Abstract
Bacterial vaginosis (BV) is a common cause of vaginal discharge in reproductive age women around the world, and is associated with several poor reproductive health outcomes, including HIV-1 acquisition. One possible mechanism for this association is the inflammatory immune response induced by BV in the cervical and vaginal mucosae. There is significant heterogeneity in reports of markers of cervicovaginal inflammation in women with bacterial vaginosis, likely due to microbial and host diversity, as well as differences in study design. In this article we review the characteristics of the mucosal immune response in BV, the potential role of lactobacilli in modulating that response, and the impact of individual BV-associated bacterial species on mucosal immunity. We focus on inflammatory markers that are proposed to increase the risk of HIV-1 acquisition.
Introduction
Bacterial vaginosis (BV) is the most prevalent cause of vaginal discharge in reproductive age women,1 is present in ~29% of women in the United States,2 and is characterized by vaginal colonization with anaerobic bacterial species and a loss of normal lactobacilli. Moreover, BV is even more common in women who live in areas of the world where HIV-1 seroprevalence is highest, particularly sub-Saharan Africa.3 The clinical presentation of BV is characterized by an odorous discharge (or no symptoms at all), without the redness, swelling or pain typical of inflammation. However, at the mucosal level this condition has a significant pro-inflammatory impact that is associated with several poor clinical outcomes, including a nearly 2-fold increased risk of HIV-1 acquisition4,5 as well as a 3-fold increased risk of HIV-1 transmission to a male partner.6
There are several hypotheses for mechanisms that link genital mucosal inflammation and increased HIV-1 acquisition, including disruption of mucosal integrity, alteration of protective innate immunity, and increased numbers of HIV-1 target cells at the mucosal surface.7,8 The early events in HIV-1 acquisition in the female genital tract appear to include9 uptake of the virus by CD4+ T cells or Langerhans cells located in the stratified squamous epithelium of the vagina and/or ectocervix, which then transfer the virus to CD4+ T cells.10,11 It is also possible that HIV transmission occurs across the upper reproductive tract epithelia, which are single-layer columnar structures in the endocervix and endometrium, though this has been difficult to evaluate.
While the potential for BV to cause genital mucosal inflammation is not in dispute, many questions remain as to how and when this actually happens, and how the effects can be mitigated. The association between BV and HIV-1 acquisition may be mediated by many different factors (Figure 1). The field is in desperate need of well-designed, longitudinal studies to provide a better understanding of the mechanisms by which the vaginal microbial community regulates and alters the host reproductive mucosal immune response.
Figure 1.
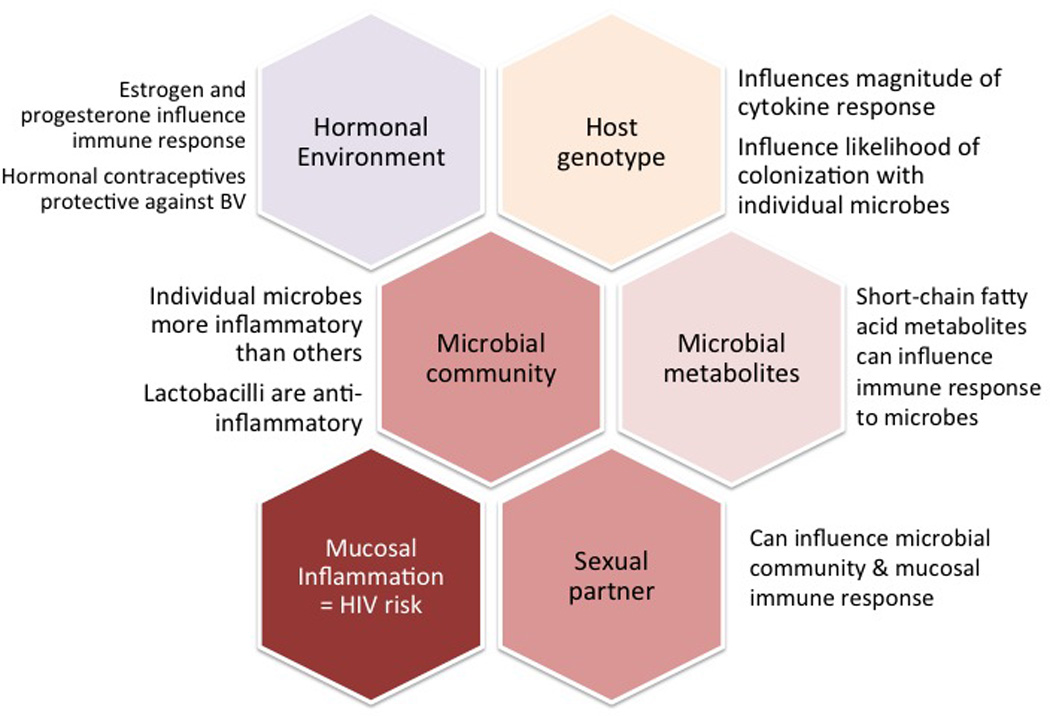
The microbial community is one component of a complex set of interactions that may influence a woman’s risk of HIV-1 acquisition.
