Abstract
Arabidopsis (Arabidopsis thaliana) SUPERMAN (SUP) plays a role in establishing a boundary between whorls 3 and 4 of flowers and in ovule development. We characterized a Petunia hybrida (petunia) homolog of SUP, designated PhSUP1, to compare with SUP. Genomic DNA of the PhSUP1 partially restored the stamen number and ovule development phenotypes of the Arabidopsis sup mutant. Two P. hybrida lines of transposon (dTph1) insertion mutants of PhSUP1 exhibited increased stamen number at the cost of normal carpel development, and ovule development was defective owing to aberrant growth of the integument. Unlike Arabidopsis sup mutants, phsup1 mutants also showed extra tissues connecting stamens, a petal tube and an ovary, and aberrancies in the development of anther and placenta. PhSUP1 transcripts occurred in the basal region of wild-type flowers around developing organ primordia in whorls 2 and 3 as well as in the funiculus of the ovule, concave regions of the placenta, and interthecal regions of developing anthers. Overexpression of PhSUP1 in P. hybrida resulted in size reduction of petals, leaves, and inflorescence stems. The shortening of inflorescence stems and petal tubes was primarily attributable to suppression of cell elongation, whereas a decrease in cell number was mainly responsible for the size reduction of petal limbs.
INTRODUCTION
Flowers of many angiosperms are composed of four kinds of organ that arise in four concentric whorls: sepal in the outermost whorl (whorl 1), petal in whorl 2, stamen in whorl 3, and carpel in the innermost whorl (whorl 4) (Smyth et al., 1990). The number of floral organs in each whorl and the arrangement of the organs within the whorl are genetically determined. The patterning of floral whorls has been explained by the ABC model; the identity of floral organs that generate in each whorl is specified by combinatorial interaction of three classes of homeotic genes, A, B, and C, each of which is expressed in two adjacent whorls (Yanofsky et al., 1990; Jack et al., 1992; Goto and Meyerowitz, 1994). The floral homeotic genes, which mostly encode MADS box–type transcription factors, are conserved among angiosperms. Several studies have demonstrated that the ABC model fundamentally applies to many plant species that have various structures and reproductive systems of flowers. By contrast, the mechanisms for determining the number and position of floral organs in each whorl have been much less studied. The size of floral meristem in respective whorls is a key determinant of the number of floral organs. For instance, Arabidopsis (Arabidopsis thaliana) clavata mutants have enlarged floral meristems, and the total number of their floral organs is increased in proportion to the size of the meristem (Clark et al., 1993). Similarly, the organ number in each whorl seems to be correlated with the size of the whorl (Meyerowitz, 1997), but less is understood about how the number of each type of floral organ is determined.
Arabidopsis superman (sup) mutants have an increased number of stamens and defective pistil (Schultz et al., 1991; Bowman et al., 1992). The SUP gene is expressed in the subdomain of whorl 3 adjacent to whorl 4 during a very early stage of flower development (Sakai et al., 1995). This expression is dependent on the floral meristem gene LEAFY and two class B homeotic genes, APETALLA3 (AP3) and PISTILLATA (PI) (Sakai et al., 2000). On the basis of expression and epistasis studies, as well as the phenotype attributable to constitutive expression of AP3 and PI in the sup background (Krizek and Meyerowitz, 1996), SUP is thought to coordinate proliferation of stamen- and carpel-specific meristematic cells, keeping the proper structure of whorls and maintaining the boundary between whorl 3 and whorl 4 at the right position (Sakai et al., 2000). Ectopic expression of SUP in Nicotiana tabacum (tobacco) plants causes a decrease in cell number in various organs, resulting in reduction in the sizes of those organs (Bereterbide et al., 2001). This observation supports the involvement of SUP in the control of cell proliferation. Another report proposes a role of SUP in cell elongation on the basis of the phenotype resulting from ectopic expression of SUP in petals and stamens of Petunia hybrida (petunia) (Kater et al., 2000). In addition to the early floral meristem function we have just described, SUP plays a role in the development of ovules (Gaiser et al., 1995). Normal ovules have a hood-like morphology because of asymmetric growth of the outer integument. By contrast, the ovule in sup mutants is nearly radially symmetrical because of the loss of asymmetry in the growth of the outer integument. SUP is expressed in developing ovule primordia, and this expression later becomes restricted to the stalks of ovules, called funiculi. This so-called late expression is thought to be responsible for SUP function in the morphogenesis of ovules. This function of SUP in ovule development also can be regarded as a control of cell division.
In angiosperms, the number of organs in each floral whorl and their arrangement within the whorl differ between plant species, as do the structures of the floral organs. Whether orthologs of SUP, if present, play the same role as in Arabidopsis or have other roles in the diversification of flower and organ structures is of particular interest. Orthologs of SUP, however, have not been reported, presumably owing to their extremely low expression level (Sakai et al., 1995). P. hybrida has been used as one of the model plants to study flower development, in part because of the ease of Agrobacterium tumefaciens–mediated transformation and the availability of transposon-inserted gene knockout mutants (Koes et al., 1995). Several floral homeotic genes, which have unique features in their expression and functioning, have been reported (van der Krol and Chua, 1991; Angenent et al., 1993; Tsuchimoto et al., 1993; Colombo et al., 1995; Kapoor et al., 2002; Ferrario et al., 2003). In Solanaceae plants, including P. hybrida, petals are fused with each other at their margins and with stamen filaments in their lower parts, whereas in Arabidopsis, all flower organs are separated from each other. The structure of the ovary differs markedly between P. hybrida and Arabidopsis. The ovule of P. hybrida has only one layer of integument, whereas the Arabidopsis ovule has two (outer and inner) integuments. Whether differences between the functioning of SUP and its P. hybrida counterpart are responsible for the differences in flower architecture between the two plant species is an attractive question.
We have isolated a SUP-like gene, designated PhSUP1, from P. hybrida and demonstrated that this gene is a P. hybrida counterpart of SUP by showing that PhSUP1 genomic DNA can partially complement the Arabidopsis sup mutation. Transposon-inserted knockout mutants for PhSUP1 displayed sup mutant–like phenotypes in stamen number and ovule morphology. In addition, however, the phsup1 mutants exhibited some unique phenotypes that have not been reported for Arabidopsis sup mutants (i.e., generation of extra tissues at the base of the stamen and defective development of anther and placenta). The distribution of PhSUP1 transcripts in the respective organs seemed to account for the distinctive mutant phenotypes. Overexpression of PhSUP1 expression affected both division and elongation of cells in various organs. In light of our results, we discuss the conservation and diversification of SUP/PhSUP1 function between P. hybrida and Arabidopsis.
RESULTS
Isolation of the PhSUP1 Gene from P. hybrida
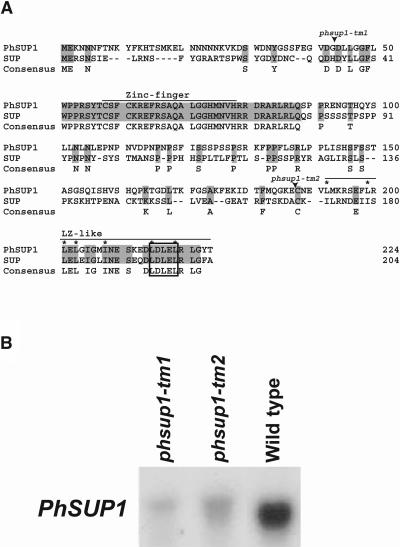
We have isolated four partial DNA sequences for SUP-like zinc-finger proteins in P. hybrida by PCR-based cloning using degenerate primers, which were designed on the basis of the conserved amino acid sequences between SUP and SUP-like proteins in Glycine max (soybean) (H. Kouchi, unpublished results). Among these clones, the one named PhSUP1 showed the highest similarity to SUP; therefore, we isolated its corresponding cDNA and genomic DNA clones. PhSUP1 cDNA is 1278 bp long and encodes a protein of 224 amino acids. Although the overall deduced amino acid sequence identity between PhSUP1 and SUP is only 39%, their zinc-finger domains and C-terminal regions are highly conserved (Figure 1A), with the zinc-finger motifs and flanking basic residues being completely identical over 38 amino acid residues. The zinc-finger motif in SUP has been demonstrated to serve as a DNA binding domain (Dathan et al., 2002). An ethylene-responsive element binding factor–associated amphiphilic repression (EAR)-like motif (Hiratsu et al., 2002) and two overlapping Leu zipper–like motifs (Sakai et al., 1995), which have been found in SUP, are also present in the C-terminal region of the deduced PhSUP1 protein. The EAR-like motif in SUP has been demonstrated to act as an active transcriptional repressor domain (Hiratsu et al., 2002). Sequence comparison between PhSUP1 cDNA and the corresponding genomic sequence revealed that the PhSUP1 gene lacks introns.
