Abstract
Recent advances in our understanding of genetic-epigenetic interactions have unraveled new mechanisms underlying the etiology of complex diseases such as autoimmune diseases. Among autoimmune diseases autoimmune thyroid diseases are highly prevalent affecting 1-5% of the population. The term autoimmune thyroid diseases (AITD) refers to the whole spectrum of thyroid autoimmunity ranging from subclinical disease manifesting by the presence of autoantibodies targeting thyroid antigens to clinical disease. The major AITD include Graves’ disease (GD) and Hashimoto’s thyroiditis (HT). While clinically these are contrasting diseases, GD manifesting by thyrotoxicosis and HT manifesting by hypothyroidism, their pathogenesis involves shared immunegenetic mechanisms. Genetic data point to the involvement of shared genes as well as unique genes for GD and HT. Among the shared susceptibility genes HLA-DR containing arginine at position β74 gives the strongest risk. Other shared immune regulatory genes predisposing to AITD include CTLA-4, PTPN22, and CD25. CD40, in contrast, is specific for GD. Two thyroid specific genes also confer susceptibility to AITD - Tg is associated with both GD and HT while the TSH receptor is associated only with GD. It is clear that other genes are involved and several additional putative genes identified by genome wide analyses have been recently reported. Epigenetic modulation is emerging as a major mechanism by which environmental factors interact with AITD susceptibility genes. Indeed, we have recently shown an epigenetic interaction between interferon alpha, a key cytokine secreted during viral infections, and a Tg promoter variant. Dissecting the genetic-epigenetic interactions underlying the pathogenesis of AITD is essential to uncover new therapeutic targets.
Keywords: Autoimmunity, thyroid, Graves’ disease, Hashimoto’s thyroiditis
2. INTRODUCTION
Autoimmune responses target the thyroid more frequently than any other organ. The prevalence of the autoimmune thyroid diseases (AITD) Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) is estimated to be 5% (1-4). The prevalence of subclinical disease manifesting by the production of anti-thyroid antibodies without clinical disease (considered a biomarker of genetic susceptibility) is even higher (5;6). Intriguingly, these two clinically distinct syndromes share common immunopathogenic mechanisms. While the hallmark of GD is thyrotoxicosis and of HT hypothyroidism, both are characterized by lymphocytic infiltration of the thyroid and the production of thyroid autoantibodies (7;8). The AITD are prototypical organ specific autoimmune diseases, but the mechanisms triggering the autoimmune response to the thyroid are still unclear. Epidemiological data point to an interaction between genetic susceptibility and environmental triggers as the key factors leading to the breakdown of tolerance and the development of the disease (9). Of the environmental factors infection, diet, iodine, and smoking probably are the most important ones (for a review see (10)). Considerable progress has been made in the past 2 decades in unraveling the genetic risk factors for AITD (reviewed in (9)). While originally only MHC class II genes have been shown to predispose to AITD, several non-MHC genes have been confirmed as susceptibility genes contributing to the etiology of AITD. It has become clear that some genes are unique for GD or HT and some are common to both diseases (Figure 1). Moreover, certain genes are common to AITD and other autoimmune diseases (11).
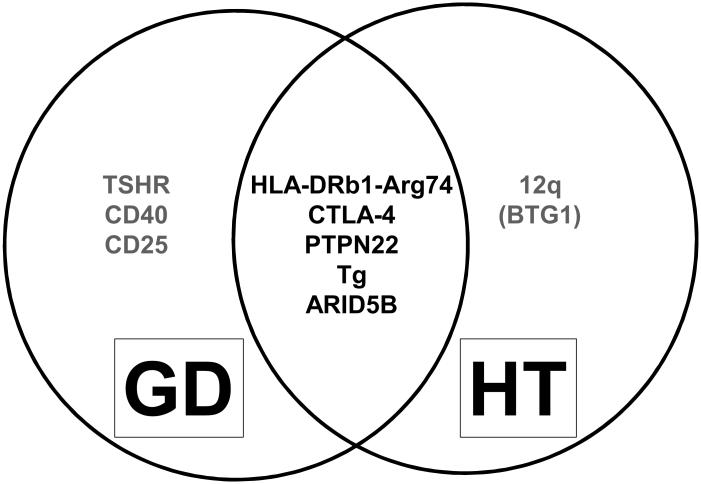
Figure 1.
