Abstract
Background and Purpose
Nuclear Overhauser Enhancement (NOE) mediated chemical exchange saturation transfer (CEST) is a novel magnetic resonance imaging (MRI) technique on the basis of saturation transfer between exchanging protons of tissue proteins and bulk water. The purpose of this study was to evaluate and compare the information provided by three dimensional NOE mediated CEST at 7 Tesla (7T) and standard MRI in glioblastoma patients.
Patients and Methods
Twelve patients with newly diagnosed histologically proven glioblastoma were enrolled in this prospective ethics committee–approved study. NOE mediated CEST contrast was acquired with a modified three-dimensional gradient-echo sequence and asymmetry analysis was conducted at 3.3ppm (B1 = 0.7 µT) to calculate the magnetization transfer ratio asymmetry (MTRasym). Contrast enhanced T1 (CE-T1) and T2-weighted images were acquired at 3T and used for data co-registration and comparison.
Results
Mean NOE mediated CEST signal based on MTRasym values over all patients was significantly increased (p<0.001) in CE-T1 tumor (−1.99±1.22%), tumor necrosis (−1.36±1.30%) and peritumoral CEST hyperintensities (PTCH) within T2 edema margins (−3.56±1.24%) compared to contralateral normal appearing white matter (−8.38±1.19%). In CE-T1 tumor (p = 0.015) and tumor necrosis (p<0.001) mean MTRasym values were significantly higher than in PTCH. Extent of the surrounding tumor hyperintensity was smaller in eight out of 12 patients on CEST than on T2-weighted images, while four displayed at equal size. In all patients, isolated high intensity regions (0.40±2.21%) displayed on CEST within the CE-T1 tumor that were not discernible on CE-T1 or T2-weighted images.
Conclusion
NOE mediated CEST Imaging at 7T provides additional information on the structure of peritumoral hyperintensities in glioblastoma and displays isolated high intensity regions within the CE-T1 tumor that cannot be acquired on CE-T1 or T2-weighted images. Further research is needed to determine the origin of NOE mediated CEST and possible clinical applications such as therapy assessment or biopsy planning.
Introduction
Magnetic resonance imaging (MRI) has become the gold standard for the assessment of intracerebral lesions and is thus the primary tool for diagnosis and follow up examination of glioblastoma [1]. Within clinical routine, diagnosis of glioblastoma is usually based on T1-weighted gadolinium contrast enhanced MRI (CE-T1) and T2-weighted images. The limitation of this approach is that CE-T1 images exclusively visualize the disruption of the blood brain barrier and hence lack identification of non-enhancing tumor portions [2], [3]. Furthermore, T2-weighted images cannot distinguish between infiltrative tumor growth and other possible causes of non-specific T2-signal increases [4]. Therefore, alternative sequences for the determination of the most malignant tumor parts and the tumor extent are highly desirable.
Chemical Exchange Saturation Transfer (CEST) imaging is a non-invasive MRI technique sensitive to endogenous mobile proteins and peptides respectively and their tissue specific concentration [5], [6]. Multiple metabolites (e.g. glutamate, creatine, myo-inositol, proteins) possess exchangeable protons and thus become endogenous agents with distinct chemical shifts making CEST a technology with the potential for frequency selective molecular imaging [5]. Signal contrast related to mobile proteins results from saturation of their exchanging protons by selective radiofrequency irradiation. Protons in the saturated state transfer to free bulk water yielding a reduction of local z-magnetization of water protons. This leads to a successive signal reduction in the water pool allowing indirect MR imaging of mobile proteins.
Biomedical applications have for example been demonstrated for the detection and grading of tumors [7], [8], [9], [10], the differentiation between tumor progress and radiation necrosis [11] and acute stroke imaging [12].
At low saturation power (e.g. 0.6–0.8 µT) CEST studies revealed that saturation transfer at −2 to −5 ppm is predominantly mediated by Nuclear Overhauser Enhancement (NOE) effects [13], [14], [15]. NOE mediated CEST effects are attributed to aliphatic and olefinic protons in mobile proteins [14]. Initial examinations of human brain tumors at 7 Tesla (7T) showed for one patient with astrocytoma WHO Grade III [14] and one patient with glioblastoma [15] that NOE mediated CEST effects significantly drop in tumor tissue.