Mucosal inflammatory markers associated with HIV-1 transmission
In the HPTN 035 Study, which evaluated two vaginal microbicides for efficacy in preventing HIV-1 acquisition, women who acquired HIV-1 were found – prior to HIV-1 seroconversion -- to have higher levels of human beta-defensin 2 (HBD2 – a cationic antimicrobial peptide) in vaginal secretions and more E. coli bactericidal activity of their vaginal fluid than non-seroconverters.12 Several studies of highly HIV-1 exposed but persistently seronegative sex workers have shown lower levels of inflammatory cytokines such as IL1α, IL1β, IL8 and RANTES in genital secretions compared to those in HIV-1 positive and HIV-1 negative controls.13,14 Analysis of cervical samples from a study of HIV acquisition in hormonal contraceptive users showed higher levels of RANTES and lower levels of SLPI in women who acquired HIV.15 In vitro, a TLR 1/2 agonist (PAM3CSK4) and TNFα increased HIV-1 transmission by Langerhans cells.16 Together, these data suggest that higher levels of vaginal inflammation, and lower levels of anti-inflammatory factors are associated with increased HIV-1 transmission across the genital mucosa.
What is a healthy vagina?
The definition of vaginal health includes both absence of symptoms and lack of risk for poor outcomes such as infertility, infection, pregnancy loss or preterm delivery. The vaginal microbiota in many women is dominated by certain Lactobacillus species, which have been associated with lower rates of these reproductive health complications.4,17 It is not certain whether the presence of these Lactobacillus species is simply a proxy for the absence of BV-associated bacterial species, or whether they themselves have immunomodulatory effects. Ravel et al performed deep sequencing on vaginal samples from reproductive age women and grouped the genital microbiota into four Lactobacillus-dominant “community state types” (CST) and one non-Lactobacillus-dominant CST.18 The presence of a diverse, heterogenous microbiota in ostensibly healthy women was used to argue that Lactobacillus-dominance is not necessarily universal and should not be used to define health. However, participants in this study were not screened for BV by either Amsel’s or Nugent’s criteria, and indeed, the majority of participants in the non-Lactobacillus-dominant CST had Nugent scores consistent with BV. As we try to understand how the presence of Lactobacillus species and the diagnosis of BV interact with the reproductive mucosal immune response to impact adverse outcomes such as HIV-1 acquisition, it is important to use consistent definitions of “health” and “disease.” A 2008 NIH-sponsored workshop on research on BV recommended consistently using Nugent score plus modified Amsel’s criteria to diagnose BV, to allow better standardization across studies.19
At the gastrointestinal mucosal surface, the presence of lactobacilli is associated with downregulation of immune response to inflammatory stimuli,20,21 but a similar process has not been defined in the genital tract. Because most of the adverse health outcomes associated with BV are presumably related to inflammatory complications of BV, understanding whether the presence of (or introduction of) Lactobacillus species could mitigate those complications is important. In a study of Swedish women without BV, the presence of L. iners by culture was negatively correlated with IL1β levels, but positively with the anti-inflammatory molecule secretory leukocyte protease inhibitor (SLPI), while L. gasseri was positively associated with IL1β.22 In a group of U.S. women, where pyrosequencing of the 16S rRNA gene was used to determine the dominant microbial species, those with vaginal microbiota dominated by L. crispatus had the lowest levels of IL1β and highest levels of SLPI.23 In pregnant Japanese women, those with Lactobacillus spp detected by culture had the lowest levels of IL8 – whether or not anaerobic species were also detected.24 In a cohort of 30 asymptomatic Belgian women, quantities of L. iners measured by qPCR were also associated with lower levels of IL8.25 This is in contrast to a study of primarily African American adolescents, where those who had Lactobacillus spp detected by culture (73% of the 89-person cohort) had no difference in cytokine concentrations compared to those that did not have Lactobacillus spp detected.26 The species of lactobacilli involved were not identified, making these results difficult to interpret. Further evaluation of the immunologic and reproductive health implications of variations in the microbial communities of women without BV would greatly advance the field.