A Venn diagram showing susceptibility genes for AITD. Most genes are common to both GD and HT while several are unique to GD and HT. Additional genes are likely associated with AITD as demonstrated by recent GWAS and immunochip studies (see text).
Current unprecedented advances in genomics including the identification of more than a million common single nucleotide polymorphisms (SNPs) and the creation of accurate linkage disequilibrium maps of these SNPS, as well as the identification numerous rare variants of the human genome and the completion of the 1000 genome project will enable us to pinpoint the genetic variants predisposing to AITD. In this review we focus on some of the susceptibility genes for AITD which our group has been studying. As more genes are being discovered our focus is shifting to the mechanisms by which they predispose to disease and how they interact epigenetically with environmental factors.
3. GENETIC SUSCEPTIBILITY PLAYS A MAJOR ROLE IN THE ETIOLOGY OF AITD
Epidemiological investigations can give clues to the relative contributions of genetic and non-genetic factors to the etiology of disease. Both family studies showing high sibling risk ratio and twin studies showing high concordance rate among monozygotic (MZ) twins supported a strong genetic influence on AITD etiology (reviewed in (9)). Our group have reported a high sibling risk ratio for both GD and HT suggesting a strong genetic susceptibility (12). Moreover, twin studies have shown a significantly higher concordance rate among MZ twins compared to dizygotic (DZ) twins for both GD (13) and HT (14). Based on twin studies it has been proposed that approximately 80% of the risk for GD is hereditary (15). Indeed, linkage and association studies in AITD have identified several major genes for AITD. These include both thyroid specific genes (thyroglobulin and TSHR) and immune regulatory genes. Not unexpectedly, the immune regulatory genes predisposing to AITD are shared with other autoimmune disease.
4. THYROID ANTIGENS AS AITD SUSCEPTIBILITY GENES
TSH (Thyrotropin) Receptor (TSHR) gene
GD is caused by the production of TSHR stimulating antibodies which stimulate the TSHR activating unregulated thyroid hormone synthesis and secretion that leads to clinical thyrotoxicosis. TSHR antibodies have been shown to fulfill Koch’s postulates for proof of causing disease including the presence of TSHR antibodies in nearly all cases of GD, direct correlation between disease severity and TSHR antibody levels, as well as transfer of disease by injection of animals with TSHR stimulating antibodies, and transfer of the disease to newborns of mothers with GD (16). In view of the fact that TSHR is the direct target of the autoimmune response in GD it was an obvious candidate gene that was tested for association with GD as soon as polymorphisms were discovered in the TSHR gene (17). These early polymorphisms were mostly missense mutations and studies did not show conclusive evidence for association with disease. With the completion of the human genome project and discovery of dense maps of SNPs it was realized that the TSHR gene is strongly associated with GD, but that the causative variant is located within intron 1 of the gene (18;19).
While the identification of intron 1 SNPs in the TSHR gene that predispose to GD has now been substantiated by many groups the question remains as to the mechanisms by which intronic SNPs can confer risk for disease. One might speculate that these SNPs may affect splicing and/or expression of the TSHR gene, possibly in the thymus (20). If indeed TSHR expression in the thymus was reduced by the intron 1 variants that would enable autoreactive T-cells targeting the TSHR to escape deletion in the thymus and to trigger disease later in life.
Thyroglobulin (Tg) gene
Thyroglobulin (Tg) is another obvious candidate gene for AITD since it accounts for about 80% of the total thyroid protein content. Moreover, Tg, which is stored in the colloid, normally leaks into the circulation and is exposed to the immune system. In mice, the best model of human autoimmune thyroiditis is induced by immunizing mice with Tg, either as protein (21) or using cDNA immunization (22).