Figure 1.
Structure of PhSUP1 Gene and Its Expression in dTph1 Insertion Alleles.
Total RNA from whorl 3 and whorl 4 flower organs was hybridized with an antisense PhSUP1 probe.
(A) Alignment of deduced PhSUP1 and SUP proteins. Identical amino acid residues are shaded. Zinc-finger and Leu zipper (LZ)–like domains are indicated. Asterisks indicate conserved Leu–isoleucine residues in the Leu zipper–like motif. An EAR-like motif is boxed.
(B) Expression of PhSUP1 in wild-type and mutant lines.
PhSUP1 Partially Complements the Arabidopsis sup Mutant
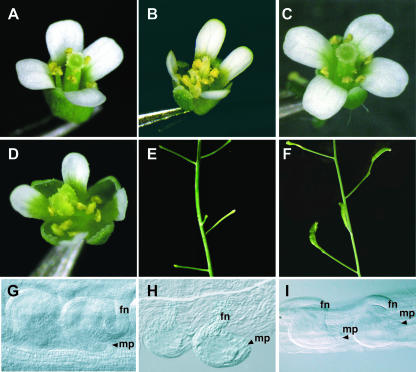
To test whether PhSUP1 is functionally equivalent to Arabidopsis SUP, we introduced a 3.8-kb genomic DNA fragment containing the 5′-upsteam (2.5 kb), coding, and 3′-untranslated (UTR) (0.46 kb) regions of PhSUP1 (gPhSUP1) into an Arabidopsis sup mutant. In wild-type Arabidopsis, a flower consists of four sepals, four petals, six stamens, and one pistil (Figure 2A). The ovules are hood-like owing to asymmetric growth of the outer integument (Figure 2G) (Gaiser et al., 1995). Almost all wild-type Arabidopsis flowers were fertile (i.e., 323 out of 324 flowers set seeds after flowering). By contrast, flowers of the sup mutant (sup2) had an increased number of stamens (8.6 ± 1.6; n = 45, from three plants) at the expense of normal pistil development (Figure 2B). Ovules in the sup mutant lacked asymmetry in the growth of the outer integument (Figure 2H) (Gaiser et al., 1995). Because of these aberrancies in the development of the pistil and ovules, the sup mutant had severely reduced fertility (i.e., only 8 of 342 flowers from three plants set seeds) (Figure 2E).
Figure 2.
Complementation of Arabidopsis sup Mutants by PhSUP1 Genomic DNA.
(A) to (D) Flowers of wild-type (A) and sup mutant (B) Arabidopsis plants and those of a sup(PhSUP1) plant that contains a PhSUP1 genomic fragment as a transgene ([C] and [D]). Flowers in the sup(PhSUP1) plants showed almost complete (C) or weak (D) recovery of flower development.
(E) and (F) Inflorescence in sup mutant (E) and sup(PhSUP1) plants (F) after flowering. The sup mutant produced no fertilized siliques (E), whereas sup(PhSUP1) plants frequently produced them (F).
(G) to (I) Ovules in wild-type (G), sup mutant (H), and sup(PhSUP1) (I) plants. Siliques in the sup(PhSUP1) plant contained both sup-like (left) and wild-type-like (right) ovules. The top of the pistil is at the right. fn, funiculus; mp, micropyle.
Introduction of the gPhSUP1 sequence markedly recovered the developmental aberrancies in sup mutants (Figures 2C and 2D). Compared with the sup mutant, the gPhSUP1-transformed sup mutants (gPhSUP1/sup) had fewer stamens (6.9 ± 0.9, n = 45, from three plants) and partially restored normal pistil morphology (Figure 2C). Whereas normal pistils are rarely formed in sup mutants, 27% of the flowers of gPhSUP1/sup plants had normal-looking pistils (Figure 2C). Stamen–pistil mosaic organs formed in many gPhSUP1/sup flowers (Figure 2D), but their staminoid feature was less prominent than that in the sup mutant. Most gPhSUP1/sup flowers contained ovules in their ovaries, whereas half of the sup flowers completely lacked ovules. In the ovaries of the gPhSUP1/sup plants, both normal-looking and sup-like ovules were present (Figure 2I), and the proportion of normal-looking ovules increased toward the apical tip of the pistil. Because of the partial restoration to a normal morphology of their pistils and ovules, the gPhSUP1/sup plants partially recovered female fertility (i.e., 58 of 343 flowers from three plants were fertile) (Figure 2F). These results indicate that PhSUP1 is an ortholog of SUP.
PhSUP1 Knockout Mutants Have a sup-Like Phenotype
To characterize the loss-of-function phenotype of PhSUP1, we screened a library of transposon (dTph1)-inserted mutant lines of P. hybrida (Koes et al., 1995) and obtained two recessive insertional alleles for PhSUP1 (phsup1-tm1 and phsup1-tm2, Figure 1A). The phsup1-tm1 allele had a dTph1 insertion immediately upstream of the zinc-finger domain, causing interruption of the reading frame. Because the phsup1-tm1 allele encodes only a short truncated protein that lacks both the zinc-finger and Leu zipper/EAR motif–like sequences, it is most likely a nonfunctional allele. The other allele, phsup1-tm2, contained a dTph1 insertion upstream of the Leu zipper/EAR–like motif, resulting in the replacement of the C-terminal 37 residues, including the putative repressor domain with an unrelated sequence. Because both mutant alleles had the same phenotype, the phsup1-tm2 also was presumed to be a null allele. RNA gel blot analysis detected low levels of PhSUP1-derived transcripts of slightly increased sizes because of the dTph1 insertion in both alleles (Figure 1B). The phenotypes observed in these mutants were multifaceted: some of them mimicked those in Arabidopsis sup mutants, but others were unique to the phsup1 mutants.
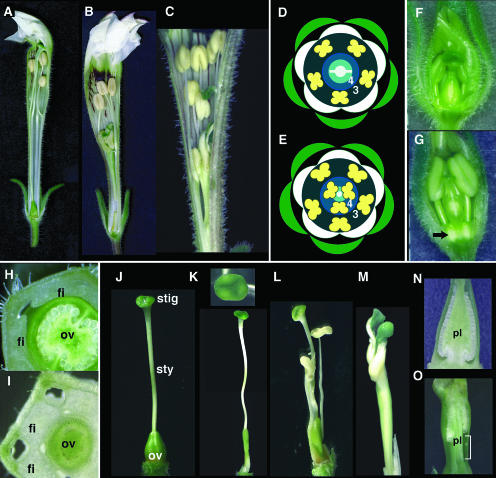
The nongenerative organs of the phsup1 mutants were apparently normal. Floral organs of the outer three whorls (sepals, petals, and stamens) were also indistinguishable from those of wild-type P. hybrida; however, whorl 4 organs showed remarkable aberrancies in their number and characteristics. Whorl 4 of wild-type P. hybrida flowers forms a pistil that generates by fusion of two carpels and consists of a stigma, a long style, and a small conical ovary (Figures 3D and 3J). In whorl 4 of phsup1 flowers, one to three extra stamens were formed, and the pistils often comprised one, three, or four carpels (Figures 3B, 3C, 3E, and 3K to 3M). The extra stamens were frequently fused with the pistil to various extents (Figures 3L and 3M). For instance, the flower in Figure 3L has two extra stamens, with one of them fused to the ovary at its base and the other fused to the pistil almost throughout the organ. In a severe case, two extra stamens were completely fused to a pistil, and they developed as antheroid sectors in a stamen–carpel mosaic organ (Figure 3M). In such a chimeric organ, a whitish stripe of stamen filament–like tissue can be distinguished below the antheroid tissue (Figure 3M). Carpels were usually incompletely fused with each other, and each was tipped by a stigma (Figures 3B, 3C, and 3M). Some mutant flowers did not contain extra stamens; instead, they contained a pistil consisting of three carpels (Figure 3K). In this case as well, the style showed a stamen filament–like feature. These phenotypes are similar to those in Arabidopsis sup mutants (Schultz et al., 1991; Bowman et al., 1992), further indicating that PhSUP1 is a P. hybrida counterpart of SUP.
Figure 3.
Phenotypes of phsup1 Mutants in Flower Development.
The wild-type flower consists of four concentric whorls of organs: five sepals, five petals, five stamens, and a pistil composed of two carpels. Whorl 3 (3) and whorl 4 (4) organs are indicated. Number and identity of organs in whorls 1, 2, and 3 in phsup1 mutant flowers are the same as those in the wild type, but two or three extra stamens were generated in whorl 4.