BV is not the same microbiologic syndrome in all women
In recent years we have come to understand that BV is not the same in all women; the composition of the vaginal microbiota and the dominant microbial species vary from woman to woman.27 In fact, some species are more associated with one or the other of the clinical signs of BV, such as altered pH or clue cells.27 While investigators have long tried to identify a profile of more “severe” BV (such as a higher Nugent score or presence of Mobiluncus morphotypes)28,29 the molecular techniques available now allow for a broad characterization of the microbial community and potentially a better assessment of what types of communities have the most significant immunologic impact. Srinivasan et al recently used molecular techniques to demonstrate that the Mobiluncus morphotypes that make up part of the Nugent scoring criteria for vaginal fluid Gram stains are more often the Clostridia-like fastidious species Bacterial Vaginosis Associated Bacterium 1 (BVAB1) than Mobiluncus species.30
The vaginal microbiota in some women is a dynamic community that changes on a daily basis.31–34 Additional studies are starting to demonstrate differential expression of genes in species such as L. iners in women with and without BV,35 though it is not clear if this is due to strain differences or if some genes are upregulated due to change in the microbial environment. Finally, although it has long been known that short-chain fatty acids such as butyrate and succinate are present in BV,36 recently it has been shown that these molecules can have an impact on the mucosal immune response.37 These data make it clear that to understand BV and its interaction with the vaginal immune response, longitudinal samples and a holistic evaluation of the vaginal environment are necessary.
In vivo measurements of cytokines and antimicrobial peptides
Many authors have measured cervicovaginal cytokine levels in BV, with disparate results. In most studies IL1β is elevated in women with BV, while SLPI is decreased (Table 1). IL6 and IL8 have also been measured in multiple studies, with much more variable results. The heterogeneity in the measurements is likely due to several factors: variation in composition of the microbial community, small sample sizes and variable methods between studies. It is difficult to say whether studies showing no difference are simply underpowered. Most critically, the cross-sectional nature of many of these studies introduces considerable uncertainty as to how representative a single “snapshot” of the vaginal microbiota and associated immune milieu is at any given time. Moreover, many of these studies evaluated pregnant women, in whom vaginal cytokines are significantly elevated compared to non-pregnant women.38,39 Longitudinal studies where cytokines are measured before and after treatment of BV provide a more dynamic look at the inflammatory response, and in the 6 studies we reviewed, IL1β decreased after treatment in five, while IL8 decreased in three and increased in two.40–45
Table 1.
Summary of studies comparing concentrations of cytokines and anti-microbial peptides in genital secretions of women with and without BV, showing the number and type of studies reviewed and how many showed an increase, decrease or no difference in the analyte of interest.
