeks demonstrated decreases in leukocyte, neutrophil, and lymphocyte counts with salsalate compared with placebo (Table 2 and Figure 3, A, C, and E). All counts remained within normal ranges.
Figure 3. Mean values and 95% CIs for leukocyte count (A), hematocrit (B), neutrophil count (C), adiponectin level (D), lymphocyte count (E), and LDL cholesterol level (F).
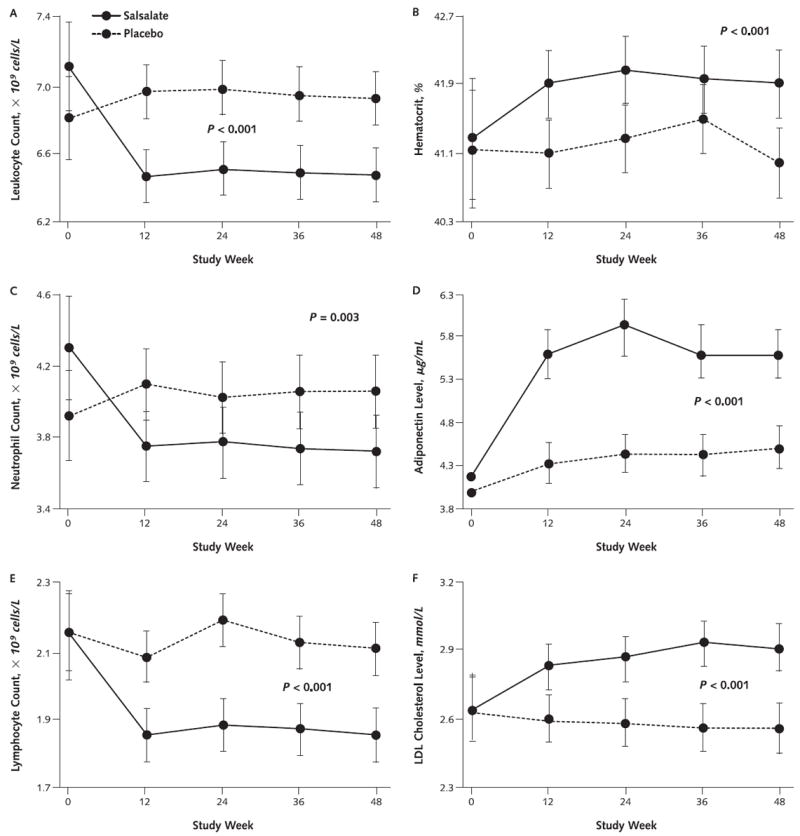
LDL = low-density lipoprotein.
Adiponectin, a potentially cardioprotective protein from adipocytes, increased by 27% over 48 weeks (P < 0.001) compared with placebo. Uric acid levels, which are associated with cardiometabolic conditions and progression of renal insufficiency, decreased by 18% in salsalate versus placebo groups (P = 0.003) (Table 2; Figure 3, D; and Figure 4, B).
Figure 4. Renal effects of salsalate.