(A) to (C) Side views of P. hybrida flowers in wild-type (A) and phsup1-tm1 ([B] and [C]) plants. A few petals and sepals have been removed to show whorl 4 organs.
(D) and (E) Flower diagrams of wild-type (D) and phsup1 mutant (E) flowers.
(F) and (G) Side views of 10-mm flower buds in wild-type (F) and phsup1-tm1 (G) plants. Extra tissues are seen at the bases of whorl 3 stamens in phsup1-tm1 flowers as indicated by an arrow (G).
(H) and (I) Transverse sections at the basal part of 10-mm flower buds in wild-type (H) and phsup1-tm1 (I) plants. fi, filament; ov, ovary.
(J) to (M) Whorl 4 organs in wild-type (J) and phsup1-tm1 ([K] to [M]) plants. Wild-type pistils consist of two fused carpels (J). In phsup1-tm1, pistils often consisted of three carpels, and the style shows a stamen filament-like feature (K). Extra stamens were frequently generated and were usually fused with a pistil to various extents ([L] and [M]) (i.e., stamens were fused with an ovary at the base of their filaments [L] or fused with a style throughout the entire filament [M]). ov, ovary; stig, stigma; sty, style.
(N) and (O) Ovaries in wild-type (N) and phsup1-tm1 (O) plants. The bottom of the placenta was elongated in phsup1-tm1 ovaries as indicated. pl, placenta.
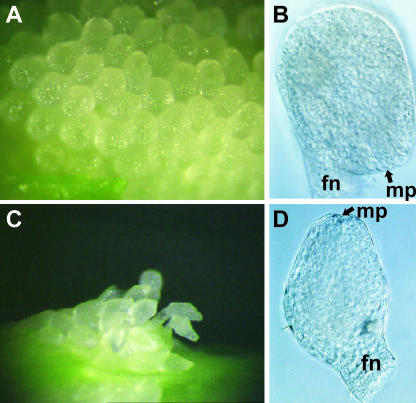
The wild-type ovary is conical and is located at the base of a flower (Figures 3A and 3J). The ovules are formed throughout the placenta (Figure 3N). By contrast, in the phsup1 mutants, the ovary is thin and elongated (Figures 3B, 3C, and 3K to 3M), and far fewer ovules were formed than in wild-type ovaries (Figure 3O). The aberrancies observed in the morphology of phsup1 ovules are similar to those reported for the Arabidopsis sup ovules (Gaiser et al., 1995). Unlike Arabidopsis ovules, which have two layers (inner and outer) of integument, P. hybrida has only a single layer of integument. The wild-type ovule has a bilaterally symmetrical hood-like morphology, and the micropyle is adjacent to the funiculus (Figures 4A and 4B) because the integument grows exclusively at the adaxial side (toward the top of the ovary). By contrast, the integument of phsup1 mutants grew evenly around a nucellus, forming nearly radially symmetrical tubular ovules (Figures 4C and 4D); consequently, the micropyle is positioned at the top of the ovule (Figure 4D).
Figure 4.
phsup1 Mutant Phenotypes in Ovules.
Stereomicroscopic images of ovules in wild-type (A) and phsup1-tm1 (C) and differential interference contrast optics of a cleared ovule in wild-type (B) and phsup1-tm1 (D). fn, funiculus; mp, micropyle.
PhSUP1 Plays a Role in the Morphogenesis of Various Floral Organs
In addition to the phenotypes that are like those of Arabidopsis sup mutants, phsup1 mutants also displayed additional aberrancies in the morphologies of various floral organs. In wild-type P. hybrida flowers, the stamen filaments are flat at their basal parts and are tightly fused with the petal tube (Figure 3H). In the phsup1 flower, unusual tissues consisting of highly vacuolated cells were generated around the filaments (Figures 3G and 3I). Presumably, these extra tissues resulted from excessive proliferation of the cells that normally form junctions between stamen filaments and petal tubes.
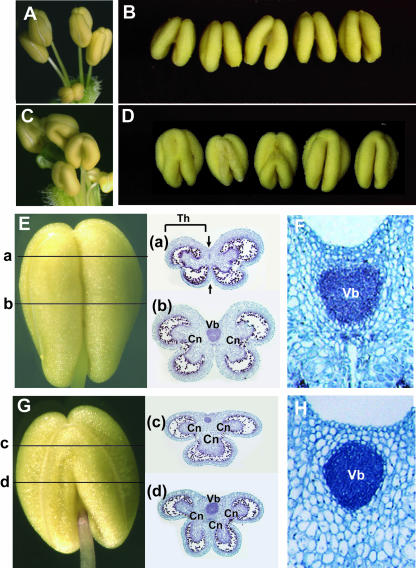
The phsup1 mutants were also defective in the shape of anthers (Figures 5A to 5D). The mature anther normally consists of two distinctively partitioned thecae with an interthecal furrow between them, and each theca consists of two locules (Figure 5E). In mature anthers in phsup1 mutants, the two thecae appear to be fused in the upper part at the adaxial side of the anther (Figure 5G, c). The lower part is apparently normal, but its transverse section revealed an abnormal development around the vascular bundle (Figures 5G, d and 5H). In wild-type anthers, interthecal furrows are well developed, which makes the anther walls closely bound to the vascular bundle (Figures 5E, a; 5E, b; and 5F). By contrast, the anther walls of phsup1 mutants were less closely bound to the vascular bundle, with excessively proliferated connective tissue intervening (Figures 5G, c; 5G, d; and 5H). Close-ups of the vascular bundles of wild-type anthers revealed that cells at the interthecal furrow at both the adaxial and abaxial sides of the vascular bundle are smaller than the cells in neighboring regions (Figure 5F). By contrast, the cells in the corresponding regions of phsup1 mutant anthers are the same size as those in neighboring regions (Figure 5H).
Figure 5.
phsup1 Mutant Phenotype in Anthers.
(A) to (D) Anthers in wild-type ([A] and [B]) and phsup1-tm1 ([C] and [D]) plants.
(E) and (G) A mature wild-type anther and its transverse sections at positions a and b (E) and a mature phsup1-tm1 anther and its transverse sections at positions c and d (G). Cn, connective tissue; Th, theca; Vb, vascular bundle.
(F) and (H) Close-up views of the transverse sections at lower parts of developing anthers around a vascular bundle in the wild-type (F) and phsup1-tm1 (H) anthers. Arrows in E-a indicate interthecal furrows. Vb, vascular bundle.
Scanning Electron Microscopy Analysis at Early-Stage Flower Development
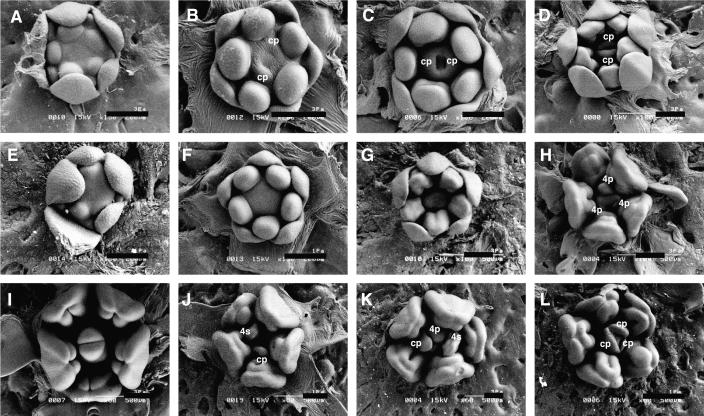
Using scanning electron microscopy, we investigated the early-stage development of stamens in wild-type and phsup1 mutant flowers. There was no difference in size and dome-shaped morphology of stamen primordia between wild-type (Figures 6A and 6B ) and phsup1 mutant (Figures 6E and 6F) flowers at early stages. Later, longitudinal hollows formed at the adaxial side of wild-type anthers (Figure 6C), and they subsequently developed into interthecal furrows (Figure 6D). The wild-type anther then separated into four locules (Figures 6I). In phsup1 mutant flowers at these stages, aberrancies in the development of interthecal furrows were observed; in particular, the top parts at the adaxial side of developing anthers failed to separate into two inner locules (Figures 6H and 6J to 6L).
Figure 6.
Scanning Electron Micrographs Depicting Flower Development.
(A) to (D) and (I) Development of wild-type flowers. In each stage, petal and sepal primordia arise (A), stamen primordia become dome-shaped and carpel primordia arise (B), stamen primordia are stalked and carpels are fused at the base (C), and then the carpel grows vertically as a slotted tube (D). Later, anther locules are formed, the gynoecium fuses at the top, and stigma and style appear (I). cp, carpel primordia.
