Abstract
The analysis of the early macrophage responses, including bacterial growth within macrophages, represents a powerful tool to characterize the virulence of clinical isolates of Mycobcaterium avium susbp. paratuberculosis (Map). The present study represents the first assessment of the intracellular behaviour in ovine monocyte-derived macrophages (MDMs) of Map isolates representing distinct genotypes (C, S and B), and isolated from cattle, sheep, goat, fallow deer, deer, and wild boar. Intracellular growth and survival of the selected isolates in ovine MDMs was assessed by quantification of CFUs inside of the host cells at 2 h p.i. (day 0) and 7 d p. i. using an automatic liquid culture system (Bactec MGIT 960). Variations in bacterial counts over 7 days from the baseline were small, in a range between 1.63 to 1.05-fold. After 7 d of infection, variations in the estimated log10 CFUs between all the tested isolates were not statistically significant. In addition, ovine MDMs exhibited enhanced anti-inflammatory, antiapoptotic and antidestructive responses when infected with two ovine isolates of distinct genotype (C and S) or with two C-type isolates from distinct hosts (cattle and sheep); which correlated with the successful survival of these isolates within ovine MDMs. A second objective was to study, based on an in vitro granuloma model, latter stages of the infection by investigating the capacity of two Map isolates from cattle and sheep to trigger formation of microgranulomas. Upon 10 d p.i., both Map isolates were able to induce the formation of granulomas comparable to the granulomas observed in clinical specimens with respect to the cellular components involved. In summary, our results demonstrated that Map isolates from cattle, sheep, goats, deer, fallow-deer and wild boar were able not only to initiate but also to establish a successful infection in ovine macrophages regardless of genotype.
Introduction
Mycobacterium avium subsp. paratuberculosis (Map) is the causal agent of paratuberculosis or Johne's disease (JD), a chronic inflammatory bowel disease of domesticated ruminants including cattle, sheep, goats and farmed deer, and wildlife worldwide [1], [2], [3]. Paratuberculosis causes major economic losses to the global dairy industry due to lower milk production and reduced slaughter value [4], [5]. Map isolates can be classified in two genotypes based on culture characteristics and genome analysis: sheep isolates (also called “S type” or “type I”) and cattle isolates (also called “C type” or “type II”) [6], [7]. Single nucleotide polymorphism (SNP) analysis of the IS1311 insertion sequence distinguishes three types of strains: S, C and B or “bison” type [8]. Because S-type isolates of Map predominate in sheep and C-type in cattle, different host specificities were assumed for both types of isolates.
Map is endocytosed by the M cells of the ileal Peyer's patches and subsequently phagocytosed by subepithelial and intraepithelial macrophages [9], [10]. Once inside host macrophages, many phagosomes containing Map fail to acquire significant amounts of lysosomal-associated membrane protein (LAMP-1) and to fuse with lysosomes allowing Map to persist within infected macrophages [11]. In addition, it has been suggested that Map alters the ability of infected macrophages to react to extracellular signals from T cells, particularly through the CD154-CD40 system [12]. Map-infected macrophages secrete cytokines and chemokines which contribute to the recruitment of blood and tissue macrophages and T-lymphocytes to the infection site. This organized aggregate of immune host cells around Map-infected macrophages is called a granuloma. Within granulomas, activated macrophages differentiate to lipid-loaded macrophages (foamy macrophages), epithelioid cells with large cytoplasms and interdigitated membranes, and/or fuse together to form multinucleated giant cells also called giant Langhans cells [13], [14]. T and B lymphocytes surround the granuloma core, and a tight coat of fibroblasts and collagen closes the structure [15].
The aim of the current study was to to identify differences in virulence between Map isolates at two different stages of the infection. Early interaction of Map isolates with ovine macrophages was assessed using an ovine monocyte-derived macrophage model. Later stages of the infection, such as the first stages of granuloma formation, were mimicked using an in vitro granuloma model. The early interaction of Map with subepithelial dome macrophages, its primary host cell, leads to the release of cytokines and chemokines. The differential release of proinflammatory and anti-inflammatory cytokines contributes to the overall cell activation, which may determine whether the pathogen is eradicated or not. Therefore, the analysis of the initial macrophage responses, including bacterial growth within macrophages, represents a powerful tool to rapidly characterize the virulence of clinical isolates of Map. By using a bovine macrophage-like cell line (BoMac) and bovine monocyte-derived macrophages (MDMs), we previously observed differences in intracellular growth and persistence in bovine macrophages between strains of Map that grouped according to the host of origin [16]. Our results demonstrated that Map isolates from goats and sheep persisted within bovine macrophages in lower CFUs than cattle, bison, deer and wild boar strains after 7 days of infection regardless of genotype. A strong correlation between the intracellular multiplication of the tested isolates and patterns of production of host IL-6, TGF-β, MMPL-6, BCL2-1 and IL1-α was observed. Consequently, we suggested that the levels of expression of these proteins might be used to discriminate between isolates of Map with differential pathogenicity in bovine macrophages [17]. The intracellular survival within ovine macrophages of Map isolates representing distinct genotypes and isolated from a diverse range of hosts has not been fully addressed. Therefore, our first objective was to identify differences in pathogenicity between distinct isolates of Map by clarifying which Map isolates could potentially initiate disease in an ovine MDM model. For this purpose, we evaluated the capacity of a panel of 10 Map isolates representing distinct genotypes to grow and survive within ovine MDMs using an automatic culture system (Bactec MGIT 960). In addition, the expression of several pro- and anti-inflammatory cytokines and genes involved in apoptosis and tissue destruction were tested by qRT-PCR in ovine MDMs infected with the selected Map isolates. Because common changes in IL10, TGF-β, and TNFα gene expression were previously observed in human and bovine peripheral blood mononuclear cells (PBMCs), MDMs, and in macrophage-like cell lines infected with Map [17], these specific genes were selected for gene expression analysis in ovine MDMs. The expression of the apoptotic inhibitor BCL2-1 and the inhibitor of tissue destruction TIMP-1 was analyzed in ovine MDMs because up-regulation of both genes was previously demonstrated in BoMac cells infected with a bovine isolate of Map [16]. Using c-DNA microarrays focused on expressed sequences from a bovine total leukocyte library (BOLT5), significant up-regulation of the antiapoptotic BCL2-A1 gene was also observed in Map-infected bovine MDMs relative to uninfected cells [18].
Although MDMs can provide insights into Map-host interactions at the very early stages of the infection (7-10 d p. i.), this cellular model is unable to mimic later stages of the infection, such as the first stages of granuloma formation. To address this issue, we have developed a three-dimensional in vitro model enabling the formation of Map-induced granulomas by incubating infected ovine PBMCs with an extracellular matrix. Since the ability to develop a well-defined granulomatous response was previously shown to correlate with the severity of mycobacterial infections [19], [20], [21], the second objective of our study was to assess whether in vitro granuloma formation was induced by two Map isolates from cattle and sheep with distinct genotypes.
By comparing the interaction of distinct Map isolates with ovine macrophages at two different stages of the infection, we are seeking to identify differences in host specificity and pathogenicity between Map isolates and to provide scientific data to support effective control management strategies against ovine paratuberculosis. This information might be very useful in situations where veterinarians and producers have to assess risks and introduce effective management strategies to control paratuberculosis in multispecies livestock operations or on farms where livestock share pastures with wildlife animals potentially infected with Map.
Materials and Methods
Ethics Statement
Experimental procedures were performed by clinical veterinarians in strict accordance with the recommendations in the Spanish Ethical Guide for the care of animals used for experimental and other scientific purposes (Royal Legislative Decree 53/2013). Blood collection procedure was approved by the Institutional Animal Care and Use Committee (IACUC) of NEIKER-Tecnalia and by the Department of Agriculture, Diputacion Foral de Bizkaia, Spain (Permit N° 14133).