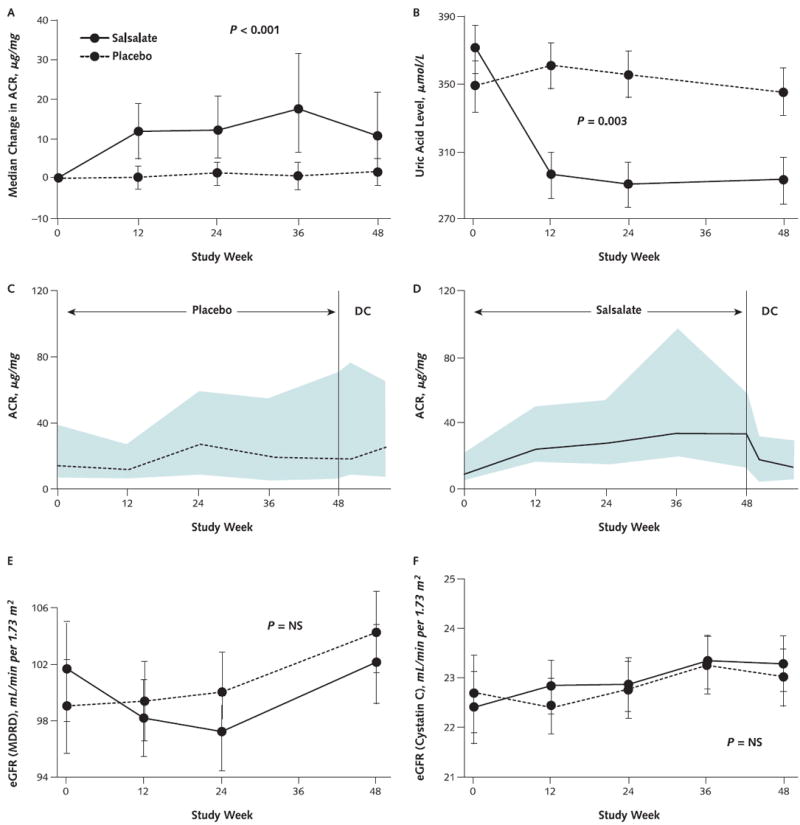
ACR = albumin–creatinine ratio; DC = discontinued; eGFR = estimated glomerular filtration rate; IQR = interquartile range; MDRD = Modification of Diet in Renal Disease; NS = not significant. A. Median changes and IQRs for urinary ACR. Error bars represent the IQRs. The 2.3-μg/mg between-group difference in ACR reported in the text and values reported in Table 2 were obtained by back-transformation of log-transformed data for ACR because ACR was not normally distributed. B. Mean changes and 95% CIs in circulating uric acid levels. C and D. ACRs for the 23 participants receiving placebo (C) and 33 receiving salsalate (D) who were asked to return at week 56, after 8-week washout period, because ACR or blood pressure was elevated at week 48. Lines are median values; shaded areas are 25th through 75th quartiles. E and F. Mean changes and 95% CIs for eGFRs, using creatinine concentrations and the MDRD equation (E) or cystatin C concentrations (F).
For salsalate recipients, there was a 1.3-kg placebo-corrected increase in weight (P < 0.001), a trend toward increased systolic blood pressure, and lower heart rate (P = 0.010) (Table 2).
Salsalate decreased median triglyceride concentrations by 9% compared with placebo (P = 0.002). In contrast, mean total and directly measured low-density lipoprotein (LDL) cholesterol levels (Figure 3, F) both increased (P < 0.001) with salsalate versus placebo, without changes in high-density lipoprotein (HDL) cholesterol levels. Increased LDL cholesterol levels in the salsalate group were independent of baseline statin use.
Alanine aminotransferase trended lower and γ-glutamyltransferase levels decreased (Table 2) with salsalate compared with placebo.
Renal Function
The urinary albumin–creatinine ratio (ACR) increased by 2.3 μg/mg (CI, 2.0 to 2.7 μg/mg; P < 0.001) in patients treated with salsalate compared with placebo (Figure 4, A). Of 248 participants with urinary ACRs less than 30 μg/mg at screening and baseline, 7 of the 120 receiving placebo compared with 24 of 128 participants receiving salsalate had greater values at week 48. However, if the urinary ACR increased to greater than 30 μg/mg at any time during randomized treatment, it tended to remain increased in the placebo group (Figure 4, C) but reversed during the 8-week washout period in the salsalate group (Figure 4, D). One participant in each group had frank albuminuria (ACR >300 μg/mg) at the end of the study. Change in weight, blood pressure, and salicylate concentrations were modestly correlated with changes in albuminuria, but estimated glomerular filtration rate (GFR), aspirin use, and antihypertensive medications—including angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers—were not correlated (data not shown).
Estimated GFR did not change within or between groups (P = 0.176) (Figure 4, E). However, serum creatinine levels were greater with salsalate than with placebo (P = 0.008). In contrast, serum cystatin C levels did not differ between groups (P = 0.50) and, similarly, estimated GFR calculated using cystatin C levels did not change between salsalate and placebo groups (P = 0.28) (Figure 4, F).