(E) to (H) and (J) to (L) Development of phsup1-tm1 mutant flowers. Stages of phsup1-tm1 mutant flower in (E), (F), (G), and (H) correspond to those of wild-type flowers in (A), (B), (C), and (D), respectively. No sign of whorl 4 organ initiation is seen in early stages ([E] to [G]). In a following stage, a ring of three organ primordia (4p) initiates interior to whorl 3 stamens (H), and various patterns of organs appear in whorl 4. Outer whorls of flowers have been removed to show inner organs. 4p, undifferentiated whorl 4 organ primordia; 4s, whorl 4 stamen primordia; cp, carpel primordia.
Bar = 200 μm.
In wild-type P. hybrida, two carpel primordia are initiated as horseshoe-shaped banks within whorl 4 when stamen primordia become dome-shaped (Figure 6B). These two carpel primordia are fused at the base (Figure 6C) and grow vertically as a slotted tube (Figure 6D). Subsequently, the bicarpellate pistil becomes fused at the top to form the style and stigma, completing the ontogeny of a wild-type pistil (Figure 6I). In the phsup1 mutant flower, initiation of whorl 4 organs was delayed (Figures 6F and 6G). Later, two to four organ primordia were initiated within a ring interior to whorl 3 (Figure 6H), and these organ primordia started to differentiate into extra stamens, carpels, or stamen–carpel mosaic organs (Figures 6J to 6L). The one or two primordia that initiated early tended to develop as extra stamens, and the other late-initiating ones develop as pistils or stamen–carpel mosaic organs (Figures 6J and 6K). In some cases, three organ primordia developed as carpels (Figure 6L) and fused to form a tricarpellate pistil (Figure 3K).
Expression of PhSUP1
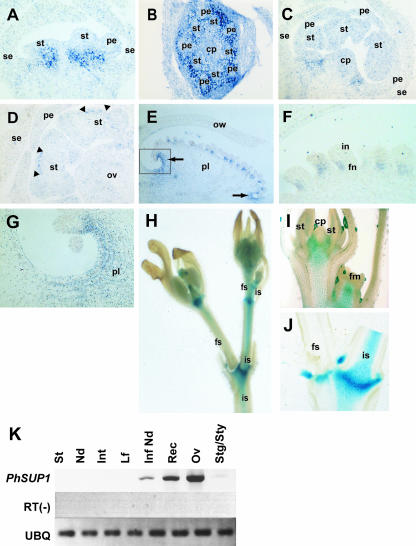
Distribution of PhSUP1 transcripts in developing flower organs was examined by in situ hybridization. At stages when organ primordia begin to differentiate, PhSUP1 transcripts were detected at the basal region of developing whorl 2 and whorl 3 organs (Figure 7A). Transverse sections showed that the expression was distributed in marginal regions between organ primordia and was excluded from the organ primordia themselves (Figures 7B). PhSUP1 transcripts also were detected in developing anthers as bands of signals in the interthecal region after the formation of thecae (Figure 7D). At later stages, PhSUP1 expression appeared in the funiculi that constitute the basal part of the ovule (Figures 7E and 7F). Distribution of PhSUP1 transcripts in the funiculi was asymmetric: they were localized at the side abaxial to that in which the growth of the integument is suppressed. PhSUP1 also was expressed at the top and bottom parts of the developing placenta, where the organ is concave and free of ovules (Figures 7E and 7G). The concave area at the bottom of the placenta remains in the mature flower, whereas that at the top of placenta has disappeared because of the outgrowth of style (Figure 3N). In the mature flower of phsup1 mutants, the concave area at the bottom of placenta is lost; instead, the corresponding regions are elongated (Figure 3O), suggesting a role of PhSUP1 in the morphogenesis of the placenta.
Figure 7.
Expression Pattern of PhSUP1 Gene.
(A) to (D) In situ hybridization of PhSUP1 transcripts in developing flower. Longitudinal ([A] and [C]) and transverse (B) sections of flower buds are shown. The stage of flower in (A) and (B) corresponds to that in Figure 6B, (C) corresponds to Figure 6C, and (D) corresponds to Figure 6I. In (D), bands of expression are indicated by pairs of closed triangles. cp, carpel; ov, ovary; pe, petal; se, sepal; st, stamen.
(E) to (G) In situ hybridization of PhSUP1 transcripts in a young ovary. Shown are a longitudinal section of an ovary (E), a higher-magnification view of ovules (F), and a close-up of basal part of placenta ([G], boxed area in [E]). In (E), top and bottom of the ovary are shown to the right and left in the panel, respectively, and PhSUP1 transcripts in the placenta are indicated by arrows. ow, ovary wall; pl, placenta; in, integument; fn, funiculus.
(H) to (J) Histochemical staining of GUS activity driven by PhSUP1 promoter in the inflorescence (H), a young flower bud (I), and an inflorescence node (J). st, stamen; cp, carpel; fm, floral meristem; fs, flower stalk; is, inflorescence stalk.
(K) RT-PCR analysis of PhSUP1 transcripts in vegetative and floral organs from 10-mm flower buds. St, shoot tip; Nd, vegetative stem node; Int, stem internode; Lf, leaf; Inf Nd, inflorescence node; Rec, receptacle; Ov, ovary; Stg/Sty, stigma/style; UBQ, ubiquitin.
To investigate the expression of PhSUP1 in other parts of plants, we constructed transgenic P. hybrida plants harboring a recombinant reporter gene that is comprised of a 2.5-kb 5′-upstream region of PhSUP1 fused upstream of the β-glucuronidase (GUS) coding sequence (PhSUP1:GUS). Three independent PhSUP1:GUS transgenic plants were characterized for promoter activity. In young flowers, the PhSUP1:GUS plants expressed GUS activity in the ovary and at boundary regions between developing stamens and carpels (Figure 7I), consistent with the results of in situ hybridization experiments (Figure 7C). In addition, the expression extended into receptacles and piths in inflorescent stems. A recent study by Ito et al. (2003) demonstrated that negative cis elements determining whorl-specific expression of SUP are located in the coding region. Because our PhSUP1:GUS construct does not include the coding region, it is quite possible that the lack of certain negative elements resulted in the ectopic promoter activity. The upstream region of SUP between −3 and −5 kb contains cis elements for early expression (Ito et al., 2003). Our PhSUP1:GUS construct contains only up to −2.5 kb of upstream sequence, which could also affect the accuracy of the promoter activity. Figures 7H and 7J show GUS activity in the inflorescence, with particularly strong activity at the nodal region. The GUS activity in the inflorescence is stronger in inflorescence stalks than in flower stalks (Figures 7H and 7J). In an RT-PCR experiment, PhSUP1 transcripts were detected in the inflorescence nodes (among vegetative tissues) and in the ovary and receptacle but not in stigmas or styles of developing flowers, consistent with the results of the promoter–GUS experiments (Figure 7K). These results suggest that PhSUP1 plays a role in the growth of inflorescences as well. However, we did not find any visible aberrancy in the growth of inflorescence in the phsup1 mutants.
Phenotype of Plants Overexpressing PhSUP1
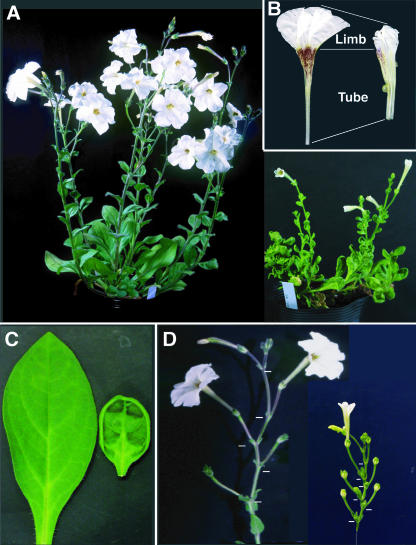
To characterize the effects of ectopic expression of PhSUP1, its cDNA was driven by the 35S promoter of Cauliflower mosaic virus (CaMV) in transgenic P. hybrida plants. Two lines were found to overexpress PhSUP1, and they both showed the same dwarf phenotype (PhSUP1-ox plants, Figure 8A). The overexpression of PhSUP1 caused reduction in the sizes of flower organs by an average of 60 to 70% compared with wild-type organs, with the most severe reduction being in the petal limbs (∼30% of wild type, Figure 8B). The leaves were also smaller than those of wild-type plants and were curled up at their margins (Figure 8C). The inflorescence of P. hybrida consists of two types of stem: the pedicel (floral stalk) terminates in a flower, and the peduncle (inflorescence stalk) constitutes a main stem. The internodes of the peduncles in PhSUP1-ox plants were shortened by ∼30% compared with those in wild-type plants, whereas pedicels were only slightly shortened (∼85% of wild type, Figure 8D).