Even though Tg antibodies are not the most sensitive or specific for diagnosing autoimmune thyroiditis, Tg has been recently recognized as the potential earliest trigger of autoimmune thyroiditis (23). Supporting a key role for Tg in triggering AITD are data showing that Tg is a major AITD susceptibility gene. The earliest indication that Tg may be an AITD susceptibility gene came from linkage studies demonstrating a significant linkage peak on chromosome 8q at the Tg gene region (24). Further sequencing of the Tg gene identified amino acid variants that were significantly associated with AITD (25). Moreover, one of the variant, a SNP in exon 33, showed significant statistical interaction with an HLA-DR variant containing arginine at position beta 74 (HLA-DRb1-Arg74)conferring together a high risk for AITD (26).
In view of the statistical interaction between the HLA-DRb1-Arg74 and the Tg SNP we hypothesized that this reflects a biological interaction between these two genes. One potential interaction is by augmenting presentation of pathogenic Tg peptides. Indeed, further studies have identified 4 Tg peptides that bind specifically to the HLA-DRb1-Arg74 pocket and not to the Gln-74 pocket which is protective (27). However, a direct link between the Tg SNPs and the pathogenic Tg peptides was not yet established.
Further analysis of the non-coding regions of the Tg gene yielded interesting and unexpected results. Sequencing of the Tg promoter up to 2.5 Kb upstream from the transcription start site identified a SNP at position -1623 (rs180195) that showed strong association with AITD both in case-control studies and in family based association studies (28). This SNP was not in linkage disequilibrium (LD) with the missense Tg SNPs found to be associated with AITD. These data demonstrated that several SNPs within the same genes can independently influence risk for disease. Moreover, rs180195 was found to interact epigenetically with IRF-1 to trigger AITD (see below).
5. IMMUNE-RESPONSE GENES AS AITD SUSCEPTIBILITY GENES
The HLA-DR class II gene complex
The HLA locus was the first locus to show association with GD and HT (reviewed in (29). HLA-DR3 has been conclusively shown to be a major GD gene conferring the strongest risk of all GD susceptibility genes identified so far. The data for HT was less conclusive but more recent data strongly suggest that HLA-DR3 is the primary HLA allele predisposing for HT (29). However, HLA-DR3 has over 30 alleles and therefore, we sequenced the HLA-DR3 in order to identify the unique sequence signature that predisposes to AITD. Our findings revealed that the presence of arginine at position 74 of the HLA-DRβ chain is critical for the development of AITD while glutamine at this position is protective (30). Moreover, HLA-DRβ-Arg74 was part of a pocket amino acid signature that was associated with the development of type 1 diabetes and autoimmune thyroiditis in the same individual (31). Thus, HLA-DRβ-Arg74 must play a key mechanistic role in the development of AITD (32;33). Since the key role of HLA class II molecules is to present peptide antigens to the T-cell receptor we hypothesized that HLA-DRβ-Arg74 may induce a pocket conformation that will enable the presentation of thyroid peptides that can trigger autoimmune thyroiditis. Indeed, computer modeling studies have shown that the presence of arginine at position 74 of the DRβ chain created a narrower more positively charged pocket P4 when compared to the pocket containing glutamine at position 74 (protective from disease) (30). Furthermore, the presence of arginine at position 74 enabled the binding and presentation of pathogenic thyroglobulin peptides (27;34) or TSHR peptides (35) that may trigger AITD. A similar mechanism has been shown to underlie the HLA class II associations with other autoimmune diseases such as type 1 diabetes, suggesting that this is a generalized mechanism in autoimmunity (36). To further explore the role of MHC II in the etiology of autoimmune thyroiditis we sequenced the murine MHC class II region, I-E (DR homolog) in 12 mouse strains that are susceptible to experimental autoimmune thyroiditis (EAT) and 10 mouse strains that are resistant. As in humans we identified a pocket amino acid signature in the I-E gene locus that was associated with autoimmune thyroiditis in mice (30). However, the murine pocket structure was found to be different than the human pocket structure that is associated with autoimmune thyroiditis. Thus, it is likely that the Tg epitopes that are associated with EAT are distinct from those associated with human AITD. One way to overcome this issue is to use humanized DR3 mice when studying human Tg epitopes that trigger AITD (34).