In the current study, we investigated if NOE-weighted CEST-MRI with high 3D spatial resolution at 7T and precise sequence co-registration provides additional information about glioblastoma imaging, specifically the visualization of surrounding tumor hyperintensities and isolated CEST high intensity regions (HIR) that do not display on CE-T1 or T2-weighted images.
Patients and Methods
Patients
Twelve patients (3 female, 9 male; age: 62.58±12.67 years) with newly diagnosed and subsequently histopathologically confirmed glioblastoma were included in this prospective study. The study was approved by the Medical Ethics Committee (Faculty of Clinical Medicine, University of Heidelberg, Germany) and written informed consent was received from all participants before enrollment.
Conventional MRI at 3T
CE-T1 weighted (TE = 4.04 ms, TR = 1710 ms, FoV 256×256, resolution 512×512, slice thickness 1 mm) and T2-weighted (TE = 89 ms, TR = 5140 ms, FoV 172×229, resolution 384×230, slice thickness 4 mm) images were acquired on a 3T whole body MR imaging system (Magnetom Verio/Trio TIM; Siemens Healthcare, Erlangen, Germany).
CEST-MRI at 7T
The CEST sequence was performed on a 7T whole body MRI scanner (Magnetom 7T; Siemens Healthcare, Erlangen, Germany) with a time delay of 1–5 days in relation to 3T MRI. CEST imaging was performed with a centric-reordered three-dimensional gradient echo sequence [16] with the following parameters: Base resolution 128, FoV phase = 78.125%, 26 slices, resolution = [1.8 mm×1.8 mm]×2 mm, TR = 12 ms, TE = 2.88 ms, BW = 320 HZ/px, FA = 10°, GRAPPA acceleration factor 3. Before each segment of the 3D stack a saturation pulse train was applied. The pulse train consisted of 5 gaussian pulses with a duration of 100 ms per pulse and a pulse-train-average amplitude of B1 = 0.7 µT. Due to SAR limitations, the interpulse delay was 100 ms leading to an effective saturation time of 900 ms. For each pixel the reduced water magnetization M was normalized by the unsaturated magnetization M0 yielding Z = M/M0. Z plotted as a function of the irradiation frequency offset Δω relative to the water resonance formed the Z-spectrum. Thirteen equidistant frequency offsets between −4 and +4 ppm and the additional M0 image were acquired, resulting in an acquisition time of 9 min 30 s. Due to B0 inhomogeneities, irradiation frequency offsets are distorted, but can be corrected by post-processing [16], [17], [18] using a B0 map. Similar to Stancanello et al. [18] we obtained a B0 map by determining the minima of the Z-spectra for each pixel employing a cubic spline interpolation. Consequently, every Z-spectrum was interpolated and shifted to assure that the minimum of the Z-spectrum, and thus the water resonance, is at 0 ppm. CEST signal intensity was defined by the magnetization transfer ratio asymmetry (MTRasym) at 3.3 ppm and B1 = 0.7 µT, which was calculated pixel-wise by MTRasym(3.3 ppm, B1 = 0.7 µT) = Z(Δω = −3.3 ppm, B1 = 0.7 µT) - Z(Δω = +3.3 ppm, B1 = 0.7 µT). The resulting CEST contrast defined by MTRasym thus shows hyperintensities where NOE mediated CEST effects are decreased and vice versa for hypointensities. The CEST contrast was windowed between MTRasym from −10% to 5%. Images of 3T sequences (CE-T1 and T2) were co-registered on CEST MTRasym using a SIEMENS Syngo Fusion Station.
Qualitative Analysis
Analyses of 3D-coregistered CE-T1, T2-weighted and CEST images were performed by two neuroradiologists (AR and PK). Discrepancies were resolved by consensus reading. CE-T1 tumor and tumor necrosis were identified on CE-T1 and peritumoral edema on T2-weighted images. Size and structure of the edema on T2-weighted images were visually compared to corresponding peritumoral hyperintensities on co-registered CEST images in three dimensions. The appearance of isolated CEST HIR on MTRasym within the area of CE-T1 tumor and within the area of peritumoral edema according to its extent on T2-weighted images were evaluated. Illustration of CEST HIR was performed in the same window (−10% to +5%) but with different color gradients for improved visualization. Finally, the appearance of tumor satellite lesions (defined as contrast enhanced lesions on CE-T1, diameter <1 cm, without connection to the main tumor) was investigated on CE-T1, T2-weighted and on corresponding CEST images.