Figure 8.
Phenotype of a PhSUP1-Overexpressing P. hybrida.
Wild-type and PhSUP1-ox phenotypes are shown at the left and right in the panels, respectively. Bars in (D) indicate positions of inflorescence nodes.
(A) Whole plants.
(B) Flowers.
(C) Leaves.
(D) Inflorescences.
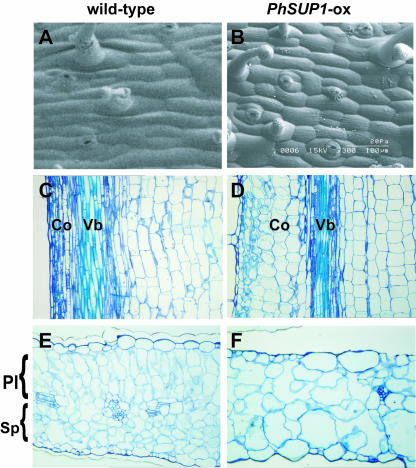
Scanning electron microscopy revealed that PhSUP1 overexpression markedly suppressed the longitudinal growth of the epidermal cells in the peduncles (Figures 9A and 9B). The length of the epidermal cells in the peduncle was ∼40% of the wild type, a difference that is nearly proportional to the suppression in the length of peduncle (Table 1). The size of the pedicel epidermal cells was 82% of the wild type (Table 1), which is also proportional to the suppression in the pedicel length. Longitudinal sections of a peduncle revealed that the elongation of inner cells also was affected in a similar manner to that in the epidermal cells (Figures 9C and 9D). These results indicate that suppression of inflorescence growth in PhSUP1-ox plants is primarily attributable to suppression of cell elongation.
Figure 9.
Effects of PhSUP1 Overexpression on Cellular Morphology.
(A) and (B) Scanning electron microscopy images of peduncle epidermal cells in wild-type (A) and PhSUP1-ox (B) P. hybrida plants. The shoot apex is oriented to the left in the panels.
(C) and (D) Longitudinal sections of peduncles in wild-type (C) and PhSUP1-ox (D) plants. Co, cortex; Vb, vascular bundle.
(E) and (F) Cross-sections of leaves in wild-type (E) and PhSUP1-ox (F) plants. Pl, palisade mesophyll layer; Sp, spongy mesophyll layer.
Table 1.
Correlation between the Sizes of Organs and Cells
| Length (mm)
|
Cell Size (μm)
|
|||||
|---|---|---|---|---|---|---|
| WT | PhSUP1-ox | %WT | WT | PhSUP1-ox | %WT | |
| Peduncle internode | 38.6 ± 3.8 | 12.7 ± 1.4 | 32.9 | 168.4 ± 27 | 66.8 ± 9.6 | 39.8 |
| Pedicel | 36.9 ± 2.7 | 31.2 ± 2.6 | 84.6 | 148.1 ± 24 | 121.63 ± 25 | 82.1 |
| Petal limb | 20.8 ± 0.9 | 8.0 ± 0.4 | 38.5 | 20.2 | 19.6 | 97.0 |
| Petal tube | 52.2 ± 1.4 | 38.4 ± 1.7 | 73.6 | 118.2 ± 23 | 91.6 ± 16 | 77.6 |
The lengths of peduncle internodes and pedicels are the mean ± 1 standard deviation of 10 samples, and the cells sizes are the mean ± 1 standard deviation of 30 cells. The lengths of petal limbs and petal tubes are the mean ± 1 standard deviation of 15 flowers, and the corresponding cell sizes were estimated from cell numbers in scanning electron micrographs (300 × 258 μm), assuming that cells are approximately round. Cell sizes are in longitudinal direction.
PhSUP1-ox, line overexpressing PhSUP1.
Scanning electron microscopy of the adaxial surface of petals revealed that the sizes of epidermal cells in the petal limb of PhSUP1-ox plants were comparable with those in wild-type plants (Table 1). These observations indicate that the shortening of the petal limb is mainly because of a decrease in cell number. By contrast, the reduction in the length of the petal tube was nearly proportional to the reduction in the length of cells (78% of the wild type), suggesting that suppression of cell elongation is the main cause for the reduced growth of petal tubes (Figure 8B).
PhSUP1 overexpression also affected the morphology and sizes of cells in leaf mesophyll tissues (Figures 9E and 9F). Wild-type leaf mesophyll tissue is distinctively differentiated into two types of cell layer: two to three layers of palisade cells at the adaxial side and four to five layers of spongy cells with increased intercellular space along the abaxial side (Figure 9E). By contrast, the leaf mesophyll tissue in the PhSUP1-ox plant was not clearly differentiated into the two cell types, instead appearing as a homogeneous tissue consisting of only spongy cells (Figure 9F). The number of mesophyll cell layers and total number of cells were clearly decreased, whereas individual cells are expanded.
DISCUSSION
PhSUP1 Is a P. hybrida Counterpart of Arabidopsis SUP
In this study, we have shown that PhSUP1 is a P. hybrida ortholog of the Arabidopsis SUP gene. Introduction of PhSUP1 genomic DNA into an Arabidopsis sup mutant resulted in partial complementation of the early phenotypes (i.e., increased stamen number and defective carpel development) and the late phenotype of loss of bilateral symmetry in ovule development. Transposon-induced mutants displayed sup-like phenotypes with respect to stamen number, carpel development, and ovule development. These results indicate that both the early floral meristem function in the floral meristem and the late function in ovule development are common between Arabidopsis SUP and P. hybrida PhSUP1.
The incompleteness of the complementation may be attributable to differences in protein function or to an imperfect match of the PhSUP1 promoter activity and the expression pattern of SUP in Arabidopsis. Considering the difference in flower structures between the two plant species and the similarity in the phenotypes resulting from overexpression of the two genes (discussed later), the faulty promoter scenario seems the more likely.
Null alleles of Arabidopsis sup mutants occasionally exhibited nearly complete loss of carpel formation and increased stamen numbers up to as many as 20. By contrast, phsup1 mutant P. hybrida plants never completely lacked carpels, and the number of extra stamens was at most 3; therefore, the effects of the mutation appear to be less severe in P. hybrida. The difference in the number of extra stamens might reflect the size of region of the floral apex, which is predestinate to form whorls 3 and 4, relative to those of stamen primordia. It has been proposed that the number of organs formed in a particular whorl is correlated with the size of each whorl (Meyerowitz, 1997). In Arabidopsis, because the size of floral apex is much larger than that of stamen primordia, more than one ring of extra stamen primordia appear in the floral apex of sup mutants. By contrast, the floral apex of P. hybrida is only a few times larger than the region occupied by stamen primordia, which might reflect the small number of extra stamens in phsup1 mutant flowers.
We detected PhSUP1 transcripts at the bases of developing whorl 2 and whorl 3 organs only after stamen primordia emerged. This distribution pattern of transcripts is different from the early-stage expression of Arabidopsis SUP, which localized in the subdomain of whorl 3 adjacent to whorl 4. Considering that the expression level of SUP is low (Sakai et al., 1995), it is possible that we missed very early expression of PhSUP1 at a stage earlier than that shown in Figure 7A. Therefore, we refrain from discussing early functions of PhSUP1, in light of its observed expression, and comparing with those of Arabidopsis sup.
PhSUP1 Function in Ovule Development
PhSUP1 plays a role in ovule development of P. hybrida, as SUP does in Arabidopsis. The conservation of SUP/PhSUP1 function in ovule development in the two plant species has implications for the evolution of the ovule structure in dicot plants. Whereas the Arabidopsis ovule has two layers of integument (bitegmy), the P. hybrida ovule has a single-layer integument (unitegmy). The unitegmic ovule of Asteridae plants, including P. hybrida, is thought to have derived from the bitegmic ovules during evolution either by loss of either the outer or inner integument or fusion of the two primordia for outer and inner integuments (Fahn, 1990). Gaiser et al. (1995) found that the role of SUP is limited to development of the outer integument. This finding and the fact that PhSUP1 is involved in the development of the unitegmic ovule in P. hybrida argue against the possibility that the P. hybrida integument originated from the inner integument.
PhSUP1 Function in Intercalary Growth at the Base of Flower Organs
In P. hybrida, stamen filaments are fused to the petal tube at their lower portion. This structure results from the intercalary growth of cells between stamen and petal primordia that begins just after emergence of the organ primordia (van der Krol et al., 1993). phsup1 mutants have some unusual tissues around the bottom of the stamen filaments. These extra tissues appear to connect stamen filaments with petal tubes and also with the ovary, and they seem to have arisen by excessive progression of the intercalary growth in interorgan regions. PhSUP1 is expressed in the region between organ primordia, which is in agreement with the phenotype observed in this region of phsup1 mutants. Unlike in P. hybrida, the stamens of Arabidopsis are separated from petals and pistil. In accordance with this difference in flower structure, this extra-tissue phenotype has not been reported for Arabidopsis sup mutants. PhSUP1 may contribute to flower morphogenesis by suppressing over-progression of intercalary growth. Presumably, this particular role of PhSUP1 has coevolved with the flower structure of P. hybrida.
