Abstract
Mesenchymal stem cells (MSCs) are adult cells with the capacity to differentiate into multiple cell types, including bone, fat, cartilage, and muscle cells. In order to effectively utilize autologous MSCs in cell-based therapies, precise genetic manipulations are required to eliminate the effects of disease-causing mutations. We previously used adeno-associated virus (AAV) vectors to target and inactivate mutant COL1A1 genes in MSCs from individuals with the brittle bone disorder, osteogenesis imperfecta (OI). Here we have used AAV vectors to inactivate mutant COL1A2 genes in OI MSCs, thereby demonstrating that both type I collagen genes responsible for OI can be successfully targeted. We incorporated improved vector designs so as to minimize the consequences of random integration, facilitate the removal of potential antigens, and avoid unwanted exon skipping. MSCs targeted at mutant COL1A2 alleles produced normal type I procollagen and formed bone, thereby demonstrating their therapeutic potential.
INTRODUCTION
Osteogenesis imperfecta (OI) is a genetic bone disease usually caused by defects in the type I collagen genes COL1A1 and COL1A2. These genes encode the proα 1(I) and proα2(I) chains, respectively, that are present at a 2:1 ratio in type I procollagen molecules.1 Severe forms of OI can be caused by mutations that affect the Gly-X-Y amino acid sequence repeat that is required for forming the long triple helix of collagen (Gly is glycine, X is frequently proline, and Y is often hydroxyproline). Changes in this repeat can disrupt the triple helix and result in life-threatening skeletal abnormalities. Because the collagen gene mutations responsible for severe OI act in a dominant-negative fashion, therapeutic strategies based on gene addition are unlikely to succeed, and inactivation of the mutant allele is a more promising approach.
Mesenchymal stem cells (MSCs) can be isolated easily from adults, and have been shown to differentiate into bone-forming osteoblasts2,3 as well as into other mesenchymal cell types that form cartilage,4 tendon,5,6 muscle,7,8 adipose tissue,9 and bone marrow stroma.10,11 They are therefore ideal candidates for use in cell-based therapies, particularly in those that seek to promote osteogenesis. Our group developed a gene-targeting method based on adeno-associated virus (AAV) vectors, and used it to make precise genetic changes in numerous human cell types including MSCs.12–15 The high fidelity and high targeting frequencies of AAV-mediated gene targeting (0.01–1% of the unselected cell population) make this a promising technique for the correction or elimination of dominant-negative mutations such as those responsible for OI.
We previously demonstrated that AAV targeting vectors can disrupt mutant COL1A1 genes in OI MSCs, which can then produce normal collagen and form bone in vivo after xenotransplantation.12 In that study, an internal ribosome entry site (IRES), neomycin resistance gene (neo), and polyadenylation sequence (IRES–neo–pA) were inserted into exon 1 of COL1A1 to simultaneously prevent collagen expression and drive expression of the selectable neo gene from the COL1A1 promoter. Here we report similar studies of the COL1A2 locus, including improvements in vector design that allow removal of the IRES–neo cassette and decrease potential genotoxicity. Given that individuals who are heterozygous for null mutations in COL1A2 are phenotypically normal,16 while those who are heterozygous for null mutations in COL1A1 have a mild form of OI,17 the COL1A2 allele is a more suitable candidate for use in initial clinical studies.
RESULTS
MSCs from individuals with OI were isolated by passaging the adherent cells that grew out of bone samples obtained during therapeutic surgical procedures. Point mutations in COL1A2 were identified in MSCs from three individuals with severe OI, designated OIMSC1, OIMSC5, and OIMSC12. In each case, a glycine codon in the triple helical domain was mutated to code for a different amino acid (Table 1).