Spectral analysis
Six regions of interest (ROI) were selected for each patient on co-registered data in a representative slice for quantitative MTRasym signal analysis. The selection of the ROIs was performed based on best visibility of the several tissues on the following sequences: 1) CE-T1 tumor and 2) tumor necrosis were selected on CE-T1 images. 3) Isolated CEST HIR within the CE-T1 tumor and 4) peritumoral CEST hyperintensites (PTCH) within T2 edema margins were directly selected on MTRasym. 5) Cerebrospinal fluid (CSF) and 6) contralateral normal appearing white matter (CLNAWM) were selected on T2-weighted images. For each ROI the average MTRasym was determined. Furthermore, the contribution of up and downfield effects on MTRasym within Z-spectra were visually compared for each ROI.
Statistical Analysis
The data from ROI analysis was used for statistical evaluation. A repeated measures analysis of variance (rm ANOVA) for all regions and patients and post hoc Holm-Sidac pairwise multiple comparisons were performed with SigmaPlot version 12.5 (Systat Software, Inc., San Jose California USA). The level of significance was set at P<0.05.
Results
CEST effects given by MTRasym were observed in a minimum-to-maximum range from −25% to +12% resulting from the asymmetry analysis based on the measured Z-spectra. 98.51% of all intracranial values were in the range from −12% to +5%. All tumors could be identified on MTRasym as hyperintense lesions since NOE mediated CEST effects decreased in all glioblastoma tumors. Highest MTRasym intensity values appear in CSF and in isolated CEST HIR of the CE-T1 tumor, both showing MTRAsym values of approximately 0. For CSF this is because no saturation transfer is apparent neither at +3.3 ppm nor at −3.3 ppm. Within the isolated CEST HIR of the CE-T1 tumor, MTRasym = 0 reflects that NOE signals (−3.3 ppm) are of equal size as saturation transfer effects at the opposite side of the Z-spectrum (+3.3 ppm).
Qualtitative analysis
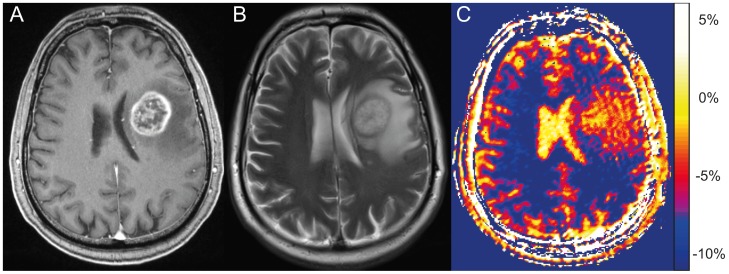
In eight out of 12 patients, the peritumoral hyperintensity on CEST was smaller than on T2-weighted images. In four patients, CEST displayed congruent areas. In two of the eight patients with smaller peritumoral hyperintensity, the CEST hyperintensity moderately exceeded the T2 edema in one direction. In comparison to the edema on T2-weighted sequences, peritumoral hyperintensities on CEST displayed an irregular border and subareas of different signal intensity. Furthermore, stria like structures could be identified on CEST images within peritumoral hyperintensities (Fig. 1).
Figure 1. Peritumoral hyperintensity on NOE mediated CEST compared to standard MRI.
Left frontal glioblastoma in a 59 year old man at 3 Tesla, CE-T1 (A) and T2-weighted images (B). On the selected slice the CEST contrast at 7 Tesla, based on MTRasym (C), displays peritumoral hyperintensities at equal extent compared to the edema on T2-weighted images. In contrast to T2-weighted images, the CEST peritumoral hyperintensity displays an irregular border and subareas of different signal intensity.
Tumor necrosis on CEST appeared predominantly hyperintense compared to average signal in peritumoral hyperintensities. Within the corresponding area of CE-T1 tumor, CEST images revealed heterogeneous signal intensities. The MTRasym signal intensity in the CE-T1 tumor varied from isointense to peritumoral hyperintensities to isolated CEST HIR.
Isolated CEST HIR on MTRasym within the CE-T1 tumor could be observed in all 12 patients. In eight patients isolated CEST HIR could be additionally identified within the edema according to its extent on T2-weighted images.