PhSUP1 Function in Placenta Morphogenesis
PhSUP1 transcripts were detected around the concave regions at the top and bottom of the placenta in the ovary. In light of this expression pattern, the concavity at the bottom of the placenta appears to be lost in phsup1 mutants with apparent elongation of the corresponding region, suggesting a role of PhSUP1 in controlling placenta morphogenesis. This loss-of-concavity phenotype has not been reported for Arabidopsis sup mutants. The structure of the ovary is very different between the two plant species: P. hybrida forms a placenta on a central axis by axial placentation, whereas in Arabidopsis, placenta originates at the sidewalls of the ovary near the junction of the two carpels. The function of PhSUP1 in placenta morphogenesis might have coevolved with the specific type of placentation.
PhSUP1 Function in the Morphogenesis of Anther
Anthers in the phsup1 mutants showed an aberrant morphology—incomplete development of the interthecal furrow and fusion of inner (adaxial) locules in the upper part. In wild-type anthers, the cells around a vascular bundle are smaller than those in surrounding regions. This control of differential cell growth, which seems to be crucial for normal anther morphogenesis, is lost in phsup1 mutants (Figure 5). The cells in the connective tissue along the adaxial side appear to be excessively proliferated in phsup1 mutants. In accordance with these phenotypes, PhSUP1 transcripts were found in anthers. The PhSUP1 transcripts are distributed in a band of cells across the interthecal region at the abaxial region of anthers, which is adjacent to the region where cell growth is suppressed in wild-type anthers. PhSUP1 may function in a non-cell-autonomous manner in anthers, as has been proposed for the early floral meristem function of SUP in floral meristems (Sakai et al., 1995). The structures of anthers in P. hybrida and Arabidopsis appear quite similar; therefore, it is striking that PhSUP1 plays a role in anther development in P. hybrida, whereas SUP does not in Arabidopsis. The function of SUP in anther morphogenesis, if any, might be masked by functional redundancy.
Phenotypes similar to those for anther development in phsup1 mutants have been reported for Arabidopsis ettin mutants (Sessions et al., 1997). In anthers, ETTIN is expressed in the vascular tissue and in four bands of cells. The adaxial interthecal furrow is poorly developed in ettin anthers, causing fusion of two thecae, similar to that seen in phsup1 mutants. Considering the similarity in the mutant phenotypes, it is of interest whether a gene orthologous to ETTIN is involved in anther development in P. hybrida and whether there is any functional interaction between PhSUP1 and the putative ETTIN homolog.
PhSUP1 Overexpression Phenotypes and Their Implications for PhSUP1 Function
CaMV 35S promoter–driven ectopic expression of PhSUP1 resulted in reduced sizes of various organs, although the cellular basis for the size reduction was different for the different organs. Cell numbers were obviously reduced in leaves and petal limbs of PhSUP1-ox plants. By contrast, shortening of the inflorescence, especially the peduncles, was primarily because of suppression of cell elongation in the longitudinal direction, whereas the effect on cell number was small. Thus, the phenotypes obtained by ectopic expression suggest that PhSUP1 functions in the control of both cell division and cell elongation. A role in cell division control is proposed for SUP on the basis of its mutant phenotypes (Sakai et al., 1995, 2000). This model perhaps holds true for the early floral meristem function of PhSUP1. However, the aberrancy in anther development in phsup1 mutants suggests a role of PhSUP1 in the control of cell growth as well because the variation in the size of cells around the vascular bundle in wild-type anthers clearly is lost in phsup1 mutants. Therefore, this loss-of-function phenotype supports a role of PhSUP1 in the control of cell growth as well as cell division.
The effects of SUP overexpression have been studied using various promoters in both dicot (Kater et al., 2000; Bereterbide et al., 2001; Yun et al., 2002) and monocot (Nandi et al., 2000) plants. Bereterbide et al. (2001) overexpressed SUP in N. tabacum under the control of the CaMV 35S promoter and observed aberrations in plant architecture and cellular morphology that were mostly similar to those observed in PhSUP1-ox P. hybrida. However, the reduction in internode cell number observed in the SUP-overexpressed (SUP-ox) N. tabacum was not observed in PhSUP1-ox P. hybrida. A similar discrepancy is present regarding the effects of SUP overexpression on the number and expansion of petal cells. The 35S promoter–driven SUP suppresses both cell division and cell elongation in the petals of both N. tabacum (Bereterbide et al., 2001) and P. hybrida (this article), whereas Floral Binding Protein1 (FBP1) promoter-driven SUP in P. hybrida petals affected cell elongation but not cell number (Kater et al., 2000). Bereterbide et al. speculated that the discrepancy between the phenotypes may be because of a difference in strength of the two promoters in these plants. A similar situation may account for the difference in the internode phenotypes between the PhSUP1-ox P. hybrida and SUP-ox N. tabacum. Although the CaMV 35S promoter was used for both transformants, the strength of this promoter is known to differ between these two plant species and may have led to the different phenotypes even when the SUP and PhSUP1 proteins are functionally equivalent. Furthermore, the timing of promoter activation may be a crucial factor that accounts for the different phenotypes resulting from 35S versus FPB1 promoter-driven SUP. Cell elongation in petals and stamens mainly occurs after cell division has completed. If the FBP1 promoter becomes active later during flower development than does the 35S promoter, the different effects of SUP overexpression between the two transformants may be ascribed to that difference in timing. On the basis of these considerations, taken together with the phsup1 mutant phenotype in anther that is related to cell growth, it seems reasonable to consider that SUP/PhSUP1 is involved in the control of both division and growth of cells. Recent studies by Yun et al. (2002) in Arabidopsis suggest that, in addition to indirect effects via cell proliferation control, SUP has a direct suppressive effect on the expression of class B homeotic genes on the basis of the observation that SUP overexpression driven by the AP1 promoter caused homeotic conversion of petals to stamen. Such homeotic effect was not observed in our PhSUP1 overexpression experiment in the P. hybrida system. The AP1 promoter is active before homeotic genes are active, whereas the 35S promoter may not be strong enough to cause the homeotic effects in early floral meristems in P. hybrida. Therefore, our results do not exclude the possibility that PhSUP1 has a direct effect on the expression of class B genes.
In summary, we have shown that the SUP gene and its early floral meristem function and late function in ovule development, originally discovered in Arabidopsis, are conserved in another dicot plant, P. hybrida, indicating the generality of this gene function. Furthermore, we have shown that PhSUP1 has some additional functions unique for P. hybrida. These specific functions have implications regarding the roles of PhSUP1 in the diversification in flower structure. Further comparative studies of SUP orthologous genes in various plant species likely will provide further insight into the roles of this important gene in the floral structure specification.
METHODS
Isolation of the PhSUP1 Gene
Nested PCR was performed to amplify partial sequences of SUP-like zinc-finger genes using P. hybrida (cv Mitchell diploid) genomic DNA as a template. The QALGGH primer [5′-CA(A/G)GCI (T/C)TIGGIGGICA(C/T)-3′], corresponding to a conserved sequence in zinc-finger domain, and the LDLELR primer [5′-A(G/A)IC(T/G)IA(G/A)(T/C)TCIA(G/A)(G/A)TC-3′], corresponding to a conserved sequence in the C-terminal region, were used for the first round of PCR, and the LGGHMN primer [5′-(T/C)TIGGIGGICA(C/T)ATGAA(C/T)-3′] and the LDLELR primer for the second round. The PCR products were separated by agarose gel electrophoresis, cloned into the pCR II vector (Invitrogen, San Diego, CA), and sequenced. A PhSUP1 cDNA clone (pSP-cPhSUP1) was isolated from a P. hybrida ovary cDNA library, which had been constructed in the pSPORT1 vector (Life Technologies, Rockville, MD), using the Gene Trapper positive selection system (Life Technologies) according to the manufacturer's instructions. A genomic DNA library of P. hybrida (cv Mitchell diploid) was constructed in the λEMBL3 vector. A PhSUP1 genomic clone (λgPhSUP1) was isolated from the genomic DNA library by plaque hybridization screening using the PhSUP1 cDNA as a probe. A 3.8-kb DNA fragment containing 5′-upstream (2.5 kb), coding, and 3′-UTR (0.46 kb) regions of PhSUP1 was excised from the λgPhSUP1 DNA using SalI and EcoRI and cloned into pBluescript SK+ (pBS SK+; Stratagene, La Jolla, CA) cleaved with the same set of restriction enzymes, yielding the subclone pBS-gPhSUP1S-E.