Map Isolates, Bacterial Culture and Preparation of Bacterial Suspensions
Nine Map isolates from cattle (Bos taurus), sheep (Ovis aries), goat (Capra hircus), red deer (Cervus elaphus), fallow deer (Dama dama), and wild boar (Sus scrofa) species were selected from the collection of isolates at the Mycobacteria laboratory, NEIKER-Tecnalia, on the basis of varied hosts and genomic profiles as per Sevilla et al., 2007 [22]. These isolates of Map were previously recovered from fecal or tissue specimens of domestic or wildlife animal species and maintained as glycerol stocks at −80°C [23], [24]. Aliquots of these glycerol stocks were utilized to directly inoculate all subsequent cultures for use in infection of macrophages. Most of the specimens were collected in several geographic areas of Spain, but three isolates from India, Portugal and The United States were also included in the study. Map reference strain K10, a sequenced and laboratory-adapted strain recovered from a clinical case of paratuberculosis, was obtained from the American Type Culture Collection (ATCC) (Manassas, VA). The 10 isolates of Map selected for our study were grown in T25 tissue culture flasks at 37±1°C for up to 3 months in 10 ml of Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) (Becton, Dickinson and Company, Franklin Lakes, NJ), 0.05% (wt/vol) Tween-80 (Sigma-Aldrich, St. Louis, MO) and 2 mg L-1 of mycobactin J (Allied Monitor Inc., Fayette, MO). Bacterial cells were harvested by centrifugation at 3,000 x rpm for 20 min in a Beckman Coulter Allegra X-12 centrifuge. Bacterial pellets were resuspended in 2 ml of Hank's balanced salt solution (HBSS), and the resultant suspension was passed 20 times through a 27-gauge needle in order to declump cells. The turbidity of the bacterial suspension was adjusted to a McFarland standard of 1 with a Densimat (bioMerieux, Marcy l'Etoile, France). Only the top fraction of the suspension containing dispersed bacteria was used for the infection assays.
Ovine Monocyte-derived Macrophages (MDMs) Culture
For isolation of ovine mononuclear cells, peripheral blood was collected from the jugular vein of a healthy Latxa sheep older than 48 months. Blood draws were separated by a period of 14 days for red blood cell renewal and a maximum of 1% of the animal's body weight was removed in each blood draw. By monitoring the hematocrit and hemoglobin of the animal, we evaluated whether the animal had sufficiently recovered from a single blood draw. The blood was collected into heparinised Vacutainer tubes (Becton, Dickinson and Company, Sparks, MD), transferred aseptically into sterile glass bottles and diluted 1∶2 in HBSS. Twenty-five millilitres of blood:HBSS were layered over 10 ml of Ficoll-Paque (1,084 g/cm3) (GE HealthCare Bio-Sciences, Uppsala, Sweden) in 50-ml centrifuge tubes. Cells were centrifuged at 900× g for 30 min to separate erythrocytes and polymorphonuclear cells from PBMCs. PBMCs were collected from the HBSS-Ficoll-Paque interface and washed with HBSS by centrifugation at 400× g for 10 min. The proportion of purified ovine monocytes was previously estimated in 35.5% of the PBMC fraction obtained by density centrifugation which in itself represents about 70% of the total white blood fraction [25]. The isolated PBMCs were resuspended in Macrophage-serum free medium (Macrophage-SFM) (Invitrogen, Carlsbad, CA) supplemented with 20 mM l-glutamine, 10% heat-inactivated lamb serum (Lonza, Basel, Switzerland), 100 U/ml of penicillin G and 100 µg/ml of streptomycin sulphate. PBMCs were seeded at a density of 1×106 PBMC/ml into 24-well tissue culture plates and incubated for 2 h at 37°C in a 5% CO2 incubator. Non-adherent cells were removed by washing twice with HBSS. This step is expected to increase the purity of the purified monocytes. Residual lymphocytes present in the cultures are not expected to influence significantly the experimental results because a normal macrophage-T cell interaction has been shown to be impaired in Map-infected macrophages [12]. Adherent cells were incubated for 7 days at 37°C in supplemented Macrophage-SFM to allow differentiation to MDMs prior to infection with Map. After 7 days at 37°C most adherent cells were stellate in shape, consistent with macrophage morphology. Incubation of monocytes in Teflon wells or with granulocyte-macrophage colony stimulating factor (GM-CSF) for macrophage maturation are standard protocols for maturing monocytes after Ficoll gradient. However, it was previously suggested that ovine mononuclear blood cells are able to proliferate and differentiate in culture without the addition of growth factors [26].
Infection of Ovine MDMs with Map Isolates from Domestic and Wild Animal Species
Ovine MDMs were inoculated in triplicate with single-cell suspensions of each of the 10 Map isolates at a MOI (bacteria:cell) of 10∶1. This level of infection did not alter cell viability over a 1-week assay, as was previously assessed by Trypan blue staining. After a 2 h infection time, the supernatant was removed and the cells were washed twice with HBSS to remove extracellular bacteria. Infected macrophages were lysed at this time point (considered as day 0) or cultured in supplemented Macrophage-SFM medium at 37°C for 7 days (day 7). At each time point, the supernatant was aspirated and infected macrophages were lysed by vigorous pipetting with 0.5 ml of 0.1% Triton X-100 (Sigma-Aldrich) in sterile water for 10 min.
Viable Map Quantification using the Bactec MGIT 960 System
Supplemented Mycobacteria Growth indicator tubes (MGIT) (Becton, Dickinson and Company, Sparks, MD) were inoculated with 0.1 ml of each initial bacterial suspension or with 0.5 ml of the cell lysates for each time point. Each MGIT tube contained 7 ml of modified Middlebrook 7H9 broth base with casein peptone and an oxygen-sensitive fluorescent compound (tris-4,7-diphenyl-1,10-phenathroline ruthenium chloride pentahydrate) embedded in silicone on the bottom of the tube. Each tube was supplemented with 800 µl of an enrichment supplement (BBL MGIT OADC growth supplement) and an antibiotic mixture (BBL MGIT PANTA Antibiotic Mixture) (Becton, Dickinson and Company). The tubes were also supplemented with 2 µg ml-1 of mycobactin J. Inoculated vials were incubated at 37±2°C for up to 41 days in the Bactec MGIT 960 instrument (Becton, Dickinson and Company) and were monitored automatically every hour for an increase of fluorescence. The earliest instrumental indication of positivity (i.e., time to detection [TTD]) for each tube was recorded. Any tube that was identified as positive was removed from the instrument, and a sample was tested by PCR to confirm the presence of Map. If a tube did not signal positive before 42 days (6 weeks) of incubation, it was removed from the instrument and determined to be negative. The predicted number of bacteria in each positive tube was calculated by using previously generated mathematical formulas which relate TTD (in days) to estimated log10 CFUs for each specific Map isolate (Table 1) [27].