Adverse Events
No serious adverse events were attributed to salsalate. Tinnitus, an expected adverse effect of high-dose salicylates, was reported by 16 (11%) patients receiving salsalate and 7 (5%) receiving placebo (P = 0.082). Two placebo recipients and 7 salsalate recipients had dose adjustments for tinnitus (P = 0.174, Fisher exact test). Tinnitus resolved or returned to baseline in all participants by the end of the study. There was no evidence of gastrointestinal bleeding by history, and hematocrit levels actually increased (P < 0.001) (Figure 3, D). The Short Form-36 questionnaire did not detect differences in quality of life. Adverse events that occurred with frequencies of 5% or greater and numerically more frequently in salsalate recipients than placebo recipients are listed in Appendix Table 3 (available at www.annals.org). Gastrointestinal side effects did not differ between groups (Appendix Table 4, available at www.annals.org).
Discussion
This trial evaluated glycemic effects of salsalate compared with placebo as add-on therapy for patients with inadequately treated, established T2DM. Salsalate reduced both HbA1c and fasting blood glucose levels at all study time points. The magnitude of glycemic improvement in the first few months was consistent with those seen in our previous trial, which also included patients with established diabetes who were using as many as 3 oral diabetes medications (8), but was lower in magnitude than the between-group 2.3% reduction in HbA1c levels seen over 12 weeks in drug-naive patients with new-onset T2DM (9). The magnitude of change in HbA1c levels with salsalate was similar to that seen in recent studies for other marketed drugs used clinically to treat T2DM in which baseline HbA1c levels were less than 8%. For example, a recent study compared linagliptin with glimepiride in patients receiving metformin. The baseline HbA1c level of 7.7% was similar to participants in our study. The mean changes in HbA1c level was −0.16% for linagliptin −0.36% for glimepiride (10). The effect of salsalate on HbA1c levels of −0.37% was of similar magnitude.
There was attenuation of HbA1c levels decreasing at 1 year. This was partially attributable to adjustments in concomitant diabetes medications, which had been discouraged during the first 24 weeks of the trial. Disease progression and attenuation of drug efficacy may contribute. Concomitant diabetes medications were adjusted in more than 25% of participants, with more reductions for participants who received salsalate and more increases for those who received placebo, thus diminishing estimates of salsalate efficacy.
Hypoglycemia is both a measure of efficacy and the most commonly seen side effect in this trial. The relative risk for mild hypoglycemia was 6-fold greater when salsalate was coadministered with sulfonylureas. These findings are consistent with the absence of hypoglycemia in persons who do not have diabetes but use salsalate for pain management.
Salicylates, including salsalate, have been used extensively for joint pain, without safety concerns specific to T2DM or cardiovascular disease (CVD). There were no major signs of increased cardiovascular risk, yet modest changes in placebo-adjusted weight and LDL cholesterol and urinary albumin levels warrant further assessment. Some diabetes medications cause weight gain through increased adiposity and fluid retention. Salicylates are not known to increase adiposity. Insulin increases weight through improved tissue glucose utilization, and salsalate increases circulating insulin levels. Salicylates may also reverse loss of muscle mass associated with diabetes and aging (11). Biological modifiers, including anti–tumor necrosis factor-α and anti–interleukin-6, also increase LDL cholesterol levels (12). Potential benefits of salicylates in CVD are being tested (ClinicalTrials.gov: NCT00624923).