CD40
CD40 plays a key role in the cross-talk between antigen presenting cells and T-cells. Expressed constitutively on antigen presenting cells, CD40 is key to their normal function. On B-cells CD40 provides crucial signal for their proliferation, differentiation, and switching to production of IgG (37). Therefore, when we first identified CD40 as a unique GD susceptibility gene (38), it came as no surprise; especially given the fact that GD is a B-cell mediated autoimmune disease. These data have now been replicated in Caucasians as well as other ethnic groups (reviewed in (37)). We have explored the mechanisms by which CD40 variants can trigger GD and found that the causative variant (the C-allele of the CD40 SNP) upregulated CD40 expression on B-cells and on thyroid cells (38). Upregulation of CD40 expression on B-cells, driven by the C-allele, could lower the threshold for their activation, thereby contributing to the onset of autoimmune disease. Another possibility is that upregulation of CD40 in thyrocytes can contribute to disease. According to this model activation of CD40 in thyrocytes will trigger cytokine secretion and activation of resident T-cells, leading to local inflammatory response and autoimmunity through bystander mechanisms. These two mechanisms are not mutually exclusive and likely are both contributing to the association of CD40 with GD. Recently, we have shown that upregulation of CD40 accelerated disease in a mouse model of GD through activation of IL-6 REF (39). Moreover, blocking IL-6 completely suppressed experimental autoimmune GD in mice (39). This may suggest that IL-6 blockade could be a potential therapeutic strategy in human GD. The analysis of CD40 in GD demonstrates how genetic studies can unravel new mechanisms leading to potential new therapeutic targets. This demonstrates the power of genetic studies when coupled with mechanistic analyses.
Interestingly, more recent data have implicated CD40 in several additional autoimmune diseases including high IgE asthma, rheumatoid arthritis, and multiple sclerosis (40-42). Since, unlike GD, these are not primarily B-cell mediated diseases it suggests that additional mechanisms may be responsible for the involvement of CD40 in autoimmunity.
6. AITD SUSCEPTIBILITY GENES INVOLVED IN T-CELL REGULATION
In addition to HLA-DR and CD40, 3 other genes involved in T-cell activation and regulation are associated with AITD: CTLA-4, PTPN22, and CD25. CTLA-4 is a co-stimulatory molecule expressed on T-cells that functions to downregulate T-cells activation through binding to B7 molecules on antigen presenting cells (APC’s) (reviewed in (43)). CTLA-4 is also constitutively expressed on Treg cells and plays an important role in their suppressive function. Moreover, recent data suggest that CTLA-4 can limit the contact between APC’s and T-cells, thereby reducing T-cell activation (reviewed in (44)). Therefore, polymorphisms that may cause decreased CTLA-4 function may confer risk for autoimmunity by reducing the suppression of T-cell activation. Several polymorphisms in the CTLA-4 gene have been reported to be associated with AITD and some of them have been shown to be associated with reduced CTLA-4 function (45-48) (reviewed in (37)). A detailed analysis of the CTLA-4 gene-locus showed that the causative variant is located in the 3’UTR region of CTLA-4 (48). However, the exact causative polymorphism at the 3’UTR of CTLA-4 is not known. One group suggested that an A/G SNP designated CT60 is the causative SNP (48) but other groups could not confirm CT60 as causative (49). Thus, the causative variant and the mechanisms by which it reduced CTLA-4 expression and/or function are yet to be determined. Interestingly, the role of reduced CTLA-4 function as a trigger of autoimmunity was recently underscored by the unusual autoimmune phenomena associated CTLA-4 inhibition by monoclonal antibodies used as a therapy for melanoma (50).