Table 1. IS1311 PCR-REA types, PFGE profiles and estimations of span, K, and plateau for the quantification of each M. avium subsp. paratuberculosis isolate in the Bactec MGIT 960 system.
| Isolate | Region/Country | Host | PCR-REA | PFGE | Span | K | Plateau |
| K10 | United States | Cattle | C | 1-1 | 11.48 | 0.0503 | −1.316 |
| 6 | Cantabria/Spain | Cattle | C | 52-1 | 16.44 | 0.0220 | −6.080 |
| P38I | Aragon/Spain | Sheep | C | 2-1 | 9.972 | 0.0211 | −0.634 |
| 2349/06-1 | Portugal | Sheep | S | - | 11.82 | 0.0724 | 3.222 |
| 334 | India | Sheep | B | 60-1 | 11.86 | 0.0298 | −0.480 |
| 711 | Bizkaia/Spain | Goat | C | 2-1 | 17.25 | 0.0301 | −8.208 |
| 311 | Menorca/Spain | Goat | S | 16- 47 | 9.511 | 0.0612 | 3.016 |
| 855 | Toledo/Spain | Deer | C | 68-1 | 10.95 | 0.1927 | 1.317 |
| 622/07 | Asturias/Spain | Fallow Deer | C | - | 21.06 | 0.0106 | −11.25 |
| 681 | Toledo/Spain | Wild boar | C | 2-1 | 8.755 | 0.0486 | 0.760 |
Growth of all the isolates in the Bactec MGIT 960 system fitted to a one-phase exponential-decay model according to the following equation [log10 inoculum size = span X e (-K X TTD) + plateau]. Span is the difference between TTD at time zero and the plateau, K is the degree of decay for the log10 CFU, and plateau is the value for log10 CFU curve flattening.
Assessment of Uptake, Intracellular Growth and Persistence of Map Isolates in Ovine MDMs
Mean estimated log10 CFUs in the initial inocula and at days 0 and 7 from three replicate assays were calculated. The percentages of uptake were calculated as the percentages of the inoculated bacteria that were recovered from each cell lysate at day 0. Growth changes between day 0 and day 7 were calculated by dividing the estimated log10 CFUs at day 7 by that at day 0 (n-fold). The ability of each isolate to persist within host cells is presented as the log10 CFUs at day 7.
RNA isolation, c-DNA Synthesis, and Detection of Several Cytokines and Proteins Involved in Apoptosis or Tissue Destruction by a Two-step Quantitative Reverse-Transcription PCR (qRT-PCR)
Ovine MDMs were inoculated with two ovine isolates of distinct genotype (C and S) or with two C-type isolates from distinct hosts (cattle and sheep) as described above. Uninfected cells were used as controls. At 4, 14 and 24 h p. i., the infected MDMs were washed in 0.5 ml of cold HBSS, mixed with 50 µl of Lysis Solution and incubated at room temperature for five minutes to allow RNA release into the Lysis Solution (PowerSYBR® Green Cells-to-CT™ Kit, Life Technologies, Carlsbad, CA). DNAse I was added to the Lysis Solution to allow genomic DNA degradation at this step. The lysis procedure simultaneously prepares cell lysates for RT-PCR and removes genomic DNA. Next, 5 µl of Stop Solution were mixed into the lysate to inactivate the lysis reagents so that they would not inhibit the reverse transcription (RT) or polymerase chain reactions (PCR). Cell lysates were then reverse transcribed to synthesize cDNA using 20 X RT Enzyme Mix and 2 X SYBR RT Buffer. The reaction mixtures contained 2.5 µl of 20 X RT Enzyme Mix, 25 µl of 2 X SYBR ® RT Buffer, 12.5 µl of Nuclease-free water and 10 µl of the lysate in a 50 µl cDNA synthesis reaction. In order to demonstrate that the template for the PCR was cDNA and not genomic DNA, minus-RT controls containing all the RT components except the 20 X RT Enzyme Mix were prepared for every RNA sample. The reaction mixtures were incubated at 37°C for 60 min and then at 95°C for 5 min to inactivate the RT enzyme. Finally, the synthesized cDNAs were amplified by real-time PCR using the Power SYBR Green PCR Master Mix and the PCR primers set for the target of interest. Real-time qPCR reactions were carried out in triplicate in 25 µl reaction mixtures containing 12.5 µl of Power SYBR Green PCR Master Mix, the optimum concentration of each pair of primers, and 4 µl of cDNA. Using c-DNA synthesized from non-infected cells as template, we had previously determined the concentration of each pair of primers that provided optimal assay performance (i.e., low Ct and maximum ΔRn), but did not produce nonspecific product formation (primer dimmer products) with no-template negative controls (NTC). Primer-dimer products are shorter than the expected amplicons, and thus will have a lower Tm. Real-time qPCR amplifications of cDNAs were accomplished using the ABIPrism 7500 detection system (Applied Biosystem, Carlsbad, CA) under the following conditions: 1 cycle of 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 60 s, and a dissociation curve to measure the specificity of the amplification. Real-time qPCR primers for the amplification of each selected host gene were designed using PrimerExpress 3.0 software and verified for theoretical non-specific annealing with Primer-Blast. Table 2 shows the list of the amplified ovine genes and the corresponding primer sequences. Since the Glyceraldehyde 3-phosphate dehydrogenase (GADPH) gene is constitutively expressed, it was used as the endogenous control gene in the assays. To determine the changes in gene expression (n-fold) or relative quantitation (RQ), the following formula was used: RQ = 2 -Δ(ΔCT) where ΔC T is C T (target gene) − C T (GADPH) and Δ(ΔC T) is ΔC T (experimental) − ΔC T (control). Results were expressed as relative quantifications of transcription compared to those of control uninfected cells.
Table 2. Genes and primer sequences used in the qRT-PCR assays.
| Code Protein | Name | Abbreviation | Primers code/sequence (5′-3) | ||
| NM001009806.1 | Interleukin 2 | IL2 | 169F/ACAACCCTTGTCTTGCATTGC 170R/CTTGAAGTAGGTGCACCGTTTG | ||
| NM0010093192 | TIMP Metallopeptidase inhibitor 1 | TIMP-1 | 173F/TGCTCATCTATCCCCTGCAAA 174R/TGGTCCGTCCACAAGCAA | ||
| NM001009327.1 | Interleukin 10 | IL10 | 191F/TTCTTTCAAATGAAGGACCAACG 192R/CCCTTAAAGTCATCCAGCAGAGA | ||
| X55152.1 | Tumor necrosis factor, member 2 | TNFα- 2 | 177F/CCATCAGCCGCATTGCA 178R/TTGATGGCAGAGAGGATGTTGA | ||
| NM001009226.1 | Mitocondrial protein | BCL2-1 | 181F/GGGCACTGTGCGTGGAA 182R/TGCGATCCGACTCACCAATA | ||
| NM0010094001 | Transforming growth factor, beta 1 | TGFβ-1 | 179F/AAGCGGAAGGGCATCGA 180R/CGAGCCGAAGTTTGGACAAA | ||
| AF030943.1 | Glyceraldehyde 3- PO4 3 dehydrogenase | GAPDH | 187F/TGCCGCCTGGAGAAACC 188R/CGCCTGCTTCACCACCTT | ||
Ovine PBMCs Infection with Map Isolates Representing Distinct Genotype and in vitro Granuloma Formation
An extracellular matrix (ECM) was prepared according to Kapoor et al. [28] by mixing: 0.8 ml of 3 mg/ml Purecoll collagen solution (Nutacon BV, Leimuden, The Netherlands), 0.1 ml of 10 X Dulbecco's Phosphate buffered saline (DPBS) (Lonza) and 4 µl of 1mg/ml Human Fibronectin (BD Biosciences). The pH of the mixture was adjusted to 7.2–7.6 using sterile 0.1 M NaOH (Sigma). The final volume of the matrix solution was adjusted to 1 ml with sterile water. To prevent gelation, the temperature of the mixture was maintained at 4°C. Fifty microliters of the matrix solution were added to individual wells of a 96-well tissue culture plate. Ovine PBMCs, prepared as described above, were seeded on the ECM at 5 x 105 cells/50 µl ECM/well of a 96-weell plate. Map K10 strain or the ovine 2349/06.1 isolate of Map were added to each well without touching the surface of the ECM at MOIs (bacteria: cell) of 1∶8, 1∶16 and 1∶33. The ECM was allowed to set by incubating at 37 °C in a 5% CO2 incubator for 2 h. The volume of each well was adjusted to 200 µl by the addition of RPMI +20% FBS. Samples were incubated at 37°C in a 5% CO2 incubator for 10 days. The media was changed on day 5. Cellular aggregation was observed under an inverted phase-contrast microscope (Olympus IX81) equipped with a Nikon DS-Fi1 digital camera. Images were edited using Fiji/Image J Software (v 1.48). In vitro generated aggregates were counted at 5 and 10 days p. i.