Table 1.
Osteogenesis imperfecta cell lines with mutations in COL1A2
| Cell line | Osteogenesis imperfecta type |
Age at isolation | DNA mutationa | Exon | Protein mutationb | Triple helix locationc |
|---|---|---|---|---|---|---|
| OIMSC1 | III | 12 yr | c.2350G>A | 39 | p.Gly784Ser | Gly694Ser |
| OIMSC5 | III/IV | 12 yr | c.2288G>T | 37 | p.Gly763Val | Gly673Val |
| OIMSC12 | III | 15 yr | c.2027G>A | 34 | p.Gly676Asp | Gly586Asp |
Numbering starts at the A of the initiator methionine codon of the messenger RNA.
Numbering starts at the first methionine of the protein.
Numbering starts at the first amino acid of the triple helical domain.
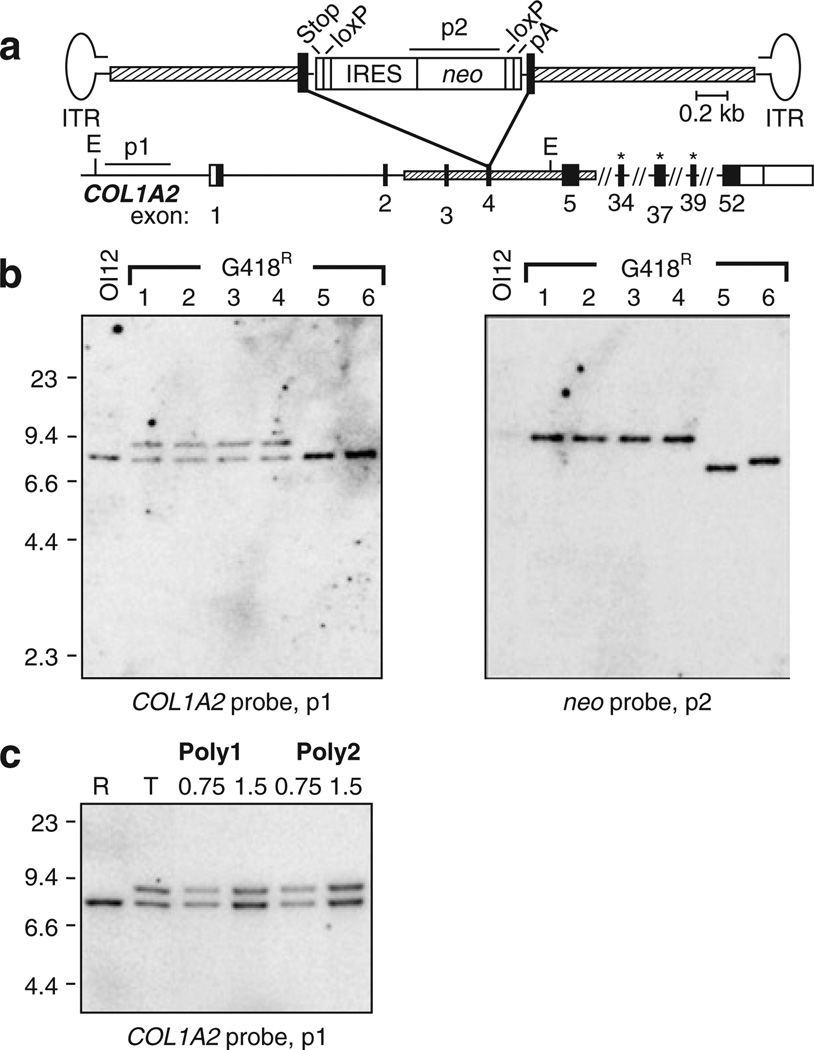
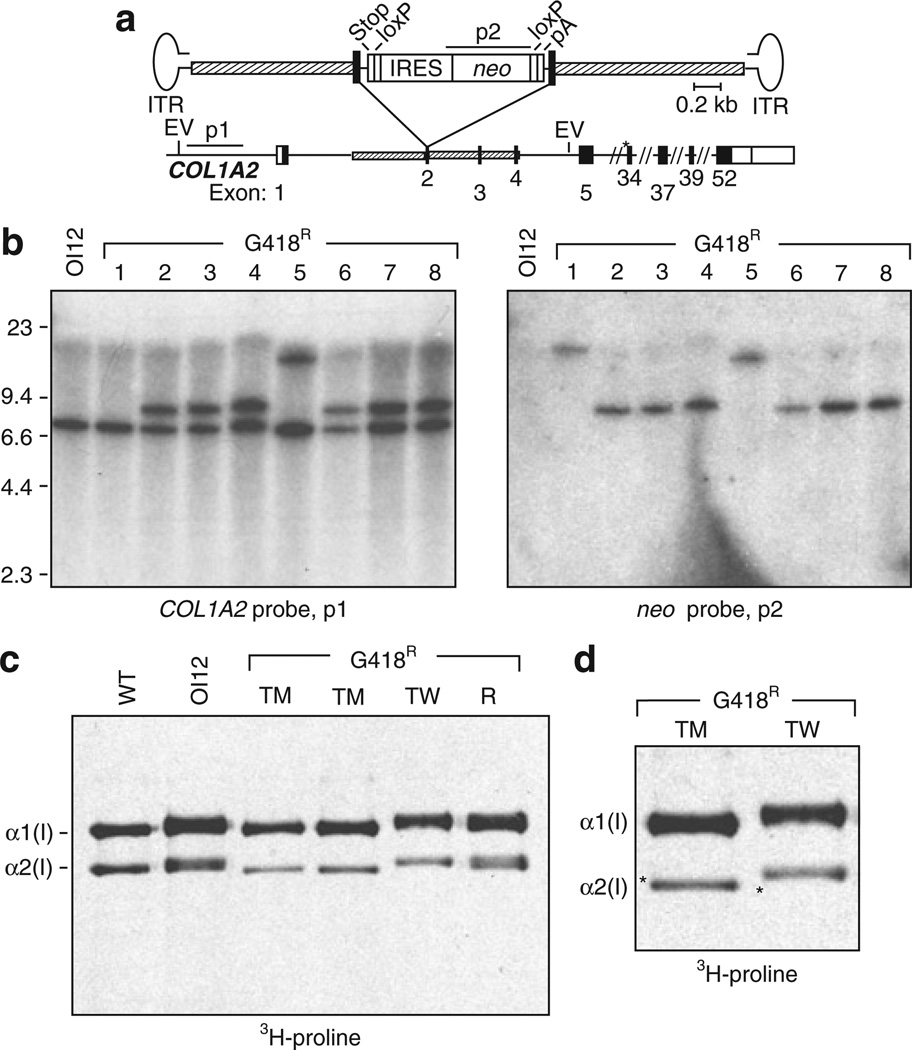
The AAV-COL2e4INloxpA targeting vector (Figure 1a) was designed to disrupt exon 4 of COL1A2 by inserting a loxP-fanked IRES-neo cassette with an upstream stop codon in each reading frame, and a downstream polyadenylation sequence (pA). This allows neo to be expressed from the upstream COL1A2 promoter and confers G418-resistance in targeted cells. We chose exon 4 so as to exclude COL1A2 promoter sequences that could activate nearby genes after random integration. The loxP sites were included so as to facilitate subsequent removal of the neo cassette by Cre recombinase.18 OI MSCs were infected with AAV-COL2e4INloxpA, and G418-resistant colonies were either isolated as single clones or pooled as polyclonal populations. These were then expanded, and their DNA was analyzed by Southern blots. Insertion of the IRES-neo cassette into exon 4 of COL1A2 by homologous recombination results in a novel 9.1 kilobase fragment after digestion with EcoRV and probing for upstream chromosomal sequences that are not present in the targeting vector (Figure 1b; clones 1–4). Colonies that contained randomly integrated vector sequences were identified by hybridization of the neo probe to fragments of sizes different from the expected 9.1 kilobase targeted fragment (Figure 1b; clones 5 and 6). On the basis of Southern blots, 60–67% of G418-resistant colonies obtained from OIMSC5 or OIMSC12 were targeted at a COL1A2 allele, representing 0.03–0.14% of the entire unselected MSC population (Table 2). Random integrants were also observed occasionally in targeted clones as additional neo-hybridizing bands (3 of 34 colonies; data not shown). Similar results were obtained when polyclonal populations were analyzed by quantitative Southern blot analysis, with 84 and 92% of G418-resistant cells undergoing targeting in OIMSC1 and OIMSC12, respectively (Figure 1c and Table 2).
Figure 1. COL1A2 gene targeting in osteogenesis imperfecta mesenchymal stem cells (OI MSCs).
(a) Schematic representation of the gene-targeting vector AAV-COL2e4INloxpA shown above the COL1A2 locus. Targeting homologies (hatched boxes), coding (black boxes), and non-coding (open boxes) regions of COL1A2 exons are indicated. Elements in the targeting vector include an internal ribosome entry site (IRES) element, neo gene, stop codons in each reading frame (stop), polyadenylation site (pA), loxP sites, and AAV inverted terminal repeats (ITRs). EcoRV sites (E) and COL1A2 and neo probes (p1 and p2) are depicted. Asterisks indicate exons containing point mutations in the three OIMSC lines used for gene targeting. (b) Southern blot of EcoRV-digested genomic DNA isolated from parental, untransduced OIMSC12 cells (OI12) and six representative transduced G418R clones. Expected fragment sizes are 7.5 kilobase (kb) and 9.1 kb with the COL1A2 probe p1 for the untargeted and targeted alleles, respectively, and 9.1 kb with the neo probe p2 for the targeted allele. (c) Southern blot of EcoRV-digested genomic DNA from G418R polyclonal populations Poly1 and Poly2 selected from AAV-COL2e4INloxpA-transduced OIMSC12 and OIMSC1 cells, respectively. R and T are DNAs from OIMSC12 clones with random and targeted integrants, respectively. The amounts of Poly1 and Poly2 DNA loaded are indicated [microgram (µg)].