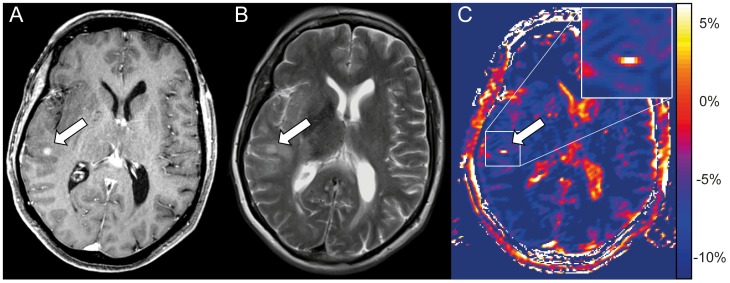
A total of eight tumor satellites were identified in the patient collective on CE-T1. Four of these eight satellites were clearly hyperintense both on T2-weighted images and on CEST, while three of the eight CE-T1 satellites barely displayed on T2-weighted images and were also clearly visible on CEST images (Fig. 2). One tumor satellite was not hyperintese on CEST and barely hyperintense on T2-weighted images.
Figure 2. Tumor satellite lesion displays hyperintense on NOE mediated CEST.
Tumor satellite of a glioblastoma subcortical temporal right in a 67 year old woman. The satellite presents a clear enhancement on CE-T1 (arrow in A) and barely displays on the T2-weighted image (arrow in B). In contrast, the satellite displays clearly hyperintense on CEST based on MTRasym (C) and matches with the area of contrast enhancement on the CE-T1 image (A). Furthermore also CSF in lateral ventricles and cerebral sulci displays hyperintense on MTRasym.
Table 1 summarizes the observations obtained from qualitative data analyses.
Table 1. Qualitative analyses of NOE-mediated CEST contrast on 3D co-registered data.
| Patient No. | Size of peritumoral hyperintensity: | Appearance of isolated high intensity regions (HIR) on MTRasym in the area of: | Satellite lesions: | |
| CEST versus T2-w. | CE–T1 tumor | T2 peritumoral edema | CEST hyperintense/Total on CE-T1 | |
| #1 | smaller | Y | Y | ∅ |
| #2 | equal | Y | N | 1/1 |
| #3 | smaller | Y | N | ∅ |
| #4 | equal | Y | Y | 2/2 |
| #5 | smaller | Y | Y | 1/1 |
| #6 | smaller | Y | Y | ∅ |
| #7 | smaller | Y | N | ∅ |
| #8 | smaller | Y | Y | ∅ |
| #9 | smaller* | Y | N | ∅ |
| #10 | equal | Y | Y | ∅ |
| #11 | smaller* | Y | Y | 2/3 |
| #12 | equal | Y | Y | 1/1 |
Peritumoral hyperintensity: Comparison of the extent of the peritumoral hyperintensity on CEST and T2-weighted images (smaller* = total extent smaller on CEST contrast but exceeding the margins of the T2 edema in one direction). Appearance of isolated high intensity regions (HIR) on MTRasym: Evaluation if isolated CEST HIR on MTRasym displayed in the area of CE-T1 tumor or T2 peritumoral edema (Y = Yes, N = No). Satellite lesions: Fraction of contrast enhanced satellite lesions identified on CE-T1 images that were also clearly hyperintense on CEST (∅ = no satellite lesion detected).