| Increase | No difference | Decrease | |||||
|---|---|---|---|---|---|---|---|
| Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | ||
| IL1ba | CVL | 726,46,55,62–64 | 343,44, 67 | 141 | |||
| VS | 138 | 142 | |||||
| CS | 265,66 | 240,45 | |||||
| IL2 | CVL | 169 | |||||
| CS | 168 | 140 | 170 | ||||
| IL4 | CVL | 226,69 | |||||
| CS | 140 | 170 | |||||
| IL6 | CVL | 426,46,38,71 | 341,67,73 | ||||
| VS | 142 | ||||||
| CS | 168 | 145 | 270, 72 | 240 | |||
| IL8b | CVL | 173 | 341,43,67 | 426,46,63,64 | 144 | ||
| VS | 138 | 176 | 142 | ||||
| CS | 274,75 | 145 | 270,72 | 140 | |||
| IL10 | CVL | 177 | 226,71 | 162 | |||
| CS | 178 | 140 | 370,72,75 | ||||
| IL12c | CVL | 226,71 | |||||
| CS | 168 | 270,75 | |||||
| TNFad | CVL | 126 | |||||
| CS | 166 | 140 | 268,70 | ||||
| IFNg | CVL | 126 | 169 (trend) | ||||
| CS | 140 | 368,70,75 | |||||
| RANTES | CVL | 143 | |||||
| CS | 179 | 170 | |||||
| SLPI | CVL | 179 | |||||
| VS | 238,80 | 142 | |||||
| HNP1–3 | CVL | 144 | |||||
| VS | 1e 81 | 147 | 1e 81 | ||||
| CS | 182 | ||||||
| HBD2 | CVL | 169 | 144 | ||||
| VS | 147 | ||||||
| HBD3 | CVL | 144 | |||||
| VS | 147 | ||||||
Marconi et al46 evaluated the association of individual species with vaginal cytokines and found that higher quantities of G. vaginalis, A. vaginae and total 16S bacterial rRNA gene copies measured by qPCR were associated with higher IL1β levels, while levels of Megasphaera spp. were inversely associated with IL8 quantity. Although IL6 and IL8 levels were correlated with IL1β, they did not also correlate with bacterial quantities. In that study, quantities of lactobacilli were not measured. In a study of pregnant women, all nine BV-associated bacterial species measured by qPCR were associated with lower quantities of HBD3.47 The variation seen between these studies suggests that in addition to the broad diagnosis of BV, the specific composition of the vaginal microbial community may have a significant impact on mucosal inflammation and the risk of poor reproductive health outcomes
In vitro studies demonstrate higher pro-inflammatory potential in some BV-associated bacterial species compared to others
Progress in understanding the mucosal immune response to BV and BV-associated bacterial species has been slow in part due to the lack of an animal model to test mechanistic hypotheses. However, in vitro experiments using co-cultured genital epithelial cells and common vaginal bacteria species offer some insight into the simplest host-microbe interactions. In models using a monolayer culture of immortalized vaginal epithelial cells, co-culture with Atopobium vaginae or Gardnerella vaginalis induce significantly higher levels of IL6 and IL8 than Lactobacillus species.48,49 In another study that compared immortalized cell lines from endocervix, ectocervix and vagina, BV-associated bacterial species such as G. vaginalis, A. vaginae, Mobiluncis curtisii, and Prevotella bivia induced IL6, IL8, G-CSF, IP-10, MIP-1β, RANTES and Gro-α from all three cell types, while Lactobacillus species did not.50 These results confirm that BV-associated species can induce an innate immune response from the genital epithelium characterized by upregulation of cytokines associated with increased risk of HIV-1 transmission, while commensal lactobacilli do not. In addition, these in vitro studies show differences in stimulatory potential between individual species, which may account for some of the heterogeneity observed in the studies summarized in Table 1.
In the gastrointestinal tract, the presence of commensal Lactobacillus and Bifidobacteria species are associated with decreased immune response to inflammatory stimuli, in part through activation of Toll-like receptor pathways.51 In a multilayer culture of immortalized vaginal epithelial cells L. jensenii suppressed the epithelial cell response to both Toll-receptor agonists FSL-1 (TLR 1/2) and PIC (TLR3), while L. crispatus did not.52 In a separate model using 3D bead-based vaginal epithelial cell aggregates, L. crispatus induced minimal epithelial immune response, while P. bivia and L. iners upregulated PRR-signalling.53 When co-cultured with HeLa cells, L. crispatus diminishes the IL-8 response to challenge with Candida species.54 While these results suggest that Lactobacillus species are an anti-inflammatory influence on the genital epithelia, the more interesting question is how the presence of lactobacilli alters (or does not alter) the mucosal inflammatory response to BV-associated species, and whether individual Lactobacillus species have different effects.
Host genotype can influence the mucosal immune response to BV
In several studies, the reproductive health risks associated with BV are modified by the presence of genetic polymorphisms in genes associated with the inflammatory response. Genc et al showed that in women heterozygous for an allele of the IL1ra gene associated with less gene function no change in vaginal IL1β when anaerobic gram negative rods or G. vaginalis were present, but women homozygous for the wildtype allele showed increased levels.55 Women carrying a TLR4 polymorphism associated with lower response to LPS had no change in IL1β when colonized with BV-associated bacterial species, and 10-fold higher quantities of G. vaginalis colonization compared to women without the polymorphic allele.56 Goepfert et al showed that women with IL1β and IL8 gene polymorphisms associated with increased cytokine response had lower prevalence of BV, while women with an IL6 gene polymorphism associated with less response had a higher prevalence of BV.57 While these types of polymorphisms have been linked to differences in risk of preterm birth in women with BV,58 their impact on HIV-1 acquisition risk has not been evaluated.