Harvesting Aggregates for Histology
After 10 days of incubation, the medium was carefully removed from each well and replaced with 0.2 ml of 10% neutral buffered formalin. Plates were incubated overnight at 37°C in a 5% CO2 incubator overnight. Next day, the formalin was removed and 0.2 ml of hematoxylin diluted 1∶1 in phosphate-buffered saline (PBS) were added to each well. After 10 min of incubation at room temperature, the stain was removed and 0.2 ml of 2% LE-2 agarose (Lonza) heated at 42–45°C was added to each well. Plates were placed at 4°C for 10–15 min to allow agarose to solidify. Agarose plugs containing cellular aggregates were removed from each well and stored in six-well dishes in 70% ethanol before processing for histopathology.
Paraffin Embedding, Sectioning and Histological Staining
A drop of eosin was applied to the agarose plugs containing aggregates. Each plug was wrapped in tissue wipers (VWR, Radnor, PA, US), placed in a marked cassette and processed on a tissue processor Shandon Citadel 2000 (Fisher Scientific Co., Pittsburgh, PA, US) for 17 h. Each plug was embedded in paraffin wax, and sectioned at 4 µm on a microtome Leica RM2035 (Leica Mycrosystems, Barcelona, Spain). Sections were mounted on treated microscope slides (Fisher Scientific Co) and stained with hematoxylin and eosin (HE) and Ziehl–Neelsen (ZN) stain for acid-fast bacteria. Aggregates were observed under a microscope Olympus BX51 equipped with an Olympus U-CMAD3 digital camera.
Statistical Analysis
Estimated log10 CFUs in the initial inoculums and 0 and 7 days after infection of ovine MDMs were compared with the General Lineal Model (GLM) procedure of the SAS statistical package version 9 (SAS Institute Inc., Cary, NC). In the analysis; time p.i. (inoculum and days 0 and 7), genotype (C, S and B), and host of origin (cattle, sheep, goat, deer, wild-boar, and fallow-deer) were the independent variables. Cytokine production at 4, 14 and 24 h p. i. was compared with the GLM procedure of the SAS Software. In the analysis; host (cattle and sheep), and time (4, 14 and 24 h p.i.) were the main effects. Numbers of in vitro generated granulomas were compared by one-way analysis of variance (ANOVA) with the Tukey-Kramer multiple-comparison post-test (GraphPad Software, San Diego, CA). In all analyses, differences were considered significant when P values were <0.05.
Results
Uptake, Growth and Persistence of Map Isolates from Cattle, Goat, Sheep, Deer, Fallow Deer and Wild Boar in Ovine MDMs
Ovine MDMs were infected in triplicate with a panel of 10 Map isolates. Isolate code, country of isolation, host of origin, and genotype for each isolate are summarized in Table 1. The log10 CFUs present in the initial inocula and after 2 h (day 0) or 7 days (day 7) of infection were estimated for each isolate in the Bactec MGIT 960 system and the corresponding results are presented in Table 3. When the individual means of all the isolates were compared, variations between the estimated log10 CFUs in the initial inocula, and at days 0 and 7 p.i. were not statistically significant. When we compared time effect on the overall means, no significant differences among the means of the estimated log10 CFUs in the initial inocula and at day 7 were obtained (P = 0.3902). In contrast, significant differences between the mean log10 CFUs in the inocula and at day 0 were observed for all the strains, which indicated that not all bacteria in the initial inocula was successfully internalized (P<0.0001). As shown in table 3, the percentages of uptake in ovine MDMs were estimated in a range between 52% and 87% of the initial inocula depending on the isolate.
Table 3. Entry and intracellular growth of Map isolates and the reference strain K10 in ovine MDMs.
| Isolate | Host-IS1311 PCR/REA type | Entry (%) a | log10 CFU (± SD) b | n-Fold d | P value | |
| Day 0 c | Day 7 | |||||
| K10 | Cattle-C | 65.32 | 4.88 (±2.59) | 7.99 (±0.35) | 1.63 | 0.0058 e |
| 681 | Wild board- C | 73.13 | 5.33 (±0.99) | 8.01 (±0.20) | 1.50 | 0.1901 |
| 6 | Cattle-C | 67.36 | 5.86 (±2.73) | 7.89 (±1.50) | 1.34 | 0.4752 |
| 334 | Sheep-B | 75.01 | 6.05(±3.28) | 7.64 (±3.47) | 1.26 | 0.9942 |
| 622/07 | Fallow deer-C | 79.56 | 7.48 (±1.66) | 8.82 (±0.19) | 1.17 | 0.9998 |
| 711P | Goat-C | 74.91 | 5.59 (±0.80) | 6.17 (±1.10) | 1.10 | 1.0000 |
| P381 | Sheep-C | 86.39 | 7.81 (±1.36) | 8.44(±0.22) | 1.08 | 1.0000 |
| 311 | Goat-S | 77.00 | 7.75 (±2.44) | 8.19 (±2.44) | 1.05 | 1.0000 |
| 2349/06-1 | Sheep-S | 51.93 | 4.93 (±0.05) | 4.90 (±0.24) | 0.99 | 1.0000 |
| 855 | Deer-C | 74.45 | 5.86(±0.73) | 5.77 (±0.86) | 0.98 | 1.0000 |
Uptake was calculated as the percentage of the inoculated bacteria that was recovered from each cell lysate at day 0.
Values shown are means of three repeated experiments ± standard deviations (SD).
Day 0 = 2 h post infection.
Growth changes (n-fold) were calculated by dividing the number of log10 CFU at day 7 by that at day 0 for each Map isolate.
Indicates a significant change between day 0 and day 7 (P<0.05).
When time post-infection was considered as the main effect, significant differences between the overall means of the estimated log10 CFUs at days 0 and 7 were observed suggesting that variations in log10 CFUs occurred during the 7-day incubation period (P<0.0001). The intracellular growth exhibited for each isolate between days 0 and 7 in ovine MDMs is individually represented in Table 3 as the calculated fold change. Of the ten isolates, seven isolates (681, 6, 334, 622/07, 711P, P381, 311) and the K10 reference strain were observed to increase in number the initial bacterial concentration (n-Fold >1). However, these variations in bacterial counts over 7 days from the baseline were small, in a range between 1.63 to 1.05-fold, with K10 reference strain proliferating more rapidly than the other tested isolates. Statistical analysis of the data indicated that only the K10 reference strain exhibited significantly increased bacterial counts after 7 days of infection when compared with baseline (P = 0.0058). Although the isolates from sheep (2349/06-1) and deer (855) were observed to minimally decrease in log10 CFUs over 7 days from baseline (n-Fold <1), this variation was not statistically significant (P = 1.000). The ability of each isolate to persist within ovine MDMs is presented as the mean log10 CFUs at day 7 in Table 3. After 7 days of infection, variations in the estimated log10 CFUs at day 7 between all the tested isolates were not statistically significant.