Vector Construction
PhSUP1:GUS
An XbaI-EcoRI fragment containing the GUS coding sequence and Nos terminator was excised from pBI121 (Clontech, Palo Alto, CA) and inserted into pUCAP (pUC-GUS:Tnos). The upstream region of PhSUP1 (2.5 kb) was amplified by PCR with the M13 forward (Stratagene) and PhSUP1-ATG-Bam primers (5′-ATTGGATCCCTCCATGCCTGCCTAC-3′) using pBS-gPhSUP1S-E as a template. The PhSUP1-ATG-Bam primer was designed to introduce a BamHI site just downstream of the PhSUP1 initiation codon. The PCR fragment was digested with SalI and BamHI and cloned into pBS SK+ (pBS-PhSUP1-Bm). The cloned fragment was partially sequenced from the 3′-end to confirm the absence of any mutation within 500 bp upstream of the PhSUP1 initiation codon. A SalI-BstXI fragment (from −2.5 kb to −360 bp upstream of PhSUP1 initiation codon) of pBS-PhSUP1-Bm was replaced with a corresponding sequence excised from pBS-gPhSUP1S-E to obtain pBS-PhSUP1:Bm, which contained the 2.5-kb PhSUP1 upstream region with a BamHI site at its 3′-end. The PhSUP1 upstream fragment was excised from pBS-PhSUP1:Bm using PstI and BamHI and inserted into pUC-GUS:Tnos upstream of the GUS coding sequence using the same sets of restriction enzymes (pUC-PhSUP1:GUS:Tnos). Subsequently, the PhSUP1:GUS-Tnos chimeric gene was excised from pUC-PhSUP1:GUS:Tnos by PacI and AscI and cloned into pBINPLUS using the same sets of restriction enzymes, thereby producing pBIN-PhSUP1:GUS:Tnos.
gPhSUP1
For the complementation test of the Arabidopsis sup mutant by PhSUP1, pBIN-gPhSUP1:Tnos, a derivative of pBINPLUS that contained the PhSUP1 genomic sequence, was constructed as follows. A 3.8-kb SalI-BamHI fragment containing the upstream (2.5 kb), coding, and 3′-UTR (0.46 kb) sequences of PhSUP1 was excised from pBS-gPhSUP1S-E and placed upstream of the nos terminator in pUCAP-nos, in which the nos terminator had been inserted into the SacI-EcoRI site of pUCAP (van Engelen et al., 1995), yielding pUC-gPhSUP1SB:Tnos. Because pUC-gPhSUP1SB:Tnos lacked part of the 3′-UTR of PhSUP1, the 3′-UTR was recruited from PhSUP1 cDNA (pSP-cPhSUP1) to form pUC-gPhSUP1:Tnos. Then, the gPhSUP1:Tnos fragment was shuttled from pUC-gPhSUP1:Tnos to pBINPLUS (van Engelen et al., 1995) to generate pBIN-gPhSUP1:Tnos.
P35S:PhSUP1
Into the pUCAP plasmid (van Engelen et al., 1995), the CaMV 35S promoter was inserted between the HindIII and BamHI sites, and nopaline synthase terminator (Tnos) sequences were inserted between the SacI and EcoRI sites to generate pUC-P35S:Tnos. A SalI-NotI fragment containing a complete PhSUP1 cDNA sequence was excised from pSP-cPhSUP1 and inserted into pUC-P35S:Tnos between the 35S promoter and the nos terminator (pUC-P35S:PhSUP:Tnos). A fragment containing the P35S:PhSUP1-Tnos chimeric gene was excised from pUC-P35S:PhSUP1:Tnos using AscI and PacI and was introduced into the binary vector pBINPLUS (van Engelen et al., 1995) to yield pBIN-P35S:PhSUP1:Tnos.
Plant Materials and Transformation
Mutant alleles for PhSUP1 were isolated by screening libraries of dTph1-inserted mutant lines using a PCR-based method (Koes et al., 1995). A primer complementary to a terminal repeat of dTph1 (Out-1) and one of two PhSUP1-specific primers (PhSUP1-UPS, 5′-ATTCTGCTAGTTGTCCCCTTGAT-3′, and PhSUP1-DS, 5′-ATTTTTGCATCTTCATACTCCTG-3′) were used for each amplification. PhSUP1 sequences in dTph1-inserted alleles were amplified by PCR with the PhSUP1-UPS and PhSUP1-DS primers, cloned into the pCR II vector (Invitrogen), and sequenced. Transformation of P. hybrida cv Mitchell diploid was performed by the A. tumefaciens–mediated method (Jorgensen et al., 1996).
All Arabidopsis plants that were used in this study were of the Columbia (Col-0) ecotype. The sup mutant used in this study carried the sup-2 (flo10, Schultz et al., 1991) allele and was obtained from the ABRC seed stock center. Complementation of the sup mutant by the PhSUP1 genomic DNA fragment was performed as follows. The binary vector pBIN-gPhSUP1-NT, which contains the upstream and coding sequences of PhSUP1, was introduced into A. tumefaciens strain GV3101. This bacterial strain was used to transform wild-type Arabidopsis plants by the vacuum infiltration method (Bechtold and Pelletier, 1998). Kanamycin-resistant transformants were crossed with the sup-2 mutant, and resulting F1 plants were self-pollinated. From among the F2 plants, homozygous sup-2 mutant plants harboring a PhSUP1 transgene were selected and characterized. The presence of the PhSUP1 transgene in each F2 plant was confirmed by PCR amplification of the PhSUP1 sequence using the PhSUP1-UPS and PhSUP1-DS primers. The genotype of the SUP locus in each F2 plant was analyzed by examining the NcoI restriction pattern of the SUP locus after PCR amplification with SUP-specific primers (AtSUP-U1, 5′-GCATAGCCAAAAAGAAAGAGC-3′, and AtSUP-D2, 5′-GGGTAAGGAGGAGAAGGTGTT-3′). NosI cleaves the wild-type SUP sequence but not that of the sup-2 allele.
Microscopy
For light microscopy, P. hybrida tissues were fixed in FAA (formalin:acetic acid:ethanol:water, 10:5:50:35), dehydrated in an ethanol series, embedded in Historesin (Leica Microsystems, Wetzlar, Germany), and sectioned at 2 to 4 μm. The sections were stained in a 1% solution of toluidine blue. For interference contrast microscopy, tissues were fixed in fixation solution (ethanol:acetic acid, 90:10) and cleared by chloral hydrate treatment. For scanning electron microscopy, tissues were frozen in liquid nitrogen and observed through a Hitachi S-2380 (Hitachi Science Systems, Hitachinaka, Japan) under low vacuum.
In Situ Hybridization
Tissues were fixed in FAA at 4°C for 48 h, dehydrated in an ethanol series and then a tertiary butanol series, and embedded in Paraplast Plus (Sigma, St. Louis, MO). The paraffin-embedded tissues were sectioned to 5-μm thickness, affixed on microscopic slides by incubating at 42°C overnight, and used for in situ hybridization. Template plasmids for probe RNA synthesis were constructed as follows. With the PhSUP1 cDNA (pSP-cPhSUP1) as a template, a 750-bp PCR fragment beginning downstream of the zinc-finger region of PhSUP1 was amplified using the T7-SPL1-2 primer (5′-TAATACGACTCACTATAGGGAGAGGCTTAGACTACAATCAC-3′, with the T7 promoter sequence underlined) and the T3-SPL1-3RV primer (5′-AATTAACCCTCACTAAAGGGCATCTAGAGAAGATAGT-3′, with the T3 promoter sequence underlined) so that sense and antisense PhSUP1–specific RNA probes could be transcribed from each end. With the PCR fragment as a template, digoxigenin (DIG)-labeled RNA probes were synthesized using a DIG RNA labeling kit (Roche Diagnostics, Basel, Switzerland). In situ hybridization procedures, from pretreatment to staining, were performed using an automatic staining module, the Discovery HX system (Ventana Medical Systems, Tucson, AZ). Pretreatment, hybridization, and washing of sections were performed using the RiboMapKit (Ventana Medical Systems) according to the manufacturer's instructions. Sections were hybridized with DIG-labeled probes in Ribohybe (Ventana) hybridization solution at 67°C for 6 h. After hybridization, the sections were washed three times in 0.1× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) (70°C, 6 min). Hybridization signals were then detected with horseradish peroxidase–conjugated antidigoxigenin antibody (Roche Diagnostics), enhanced with an AmpMapKit (Ventana), which is based on the tyramide signal amplification reaction, and developed using the BlueMapKit (Ventana). The stained slides were dehydrated through an ethanol series, washed twice in xylene, and mounted in MX mounting medium (Matsunami Glass, Osaka, Japan).