Table 2.
Results of adeno-associated virus-mediated gene targeting at COL1A2
| Cell line | Exon Targetedb | Multiplicity of infection |
Plating efficiency (%)c | G418R colonies (%)d | G418R colonies analyzede |
G418R colonies targeted (#)f |
Unselected colonies targeted (%)g |
|---|---|---|---|---|---|---|---|
| OIMSC12 | 4 | 2,000 | 70 | 0.16 | 20 | 65% (13) | 0.10 |
| OIMSC12 | 4 | 20,000 | 25 | 0.24 | 15 | 60% (9) | 0.14 |
| OIMSC12pa | 4 | 2,000 | 49 | 0.04 | 71 | 92%* | 0.04 |
| OIMSC5 | 4 | 2,000 | 69 | 0.04 | 24 | 67% (16) | 0.03 |
| OIMSC1pa | 4 | 2,000 | 65 | 0.03 | 55 | 84%* | 0.03 |
| OIMSC12 | 2 | 2,000 | 42 | 0.28 | 87 | 82% (71) | 0.23 |
Polyclonal population.
Vectors AAV-COL2e4INloxpA (exon 4) or AAV-COL2e2INloxpA (exon 2).
Colonies obtained without selection per cell plated.
G418-resistant colonies/total number of colonies obtained.
For polyclonal populations, this represents the number of pooled colonies.
(Number of targeted colonies)/(total number of colonies analyzed) × 100.
Polyclonal results based on quantitative Southern blot analysis.
(% G418R colonies targeted) × (% G418R colonies).
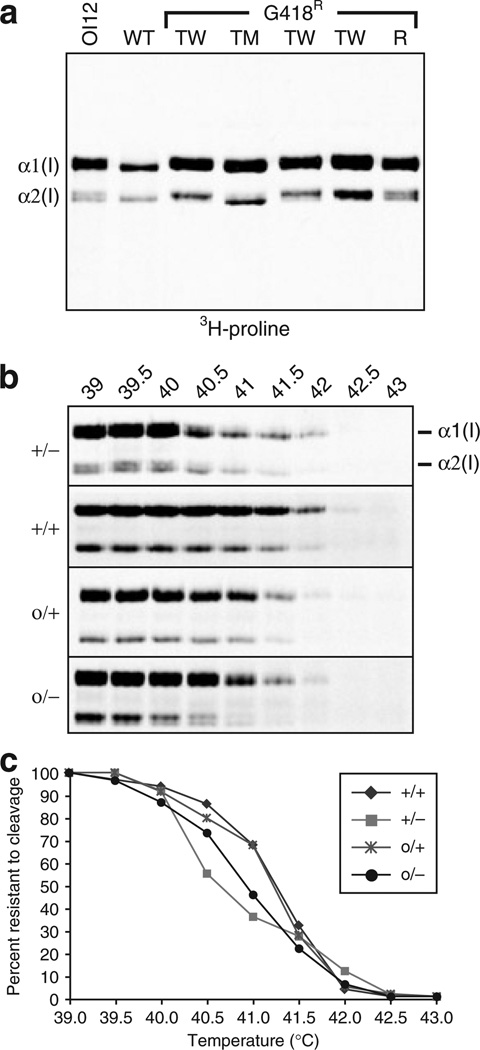
In order to determine whether it was the mutant or the wild-type (WT) COL1A2 allele that was being targeted, transduced MSC clones were cultured in the presence of [3H]-proline to preferentially label collagens and allow identification of radioactive α1(I) and α2(I) collagen polypeptides.19 Mutations that disrupt triple helix formation affect protein folding and increase the time available for hydroxylases to modify proline and lysine residues.20 This overmodification slows the migration of collagen polypeptides during sodium dodecyl sulfate polyacrylamide gel electrophoresis. In untransduced OIMSC12 cells, a subset of cells in the labeled α1(I) and α2(I) chains were overmodified, and migrated more slowly than those in WT MSC cells (Figure 2a). In targeted clones, the pattern was either similar to that in WT cells, or showed predominantly overmodified polypeptides, representing targeted knockouts of the mutant (TM) or normal (TW) alleles, respectively. In addition, the loss of one allele expressing α2(I) chains leads to a relative excess of α1(I) chains, and these presumably assemble as homotrimers in targeted cells. A similar phenomenon occurs in patients with homozygous null mutations in COL1A2, with no deleterious impact on bone formation.16 Random integrants (R) produced the same peptide pattern as the parental OI MSCs did, because neither COL1A2 allele was targeted. Based on this analysis, three of nine G418-resistant clones from transduced OIMSC12 were targeted at the mutant allele, and were observed to express WT α2(I) collagen polypeptides.
Figure 2. Protein analysis of type I collagen from targeted OIMSCs.
(a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) separation of [3H]-proline-labeled collagen peptides from untransduced OIMSC12 cells (OI1 2), wild-type (WT) mesenchymal stem cells (MSCs), transduced OIMSC12 clones targeted at the wild-type (TW) or mutant (TM) COL1A2 alleles, and a random G418R integrant (R). The α1 (I) and α2(I) chains are indicated, and their positions determine which allele was targeted. (b) SDS-PAGE analysis of [3H]-proline-labeled collagen peptides following protease digestion of molecules from WT MSCs (+/+), untransduced OIMSC12 cells (+/−), an OIMSC12 clone targeted at the mutant allele (o/+), and an OIMSC12 clone targeted at the WT allele (o/−). Temperatures used prior to protease treatment are indicated above each lane (39–43 °C). Denatured α1(I) and α2(I) peptides are indicated at the right of samples from OIMSC12. (c) Melting curves of collagen produced by MSCs with the genotypes indicated in b. The thermostability of collagen from a clone targeted at the mutant allele (o/+) is better than that of parental, untransduced OIMSC12 cells (+/−), as indicated by a shift toward degradation at higher temperatures.