Gene Expression in Ovine MDMs Infected with Two C-type Isolates from Distinct Hosts or with Two Ovine Isolates of Map with Distinct Genotypes
In order to determine whether the bacteria's host of origin affects gene expression in Map-infected MDMs, two C-type isolates from cattle and sheep were selected for gene expression analysis. The same test was run separately with two ovine isolates with distinct genotypes (C or S) to assess whether the bacteria's genotype would significantly change gene expression in ovine MDMs. Mean fold changes in gene expression between infected and non-infected cells were determined through real-time qRT-PCR analysis and are shown in figures 1A and 1B, respectively. Variations in the expression levels of several cytokines (IL10, TGFβ-1, IL2 and TNFα-2), the anti-apoptotic gene BCL2-1, and the tissue destruction inhibitory gene TIMP-1 in ovine MDMs infected with the two C-type isolates from cattle and sheep or with the two ovine isolates with distinct genotypes (C or S) were not statistically significant. Although from the Figure 1B it looks like that there are variations between the IL10 expression levels in ovine MDM infected with C- and S-type ovine isolates, statistical analysis of the data indicated that these variations were not statistically significant at 4 (P = 0.2568), 14 (P = 0.3697) and 24 h p.i. (P = 0.0914).When the expression of each gene was compared individually at the three time points assessed, ovine MDMs infected with C-type isolates from sheep and cattle for 4 and 14 h p.i. showed significantly increased levels of expression of the apoptotic inhibitor BCL2-1 when compared with the expression of this gene at 24 h p.i.. In general, up-regulation of the anti-inflammatory cytokines IL10 and TGFβ-1, and downregulation of the proinflammatory cytokines IL2 and TNFα-2 were observed in ovine MDMs infected with the selected Map isolates. The observed increased expression of the antiapoptotic BCL2-1 gene and of the tissue destruction inhibitory gene TIMP-1 might cause low levels of apoptosis and cellular destruction and allow Map persistence in the infected MDMs. In summary, ovine isolates of distinct genotype (C and S) and C-type isolates from sheep or cattle induced anti-inflammatory, antiapoptotic and antidestructive responses in ovine MDMs.
Figure 1. Expression of cytokines and proteins involved in inhibition of apoptosis or tissue destruction in ovine MDMs infected with bovine and ovine isolates of Map.
Ovine MDMs were infected with (A) C-type Map isolates from cattle (K10 strain) and sheep (p38I isolate), or with (B) two ovine isolates with C- (p38I isolate) or S-genotype (2349/06-1 isolate). At 4, 14 and 24 h p.i. gene expression was assessed by qRT-PCR. Isolates are identified in the figure by their corresponding host of origin and IS1311 PCR-REA type (C or S). Bars represent the average results of two independent infection experiments (± SD). No statistically significant differences in the expression of the indicated genes between the tested isolates were observed.
Infection of Ovine PBMCs with Two Map Isolates from Cattle and Sheep with Distinct Genotypes (C and S) Resulted in the Formation of Three-dimensional Microgranulomas
Ovine PBMCs seeded on a collagen matrix were infected with the K10 Map reference strain or with the ovine isolate (2349/06-1) at three different MOIs (bacteria: cell) (1∶8, 1∶16, and 1∶33). Previous studies demonstrated that infection of PBMCs with M. tuberculosis and M. bovis at a MOI 1∶1 or lower resulted in the formation of microgranulomas [28], [29]. Cellular recruitment around the bacteria was followed by light microscopy over a 10-day period. The experiment was repeated tree times with a conserved time course of around 3–5 days for cellular aggregation of lymphocytes around infected macrophages and 5–10 days for the formation of a multilayer, microscopic, granuloma-like aggregate. Both Map isolates formed rounded granuloma-like aggregates by day 10 as shown in Figure 2. In control, uninfected PBMCs from the same healthy donor, the formation of aggregates was not observed indicating that aggregation occurs only in response to Map infection (data not shown).
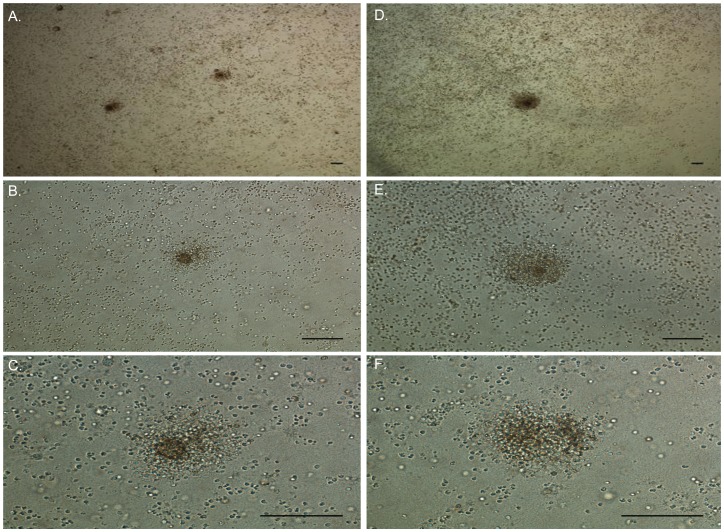
Figure 2. Phase contrast images showing the presence of day-10 granuloma-like aggregates after the infection of ovine PBMCs with bovine and ovine Map isolates.
Ovine PBMCs (5 x 105) seeded on an extracellular matrix were infected with the bovine K-10 reference strain (A, B and C) or with an ovine isolate of Map (2349/06-1) (D, E and F) at MOI (bacteria:cells) of 1:8. Magnification in A and D = 4X, in B and E = 20X and in C and F = 40X. Bars = 300 µm.
The number of granuloma-like aggregates formed in response to the infection with both Map isolates at 5 and 10 days post-infection is shown in Figure 3. Statistical analysis of the data showed significant variations between the number of aggregates generated after 5 days of infection with the K10 reference strain and the 2349/06 isolate at the highest MOI (1∶8), with the K10 strain inducing the formation of more aggregates than the ovine isolate (P<0.01). However, after 10 days of infection the number of aggregates formed by both strains was not significantly different at any of the 3 assessed MOIs. The number of aggregates generated by both strains at the highest and lowest MOI (1∶8 and 1∶33) was significantly different at 10 days p. i. Significant differences in the number of aggregates triggered by both strains between days 5 and 10 were not observed which suggested that most of the granulomas were formed during the first five days of the infection with both isolates. Uninfected cells showed no granuloma formation.
Figure 3. Number of microgranulomes generated in vitro 5 and 10 days after infection of ovine PBMCs with (A) the K10 reference strain and with (B) the ovine 2349/06-1 isolate.
Aggregate numbers were estimated under a light microscope. The mean aggregate number estimated by triplicate is shown for each MOI (1:8, 1:16 and 1:33) ± SD.
After 10 days of culture, the morphological characterization of the granuloma-like aggregates was confirmed by histological staining. Figure 4 shows the morphological characterization of the aggregates formed by primary ovine PBMCs infected with the K10 Map reference strain and with the ovine isolate of Map (2349/06-1) at MOI 1∶8. As shown in figures 4B and 4E, granulomas exhibited aggregation of lymphocytes around infected macrophages. When granuloma sections were stained with ZN, Map cells could be observed residing within the granulomas.