RNA Gel Blot Hybridization
Total RNA was isolated from tissues homogenized in liquid nitrogen using the RNeasy plant kit (Qiagen, Hilden, Germany). Aliquots (10 μg) of total RNA were separated on a 1.2% agarose gel containing 0.4 M formaldehyde and blotted onto GeneScreen membranes (NEN Life Science Products, Boston, MA). Antisense DIG-labeled probe was synthesized as described for the in situ hybridization procedure. The blotted membranes were hybridized with the DIG-labeled probe, and signals were detected by a chemiluminescence reaction using a DIG nucleic acid detection kit (Roche Diagnostics) and CDP-STAR (Roche Diagnostics). The chemiluminescence signals were detected by exposing the treated membranes to Lumi-films (Roche Diagnostics).
RT-PCR
Poly(A)+ RNA was purified from total RNA using the QuickPrep Micro mRNA purification kit (Amersham Pharmacia Biotech, Buckinghamshire, UK) and was treated with DNase I. First-strand cDNA was synthesized from an aliquot of DNased poly(A)+ RNA using the Superscript preamplification system (Gibco BRL, Cleveland, OH). An aliquot of first-strand cDNA was subjected to 30 cycles of PCR amplification with PhSUP1-specific primers (SPL1U2-23, 5′-GGCAGGCATGGAGAAAAACAATA-3′, and SPL1D2-24, 5′-TTCAGACCTCTTCATCAACACTTC-3′). A negative-control reaction lacking reverse transcriptase was performed to assess contamination with genomic DNA. The amount of template cDNA was normalized by PCR with a ubiquitin-specific primer pair (PetUBQ1-5′, 5′-GCCACTCTTCTCCTTCTATTC-3′, and PetUBQ1-3′, 5′-CTTCTTCTTACGCTTCTTTGC-3′).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AB117749.
Acknowledgments
We thank Hiroshi Kouchi for providing unpublished sequence information of a G. max zinc-finger gene. This work was supported by a Center of Excellence Promotion Fund from the Science and Technology Agency of Japan and by a PROBRAIN grant from the Bio-Oriented Technology Research Advancement Institution of Japan.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Hiroshi Takatsuji (takatsuh@nias.affrc.go.jp).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.018838.
References
- Angenent, G.C., Franken, J., Busscher, M., Colombo, L., and van Tunen, A.J. (1993). Petal and stamen formation in petunia is regulated by the homeotic gene fbp1. Plant J. 4, 101–112. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Bereterbide, A., Hernould, M., Castera, S., and Mouras, A. (2001). Inhibition of cell proliferation, cell expansion and differentiation by the Arabidopsis SUPERMAN gene in transgenic tobacco plants. Planta 214, 22–29. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Sakai, H., Jack, T., Weigel, D., Mayer, U., and Meyerowitz, E.M. (1992). SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development 114, 599–615. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418. [DOI] [PubMed] [Google Scholar]
- Colombo, L., Franken, J., Koetje, E., van Went, J., Dons, H.J., Angenent, G.C., and van Tunen, A.J. (1995). The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7, 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathan, N., Zaccaro, L., Esposito, S., Isernia, C., Omichinski, J.G., Riccio, A., Pedone, C., Di Blasio, B., Fattorusso, R., and Pedone, P.V. (2002). The Arabidopsis SUPERMAN protein is able to specifically bind DNA through its single Cys2-His2 zinc finger motif. Nucleic Acids Res. 30, 4945–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn, A. (1990). Reproductive organs. In Plant Anatomy, A. Fahn, ed (Oxford, UK: Pergamon Press), pp. 411–488.
- Ferrario, S., Immink, R.G., Shchennikova, A., Busscher-Lange, J., and Angenent, G.C. (2003). The MADS box gene FBP2 is required for SEPALLATA function in petunia. Plant Cell 15, 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiser, J.C., Robinson-Beers, K., and Gasser, C.S. (1995). The Arabidopsis SUPERMAN gene mediates asymmetric growth of the outer integument of ovules. Plant Cell 7, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, K., and Meyerowitz, E.M. (1994). Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 8, 1548–1560. [DOI] [PubMed] [Google Scholar]
- Hiratsu, K., Ohta, M., Matsui, K., and Ohme-Takagi, M. (2002). The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett. 514, 351–354. [DOI] [PubMed] [Google Scholar]
- Ito, T., Sakai, H., and Meyerowitz, E.M. (2003). Whorl-specific expression of the SUPERMAN gene of Arabidopsis is mediated by cis elements in the transcribed region. Curr. Biol. 13, 1524–1530. [DOI] [PubMed] [Google Scholar]
- Jack, T., Brockman, L.L., and Meyerowitz, E.M. (1992). The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68, 683–697. [DOI] [PubMed] [Google Scholar]
- Jorgensen, R.A., Cluster, P.D., English, J., Que, Q., and Napoli, C.A. (1996). Chalcone synthase cosuppression phenotypes in petunia flowers: Comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol. Biol. 31, 957–973. [DOI] [PubMed] [Google Scholar]
- Kapoor, M., Tsuda, S., Tanaka, Y., Mayama, T., Okuyama, Y., Tsuchimoto, S., and Takatsuji, H. (2002). Role of petunia pMADS3 in determination of floral organ and meristem identity, as revealed by its loss of function. Plant J. 32, 115–127. [DOI] [PubMed] [Google Scholar]
- Kater, M.M., Franken, J., van Aelst, A., and Angenent, G.C. (2000). Suppression of cell expansion by ectopic expression of the Arabidopsis SUPERMAN gene in transgenic petunia and tobacco. Plant J. 23, 407–413. [DOI] [PubMed] [Google Scholar]
- Koes, R., et al. (1995). Targeted gene inactivation in petunia by PCR-based selection of transposon insertion mutants. Proc. Natl. Acad. Sci. USA 92, 8149–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek, B.A., and Meyerowitz, E.M. (1996). The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22. [DOI] [PubMed] [Google Scholar]
- Meyerowitz, E.M. (1997). Genetic control of cell division patterns in developing plants. Cell 88, 299–308. [DOI] [PubMed] [Google Scholar]
- Nandi, A.K., Kushalappa, K., Prasad, K., and Vijayraghavan, U. (2000). A conserved function for Arabidopsis SUPERMAN in regulating floral-whorl cell proliferation in rice, a monocotyledonous plant. Curr. Biol. 10, 215–218. [DOI] [PubMed] [Google Scholar]
- Sakai, H., Krizek, B.A., Jacobsen, S.E., and Meyerowitz, E.M. (2000). Regulation of SUP expression identifies multiple regulators involved in Arabidopsis floral meristem development. Plant Cell 12, 1607–1618.11006335 [Google Scholar]
- Sakai, H., Medrano, L.J., and Meyerowitz, E.M. (1995). Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature 378, 199–203. [DOI] [PubMed] [Google Scholar]
- Schultz, E.A., Pickett, F.B., and Haughn, G.W. (1991). The FLO10 gene product regulates the expression domain of homeotic genes AP3 and PI in Arabidopsis flowers. Plant Cell 3, 1221–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions, A., Nemhauser, J.L., McColl, A., Roe, J.L., Feldmann, K.A., and Zambryski, P.C. (1997). ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124, 4481–4491. [DOI] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto, S., van der Krol, A.R., and Chua, N.-H. (1993). Ectopic expression of pMADS3 in transgenic petunia phenocopies the petunia blind mutant. Plant Cell 5, 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol, A.R., Brunelle, A., Tsuchimoto, S., and Chua, N.-H. (1993). Functional analysis of petunia floral homeotic MADS box gene pMADS1. Genes Dev. 7, 1214–1228. [DOI] [PubMed] [Google Scholar]
- van der Krol, A.R., and Chua, N.-H. (1991). The basic domain of plant B-ZIP proteins facilitates import of a reporter protein into plant nuclei. Plant Cell 3, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen, F.A., Molthoff, J.W., Conner, A.J., Nap, J.P., Pereira, A., and Stiekema, W.J. (1995). pBINPLUS: An improved plant transformation vector based on pBIN19. Transgenic Res. 4, 288–290. [DOI] [PubMed] [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Yun, J.Y., Weigel, D., and Lee, I. (2002). Ectopic expression of SUPERMAN suppresses development of petals and stamens. Plant Cell Physiol. 43, 52–57. [DOI] [PubMed] [Google Scholar]