In order to examine the structural integrity of the collagen triple helix, we tested the thermostability of the collagen produced after targeting COL1A2 in OIMSC12 cells. [3H]-proline-labeled collagens were treated with proteases at temperatures ranging from 39 to 43 °C. Denaturing, non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis showed that type I collagen α chains from cells targeted at the mutant allele were more resistant to proteolysis at higher temperatures than those from parental OIMSC12 cells were (Figure 2b). The relative quantitation of the loss of α2(I) chains from WT MSCs, parental OIMSC12 cells, and OIMSC12 cells targeted at the mutant or WT allele is depicted by melting curves in Figure 2c. The overlapping curves, representing values from WT MSCs and OIMSC12 cells targeted at the mutant allele, indicate increased thermostability of collagen in targeted OI MSCs.
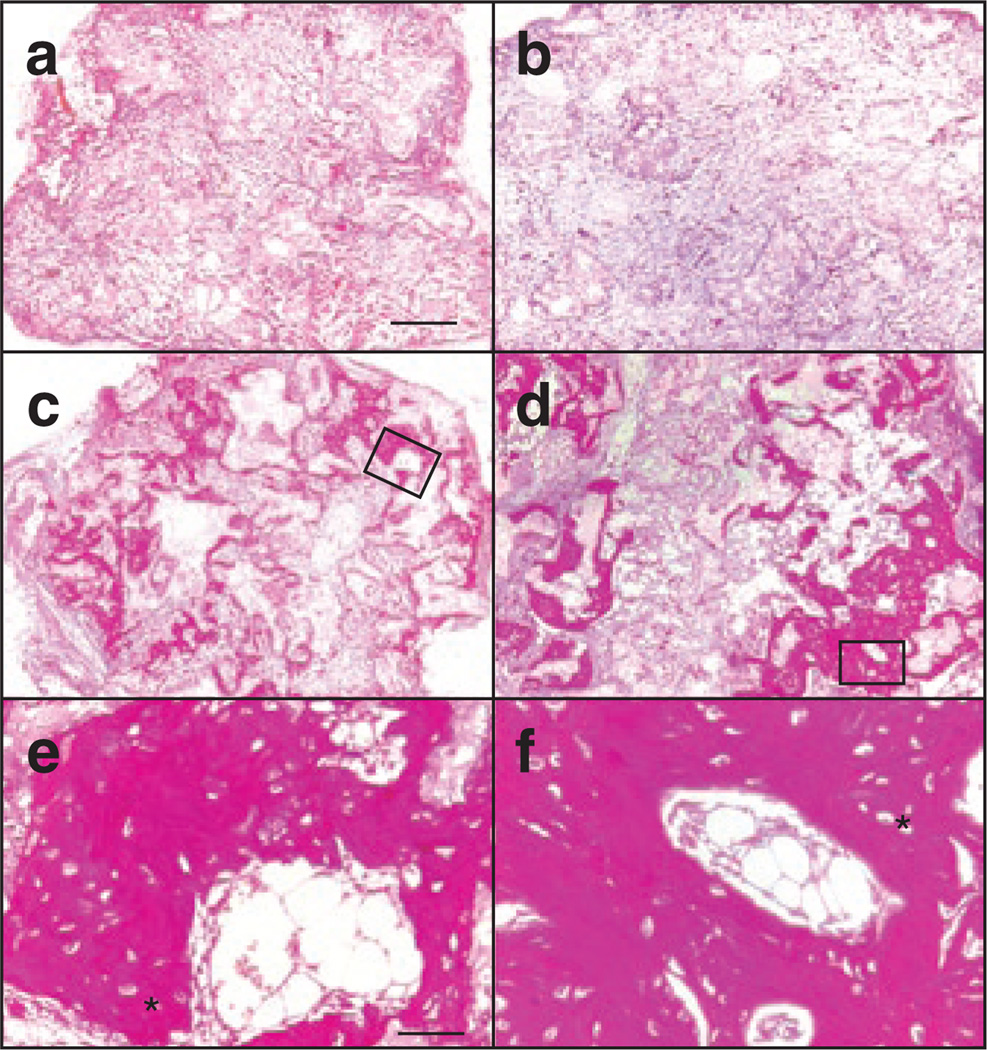
If modified MSCs are to be used as a treatment for OI, it is important that they retain the ability to form bone after ex vivo manipulation. In order to assess bone formation, transduced MSC clones were cultured for 2 weeks in bone induction media containing dexamethasone and ascorbic acid, seeded in hydroxyapatite/tricalcium phosphate matrices, and implanted subcutaneously into immunodeficient mice.3 At 8 weeks after implantation the matrices were surgically removed, sectioned, and examined histologically for the presence of bone (Supplementary Table S1; Figure 3). Three of the six clones that were analyzed produced bone, one targeted at the mutant allele and two targeted at the WT allele, while control implants with no seeded cells did not contain bone. This showed that many MSCs retained the ability to differentiate into bone despite the manipulations involved in the transduction and expansion of clonal populations.
Figure 3. Gene-targeted OIMSCs form bone in immunodeficient mice.
Histological sections of hydroxylapatite/tricalcium phosphate matrices (a) lacking mesenchymal stem cells (MSCs); (b) seeded with normal human fibroblasts; (c, e) OIMSCs targeted at exon 4 of the mutant COL1A2 allele; and (d, f) OIMSCs targeted at exon 2 of the mutant COL1A2 allele, 8 weeks after implantation under the skin of non-obese diabetic/severe combined immunodeficiency mice. Implants were cut into 5 µm sections, stained with hematoxylin and Van Giesonis picric acid fuchsin, and photographed. Regions of dark staining with osteocytes indicate bone. Higher power views are outlined in c and d and shown in e and f, respectively. Lacuna containing osteocytes can be seen, and are indicated by (*). Scale bars = 0.4 mm (a, b, c, d), and 50 µm (e, f).