Cellular immunity
In addition to increasing levels of pro-inflammatory cytokines like IL1β, BV has been associated with an increase in HIV-1 target cells in the genital mucosa. In a cohort of Kenyan sex workers, treatment of BV was associated with a decrease in numbers of CD4+, CD4+CCR5+ and CD4+CD69+ T cells in the cervix.43 In a cohort of Brazilian women, those with BV had fewer CD4+ T cells in cervicovaginal fluid than women with no vaginal infections.59 Conversely, in 30 healthy Belgian women, the presence of both L. crispatus and L. jensenii by qPCR was associated with lower numbers of cervical CD3+HLADR+ or CD3+CD4+CCR5+ cells.25 These results suggest that further evaluation of cellular immunity in BV will be important. Although BV is not associated with clinical inflammation in the lower genital tract, it has been associated with inflammatory clinical sequelae in the upper genital tract. Both cervicitis60 and pelvic inflammatory disease61 are linked to bacterial vaginosis, and could increase the risk of HIV-1 acquisition.
Conclusion
Studying BV and the mucosal immune response is challenging: there is no animal model, there are multiple factors that could alter the vaginal microbial and immune environments in a highly dynamic fashion over time, and previous literature has used a range of samples and assays, making generalization difficult. However, understanding how the reproductive mucosa interacts with and responds to the vaginal microbial community is an important component of developing strategies to prevent reproductive health complications, particularly HIV-1 acquisition.
Acknowledgements
Dr. Mitchell is supported by Career Development Awards from NIAID (1K08AI087969 - 01) and the Doris Duke Charitable Foundation.
References
- 1.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. American journal of obstetrics and gynecology. 1988 Apr;158(4):819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 2.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sexually transmitted diseases. 2007 Nov;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. American journal of obstetrics and gynecology. 2013 Dec;209(6):505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. The Journal of infectious diseases. 1999 Dec;180(6):1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 5.Taha TE, Gray RH, Kumwenda NI, et al. HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Jan 1;20(1):52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS medicine. 2012;9(6):e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirmonsef P, Krass L, Landay A, Spear GT. The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr HIV Res. 2012 Apr;10(3):202–210. doi: 10.2174/157016212800618165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011 Feb;65(2):89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 9.Carias AM, McCoombe S, McRaven M, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of virology. 2013 Nov;87(21):11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007 Feb;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushic C, Ferreira VH, Kafka JK, Nazli A. HIV infection in the female genital tract: discrete influence of the local mucosal microenvironment. Am J Reprod Immunol. 2010 Jun;63(6):566–575. doi: 10.1111/j.1600-0897.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 12.Dezzutti CS, Richardson BA, Marrazzo JM, et al. Mucosal Escherichia coli bactericidal activity and immune mediators are associated with HIV-1 seroconversion in women participating in the HPTN 035 trial. The Journal of infectious diseases. 2012 Dec 15;206(12):1931–1935. doi: 10.1093/infdis/jis555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajoie J, Juno J, Burgener A, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012 May;5(3):277–287. doi: 10.1038/mi.2012.7. [DOI] [PubMed] [Google Scholar]
- 14.Yao XD, Omange RW, Henrick BM, et al. Acting locally: innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol. 2013 Jun 26; doi: 10.1038/mi.2013.44. [DOI] [PubMed] [Google Scholar]
- 15.Morrison C, Fichorova R, Mauck C, et al. Cervical Inflammation and Immunity Associated with Hormonal Contraception, Pregnancy and HIV-1 Seroconversion. Journal of acquired immune deficiency syndromes (1999) 2014 Jan 9; doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 16.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB. TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. The Journal of clinical investigation. 2008 Oct;118(10):3440–3452. doi: 10.1172/JCI34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martius J, Krohn MA, Hillier SL, Stamm WE, Holmes KK, Eschenbach DA. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstetrics and gynecology. 1988 Jan;71(1):89–95. [PubMed] [Google Scholar]