The anti-inflammatory properties of salsalate were evidenced by reductions in circulating leukocyte, neutrophil, and lymphocyte counts. These are previously unrecognized clinical effects of salicylates, including those used for rheumatologic conditions. Although decreased leukocyte counts could signify a bone marrow effect, hematocrit levels increased, making general bone marrow depression unlikely. Leukocyte and differential counts are elevated in obesity and the metabolic syndrome (13) and predict incident T2DM (14, 15), CVD, and poor outcomes in CVD (16-18). We speculate that reductions from higher to lower normal ranges may benefit patients at cardiometabolic risk and that nuclear factor-κB inhibition is the likely molecular mechanism for these reductions (6, 19-22). Cyclooxygenase inhibitors are not known to decrease leukocyte and differential counts, distinguishing these drug classes and potential mechanisms. In addition, statins do not decrease leukocyte counts and this effect occurred in patients receiving statins, thus demonstrating anti-inflammatory effects of salsalate independent from and in addition to those of statins. By contrast, salsalate had little effect on high-sensitivity C-reactive protein levels, which are decreased by statins, further supporting different mechanisms of action.
Salsalate also increased adiponectin and decreased uric acid levels, as seen previously (8), suggesting improved cardiometabolic risk (23). Reductions in liver aminotransferases, γ-glutamyltransferase and a trend for alanine aminotransferase, are also consistent with metabolic improvements and anti-inflammatory efficacy.
Cyclooxygenase inhibitors have been associated with acute kidney injury, especially in patients with diabetes or those using diuretics. Renal side effects of salsalate, an NSAID with distinct mechanisms of action, are lower than nonselective COX inhibitors (24). Renal safety signals were mixed. Urinary albumin levels increased more frequently with salsalate than with placebo, although this was reversible in the salsalate group. The magnitude of change is of unclear clinical relevance, particularly when estimated GFR was unchanged, calculated using either creatinine or cystatin C levels.
Mechanisms of salsalate action differ considerably from the COX inhibitors. Salsalate does not alter platelet or renal prostaglandins, which are suppressed by aspirin and other NSAIDs (25, 26), or alter prothrombin times, bleeding times, or platelet aggregation (27-29). Salsalate is also less prone than aspirin and other NSAIDs to cause gastric irritation (30-33).
Although decreases in leukocyte counts are probably nuclear factor-κB–mediated effects, mechanisms for decreases in glucose levels are more difficult to pinpoint. Inflammation seems to participate in the pathogenesis of insulin resistance and T2DM, suggesting that there may be tractable anti-inflammatory strategies for decreasing glucose levels (34, 35). Although salsalate decreases both glucose levels and inflammation and these are associated in our trial, we have not proven a mechanistic link. The glucose level–decreasing effects of interleukin-1β blockade support a general strategy to targeting inflammation, albeit using an independent anti-inflammatory approach (36, 37). Salicylate has additional potential mechanisms to consider, including effects on mitochondrial dehydrogenases (38, 39), transcription factors in addition to nuclear factor-κB (40-42), and cellular kinases (43-49). Recently, salicylate has also been shown to inhibit 11-β hydroxysteroid dehydrogenase type 1 in adipose tissue (50) and to stimulate adenosine monophosphate–activated protein kinase (51). Relative contributions for these potential mechanisms have not been distinguished.
Elevated circulating insulin levels may contribute to decreasing glucose levels. However, the reduced insulin clearance seen in humans receiving salicylates is not observed in rodents, making mechanistic evaluations more difficult (7, 8, 52, 53).
Limitations of our study include the relatively small number of patients and short trial duration, which restricts assessments of long-term durability and cardiovascular outcomes. Current results do not distinguish whether decreases in glucose levels are greater for salsalate alone or in certain therapeutic combinations. In addition, changes in concomitant diabetes drugs confound estimates of the efficacy of salsalate.
To our knowledge, this was the first evaluation of either salicylate or salsalate conducted using a multicenter, randomized, double-blinded, placebo-controlled trial format lasting longer than 3 months. The drug was well-tolerated and the primary end point of HbA1c level decreasing was achieved at all points tested. The magnitude of effect was similar to other oral diabetes therapies currently in use when added to metformin. Anti-inflammatory effects of salsalate were readily apparent at all time points as reductions in leukocyte and differential counts. Glucose level–decreasing and anti-inflammatory effects were associated, although this does not prove a mechanistic connection. Changes in renal function or LDL cholesterol levels and associated long-term cardiorenal safety and outcomes require continued evaluation before salsalate can be recommended for widespread use in T2DM.