Spectral analysis
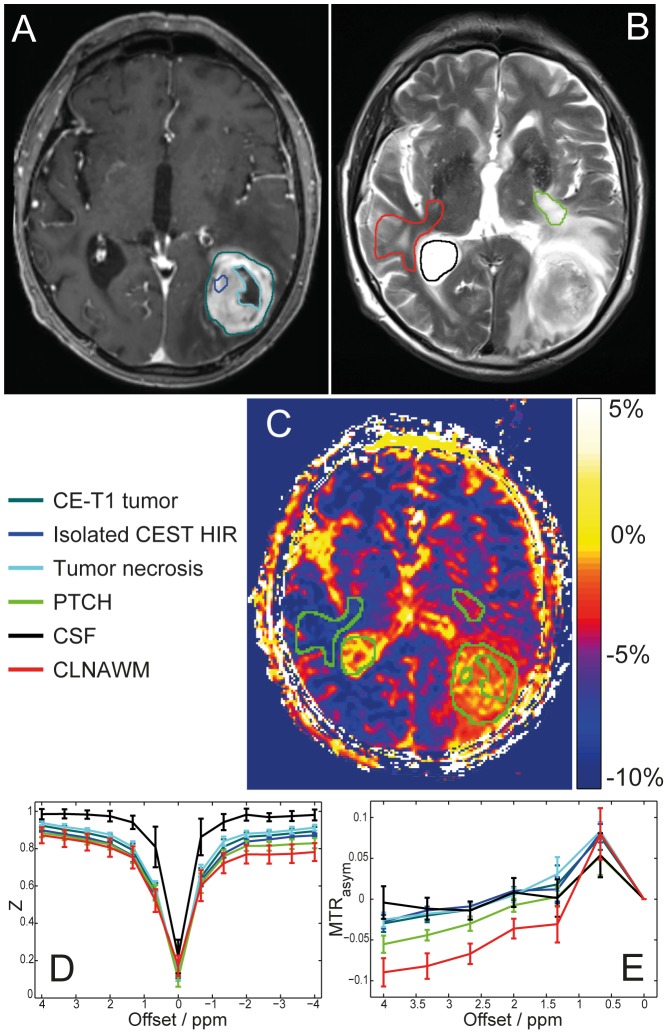
Mean MTRasym in CE-T1 tumor was −1.99±1.22% and −1.36±1.30% in tumor necrosis. For isolated CEST HIR on MTRasym within CE-T1 tumor the mean signal strength was 0.40±2.21% and −3.56±1.24% in PTCH within T2 edema margins. In CSF average MTRasym value was 0.76±1.29% and −8.38±1.19% in CLNAWM (Fig. 3). In the tumor and peritumoral tissue of all patients (CE-T1 tumor, tumor necrosis, PTCH within T2 edema margins and isolated CEST HIR within CE-T1 tumor), a clear decrease of the Z-values around -3.3 ppm was observed compared to CLNAWM. At +3.3 ppm, no clear change in saturation transfer effect could be identified in any tissue and in any patient. A representative Z-spectrum analysis is given in Fig. 3D.
Figure 3. Regions of interest (ROI) selection for spectral analysis and MTRasym quantification.
Left occipital glioblastoma of a 79 year old patient, CE-T1 (A) and T2-weighted images (B) with color coded ROIs: CE-T1 tumor, isolated CEST HIR within CE-T1 margins, tumor necrosis, PTCH within T2 edema margins, CSF and CLNAWM. CEST contrast based on MTRasym (C): Same ROIs illustrated in green for improved visualization. Z-spectrum (D) and asymmetry analysis (E) shown. Analyses of Z-spectra reveals that a decrease of NOE upfield effects at −3.3 ppm causes the hyperintense MTRasym contrast in the tumor regions, while no clear APT peak around +3.3 ppm could be identified in any of the analyzed tissues. Even though MTRasym shows high intensities both in CSF and isolated CEST HIR within CE-T1 tumor, Z-spectrum analysis reveals that the underlying asymmetry has a different origin: no saturation transfer is apparent in CSF at ±3.3 ppm (D black line) while in tumor regions (D dark green, dark blue and light blue lines) MTRasym = 0 reflects that NOE signals (−3.3 ppm) and saturation transfer effects at the opposite side of the Z-spectrum (+3.3 ppm) are of equal size. Furthermore the width of the Z-spectrum of CSF is decreased due to the longer T2 relaxation time.