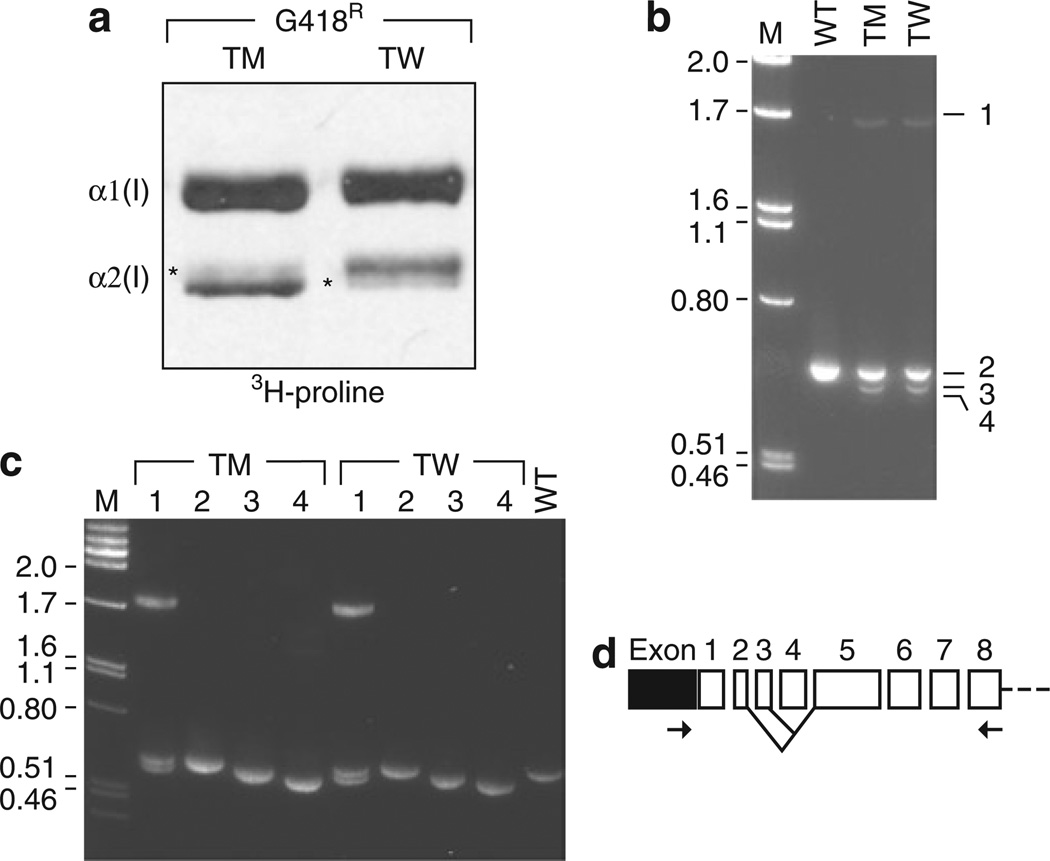
Close inspection of the [3H]-proline-labeled α2(I) collagen chains from targeted MSCs revealed the presence of faint bands that should have been eliminated by gene targeting, thereby suggesting that messenger RNA (mRNA) was still being expressed from the targeted allele (Figure 4a). In order to identify messages that were expressed from the targeted allele, we amplified COL1A2 complementary DNAs with primers located in the 5’-untranslated region and in exon 8, and detected mRNA species smaller than the full-length COL1A2 transcript (Figure 4b). We analyzed each additional message by sequence determination after reamplification of gel-purified reverse transcriptase-polymerase chain reaction (PCR) products (Figure 4c). Reverse transcriptase-PCR product 2 corresponded to the expected full-length spliced product from the untargeted allele, and the shorter messages present in targeted cells lacked exon 4 (product 3) or both exons 3 and 4 (product 4). Product 1 was a heteroduplex of products 2 and 3. On the basis of this analysis, we conclude that alternative splicing with skipping of exon 4 or exons 3 and 4 at targeted alleles can produce mRNAs that do not contain the IRES-neo cassette, and thereby result in the production of in-frame messages that express proα2(I) chains.
Figure 4. Analysis of exon skipping after gene targeting at COL1A2 exon 4.
(a) Sodium dodecyl sulfate polyacrylamide gel electrophoresis separation of [3H]-proline-labeled collagen peptides from OIMSC12 clones targeted at the mutant (TM) or wild-type (TW) COL1A2 allele. Light bands indicated by asterisks (*) are α2(I) chains produced from the targeted alleles. (b) Reverse transcriptase-PCR (RT-PCR) of transcripts of the 5’-untranslated region (UTR)-exon 8 region of COL1A2 from wild-type (WT) and gene-targeted clones, labeled as in a. Numbers at right correspond to PCR products from (1) a heteroduplex product composed of WT and exon 4-skipped complementary DNA (cDNA); (2) the normal, full-length messenger RNA (mRNA); (3) mRNA missing exon 4; and (4) mRNA missing exons 3 and 4. (c) Reamplification products of excised cDNA species 1–4 from b. (d) Schematic drawing of mRNA with lines indicating alternative-spliced forms detected, and PCR primers used for RT-PCR (arrows). 5’-UTR (black box) and coding exons (open boxes) are shown.
In order to overcome exon skipping, we targeted exon 2 of COL1A2. Exon 2 contains 11 nucleotides; therefore, if it is skipped after targeting, the resulting mRNA will be an out-of-frame message, terminate translation prematurely, and fail to produce protein. Vector AAV-COL2e2INloxpA was constructed in order to insert into exon 2 the same IRES-neo cassette that was used previously for targeting exon 4 (Figure 5a). When OIMSC12 cells were infected with AAV-COL2e2INloxpA, 82% of the G418-resistant colonies and 0.23% of the entire unselected cell population underwent targeting at one allele of COL1A2 (Figure 5b and Table 2). Other G418-resistant colonies contained randomly integrated vector sequences that were identified as neo-hybridizing fragments of different sizes (clone 1 in Figure 5b). In 3 of 80 clones analyzed (3.8%), a larger fragment hybridized to the upstream COL1A2 probe (clone 5 in Figure 5b), consistent with insertion of multimeric vector genomes. These types of insertions were previously shown to consist of head-to-tail concatamers of the targeting vector, and were presumably formed prior to homologous recombination with the chromosome.12 Protein analysis showed that 13 of 27 G418-resistant OIMSC12 clones had undergone targeting of the mutant allele, and expressed only WT α2(I) chains (Figure 5c). This finding is an argument against an allele-specific targeting preference with this vector. Importantly, only one α2(I) band was observed on these gels (Figure 5d), thereby demonstrating the absence of protein expression in the targeted allele. A bone formation assay confirmed that MSCs targeted at exon 2 of the mutant COL1A2 allele maintained the ability to differentiate into osteocytes and produce bone (Figure 3d and f).
Figure 5. Gene targeting at exon 2 of COL1A2.