The protein tyrosine phosphatase-22 (PTPN22) gene encodes the lymphoid tyrosine phosphatase (LYP) protein. LYP is a powerful negative regulator of T-cell receptor signaling through interaction with the Csk protein and dephosphorylation of Lck and Fyn kinases which are involved in T-cell receptor signaling (51). A SNP at position 1858 resulting in a change of amino acid 620 in LYP from arginine to tryptophan (R620W) is associated with AITD (52), as well as with other autoimmune diseases (53;54). Interestingly, the disease associated allele is a gain of function variant that would result in suppression of T-cell receptor signaling. It is unclear how suppression of T-cell receptor signaling could predispose to autoimmunity but some have suggested that it may allow escape from central tolerance in the thymus (55).
The CD25 gene [interleukin-2 receptor alpha chain (IL2RA)] has been shown to be associated with GD (56). CD25 is constitutively expressed on Treg cells and is critical to their function (57;58). Therefore, it is plausible that polymorphisms in CD25 reduce Treg function thereby promoting autoimmunity (59). Interestingly, CTLA-4, another AITD gene is also expressed on Treg’s. These data suggest that inherited defects in Treg function may be a major mechanism predisposing to AITD. Indeed, we have previously shown association between FOXP3 variants and AITD (60).
7. NEW GENOME-WIDE STUDIES IN AITD
Recently, the first genome wide association study (GWAS) was reported in GD. In this large study from China 1,536 GD patients and 1,516 controls were genotyped for approximately 660,000 SNPs (61). In addition, to confirming previously identified GD loci the investigators mapped two new GD loci, on chromosomes 6q27 and 4p14. Both of these loci contain several genes and it is currently unclear which genes in these loci are associated with GD (61). Another recent study used a different genome-wide approach. In this study the immunochip was utilized to genotype a large cohort of 2,374 GD patients, 474 HT patients, and 6,894 controls (62). The immunochip contains approximately 200,000 SNPs and is enriched for SNPs within genes-loci previously shown to be associated with immune-mediated diseases. This study successfully genotyped 103,875 SNPS of the immunochip and identified 7 new loci that were associated with both GD and HT (62). These new exciting data shed new light on the pathogenesis of AITD; however, these genome-wide data await replication.
8. EPIGENETIC INFLUENCES ON THE ETIOLOGY OF AITD
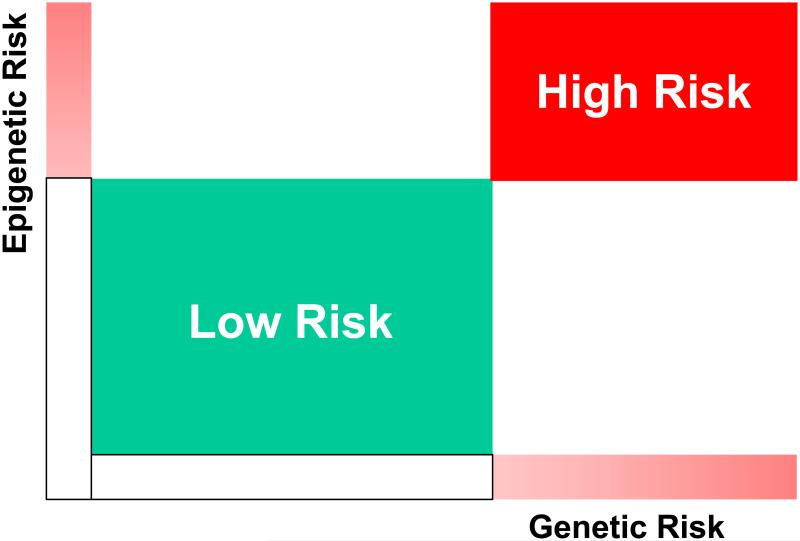
One of the intriguing surprises of GWAS studies in complex diseases, such as AITD, was the very low odds ratios obtained for most loci that were identified and replicated. Indeed, with the exception of the HLA-DRβ1-Arg74 variant most loci that are associated with AITD give low odds ratios. These low odds ratios do not indicate that genetic susceptibility is not an important risk factor for AITD. As indicated by twin studies (15) genetic factors confer a significant risk for AITD. Thus, the low odds ratios most likely indicate that genetic risk factors alone cannot trigger disease without other non-genetic modifiers (Figure 2). One major modifier that has emerged in recent years is epigenetics. While the definition of epigenetic effects is somewhat controversial it generally indicates non-coding effects on gene expression and function that are mitotically stable. Thus, epigenetic effects are long lasting. Epigenetic effects include DNA methylation, histone modifications, and RNA interference by microRNA (miR) (63). Epigenetic modifications can amplify a risk conferred by an inherited polymorphism resulting in a combined high risk for disease (Figure 2). This is exemplified by our recent findings of an epigenetic modulation of histone methylation patterns in the Tg promoter containing a variant that is associated with AITD (28). We found that the transcription factor IRF-1 binds to the Tg promoter only in the presence of the disease-associated variant and that this binding is modulated by changes in histone methylation patterns. IRF-1 is a transcription factor triggered by interferon alpha, a cytokine that is secreted locally during viral infections. Therefore, this could be a plausible mechanism for an interaction between an environmental factor, namely viral infection, and susceptibility variant in the Tg gene. The susceptibility allele in the Tg promoter is permissive for the binding of IRF-1 during viral infections of the thyroid, when interferon alpha levels increase, triggering an increase in IRF-1 (28). We believe that similar genetic-epigenetic interactions operate in the case of most susceptibility variants that are associated with AITD.