Statistical Analysis
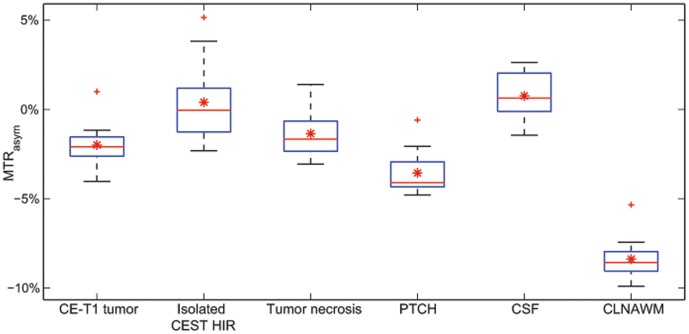
The analysis of variance (ANOVA) with repeated measures was p<0.001 for statistically significant differences among the six groups by ROI analysis. Post hoc Holm-Sidac pairwise multiple comparisons showed that average MTRasym of CE-T1 tumor, isolated CEST HIR within the CE-T1 tumor, tumor necrosis, PTCH within T2 edema margins and CSF were all significantly higher than MTRasym of CLNAWM (p<0.001). Mean MTRasym in PTCH within T2 edema margins was significantly increased (p<0.001) compared to CLNAWM and significantly lower than in CE-T1 tumor (p = 0.015) and tumor necrosis (p<0.001). Average MTRasym in isolated CEST HIR within CE-T1 tumor was significantly higher than in the whole CE-T1 tumor (p<0.001) and PTCH within T2 edema margins (p<0.001). In tumor necrosis, MTRasym was significantly lower than in isolated CEST HIR within CE-T1 tumor (p = 0.007) and CSF (p = 0.001) but not significantly different compared to CE-T1 tumor (p = 0.42) (Fig. 4).
Figure 4. Boxplots of MTRAsym quantification from regions of interest (ROI) analysis over all glioblastoma patients.
Boxplots of mean MTRAsym values on CEST contrast over all patients (N = 12). Overall mean MTRasym (red stars) and outliers (red crosses) are additionally illustrated. MTRasym values in all tumor areas (CE-T1 tumor, isolated CEST HIR in CE-T1 tumor, tumor necrosis) and CSF are significantly higher than in CLNAWM (p<0.001). Average signal intensity in PTCH within T2 edema margins is significantly higher (p<0.001) than in CLNAWM and significantly lower (p = 0.015) than in CE-T1 tumor and tumor necrosis (p<0.001). The whiskers of the boxplot for isolated CEST HIR indicate a high variance within this group, which is due to smaller ROI size and the fact that the isolated CEST HIR were visually selected relative to surrounding signal intensity in CE-T1 tumor.
Discussion
We demonstrated that a contrast in glioblastoma can be obtained by NOE mediated CEST imaging at 7T in terms of structure and extent of peritumoral hyperintensities and isolated CEST HIR that cannot be acquired with conventional CE-T1 and T2-weighted images.
As a principle finding of this study, we proofed, that the NOE-effects in glioblastoma CE-T1 tumors, as well as in the tumor necrosis and the surrounding PTCH within T2 edema margins are decreased in comparison to CLNAWM. This is in agreement with previously published studies [14], [15]. However, the cause of the NOE drop within tumor tissue is still under discussion. Due to the exchange relayed mechanism of NOE, changes in pH may result in altered NOE contrast [22]. Nevertheless, intracellular changes in pH are supposed to be small in gliomas and only a subtle pH increase (up to approximately 0.1 pH) was reported [23], [24]. Since the increase of NOE was shown to be smaller than 0.7% per pH [14], [25] we assume that pH changes are not the dominant origin of the observed effect.
A lowered protein concentration might explain the NOE drop since water content in glioblastoma is supposed to be higher and extravasated serum proteins are reported to be lower than in healthy tissue [26], [27]. Accordingly, the significantly decreased NOE in the CE-T1 tumor compared to the PTCH within T2 edema margins could be explained as protein concentration within the enhancement is supposed to be lower [27]. NOE results from dipolar interactions between protons that highly depend on the molecular structure. Therefore, an additional explanation would be a higher mobility of proteins in tumor tissue which would lower the dipolar cross-relaxation rates. Recently, NOE effects also turned out to be correlated with protein structure [15]. Thus, a further mechanism could be that the protein structure itself is less compact due to increased misfolding of the rapidly produced proteins within the area of highest proliferation. Based on the given data, it is not possible to identify the contribution of each possible cause.
Since NOE mediated CEST imaging provides additional information compared to standard MR sequences, there are numerous possible clinical applications that need to be evaluated. For biopsy guidance, the commonly used CE-T1 images lack specificity, because they only visualize the extravasation of contrast agent due to a disrupted blood brain barrier. Subareas of different signal intensity within glioblastoma on NOE mediated CEST may therefore contribute to identify tumor parts of different malignity by adding information about protein concentration or protein folding.