- 18.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2010 Mar 15;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sexually transmitted diseases. 2010 Dec;37(12):732–744. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton KA, Honkala A, Auchtung TA, Britton RA. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infection and immunity. 2011 Jan;79(1):185–191. doi: 10.1128/IAI.00880-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo NA, de Moreno de LeBlanc A, C MG, Perdigon G. Comparative study of the protective capacity against Salmonella infection between probiotic and nonprobiotic Lactobacilli. J Appl Microbiol. 2013 Mar;114(3):861–876. doi: 10.1111/jam.12074. [DOI] [PubMed] [Google Scholar]
- 22.Nikolaitchouk N, Andersch B, Falsen E, Strombeck L, Mattsby-Baltzer I. The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH) Apmis. 2008 Apr;116(4):263–277. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 23.Orfanelli T, Jayaram A, Doulaveris G, Forney LJ, Ledger WJ, Witkin SS. Human Epididymis Protein 4 and Secretory Leukocyte Protease Inhibitor in Vaginal Fluid: Relation to Vaginal Components and Bacterial Composition. Reprod Sci. 2013 Sep 10; doi: 10.1177/1933719113503416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai M, Ishiyama A, Tabata M, et al. Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am J Reprod Immunol. 2004 Aug;52(2):106–112. doi: 10.1111/j.1600-0897.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 25.Kyongo JK, Jespers V, Goovaerts O, et al. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One. 2012;7(8):e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Olmos MI, Barousse MM, Rajan L, et al. Vaginal lactobacilli in adolescents: presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sexually transmitted diseases. 2004 Jul;31(7):393–400. doi: 10.1097/01.olq.0000130454.83883.e9. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culhane JF, Nyirjesy P, McCollum K, Goldenberg RL, Gelber SE, Cauci S. Variation in vaginal immune parameters and microbial hydrolytic enzymes in bacterial vaginosis positive pregnant women with and without Mobiluncus species. American journal of obstetrics and gynecology. 2006 Aug;195(2):516–521. doi: 10.1016/j.ajog.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Allsworth JE, Peipert JF. Severity of bacterial vaginosis and the risk of sexually transmitted infection. American journal of obstetrics and gynecology. 2011 Aug;205(2):113 e111–116 e111. doi: 10.1016/j.ajog.2011.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S, Morgan MT, Liu C, et al. More than meets the eye: associations of vaginal bacteria with gram stain morphotypes using molecular phylogenetic analysis. PLoS One. 2013;8(10):e78633. doi: 10.1371/journal.pone.0078633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert JA, John S, Sobel JD, Akins RA. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: recognition of the conversion process. PLoS One. 2013;8(12):e82599. doi: 10.1371/journal.pone.0082599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravel J, Brotman RM, Gajer P, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1(1):29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwebke JR, Morgan SC, Weiss HL. The use of sequential self-obtained vaginal smears for detecting changes in the vaginal flora. Sexually transmitted diseases. 1997 Apr;24(4):236–239. doi: 10.1097/00007435-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome. 2013;1(1):12. doi: 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bump RC, Zuspan FP, Buesching WJ, 3rd, Ayers LW, Stephens TJ. The prevalence, six-month persistence, and predictive values of laboratory indicators of bacterial vaginosis (nonspecific vaginitis) in asymptomatic women. American journal of obstetrics and gynecology. 1984 Dec 15;150(8):917–924. doi: 10.1016/0002-9378(84)90381-8. [DOI] [PubMed] [Google Scholar]
- 37.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol. 2012 May;67(5):391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balkus J, Agnew K, Lawler R, Mitchell C, Hitti J. Effects of pregnancy and bacterial vaginosis on proinflammatory cytokine and secretory leukocyte protease inhibitor concentrations in vaginal secretions. Journal of pregnancy. 2010;2010:385981. doi: 10.1155/2010/385981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beigi RH, Yudin MH, Cosentino L, Meyn LA, Hillier SL. Cytokines, pregnancy, and bacterial vaginosis: comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. The Journal of infectious diseases. 2007 Nov 1;196(9):1355–1360. doi: 10.1086/521628. [DOI] [PubMed] [Google Scholar]
- 40.Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sexually transmitted infections. 2008 Feb;84(1):57–61. doi: 10.1136/sti.2007.026625. [DOI] [PubMed] [Google Scholar]