Supplementary Material
Context
Salicylate is one of the oldest drugs in clinical practice. Neither salicylate nor its prodrug forms, including salsalate, have been tested for efficacy and safety according to currently accepted regulatory practices. Preliminary data suggest that salsalate may improve glycemic control in type 2 diabetes.
Contribution
In this randomized trial of patients with type 2 diabetes and inadequate glycemic control, salsalate improved hemoglobin A1c levels and decreased inflammatory markers over 1 year compared with placebo. Increases in weight and total and low-density lipoprotein cholesterol levels were seen, as was a reversible increase in albuminuria.
Implication
Salsalate may be an effective treatment for glucose control in type 2 diabetes, but further study on the effects on cardiac and renal disease is warranted.
—The Editors
Acknowledgments
The authors thank Elizabeth Tatro for her coordinating roles in the trial and Yeheng Liu, MPH, Cedars-Sinai Medical Center, for laboratory measurements.
Grant Support: By National Institutes of Health (U01 DK74556, P50 HL83813, P30 DK03836, and General Clinical Research Center and Clinical and Translational Science Award at several sites) and the Tullis-Tulane (Dr. Fonseca) and Helen and Morton Adler (Dr. Shoelson) Chairs. Caraco Pharmaceutical Laboratories (Detroit, Michigan) supplied the salsalate and placebo, LifeScan (Milpitas, California) supplied the home glucose-monitoring kits, and Mercodia (Uppsala, Sweden) supplied the insulin assay kits.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-2782.
Reproducible Research Statement: Study protocol: Available from Dr. Goldfine (e-mail, allison.goldfine@joslin.harvard.edu). Data set and statistical code: Not available.
Current author addresses and author contributions are available at www.annals.org.
References
- 1.Jack DB. One hundred years of aspirin. Lancet. 1997;350:437–9. doi: 10.1016/S0140-6736(97)07087-6. [DOI] [PubMed] [Google Scholar]
- 2.MacLagan TJ. The treatment of acute rheumatism by salicin. Lancet. 1876;107:342–3. 383–4. [PubMed] [Google Scholar]
- 3.Broadbent WH. Treatment of rheumatic fever by salicylic acid. Lancet. 1876;107:530–2. [Google Scholar]
- 4.Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine and 100 Years of Rampant Competition. Boston: Harvard Business School Pr; 1991. [Google Scholar]
- 5.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–43. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 6.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 7.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0329-2. [DOI] [PubMed] [Google Scholar]
- 10.Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–83. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Vis M, Nurmohamed MT, Wolbink G, Voskuyl AE, de Koning M, van de Stadt R, et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol. 2005;32:252–5. [PubMed] [Google Scholar]
- 13.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, < or = 30) Am J Cardiol. 2002;89:1441–3. doi: 10.1016/s0002-9149(02)02366-4. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 15.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–61. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290:1275–8. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 17.Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol. 2005;15:266–71. doi: 10.1016/j.annepidem.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254:1932–7. [PubMed] [Google Scholar]
- 19.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 20.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996;156:3961–9. [PubMed] [Google Scholar]
- 21.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–5. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 22.Baichwal VR, Baeuerle PA. Activate NF-kappa B or die? Curr Biol. 1997;7:R94–6. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 23.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafrance JP, Miller DR. Selective and non-selective non-steroidal anti-inflammatory drugs and the risk of acute kidney injury. Pharmacoepidemiol Drug Saf. 2009;18:923–31. doi: 10.1002/pds.1798. [DOI] [PubMed] [Google Scholar]