Since we detected seven out of eight tumor satellites on MTRasym images as hyperintense, an additional use of NOE mediated CEST as endogenous contrast might increase sensitivity for the identification of tumor satellites. However, as CSF in brain sulci and ventricles as well as blood vessels also display hyperintense on MTRasym, specificity of NOE mediated CEST is limited and requires comparison to anatomic sequences.
Another major problem in glioblastoma imaging within daily clinical decision making is that it is not possible to differentiate a T2-signal increase caused by tumor infiltration from a non-specific cause of T2-signal increase (e.g. edema, radiation effects, decreased corticosteroid dosing, seizures, postoperative changes) [2], [28]. In this context, CEST images showed peritumoral hyperintensities which tended to be smaller than T2 edema extent and revealed substructures that were not discernible on T2-weighted images. A potential explanation for this finding might be that NOE mediated CEST displays the tumor infiltration more accurately than non-specific T2-weighted images. However, comparing CEST at 7T with T2-weighted images at 3T might be inappropriate, especially concerning heterogeneities within the tumor and its peritumoral edema. Within future examinations, comparisons should be performed with T2-weighted images also acquired at 7T. Furthermore, all assumptions based on variations in signal intensity are hypothetical and should be investigated in animal studies with precise histopathological correlation.
Other CEST studies on high grade glioma patients were performed by Wen et al. [7] and Zhou et. al [10] using APT mediated CEST at 3T based on asymmetry approaches. They reported increased APT effects within the tumor and described peritumoral hyperintensities that also tended to be smaller than on T2 weighted images. In contrast to our NOE weighted approach yielding negative MTRasym values, their APT weighted CEST yielded positive MTRasym values in all tissues. Advantages of the 7T field strength in our study are higher spectral resolution and higher SNR or shorter measurement times, respectively. Consequently APT mediated CEST image contrast between the tumor and non-tumorous parenchyma is only ∼1.5–2% on MTRasym [7] whereas we measured differences of ∼6–8% between CE-T1 tumor and CLNAWM on MTRasym. Interestingly, by comparing only changes in asymmetry values, we see the same trend towards higher MTRasym values within the tumor as APT-weighted studies.
To evaluate therapeutic response effects of chemotherapy with temozolomide in mice with human glioblastoma, Sagiyama et al. recently performed asymmetry based APT imaging on a 7T small animal MR system [29]. They were able to show that APT signal in treatment resistant tumors increased significantly within one week compared to tumors that responded to treatment. Furthermore, they found a high correlation between the histopathologically determined KI67 labeling index and APT signal intensity in the tumors. These results indicate that CEST imaging might contribute to differentiate tumor progression from treatment effects in glioma.
Generally, when performing CEST asymmetry approaches, it has to be verified that the implicit assumption of competitive CEST effects is valid. At +3.5 ppm amide proton transfer (APT) of proteins was reported, while exchange relayed NOE occurs in the range from −2 to −5 ppm [6], [19]. In the current study we chose 3.3 ppm to reduce APT effects in the asymmetry analysis. Furthermore, we used a saturation field amplitude of 0.7 µT where NOE effects are much stronger than APT effects that peak at 2.1 µT [13], [15]. These theoretical considerations were confirmed in our spectral analysis since we could identify a clear decrease of effects at −3.3 ppm in all tumor parts compared to CLNAWM, while only small background effects at +3.3 ppm most probably due to exchanging amide protons could be detected. A confounding factor in asymmetry analysis is the semi-solid magnetization transfer. However, for the irradiation scheme used in this study contributing effects should be small (<1–2% in MTRasym) since magnetization transfer only becomes dominant for higher irradiation powers [19], [20], [21].
Finally, the clinical benefit of the newly introduced NOE mediated CEST contrast still needs to be proven within larger patient collectives, including follow up examinations and bioptical correlations. Ultimately, beyond MTRasym approaches, sophisticated fitting models performed on Z-spectra with additional frequency offsets may help to separate competitive CEST effects yielding a metabolite specific CEST contrast.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the Institutional Data Access/Ethics Committee of the University of Heidelberg for researchers who meet the criteria for access to confidential data.