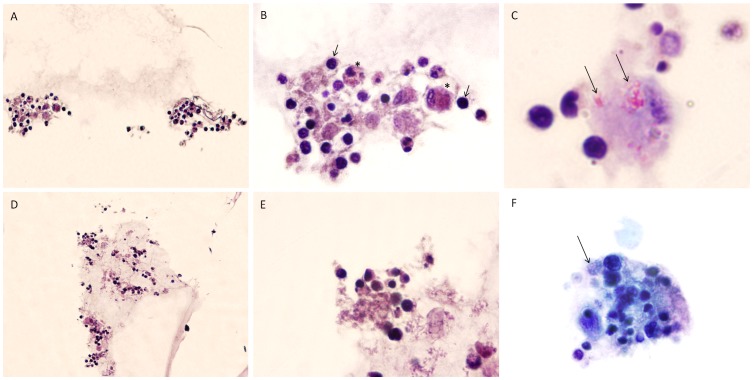
Figure 4. Morphological characterization of the cell populations recruited within in vitro- ovine granulomas.
Primary ovine PBMCs (5x105) were seeded on an extracellular matrix and subsequently infected with the K10 reference strain (A, B and C) or with an ovine isolate of Map (2349/06-1) (D, E and F) at MOI (Bacteria:cells) 1:8. At 10 days p.i., the granuloma-like aggregates were harvested, processed for histopathology and stained with HE (A, B, D and E) and ZN stains (C and F). Original magnification in A and D = 200X and in B, C, E and F = 1000X. As shown in image B, macrophages (asterisk) and lymphocytes (arrows) were present in the granulomas. In images C and F, acid-fast bacilli (arrows) were observed within macrophages by ZN staining.
Discussion
The identification of Map isolates with differential virulence may assist in further elucidating the pathogenesis of paratuberculosis and in the design of better strategies for controlling this infection. The analysis of the initial macrophage responses, including bacterial growth and survival within macrophages, represents a powerful tool to rapidly characterize the virulence of clinical isolates of Map. In the current study, we examined the intracellular growth and survival within an ovine MDM model of a panel of Map isolates representing distinct genotypes and isolated from cattle, sheep, goats, deer, fallow deer, and wild boar. Woo et al, previously demonstrated that following ingestion by bovine MDMs the number of viable Map cells increased during the first 4 days and then declined between days 4 and 8 after infection, as determined by a radiometric method [30]. In accordance with these results, we previously assessed intracellular growth and survival of our panel of Map isolates in a bovine macrophage-cell line (BoMac) and in bovine MDMs after 7 days of infection. To ensure consistency across in vitro models and to provide enough time for bacteria to grow, growth and survival of Map isolates within ovine macrophages was evaluated on day 7. Growth changes between days 0 and 7 were calculated by dividing the estimated log10 CFUs at day 7 by that at day 0 (n-fold). Of the ten isolates, seven isolates (681, 6, 334, 622/07, 711P, P381, 311) and the K10 reference strain were observed to increase in number the initial bacterial concentration (n-Fold >1). However, these variations in bacterial counts over 7 days from the baseline were small, in a range between 1.63 to 1.05-fold. Although the isolates from sheep (2349/06-1) and deer (855) were observed to minimally decrease in log10 CFUs over 7 days from baseline (n-Fold <1), this variation was not statistically significant (P = 1.000). Statistical analysis of the data indicated that only the K10 reference strain exhibited significantly increased bacterial counts after 7 days of infection when compared with baseline (P = 0.0058). The K10 is a laboratory-adapted strain while the other strains are recently isolated, low passage Map isolates. Different levels of aggregation or clumping between Map strains can affect the intramacrophage growth of each strain. However, we used a low bacillary inoculum which minimizes mycobacterial clumping during initial infection and more closely mimics conditions in vivo, where small numbers of Map cells can establish infection.
All the isolates, including the K10 strain, were able to survive in equivalent log10 CFUs within ovine MDMs after 7 days of infection. Therefore, an association between a specific host of origin and intracellular survival of the tested isolates in ovine MDMs was not observed. Consequently, we can hypothesize that the conditions encountered by the tested Map isolates within macrophages of their respective hosts did not differentially alter the phenotype of the bacteria and their subsequent persistence within ovine MDMs. In contrast, we previously showed that type S and type C isolates from sheep and goats showed a significant attenuated phenotype in a macrophage-like cell line of bovine origin (BoMac) after 7 days of infection, when compared with type C isolates form cattle, deer, fallow deer, wild boar and bison [16]. These observed variations between isolates in bovine macrophages grouped according to the host from which the isolates were isolated and were not associated to the genotype of the isolate. Similarly, strains of environmental Mycobacteria from fish and humans including strains of Mycobacterium avium, Mycobacterium peregrinum, Mycobacterium chelonae, and Mycobacterium salmoniphilum, had different abilities to grow within macrophages lines from humans, mice and carp; which grouped according to the host from which the isolates were isolated [31].
Our results suggested that sheep might be susceptible to infection with Map isolates not only from sheep and cattle but also from goats, deer, fallow deer and wild boar as well. Therefore, the importance of these isolates in the pathogenesis of Map shouldn't be underestimated. The successful survival within ovine MDMs of the sheep isolates correlates well with epidemiological data and clinical evidence of virulence, as suggested by the capacity of sheep isolates to cause numerous outbreaks in sheep [7]. The successful survival phenotype of the bovine isolates within ovine MDMs correlated well with clinical evidence of virulence, as suggested by the capacity of bovine isolates to infect sheep in experimental conditions [32]. However, it should be pointed out that experimental infections typically involve high doses of Map and therefore may not accurately assess Map transmission in field conditions.
When the estimated log10 CFU numbers within ovine MDMs at 0 and 7 days p.i. were statistically analysed, the intracellular behaviour of the tested isolates varied depending on the time p. i. (P<0.001). Small increases in the estimated log10 CFU numbers from days 0 to 7 were observed for most of the isolates. In contrast, the genotype did not seem to significantly affect the behaviour of the selected isolates within ovine MDMs with types S, B and C showing similar survival phenotype after 7 days of infection (P = 0.635). A lack of correlation between genotype and intracellular behaviour of Map isolates was also previously observed in bovine macrophages [16]. Similarly, a lack of correlation between genotype and growth rate in the Bactec MGIT 960 system was observed for the ovine isolates; with C, S and B isolates from sheep growing at equivalent rates in MGIT cultures [27].
Previously, it was suggested that a bovine, a bison, and a human type-C isolates induced anti-inflammatory and antiapoptotic responses in bovine MDMs, which would favour bacterial survival and persistence [33]. In the current study, we showed that the successful survival of bovine and ovine isolates of Map within ovine MDMs correlated with an increased expression of BCL2-1, TIMP-1, TGFβ-1, and IL10. In addition, a reduced proinflammatory immune response mediated by IL2 and TNFα-2 was observed in the infected cells. Our results also demonstrated that ovine macrophages infected with ovine isolates of distinct genotype (C or S) did not differentially express BCL2-1, TIMP-1, TGFβ-1, IL10, IL2 and TNFα-2. Previously, the bovine isolate-6 of Map that grew within BoMac cells was reported to induce the expression of the anti-inflammatory cytokines TGFβ-1 and IL-10 in BoMac cells that antagonized the proinflammatory response by down-regulating the production of TNF-α which favour bacterial survival [16]. In regard to the apoptotic response, BoMac cells infected with the bovine isolate-6 had increased levels of expression of the apoptotic inhibitor BCL2-1 and of the inhibitor of tissue destruction TIMP-1 which might cause low levels of apoptosis. Overall, a strong correlation between the successful survival of Map isolates within bovine and ovine macrophages and the patterns of production of BCL2-1, TIMP-1, TGFβ-1, TNFα-2 and IL10 was observed. Our results also indicated that the proinflammatory cytokines IL1α and IL2 were down-regulated in BoMac cells and in ovine MDMs infected with bovine isolates of Map, respectively. It is well known that the expression of these cytokines in the presence of intracellular bacteria is one of the first steps leading to activation of macrophages and effective bacteria killing. Consistently with our results, other authors also observed an up-regulation of TGFβ and/or IL10 after the infection of bovine MDMs with live Map that down-regulated the production of TNFα [33]-[38]. Similarly, rapid intracellular macrophage growth rates by strains of M. tuberculosis strains correlated with rapid production of IL10 that antagonizes the proinflammatory response by down-regulating the production of TNFα in THP-1 cells during the early stages of infection [39].