- 25.Morris HG, Sherman NA, McQuain C, Goldlust MB, Chang SF, Harrison LI. Effects of salsalate (nonacetylated salicylate) and aspirin on serum prostaglandins in humans. Ther Drug Monit. 1985;7:435–8. doi: 10.1097/00007691-198512000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Ryan J. Effects of salsalate, aspirin and naproxen on plasma renin activity and platelet thromboxane synthesis. Arthritis Rheum. 1986;29(Suppl):S103. [Google Scholar]
- 27.Estes D, Kaplan K. Lack of platelet effect with the aspirin analog, salsalate. Arthritis Rheum. 1980;23:1303–7. doi: 10.1002/art.1780231113. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney JD, Hoernig LA. Hemostatic effects of salsalate in normal subjects and patients with hemophilia A. Thromb Res. 1991;61:23–7. doi: 10.1016/0049-3848(91)90165-s. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney JD. The effect of salsalate on bleeding time, platelet aggregation in whole blood and their release reaction. Blood. 1988;72(Suppl 1):311A. [Google Scholar]
- 30.Edmar D. Effects of salicylates on the gastric mucosa as revealed by roentgen examination and the gastrocamera. Acta Radiol Diagn (Stockh) 1971;11:57–64. doi: 10.1177/028418517101100107. [DOI] [PubMed] [Google Scholar]
- 31.Roth S, Bennett R, Caldron P, Hartman R, Mitchell C, Doucette M, et al. Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study. Semin Arthritis Rheum. 1990;19:11–9. [PubMed] [Google Scholar]
- 32.Lanza F, Rack MF, Doucette M, Ekholm B, Goldlust B, Wilson R. An endoscopic comparison of the gastroduodenal injury seen with salsalate and naproxen. J Rheumatol. 1989;16:1570–4. [PubMed] [Google Scholar]
- 33.Scheiman JM, Elta GH. Gastroduodenal mucosal damage with salsalate versus aspirin: results of experimental models and endoscopic studies in humans. Semin Arthritis Rheum. 1990;20:121–7. doi: 10.1016/0049-0172(90)90025-b. [DOI] [PubMed] [Google Scholar]
- 34.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 36.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 38.Smith MJ, Bryant C, Hines WJ. Reversal by nicotinamide adenine dinucleotide of the inhibitory action of salicylate on mitochondrial malate dehydrogenase. Nature. 1964;202:96–7. doi: 10.1038/202096a0. [DOI] [PubMed] [Google Scholar]
- 39.Hines WJ, Smith MJ. Inhibition of dehydrogenases by salicylate. Nature. 1964;201:192. doi: 10.1038/201192a0. [DOI] [PubMed] [Google Scholar]
- 40.Aceves M, Duenñas A, Gómez C, San Vicente E, Crespo MS, García-Rodríguez C. A new pharmacological effect of salicylates: inhibition of NFAT-dependent transcription. J Immunol. 2004;173:5721–9. doi: 10.4049/jimmunol.173.9.5721. [DOI] [PubMed] [Google Scholar]
- 41.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–5. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 42.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 43.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278:24944–50. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 44.Alpert D, Vilcek J. Inhibition of IkappaB kinase activity by sodium salicylate in vitro does not reflect its inhibitory mechanism in intact cells. J Biol Chem. 2000;275:10925–9. doi: 10.1074/jbc.275.15.10925. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson MA, Zhao MJ, Asea A, Coleman CN, Calderwood SK. Salicylic acid and aspirin inhibit the activity of RSK2 kinase and repress RSK2-dependent transcription of cyclic AMP response element binding protein- and NF-kappa B-responsive genes. J Immunol. 1999;163:5608–16. [PubMed] [Google Scholar]
- 46.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 47.Schwenger P, Alpert D, Skolnik EY, Vilcek J. Activation of p38 mitogenactivated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci U S A. 1997;94:2869–73. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frantz B, O’Neill EA. The effect of sodium salicylate and aspirin on NFkappa B [Letter] Science. 1995;270:2017–9. doi: 10.1126/science.270.5244.2017. [DOI] [PubMed] [Google Scholar]
- 50.Nixon M, Wake DJ, Livingstone DE, Stimson RH, Esteves CL, Seckl JR, et al. Salicylate downregulates 11β-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization. Diabetes. 2012;61:790–6. doi: 10.2337/db11-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–93. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