Funding Statement
This study was supported by a grant from the “Intramurales Förderprogramm” of the German Cancer Research Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. DeAngelis LM (2001) Brain Tumors. New England Journal of Medicine 344: 114–123. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, et al. (2010) Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. Journal of Clinical Oncology 28: 1963–1972. [DOI] [PubMed] [Google Scholar]
- 3. Scott JN, Brasher PMA, Sevick RJ, Rewcastle NB, Forsyth PA (2002) How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 59: 947–949. [DOI] [PubMed] [Google Scholar]
- 4. Radbruch A, Lutz K, Wiestler B, Bäumer P, Heiland S, et al. (2012) Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro-Oncology 14: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu G, Song X, Chan KWY, McMahon MT (2013) Nuts and bolts of chemical exchange saturation transfer MRI. NMR in Biomedicine 26: 810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaiss M, Bachert P (2013) Chemical exchange saturation transfer (CEST) and MRZ-spectroscopyin vivo: a review of theoretical approaches and methods. Physics in Medicine and Biology 58: R221–R269. [DOI] [PubMed] [Google Scholar]
- 7. Wen Z, Hu S, Huang F, Wang X, Guo L, et al. (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. NeuroImage 51: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, et al. (2011) Amide proton transfer MR imaging of prostate cancer: A preliminary study. Journal of Magnetic Resonance Imaging 33: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rivlin M, Horev J, Tsarfaty I, Navon G (2013) Molecular imaging of tumors and metastases using chemical exchange saturation transfer (CEST) MRI. Scientific Reports 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, et al. (2013) Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. Journal of Magnetic Resonance Imaging 38: 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, et al. (2010) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature Medicine 17: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tietze A, Blicher J, Mikkelsen IK, Østergaard L, Strother MK, et al. (2014) Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR in Biomedicine 27: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou J, Hong X, Zhao X, Gao J-H, Yuan J (2013) APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magnetic Resonance in Medicine 70: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones CK, Huang A, Xu J, Edden RAE, Schär M, et al. (2013) Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage 77: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaiss M, Kunz P, Goerke S, Radbruch A, Bachert P (2013) MR imaging of protein folding in vitro employing Nuclear-Overhauser-mediated saturation transfer. NMR in Biomedicine n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 16. Schmitt B, Zbýň Š, Stelzeneder D, Jellus V, Paul D, et al. (2011) Cartilage Quality Assessment by Using Glycosaminoglycan Chemical Exchange Saturation Transfer and 23Na MR Imaging at 7 T. Radiology 260: 257–264. [DOI] [PubMed] [Google Scholar]
- 17. Zhou J, Payen J-F, Wilson DA, Traystman RJ, van Zijl PCM (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 18. Stancanello J, Terreno E, Castelli DD, Cabella C, Uggeri F, et al. (2008) Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast Media & Molecular Imaging 3: 136–149. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PCM (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magnetic Resonance in Medicine 50: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 20. Liu D, Zhou J, Xue R, Zuo Z, An J, et al. (2013) Quantitative characterization of nuclear overhauser enhancement and amide proton transfer effects in the human brain at 7 tesla. Magnetic Resonance in Medicine 70: 1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henkelman RM, Stanisz GJ, Graham SJ (2001) Magnetization transfer in MRI: a review. NMR in Biomedicine 14: 57–64. [DOI] [PubMed] [Google Scholar]
- 22. van Zijl PCM, Zhou J, Mori N, Payen J-F, Wilson D, et al. (2003) Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magnetic Resonance in Medicine 49: 440–449. [DOI] [PubMed] [Google Scholar]
- 23. Vaupel P, Kallinowski F, Okunieff P (1989) Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Research 49: 6449–6465. [PubMed] [Google Scholar]
- 24. Griffiths JR (1991) Are cancer cells acidic? Br J Cancer 64: 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin T, Wang P, Zong X, Kim S-G (2013) MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magnetic Resonance in Medicine 69: 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson SJ (2011) Assessment of therapeutic response and treatment planning for brain tumors using metabolic and physiological MRI. NMR Biomed 24: 734–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bodsch W, Rommel T, Ophoff BG, Menzel J (1987) Factors responsible for the retention of fluid in human tumor edema and the effect of dexamethasone. Journal of Neurosurgery 67: 250–257. [DOI] [PubMed] [Google Scholar]
- 28. Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK (2008) Response criteria for glioma. Nat Clin Pract Oncol 5: 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, et al. (2014) In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proceedings of the National Academy of Sciences 111: 4542–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the Institutional Data Access/Ethics Committee of the University of Heidelberg for researchers who meet the criteria for access to confidential data.