Although MDMs can provide insights into Map-host interactions at the very early stages of the infection, this cellular model is unable to mimic later stages of the infection, such as early granuloma formation. To address this issue, three-dimensional in vitro models of granuloma have been recently developed. The formation of small, rounded granuloma-like structures was previously reported by coculture of human blood lymphocytes with autologous macrophages infected with live M. tuberculosis, M. leprae, or M. bovis or stimulation with mycobacterial antigens such as purified protein derivatives or lipomannan [21], [29], [40], [41]. In the present study, we reported for the first time the development of an in vitro model of ovine granuloma using Map-infected PBMCs cultured in an extracellular matrix composed of fibronectin and collagen, components of the surrounding tissue in which the natural granuloma is anchored. It was previously shown that the ability to develop a well-defined granulomatous response correlated with the severity of mycobacterial infections. When human PBMCs and macrophages were treated with 105 heat-killed M. tuberculosis strain H37Rv they formed aggregates that remained very small and loose [21]. Similarly, human PBMCs infected with avirulent mycobacteria such as M. smegmatis and M. avium formed loose aggregates [20]. We observed that ovine PBMCs infected with Map isolates from sheep and cattle formed well defined aggregates after 10 days of incubation at 37 °C. Map-induced aggregates displayed morphological characteristics similar to natural granulomas, such as three-dimensional aggregation of lymphocytes around macrophages In accordance with our results, granulomatous lesions consistent with Map infection have been recently found in tissue sections from lambs experimentally infected with bovine (C-type) and ovine (S-type) isolates of Map [42]. The advantages of in vitro models of granuloma include reduced cost, increased control, and that they can provide insights into host-mycobacteria interactions at stages of granuloma formation too early to address with animal models. However, the granuloma constitutes a complex immune microenvironment highly affected by additional physiological signals (ie. growth factors and cytokines) which are exclusively produced in infected tissues. As consequence, certain aspects of in vivo granulomas may be different or absent in in vitro models, including intra-granulomatous necrosis, accumulation of fibrin and collagen, and presence and distribution of bacilli. Three-dimensional in vitro models of granuloma may be very useful to: (i) understand what factors or molecules play a role in granuloma formation and in its continued integrity, (ii) evaluate the granuloma-inducing activity of particular antigens or attenuated mutants, and (iii) provide a platform for testing vaccine and drug candidates.
In order to protect paratuberculosis-free herds, control programs have been developed in some countries. The success of these control programs depends on the ability to make decisions regarding on-farm management practices and the movement of animals between regions. For instance, policies regarding mixed farming of cattle and sheep have been based on the apparent host specificity of Map. However, our results suggested that sheep might be susceptible to infection with Map isolates not only from sheep but from cattle, goats, deer, fallow deer and wild boar as well. Therefore, the implementation of measures to prevent the risk of Map transmission from these animal species to sheep in multispecies livestock operations or on farms where sheep share pastures with wildlife animal should be recommended. Because a lack of correlation between genotype and intracellular phenotype of Map isolates in ovine macrophages was observed, destocking polices that aim to eliminate Map should assume equivalence of strains and should not be based on genotype distinction.
Conclusion
Map isolates from cattle, sheep, goats, fallow deer, deer and wild boar showed successful survival phenotype within ovine macrophages regardless of genotype. This phenotype correlated with stimulation of anti-inflammatory, antiapoptotic and antidestructive responses within ovine macrophages. In addition, we showed that two Map isolates from cattle and sheep with distinct genotypes (C and S) were able to induce the formation of in vitro granulomas with a well-defined edge and comparable to the granulomas observed in clinical specimens with respect to the cellular components involved. All together our findings reinforce the hypothesis that Map isolates from cattle, goats, deer, and wild boar may have similar clinical consequences in sheep than sheep isolates of Map which according to our results are not expected to have a selective advantage in causing ovine paratuberculosis.
Acknowledgments
Samples from fallow deer were provided by Dr. Prieto from the Department of Agriculture of the Regional Government of the Principality of Asturias (Spain). Samples from wild-boar and deer were provided by the Instituto de Investigación en Recursos Cinegéticos (IREC). Samples from India, Portugal and USA were kindly provided by S. V. Singh, A.C. Coelho and R. Whitlock, respectively. We thank Dr. Esmeralda Minguijon and Nieves Gomez at the Department of Histopathology (NEIKER-Tecnalia) for excellent technical assistance. Technical and human support provided by SGIker (UPV/EHU, MINECO, GV/EJ, ERDF and ESF) is gratefully acknowledged. We thank Dr. Luiz Bermudez from Oregon State University for helpful discussions. We are grateful to Kyle Hearn and Liam Fitzgerald for the careful editing of the manuscript.
Funding Statement
Financial support for this work was provided by a grant from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) and by European Funds for Regional Development (FEDER) (RTA2011-00049). Naiara Abendaño has a fellowship from the department of Agriculture of the Basque Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bakker D, Willemsen PT, van Zijderveld FG (2000) Paratuberculosis recognized as a problem at last: a review. Vet Q 22: 200–204. [DOI] [PubMed] [Google Scholar]
- 2. Sergeant ES (2001) Ovine Johne's disease in Australia—the first 20 years. Aust Vet J 79: 484–491. [DOI] [PubMed] [Google Scholar]
- 3. Juste RA, Garrido JM, Geijo M, Elguezabal N, Aduriz G, Atxaerandio R, Sevilla I (2005) Comparison of blood polymerase chain reaction and enzyme-linked immunosorbent assay for detection of Mycobacterium avium subsp. paratuberculosis in cattle and sheep. J Vet Diagn Invest 17: 354–359. [DOI] [PubMed] [Google Scholar]
- 4. Ott SL, Wells SJ, Wagner BA (1999) Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med 40: 179–92. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen SS, Toft N (2009) A review of prevalences of paratuberculosis in farmed animals in Europe. Prev Vet Med 88: 1–14. [DOI] [PubMed] [Google Scholar]
- 6. Collins DM, Gabric DM, de Lisle GW (1990) Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol 28: 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevenson K, Hughes VM, de Juan L, Inglis NF, Wright F, et al. (2002) Molecular characterization of pigmented and non-pigmented isolates of Mycobacterium avium subspecies paratuberculosis. J. Clin. Microbiol 40: 1798–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittington RJ, Marsh IB, Whitlock RH (2001) Typing of IS 1311 polymorphisms confirms that bison (Bison bison) with paratuberculosis in Montana are infected with a strain of Mycobacterium avium subsp. paratuberculosis distinct from that occurring in cattle and other domesticated livestock. Mol Cell Probes 15: 139–145. [DOI] [PubMed] [Google Scholar]