Figure 2.
Genetic risk alone can confer a moderate risk, but this risk is amplified when combined with synergistic epigenetic modifications of regulatory regions controlling gene expression.
9. CONCLUSIONS
The etiology of AITD is multifactorial involving genetic and environmental factors. In recent years the pieces of the puzzle are slowly fitting together to reveal a picture of interacting risk factors. Genetic variants provide the primary risk for AITD. The AITD susceptibility genes include both immune-regulatory genes and target tissue genes. When this primary genetic risk interacts with an environmental factor (e.g. infection, diet, iodine exposure) there is a synergistic effect that can trigger disease. The key to genetic-environmental interactions is epigenetic modulation. Identifying the genetic-epigenetic interactions leading to AITD and other autoimmune diseases will lead to new promising therapeutic targets.
Acknowledgements
This work was supported in part by National Institutes of Health Grants DK61659, DK067555, and DK073681 and by the Department of Veterans Affairs, Veterans Health Administration.
11. REFERENCES
- (1).Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- (2).Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- (3).Tunbridge WMG, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol Oxf. 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- (4).Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- (5).Kabelitz M, Liesenkotter KP, Stach B, Willgerodt H, Stablein W, Singendonk W, et al. The prevalence of anti-thyroid peroxidase antibodies and autoimmune thyroiditis in children and adolescents in an iodine replete area. Eur J Endocrinol. 2003;148(3):301–307. doi: 10.1530/eje.0.1480301. [DOI] [PubMed] [Google Scholar]
- (6).Pearce SH, Leech NJ. Toward precise forecasting of autoimmune endocrinopathy. J Clin Endocrinol Metab. 2004;89(2):544–547. doi: 10.1210/jc.2003-032142. [DOI] [PubMed] [Google Scholar]
- (7).Davies TF. Graves' Diseases: Pathogenesis. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid: A fundamental and clinical text. eighth Lippincott Williams & Wilkens; Philadelphia: 2000. pp. 518–530. [Google Scholar]
- (8).Weetman AP. Chronic autoimmune thyroiditis. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The thyroid. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 721–732. [Google Scholar]
- (9).Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29(6):697–725. doi: 10.1210/er.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. 2009;32(3-4):231–239. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Tomer Y, Menconi F. Type 1 diabetes and autoimmune thyroiditis: the genetic connection. Thyroid. 2009;19(2):99–102. doi: 10.1089/thy.2008.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003;13:761–764. doi: 10.1089/105072503768499653. [DOI] [PubMed] [Google Scholar]
- (13).Brix TH, Christensen K, Holm NV, Harvald B, Hegedus L. A population-based study of Graves' diseases in Danish twins. Clin Endocrinol. 1998;48:397–400. doi: 10.1046/j.1365-2265.1998.00450.x. [DOI] [PubMed] [Google Scholar]
- (14).Brix TH, Kyvik KO, Hegedus L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85(2):536–539. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- (15).Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86(2):930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- (16).Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Tonacchera M, Pinchera A. Thyrotropin receptor polymorphisms and thyroid diseases. J Clin Endocrinol Metab. 2000;85(8):2637–2639. doi: 10.1210/jcem.85.8.6801. [DOI] [PubMed] [Google Scholar]
- (18).Dechairo BM, Zabaneh D, Collins J, Brand O, Dawson GJ, Green AP, et al. Association of the TSHR gene with Graves' disease: the first disease specific locus. Eur J Hum Genet. 2005;13(11):1223–1230. doi: 10.1038/sj.ejhg.5201485. [DOI] [PubMed] [Google Scholar]