(a) Schematic representation of the gene targeting vector AAV2-COL2e2INloxpA depicted as in Figure 1a. (b) Southern blot of EcoRV-digested genomic DNA isolated from parental, untransduced OIMSC12 cells (Ol12) and eight representative G418R clones. Expected fragment sizes are 7.5 kilobase (kb) and 9.1 kb with the COL1A2 probe p1 for the untargeted and targeted alleles, respectively, 9.1 kb with the neo probe p2 for the targeted allele, and various sizes for random insertions. A fragment larger than 9.1 kb, that can be seen with both the COL1A2 and neo probes indicates targeted insertion of vector multimers at exon 2 (clone 5). (c) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of [3H]-proline-labeled collagen peptides from wild-type (WT) mesenchymal stem cells (MSCs), untransduced OIMSC12 cells (OI12), and G418R transduced clones targeted at the mutant COL1A2 allele (TM), targeted at the WT allele (TW), or containing a randomly integrated vector genome (R). (d) Enlarged view of the protein analysis from c showing peptides from G418R clones targeted at the mutant (TM) or wild-type (TW) COL1A2 alleles. Asterisks indicate the absence of residual α2(I) proteins from the targeted alleles (in contrast to clones targeted at exon 4: Figure 4a).
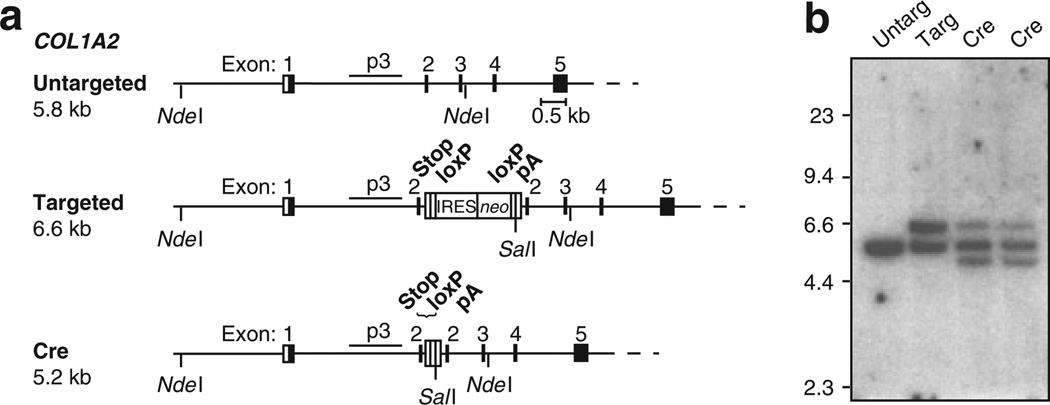
Before modified MSCs can be transplanted, it is important to eliminate expression of the foreign neo gene and avoid eliciting an immune response. We designed our IRES-neo cassette with flanking loxP sites to allow for Cre-mediated excision of the neo gene without removal of the stop codons and pA signal that are intended to disrupt COL1A2 expression. A recombinant Cre fusion protein (HNCM) containing an N-terminal hexahistidine tag (H), nuclear localization sequence from simian virus 40 large-T antigen (N), and a C-terminal membrane translocation sequence (M), was expressed in Escherichia coli and purified by Ni2 + affinity chromatography18 MSCs targeted at exon 2 of COL1A2 were incubated in the presence of HNCM for 2 hours, expanded, and then analyzed by Southern blots. Cre-mediated excision of the IRES-neo cassette decreased the size of the 6.6 kilobase NdeI-SalI fragment from the targeted allele to a 5.2 kilobase fragment (Figure 6). On the basis of quantitation of the Southern data, it was found that transient exposure to Cre excised the IRES-neo cassette from 58 or 64% of targeted alleles in two different MSC clones.
Figure 6. Cre-mediated excision of the internal ribosome entry site (IRES)–neo targeting cassette.
(a) Schematic representation of the COL1A2 locus depicting the untargeted, targeted, and Cre-excised targeted alleles. The locations of the stop codons (Stop), IRES-neo, loxP sites, and pA signal are shown. (b) Southern blot of NdeI/SalI-digested genomic DNA isolated from untransduced OIMSC12 cells (Untarg), a clone targeted at exon 2 of COL1A2 (Targ), and two targeted clones, after treatment with Cre recombinase (Cre). Expected fragment sizes obtained with probe p3 are 5.8 kilobase (kb) (untargeted alleles), 6.6 kb (targeted alleles), and 5.2 kb (Cre-excised targeted alleles). After Cre-mediated excision, the targeted allele retains stop codons in all three reading frames, a single loxP site, and a pA signal.
DISCUSSION
We have shown previously that an AAV gene-targeting vector can disrupt the dominant-negative mutant COL1A1 allele in MSCs from patients with OI.12 In the studies reported here, we have used improved AAV vectors to target the COL1A2 gene, which is a more appropriate locus for initial clinical trials. These new AAV vectors efficiently disrupted mutant COL1A2 genes in three different OI MSC lines, the targeted MSCs produced normal type I collagen, and they formed bone in vivo. The targeting frequencies were high, and in over 60% of G418-selected colonies there was accurate disruption of one COL1A2 allele.
We initially attempted to target exon 4 of COL1A2, but a small amount of mRNA lacking exon 4 (and IRES-neo) was produced from the targeted allele. The presence of aberrant mRNA species in clones targeted at the WT or mutant allele, but not in parental, untargeted cells, suggests that the insertion of the targeting cassette contributed to the skipping of exon 4. OI-causing mutations located at splice junctions in COL1A1 and COL1A2 can disrupt mRNA splicing. In view of the fact that abnormal proα2(I) chains could still be produced from messages lacking exon 4, we modified our vector to target exon 2 which, if deleted by exon-skipping, would result in a frame-shift mutation and premature termination codon preventing protein expression. We found that gene-targeting at exon 2 of the mutant COL1A2 allele completely eliminated production of the abnormal proα2(I) chains in the MSCs. Because it is difficult to predict whether a particular modification will result in exon skipping, targeting an exon that changes the reading frame should be considered as a general strategy to adopt when attempting to eliminate gene expression from targeted alleles.
A clinical trial for OI consisting of allogeneic bone marrow transplantation and MSC infusion resulted in low engraftment levels of MSCs.21–23 This may have been, in part, caused by immune rejection of MSCs expressing foreign antigens, as supported by the finding that cells expressing a neo transgene were not detected in any of the patients, and one individual demonstrated a cytotoxic T-lymphocyte response to MSCs expressing neo.21 We have demonstrated that Cre recombinase can efficiently remove the potentially antigenic IRES-neo cassette from gene-targeted MSCs. Because Cre is delivered transiently as a cell-permeable protein, the potential genotoxic risks of Cre expression are minimized. Although the efficacy of the Cre recombinase system was not 100%, the majority of the cells had eliminated the IRES-neo cassette. Our strategy of using autologous MSCs, combined with removal of the neo gene, should improve the engraftment level.