- 41.Losikoff P, Fichorova R, Snyder B, et al. Genital tract interleukin-8 but not interleukin-1beta or interleukin-6 concentration is associated with bacterial vaginosis and its clearance in HIV-infected and HIV-uninfected women. Infectious diseases in obstetrics and gynecology. 2007;2007:92307. doi: 10.1155/2007/92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell C, Balkus J, Agnew K, Lawler R, Hitti J. Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. Journal of women's health (2002) 2009 Nov;18(11):1817–1824. doi: 10.1089/jwh.2009.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebbapragada A, Howe K, Wachihi C, et al. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. Journal of acquired immune deficiency syndromes (1999) 2008 Dec 15;49(5):520–522. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 44.Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infection and immunity. 2006 Oct;74(10):5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yudin MH, Landers DV, Meyn L, Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstetrics and gynecology. 2003 Sep;102(3):527–534. doi: 10.1016/s0029-7844(03)00566-0. [DOI] [PubMed] [Google Scholar]
- 46.Marconi C, Donders GG, Parada CM, Giraldo PC, da Silva MG. Do Atopobium vaginae, Megasphaera sp. and Leptotrichia sp. change the local innate immune response and sialidase activity in bacterial vaginosis? Sexually transmitted infections. 2013 Mar;89(2):167–173. doi: 10.1136/sextrans-2012-050616. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell C, Gottsch ML, Liu C, Fredricks DN, Nelson DB. Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. American journal of obstetrics and gynecology. 2013 Feb;208(2):132 e131–137 e131. doi: 10.1016/j.ajog.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes and infection / Institut Pasteur. 2008 Apr;10(4):439–446. doi: 10.1016/j.micinf.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Fichorova RN, Buck OR, Yamamoto HS, et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sexually transmitted infections. 2013 Sep;89(6):460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eade CR, Diaz C, Wood MP, et al. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One. 2012;7(11):e50106. doi: 10.1371/journal.pone.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004 Jul 23;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Rose WA, 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One. 2012;7(3):e32728. doi: 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Vaginal microbiota alter the innate immune and barrier properties of the human vaginal epithelia in a species-specific manner. The Journal of infectious diseases. 2014 Jan 7; doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 54.Rizzo A, Losacco A, Carratelli CR. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human beta-defensins 2 and 3. Immunology letters. 2013 Nov-Dec;156(1–2):102–109. doi: 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Genc MR, Onderdonk AB, Vardhana S, et al. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. American journal of obstetrics and gynecology. 2004 Oct;191(4):1324–1330. doi: 10.1016/j.ajog.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 56.Genc MR, Vardhana S, Delaney ML, et al. Relationship between a toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. European journal of obstetrics, gynecology, and reproductive biology. 2004 Oct 15;116(2):152–156. doi: 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Goepfert AR, Varner M, Ward K, et al. Differences in inflammatory cytokine and Toll-like receptor genes and bacterial vaginosis in pregnancy. American journal of obstetrics and gynecology. 2005 Oct;193(4):1478–1485. doi: 10.1016/j.ajog.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 58.Gomez LM, Sammel MD, Appleby DH, et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. American journal of obstetrics and gynecology. 2010 Apr;202(4):386 e381–386 e381. doi: 10.1016/j.ajog.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 59.Giraldo PC, de Carvalho JB, do Amaral RL, da Silveira Goncalves AK, Eleuterio J, Jr, Guimaraes F. Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am J Reprod Immunol. 2012 Mar;67(3):198–205. doi: 10.1111/j.1600-0897.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 60.Marrazzo JM, Wiesenfeld HC, Murray PJ, et al. Risk factors for cervicitis among women with bacterial vaginosis. The Journal of infectious diseases. 2006 Mar 1;193(5):617–624. doi: 10.1086/500149. [DOI] [PubMed] [Google Scholar]
- 61.Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sexually transmitted diseases. 2013 Feb;40(2):117–122. doi: 10.1097/OLQ.0b013e31827c5a5b. [DOI] [PubMed] [Google Scholar]
- 62.Anton G, Rid J, Mylonas I, Friese K, Weissenbacher ER. Evidence of a TH1-shift of local vaginal inflammatory response during bacterial vaginosis. Infection. 2008 Mar;36(2):147–152. doi: 10.1007/s15010-007-7152-2. [DOI] [PubMed] [Google Scholar]