- (19).Tomer Y, Hasham A, Davies TF, Stefan M, Concepcion E, Keddache M, et al. Fine mapping of loci linked to autoimmune thyroid disease identifies novel susceptibility genes. J Clin Endocrinol Metab. 2013;98(1):E144–E152. doi: 10.1210/jc.2012-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Graves PN, Tomer Y, Davies TF. Cloning and sequencing of a 1.3 kb variant of human thyrotropin receptor mRNA lacking the transmembrane domain. Biochem Biophys Res Commun. 1992;187:1135–1143. doi: 10.1016/0006-291x(92)91315-h. [DOI] [PubMed] [Google Scholar]
- (21).Charreire J. Immune mechanisms in autoimmune thyroiditis. Adv Immunol. 1989;46:263–334. doi: 10.1016/s0065-2776(08)60656-2. [DOI] [PubMed] [Google Scholar]
- (22).Jacobson EM, Concepcion E, Ho K, Kopp P, Toniolo JV, Tomer Y. cDNA Immunization of Mice with Human Thyroglobulin Generates Both Humoral and T Cell Responses: A Novel Model of Thyroid Autoimmunity. Plos One. 2011;6(4):e19200. doi: 10.1371/journal.pone.0019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, et al. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151(9):4583–4593. doi: 10.1210/en.2010-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, et al. Common and unique susceptibility loci in Graves and Hashimoto diseases: Results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73:736–747. doi: 10.1086/378588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, et al. Possible Interaction Between HLA-DRbeta1 and Thyroglobulin Variants in Graves' Disease. Thyroid. 2006;16(4):351–355. doi: 10.1089/thy.2006.16.351. [DOI] [PubMed] [Google Scholar]
- (27).Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, et al. Employing a recombinant HLA-DR3 expression system to dissect MHC II-thyroglobulin peptide dynamism: A genetic, biochemical, and reverse immunological perspective. J Biol Chem. 2009;284:34231–34243. doi: 10.1074/jbc.M109.041574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Stefan M, Jacobson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L, et al. Novel Variant of Thyroglobulin Promoter Triggers Thyroid Autoimmunity through an Epigenetic Interferon alpha-modulated Mechanism. J Biol Chem. 2011;286(36):31168–31179. doi: 10.1074/jbc.M111.247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: From epidemiology to etiology. J Autoimmun. 2008;30(1-2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, et al. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci U S A. 2008;105(37):14034–14039. doi: 10.1073/pnas.0806584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Menconi F, Osman R, Monti MC, Greenberg DA, Concepcion ES, Tomer Y. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci U S A. 2010;107(39):16899–16903. doi: 10.1073/pnas.1009511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, et al. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves' disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- (33).Simmonds MJ, Howson JM, Heward JM, Cordell HJ, Foxall H, Carr-Smith J, et al. Regression Mapping of Association between the Human Leukocyte Antigen Region and Graves Disease. Am J Hum Genet. 2005;76(1):157–163. doi: 10.1086/426947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Menconi F, Huber A, Osman R, Concepcion E, Jacobson EM, Stefan M, et al. Tg.2098 is a major human thyroglobulin T-cell epitope. J Autoimmun. 2010;35:45–51. doi: 10.1016/j.jaut.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Inaba H, Martin W, Ardito M, De Groot AS, De Groot LJ. The role of glutamic or aspartic acid in position four of the epitope binding motif and thyrotropin receptor-extracellular domain epitope selection in Graves' disease. J Clin Endocrinol Metab. 2010;95(6):2909–2916. doi: 10.1210/jc.2009-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2(6):501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- (37).Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jacobson EM, Concepcion E, Oashi T, Tomer Y. A Graves' disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146(6):2684–2691. doi: 10.1210/en.2004-1617. [DOI] [PubMed] [Google Scholar]