Another potential problem with our approach is the presence of random integrants in up to 40% of G418-resistant MSCs. If they are to be re-infused into patients during therapy, it is important to ensure that these cells do not activate neighboring oncogenes by insertional mutagenesis. In order to minimize this risk, we have used homology arms that are downstream of exon 1, and do not include known promoter or enhancer sequences. This strategy decreases the recovery of random integrants, and also reduces the chance of activating neighboring genes when random integration does occur.
Isolating individual MSC clones is not a viable approach in a clinical trial, because they would require extensive expansion prior to re-infusion, and clone-to-clone variation would make the results unpredictable. The use of a polyclonal, transduced cell population is a more realistic approach, given that the majority of these cells will have undergone targeting, ex vivo cell manipulations would be minimized, and many more MSCs could ultimately be transplanted. This polyclonal population would consist of cells targeted at either the mutant or the WT allele, and should still provide a therapeutic effect. This is because mosaics for severe forms of OI have mild phenotypes,24–27 individual cells may synthesize entire collagen fibrils without mixing of procollagen from other cells,28 and the cells expressing normal collagen have a growth advantage.25,29,30 Although OI is caused by mutations in COL1A1 or COL1A2, a clinical treatment targeting COL1A2 would be a better option for an initial trial, because individuals heterozygous for null mutations in COL1A2 are phenotypically normal,16 whereas haploinsufficiency at COL1A1 results in a mild form of OI.
MATERIALS AND METHODS
MSC isolation and culture
MSC cultures were established from discarded bone fragments of affected individuals undergoing corrective surgery under Institutional Review Board approval, as described previously.12 Normal human MSCs were provided by the National Hematopoietic Cell Processing Core of the NHLBI Programs in Excellence for Gene Therapy (Fred Hutchinson Cancer Research Center, Seattle, WA) and were derived from cadaver bone marrow.
OI MSC cell line mutation identification
Genomic DNA was isolated from MSC cultures, individual exons were amplified by PCR from COL1A2, and exon-derived DNA heteroduplexes were subjected to conformation-sensitive gel electrophoresis to identify variants.31 Individual variants were then sequenced to identify the specific mutations in COL1A2 from OIMSC1 and OIMSC5. The mutation in OIMSC12 was identified by sequencing COL1A2 complementary DNA obtained by reverse transcription of total RNA isolated from cultured cells, using the RNeasy mini kit (Qiagen, Valencia, CA). complementary DNA was synthesized by priming with random hexamers using Superscript II (Invitrogen, Carlsbad, CA).16
Vector stock preparation
The vector AAV-COL2e4INloxpA includes genomic COL1A2 nucleotides +3,378 to +6,148 relative to the mRNA translation start site, and AAV-COL2e2INloxpA includes nucleotides +1,196 to +3,827, corresponding to nucleotides chr7:93,865,187 to 93,867,956 and 93,863,478 to 93,866,107, respectively, of the March 2006 UCSC human genome sequence assembly. An IRES–neo gene fusion was flanked by bacterial loxP sites, with three all-frame stop codons and a synthetic polyadenylation site at the 5′- and 3′-ends respectively. The cassette was inserted at genomic COL1A2 nucleotides +4,491 or +2,720 in AAV-COL2e4INloxpA and AAV-COL2e2INloxpA, respectively. Vector stocks were prepared as described,32,33 and titers were determined by quantifying full-length vector genomes on alkaline Southern blots.34
Gene targeting
Targeted gene insertion by AAV vectors was performed as previously described.12 Briefly, 2.5–5.0 × 104 MSCs per well of 24-well plates were infected with AAV vectors the day after plating, at a multiplicity of infection of 2,000 or 20,000 vector particles/cell. They were then replated into three 10 cm dishes the next day, and selected in G418 3–4 days later for 10–18 additional days. Colonies initiated in G418 selection were selected and expanded as individual clones. Polyclonal populations were isolated similarly, except that G418-selected cells were pooled when no unselected cells remained (after 7–12 days of selection). Colony counts were determined by staining with Coomassie blue.
DNA isolation and analysis
Genomic DNA was isolated using the Puregene DNA purification protocol (Gentra, Minneapolis, MN), and analyzed by Southern blots according to standard protocols with modifications. Each electrophoresed sample contained 500–1,000 ng of digested genomic DNA. DNA was transferred to nylon membranes (ZetaProbe; Bio-Rad, Hercules, CA). The COL1A2 probes, p1 and p3, were prepared by PCR amplification of COL1A2 genomic DNA spanning –2,041 to –1,117 and +1,827 to +2,890, respectively, relative to the start of transcription. The neo probe (p2) spanned base pairs 1,929–3,092 of GenBank UO2434. Quantitation of the targeted alleles in the polyclonal populations was performed on a PhosphorImager (Storm 820; Amersham Biosciences, Piscataway, NJ). Genomic DNA from a targeted clone was mixed with DNA from an untargeted clone to prepare standards for quantitative Southern blots.
RNA isolation and analysis
Total RNA was isolated and reverse transcribed as above in preparation for OI MSC cell line mutation analysis. The primers used for amplification of COL1A2 5′-untranslated region to exon 8 region were 5′-GCACCACGGCAGCAGGAG-3′ (forward primer) and 5′-TTTGACCAGGTTCACCAGGCTC-3′ (reverse primer). PCR conditions were 95 °C for 10 seconds, 60 °C for 30 seconds, and 72 °C for 90 seconds, for 30 cycles. Products for a second amplification were obtained by excision of individual bands after separation in 6% polyacrylamide gels. The conditions and primers for the second PCR were the same as for the first.
Protein analysis of collagen
MSC proteins were metabolically labeled with [3H]-proline, harvested from the medium and from the cell layer separately, treated with pepsin to remove terminal propeptides, separated by denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis without reduction, and visualized by autoradiography as described.9 Collagen melting curves were generated by metabolically labeling with [3H]-proline and digesting with trypsin and chymotrypsin after heating over a range of temperatures as described previously.35 Collagen digestion fragments were analyzed by 5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and quantitated with ID Digital Analysis Software (Kodak Digital Science, Eastman Kodak Company, Rochester, NY).