- 63.Cauci S, Guaschino S, De Aloysio D, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Molecular human reproduction. 2003 Jan;9(1):53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 64.Cauci S, Guaschino S, Driussi S, De Santo D, Lanzafame P, Quadrifoglio F. Correlation of local interleukin-8 with immunoglobulin A against Gardnerella vaginalis hemolysin and with prolidase and sialidase levels in women with bacterial vaginosis. The Journal of infectious diseases. 2002 Jun 1;185(11):1614–1620. doi: 10.1086/340417. [DOI] [PubMed] [Google Scholar]
- 65.Mattsby-Baltzer I, Platz-Christensen JJ, Hosseini N, Rosen P. IL-1beta, IL-6, TNFalpha, fetal fibronectin, and endotoxin in the lower genital tract of pregnant women with bacterial vaginosis. Acta obstetricia et gynecologica Scandinavica. 1998 Aug;77(7):701–706. [PubMed] [Google Scholar]
- 66.Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ. High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. The Journal of infectious diseases. 2000 Aug;182(2):467–473. doi: 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- 67.Basso B, Gimenez F, Lopez C. IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infectious diseases in obstetrics and gynecology. 2005 Dec;13(4):207–211. doi: 10.1080/10647440500240664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campos AC, Murta EF, Michelin MA, Reis C. Evaluation of Cytokines in Endocervical Secretion and Vaginal pH from Women with Bacterial Vaginosis or Human Papillomavirus. ISRN Obstet Gynecol. 2012;2012:342075. doi: 10.5402/2012/342075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan SR, Liu XP, Liao QP. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2008 Oct;103(1):50–54. doi: 10.1016/j.ijgo.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 70.Ryckman KK, Williams SM, Krohn MA, Simhan HN. Racial differences in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. Journal of reproductive immunology. 2008 Jul;78(2):166–171. doi: 10.1016/j.jri.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weissenbacher T, Walter C, Mylonas I, Scholz C, Gingelmaier A, Friese K. Interleukin-6, interleukin-10 and interleukin-12 in vaginal fluid from women with bacterial vaginosis. Archives of gynecology and obstetrics. 2009 Apr 14; doi: 10.1007/s00404-009-1072-6. [DOI] [PubMed] [Google Scholar]
- 72.Tavares-Murta BM, de Resende AD, Cunha FQ, Murta EF. Local profile of cytokines and nitric oxide in patients with bacterial vaginosis and cervical intraepithelial neoplasia. European journal of obstetrics, gynecology, and reproductive biology. 2008 May;138(1):93–99. doi: 10.1016/j.ejogrb.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 73.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. The Journal of infectious diseases. 2006 Feb 15;193(4):556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 74.Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. The Journal of reproductive medicine. 2001 Sep;46(9):806–810. [PubMed] [Google Scholar]
- 75.Nenadic DB, Pavlovic MD. Cervical fluid cytokines in pregnant women: Relation to vaginal wet mount findings and polymorphonuclear leukocyte counts. European journal of obstetrics, gynecology, and reproductive biology. 2008 Oct;140(2):165–170. doi: 10.1016/j.ejogrb.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 76.Diaz-Cueto L, Cuica-Flores A, Ziga-Cordero F, Arechavaleta-Velasco ME, Arechavaleta-Velasco F. Genetic variation in the interleukin-8 gene promoter and vaginal concentrations of interleukin-8 are not associated with bacterial vaginosis during pregnancy. Journal of reproductive immunology. 2005 Aug;66(2):151–160. doi: 10.1016/j.jri.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, Krishna TP. Local immunity in Indian women with bacterial vaginosis. Journal of reproductive immunology. 2006 Jun;70(1–2):133–141. doi: 10.1016/j.jri.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS (London, England) 1999 Feb 25;13(3):327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 79.Novak RM, Donoval BA, Graham PJ, et al. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007 Sep;14(9):1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. American journal of obstetrics and gynecology. 2000 Nov;183(5):1243–1248. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 81.Xu J, Holzman CB, Arvidson CG, Chung H, Goepfert AR. Midpregnancy vaginal fluid defensins, bacterial vaginosis, and risk of preterm delivery. Obstetrics and gynecology. 2008 Sep;112(3):524–531. doi: 10.1097/AOG.0b013e318184209b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sawada M, Otsuki K, Mitsukawa K, Yakuwa K, Nagatsuka M, Okai T. Cervical inflammatory cytokines and other markers in the cervical mucus of pregnant women with lower genital tract infection. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006 Feb;92(2):117–121. doi: 10.1016/j.ijgo.2005.10.004. [DOI] [PubMed] [Google Scholar]