- (39).Huber AK, Finkelman FD, Li CW, Concepcion E, Smith E, Jacobson E, et al. Genetically Driven Target Tissue Overexpression of CD40: A Novel Mechanism in Autoimmune Disease. J Immunol. 2012;189(6):3043–3053. doi: 10.4049/jimmunol.1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Park JH, Chang HS, Park CS, Jang AS, Park BL, Rhim TY, et al. Association Analysis of CD40 Polymorphisms with Asthma and the Level of Serum Total IgE. Am J Respir Crit Care Med. 2007;175(8):775–782. doi: 10.1164/rccm.200609-1286OC. [DOI] [PubMed] [Google Scholar]
- (41).van der Linden MP, Feitsma AL, le Cessie S, Kern M, Olsson LM, Raychaudhuri S, et al. Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2242–2247. doi: 10.1002/art.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).2009;41(7):824–828. [Google Scholar]
- (43).Tomer Y. Unraveling the genetic susceptibility to autoimmune thyroid diseases: CTLA-4 takes the stage. Thyroid. 2001;11:167–169. doi: 10.1089/105072501300042884. [DOI] [PubMed] [Google Scholar]
- (44).Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kouki T, Sawai Y, Gardine CA, Fisfalen ME, Alegre ML, DeGroot LJ. CTLA-4 Gene Polymorphism at Position 49 in Exon 1 Reduces the Inhibitory Function of CTLA-4 and Contributes to the Pathogenesis of Graves' Disease. J Immunol. 2000;165(11):6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- (46).Takara M, Kouki T, DeGroot LJ. CTLA-4 AT-repeat polymorphism reduces the inhibitory function of CTLA-4 in Graves' disease. Thyroid. 2003;13(12):1083–1089. doi: 10.1089/10507250360731479. [DOI] [PubMed] [Google Scholar]
- (47).Ban Y, Davies TF, Greenberg DA, Kissin A, Marder B, Murphy B, et al. Analysis of the CTLA-4, CD28 and inducible co-stimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun. 2003;4:586–593. doi: 10.1038/sj.gene.6364018. [DOI] [PubMed] [Google Scholar]
- (48).Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- (49).Mayans S, Lackovic K, Nyholm C, Lindgren P, Ruikka K, Eliasson M, et al. CT60 genotype does not affect CTLA-4 isoform expression despite association to T1D and AITD in northern Sweden. BMC Med Genet. 2007;8:3. doi: 10.1186/1471-2350-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol. 2012;167(1):1–5. doi: 10.1530/EJE-12-0167. [DOI] [PubMed] [Google Scholar]
- (51).Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585(23):3689–3698. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]
- (52).Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004;89(11):5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- (53).Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36(4):337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- (55).Vang T, Miletic AV, Bottini N, Mustelin T. Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity. 2007;40(6):453–461. doi: 10.1080/08916930701464897. [DOI] [PubMed] [Google Scholar]
- (56).Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, et al. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 2007;66(4):508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- (57).Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- (58).Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28(2-3):59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Andre S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174(5):1575–1587. doi: 10.2353/ajpath.2009.080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ban Y, Tozaki T, Tobe T, Ban Y, Jacobson EM, Concepcion ES, et al. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: An association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28:201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- (61).Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011;43(9):897–901. doi: 10.1038/ng.898. [DOI] [PubMed] [Google Scholar]
- (62).Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, et al. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012;21(23):5202–5208. doi: 10.1093/hmg/dds357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jungel A, Ospelt C, Gay S. What can we learn from epigenetics in the year 2009? Curr Opin Rheumatol. 2010;22(3):284–292. doi: 10.1097/BOR.0b013e3283389641. [DOI] [PubMed] [Google Scholar]