Bone formation assay
MSCs were induced to form bone in collagen matrices as described previously.12 The human fibroblast control used MHF2 cells (Coriell Institute for Medical Research, #GM05387; Camden, NJ). Sections of thickness 5 µm each were taken from the outer edge of each implant and analyzed for bone formation by staining with hematoxylin and Van Giesonis picric acid fuchsin stain. Sections were taken at 125 µm intervals throughout the implant until bone with clearly delineated osteocytes was found.
Cre recombinase
A recombinant Cre fusion protein (HNCM) was expressed in E. coli strain BL21 from a pET28a(+) plasmid (kindly provided by Dr. H. Earl Ruley, Vanderbilt University, Nashville, TN) and purified as described previously.36 For delivery to MSCs, targeted clones were seeded at 2 × 104 cells/well in a 24-well plate. The following day, the cells were washed twice with phosphate-buffered saline, incubated at 37 °C for 2 hours with Cre recombinase (16 µmol/l) in serum-free Dulbecco’s modified Eagle’s medium, washed once with phosphate-buffered saline, then cultured overnight in Dulbecco’s modified Eagle’s medium containing 10% serum. The cells were then transferred to 10 cm dishes and allowed to grow to confluence before genomic DNA was isolated.
Supplementary Material
ACKNOWLEDGMENTS
We thank Richard Newton (University of Washington, Seattle, WA) and Dorothy Li (University of Washington, Seattle, WA) for excellent technical assistance, and Thalia Papayannopoulou (University of Washington, Seattle, WA) and Greg Priestly (University of Washington, Seattle, WA) for providing non-obese diabetic/severe combined immunodeficiency mice. This work was supported by grants from the U.S. National Institutes of Health and Children’s Brittle Bone Foundation.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1. Bone formation by targeted OIMSCs implanted in NOD/SCID mice.
REFERENCES
- 1.Byers PH. Disorders of collagen biosynthesis and structure. In: Schriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 5241–5285. [Google Scholar]
- 2.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo . J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 4.Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro . Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 5.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 6.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 8.Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- 9.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 11.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 13.Inoue N, Dong R, Hirata RK, Russell DW. Introduction of single base substitutions at homologous chromosomal sequences by adeno-associated virus vectors. Mol Ther. 2001;3:526–530. doi: 10.1006/mthe.2001.0283. [DOI] [PubMed] [Google Scholar]
- 14.Inoue N, Hirata RK, Russell DW. High-fidelity correction of mutations at multiple chromosomal positions by adeno-associated virus vectors. J Virol. 1999;73:7376–7380. doi: 10.1128/jvi.73.9.7376-7380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell DW, Hirata RK. Human gene targeting by viral vectors. Nat Genet. 1998;18:325–330. doi: 10.1038/ng0498-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarze U, Hata R, McKusick VA, Shinkai H, Hoyme HE, Pyeritz RE, et al. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am J Hum Genet. 2004;74:917–930. doi: 10.1086/420794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willing MC, Deschenes SP, Slayton RL, Roberts EJ. Premature chain termination is a unifying mechanism for COL1A1 null alleles in osteogenesis imperfecta type I cell strains. Am J Hum Genet. 1996;59:799–809. [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Q, Jo D, Gebre-Amlak KD, Ruley HE. Enhanced cell-permeant Cre protein for site-specific recombination in cultured cells. BMC Biotechnol. 2004;4:25. doi: 10.1186/1472-6750-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonadio J, Holbrook KA, Gelinas RE, Jacob J, Byers PH. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985;260:1734–1742. [PubMed] [Google Scholar]
- 20.Raghunath M, Bruckner P, Steinmann B. Delayed triple helix formation of mutant collagen from patients with osteogenesis imperfecta. J Mol Biol. 1994;236:940–949. doi: 10.1006/jmbi.1994.1199. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 24.Cabral WA, Marini JC. High proportion of mutant osteoblasts is compatible with normal skeletal function in mosaic carriers of osteogenesis imperfecta. Am J Hum Genet. 2004;74:752–760. doi: 10.1086/383252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantinou CD, Pack M, Young SB, Prockop DJ. Phenotypic heterogeneity in osteogenesis imperfecta: the mildly affected mother of a proband with a lethal variant has the same mutation substituting cysteine for alpha 1-glycine 904 in a type I procollagen gene (COL1A1) Am J Hum Genet. 1990;47:670–679. [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards MJ, Wenstrup RJ, Byers PH, Cohn DH. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a mutation in the COL1A2 gene of type I collagen. The mosaic parent exhibits phenotypic features of a mild form of the disease. Hum Mutat. 1992;1:47–54. doi: 10.1002/humu.1380010108. [DOI] [PubMed] [Google Scholar]
- 27.Wallis GA, Starman BJ, Zinn AB, Byers PH. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990;46:1034–1040. [PMC free article] [PubMed] [Google Scholar]
- 28.Ploetz C, Zycband EI, Birk DE. Collagen fibril assembly and deposition in the developing dermis: segmental deposition in extracellular compartments. J Struct Biol. 1991;106:73–81. doi: 10.1016/1047-8477(91)90064-4. [DOI] [PubMed] [Google Scholar]
- 29.Fedarko NS, Moerike M, Brenner R, Robey PG, Vetter U. Extracellular matrix formation by osteoblasts from patients with osteogenesis imperfecta. J Bone Miner Res. 1992;7:921–930. doi: 10.1002/jbmr.5650070809. [DOI] [PubMed] [Google Scholar]
- 30.Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korkko J, Ala-Kokko L, De Paepe A, Nuytinck L, Earley J, Prockop DJ. Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet. 1998;62:98–110. doi: 10.1086/301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata R, Chamberlain J, Dong R, Russell DW. Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol. 2002;20:735–738. doi: 10.1038/nbt0702-735. [DOI] [PubMed] [Google Scholar]
- 33.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 34.Inoue N, Russell DW. Packaging cells based on inducible gene amplification for the production of adeno-associated virus vectors. J Virol. 1998;72:7024–7031. doi: 10.1128/jvi.72.9.7024-7031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace JM, Kuslich CD, Willing MC, Byers PH. Disruption of one intra-chain disulphide bond in the carboxyl-terminal propeptide of the proalpha1(I) chain of type I procollagen permits slow assembly and secretion of overmodified, but stable procollagen trimers and results in mild osteogenesis imperfecta. J Med Genet. 2001;38:443–449. doi: 10.1136/jmg.38.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo D, Nashabi A, Doxsee C, Lin Q, Unutmaz D, Chen J, et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat Biotechnol. 2001;19:929–933. doi: 10.1038/nbt1001-929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.