- 9. Harris NB, Barletta RG (2001) Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin Microbiol Rev 14: 489–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bermudez LE, Petrofsky M, Sommer S, Barletta RG (2010) Peyer's patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination. Infect Immun 78: 3570–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hostetter J, Steadham E, Haynes J, Bailey T, Cheville N (2003) Phagosomal maturation and intracellular survival of Mycobacterium avium subsp. paratuberculosis in J774 cells. Comparative Immunology, Microbiology and Infectious Diseases 26: 269–283. [DOI] [PubMed] [Google Scholar]
- 12. Sommer S, Pudrith CJ, Colvin CJ, Coussens PM (2009) Mycobacterium avium subsp. paratuberculosis suppresses expression of IL-12p40 and iNOS gene induced by signalling through CD40 in bovine monocyte-derived macrophages. Vet Immunol and Immunopathol 128: 269–283. [DOI] [PubMed] [Google Scholar]
- 13. Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F (2009) Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramakrishnan L (2012) Revisiting the role of the granuloma in tuberculosis. Nature Reviews Immunology 5: 352–366. [DOI] [PubMed] [Google Scholar]
- 15. Saunders BM, Cooper AM (2000) Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol Cell Biol 78: 334–341. [DOI] [PubMed] [Google Scholar]
- 16. Abendaño N, Sevilla IA, Prieto JM, Garrido JM, Juste RA, et al. (2013) Mycobacterium avium subspecies paratuberculosis isolates from sheep and goats show reduced persistence in bovine macrophages than cattle, bison, deer and wild boar strains regardless of genotype. Vet Microbiol 163: 325–334. [DOI] [PubMed] [Google Scholar]
- 17. Abendaño N, Juste RA, Alonso-Hearn M (2013) Review. Anti-inflammatory and antiapoptotic responses to infection: a common denominator of human and bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis . Biomed Res Int 2013: 908348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kabara E, Kloss CC, Wilson M, Tempelman RJ, Sreevatsan S, et al. (2010) A large-scale study of differential gene expression in monocyte-derived macrophages infected with several strains of Mycobacterium avium subspecies paratuberculosis . Briefings in Functional Genomics 9: 220–237. [DOI] [PubMed] [Google Scholar]
- 19. Emile JF, Patey N, Altare F, Lamhamedi S, Jouanguy E, et al. (1997) Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J Pathol 181: 25–30. [DOI] [PubMed] [Google Scholar]
- 20. Lay G, Poquet Y, Salek-Peyron P, Puissegur MP, Botanch C, et al. (2007) Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol 211: 76–85. [DOI] [PubMed] [Google Scholar]
- 21. Birkness KA, Guarner J, Sable SB, Tripp RA, Kellar KL, et al. (2007) An in vitro model of the leukocyte interactions associated with granuloma formation in Mycobacterium tuberculosis infection. Immunol Cell Biol 85: 160–168. [DOI] [PubMed] [Google Scholar]
- 22. Sevilla I, Garrido JM, Guijo M, Juste RA (2007) Pulsed-field gel electrophoresis profile homogeneity of Mycobacterium avium subsp. paratuberculosis isolates from cattle and heterogeneity of those from sheep and goats. BMC Microbiol 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aduriz JJ, Juste RA, Cortabarría N (1995) Lack of mycobactin dependence of mycobacteria isolated on Middlebrook 7H11 from clinical cases of ovine paratuberculosis. Vet Microbiol 45: 211–217. [DOI] [PubMed] [Google Scholar]
- 24. Sevilla I, Singh SV, Garrido JM, Aduriz G, Rodríguez S, et al. (2005) Molecular typing of Mycobacterium avium subspecies paratuberculosis strains from different hosts and regions. Rev Sci Tech 24: 1061–1066. [PubMed] [Google Scholar]
- 25. Peterson K, Koets A, Rutten VPMG, Colenbrander B, Houers DJ (2006) Flow cytometric assessment of circulating peripheral blood monocytes in small ruminants. Small Ruminant Research 65: 136–141. [Google Scholar]
- 26. Olivier M, Berthon P, Chastang J, Cordier G, Lantier F (2001) Establishment and characterisation of ovine blood monocyte-derived cell lines. Vet Immnunol and Immunopathol 8: 139–151. [DOI] [PubMed] [Google Scholar]
- 27. Abendaño N, Sevilla IA, Garrido JM, Prieto JM, Juste RA, et al. (2012) Quantification of Mycobacterium avium subsp. paratuberculosis strains with distinct genotypes and isolated from domestic and wild-life animal species using an Automatic Liquid Culture System. J Clin Microbiol 50: 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapoor N, Pawar S, Sirakova TD, Deb C, Warren WL, et al. (2013) Human granuloma in vitro model for TB dormancy and resuscitation. PLOS One 8: 1.e35657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steitzer U, Gerdes J (2003) Generation and characterization of multicellular heterospheroids formed by human peripheral blood mononuclear cells. Cells Tissue Organs 174: 110–116. [DOI] [PubMed] [Google Scholar]
- 30. Woo SR, Sotos J, Hart AP, Barletta RG, Czuprynski CJ (2006) Bovine monocytes and a macrophage cell line differ in their ability to phagocytose and support the intracellular survival of Mycobacterium avium subspecies paratuberculosis. Vet Immunol Immunopathol 110: 109–120. [DOI] [PubMed] [Google Scholar]
- 31. Harriff MJ, Wu M, Kent ML, Bermudez LE (2008) Species of environmental mycobacteria differ in their abilities to grow in human, mouse, and carp macrophages and with regard to the presence of mycobacterial virulence genes, as observed by DNA microarray hybridization. Appl Environ Microbiol 74: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verna A E, Garcia-Pariente C, Muñoz M, Moreno O, Garcia-Marín et al (2007) Variation in the immune-pathological responses of lambs after experimental infection with different strains of Mycobacterium avium susbp. paratuberculosis. . Zoonoses Public Health 54: 243–252. [DOI] [PubMed] [Google Scholar]
- 33. Janagama HK, Jeong KI, Kapur V, Coussens P, Sreevatsan S (2006) Cytokine responses of bovine macrophages to diverse clinical Mycobacterium avium subspecies paratuberculosis strains. BMC Microbiol 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiss DJ, Evanson OA, Moritz A, Deng MQ, Abrahamsen MS (2002) Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium . Infect Immun 70: 5556–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coussens PM, Colvin CJ, Rosa GJ, Perez Laspiur J, Elftman MD (2003) Evidence for a novel gene expression program in peripheral blood mononuclear cells from Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun 71: 6487–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiss DJ, Evanson OA, Deng M, Abrahamsen MS (2004) Gene expression and antimicrobial activity of bovine macrophages in response to Mycobacterium avium subsp. paratuberculosis. . Vet Pathol 4: 326–337. [DOI] [PubMed] [Google Scholar]
- 37. Murphy JT, Sommer S, Kabara EA, Verman N, Kuelbs MA, et al. (2006) Gene expression profiling of monocyte-derived macrophages following infection with Mycobacterium avium subspecies avium and Mycobacterium avium subspecies paratuberculosis . Physiol Genomics 28: 67–75. [DOI] [PubMed] [Google Scholar]
- 38. Machugh DE, Taraktsoglou M, Killick KE, Nalpas NC, Browne JA, et al. (2012) Pan-genomic analysis of bovine monocyte-derived macrophage gene expression in response to in vitro infection with Mycobacterium avium subspecies paratuberculosis . Vet Res 43: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Theus SA, Cave MD, Eisenach KD (2005) Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J Infect Dis 191: 453–460. [DOI] [PubMed] [Google Scholar]
- 40. Puissegur MP, Botanch C, Duteyrat JL, Delsol G, Caratero C, et al. (2004) An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell Microbiol 6: 423–433. [DOI] [PubMed] [Google Scholar]
- 41. Puissegur MP, Lay G, Gilleron M, Botella L, Nigou J, et al. (2007) Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent, ADAM9- and beta1 integrin-mediated pathway. J Immunol 178: 3161–9. [DOI] [PubMed] [Google Scholar]
- 42. Fernández M, Benavides J, Sevilla IA, Fuertes M, Castaño P, et al. (2014) Experimental infection with C and S-type strains of Mycobacterium avium subspecies paratuberculosis: immunological and pathological findings. Veterinary Research 45: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]