Abstract
The Medicago truncatula Does not Make Infections (DMI2) mutant is mutated in the nodulation receptor-like kinase, NORK. Here, we report that NORK-mutated legumes of three species show an enhanced touch response to experimental handling, which results in a nonsymbiotic root hair phenotype. When care is taken not to induce this response, DMI2 root hairs respond morphologically like the wild type to nodulation factor (NF). Global NF application results in root hair deformation, and NF spot application induces root hair reorientation or branching, depending on the position of application. In the presence of Sinorhizobium meliloti, DMI2 root hairs make two-dimensional 180° curls but do not entrap bacteria in a three-dimensional pocket because curling stops when the root hair tip touches its own shank. Because DMI2 does not express the promoter of M. truncatula Early Nodulin11 (ENOD11) coupled to β-glucuronidase upon NF application, we propose a split in NF-induced signaling, with one branch to root hair curling and the other to ENOD11 expression.
INTRODUCTION
The symbiosis between legume plants and soil-living rhizobia provides by far the largest amount of organic nitrogen in the global nitrogen cycle. Therefore, in agriculture, systems of crop rotation are widely used, in which legumes are used as a valuable green fertilizer. The processes leading to symbiotic nitrogen fixation have attracted the interest of researchers, not only for their environmental and economical relevance but also for a better understanding of plant development and signal transduction (reviewed in Lhuissier et al., 2001; D'Haeze and Holsters, 2002; Geurts and Bisseling, 2002). One of the research milestones in rhizobia–legume symbiosis was the discovery and characterization of the nodulation factors (NF) as the key signal molecules excreted by rhizobia that lead to a successful symbiosis (Lerouge et al., 1990; Roche et al., 1991; Spaink et al., 1991; Truchet et al., 1991; Fisher and Long, 1992). Global application of purified NF to legume roots leads to various responses, such as changes in ion fluxes (Ehrhardt et al., 1992; Felle et al., 1995, 1996, 1998, 1999a, 1999b; Cárdenas et al., 1999; reviewed in Cárdenas et al., 2000; Shaw and Long, 2003a), changes in the root hair actin cytoskeleton (Cárdenas et al., 1998; Miller et al., 1999; de Ruijter et al., 1999; reviewed in Esseling et al., 2000), calcium (Ca2+) spiking (Ehrhardt et al., 1996; Wais et al., 2000; Walker et al., 2000; Shaw and Long, 2003a), root hair deformation (RHD; Heidstra et al., 1994; reviewed in Miller et al., 1997), increase of Ca2+ before RHD (de Ruijter et al., 1998), expression of various early nodulin genes (ENODs; Scheres et al., 1990; Pichon et al., 1992; Yang et al., 1993; Pingret et al., 1998; Compaan et al., 2001; Journet et al., 2001), and cell divisions in the root cortex (reviewed in Kijne, 1992; Timmers et al., 1999).
Mutagenesis screens in Drosophila melanogaster (Nusslein-Volhard and Wieschaus, 1980), zebrafish (Driever et al., 1996; Haffter et al., 1996), and Arabidopsis (Arabidopsis thaliana; Bechtold et al., 1993; reviewed in Meinke et al., 1998; Somerville and Somerville, 1999) have proven to be a successful tool to dissect the genetic basis of signal perception and transduction in those organisms. Therefore, extensive screens in the model legumes Lotus japonicus and Medicago truncatula have been performed (Penmetsa and Cook, 1997, 2000; Sagan et al., 1995; Szczygłowski et al., 1998). From these screens, mutants that are blocked in the early steps of symbiosis have been isolated (M. truncatula, Sagan et al., 1995; Penmetsa and Cook, 1997; L. japonicus, Szczygłowski et al., 1998). The most recent addition to the NF signal transduction pathway is the cloning and description of LysM domain containing proteins, which could be NF receptor proteins involved in signaling and initiation of infection (Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003), as has been proposed before (Ardourel et al., 1994). NFR1 and NFR5 from L. japonicus (Madsen et al., 2003; Radutoiu et al., 2003) most probably constitute a (or the) signaling receptor because nfr1 and nfr5 mutants lack all early NF-induced responses (Madsen et al., 2003; Radutoiu et al., 2003), such as the earlier-described nfp mutant of M. truncatula (Ben Amor et al., 2003). The other protein, LYK3 from the M. truncatula SYM2 region (Limpens et al., 2003), most likely codes for the NF structure–specific entry receptor.
Earlier, the two nonnodulating mutant genes, NORK and SymRK, have been positionally cloned and sequenced in M. sativa and L. japonicus, respectively (Endre et al., 2002; Stracke et al., 2002). The two genes turned out to encode homologous receptor-like kinases and to have homologs in various other legumes (Endre et al., 2002; Stracke et al., 2002). Complementation studies show that targeted expression of the M. truncatula NORK homolog in the roots of the Does not Make Infections (DMI2) TR25/dmi2-1 mutant (Sagan et al., 1995) rescues the nonnodulation phenotype (Endre et al., 2002), indicating that DMI2 codes for a receptor-like kinase. The TR25/dmi2-1 M. truncatula mutant is one of the eight nodulation defective mutants from the three complementation groups, DMI1, DMI2, and DMI3 (doesn't make infections 1, 2, and 3), which have been described by Catoira et al. (2000) and Wais et al. (2000). In general, upon global application of purified NF, all the mutants show altered RHD (Catoira et al., 2000), indicating a disruption of root hair tip growth, and DMI1 and DMI2 are defective for or show aberrant Ca2+ spiking (Wais et al., 2000).
We monitored the effects NF application elicits in M. truncatula wild-type and TR25/dmi2-1 root hairs. The results show that medium refreshment induces a touch response, which is enhanced in the mutant, thus unveiling a nonsymbiotic root hair phenotype. This enhanced touch response is correlated with the NORK mutation, also in M. sativa and L. japonicus (MNNN-1008 and SYMRK, respectively). When care is taken not to induce this touch response, M. truncatula TR25/dmi2-1 root hairs respond in a wild-type fashion to NF and curl around rhizobia but do not make a closed root hair pocket. Together with the absence of Ca2+ spiking (Wais et al., 2000) and NF-induced M. truncatula Early Nodulin11 (ENOD11) expression (Vernoud et al., 1999; Catoira et al., 2000; this study), our results point to an early split in the NF-induced signaling cascade upstream of DMI2/NORK.
RESULTS
Growing M. truncatula dmi2-1 Root Hairs Are More Sensitive to Medium Refreshment than Wild-Type Root Hairs
Immediately upon draining and replacing the growth medium of M. truncatula roots in the Fåhraeus slide (Fåhraeus, 1957), we observed the appearance of vacuoles in the cytoplasmic dense region (Figure 1A) at the tips of growing root hairs; this appearance resembles the cytoarchitecture of late growth-terminating root hairs (Figures 1B and 1C). Medium refreshment was done by draining away the medium by placing the Fåhraeus slide (Fåhraeus, 1957) vertically on top of filter paper and pipetting in new medium, a method that works well for global NF application to Vicia sativa root hairs (Heidstra et al., 1994; de Ruijter et al., 1998).
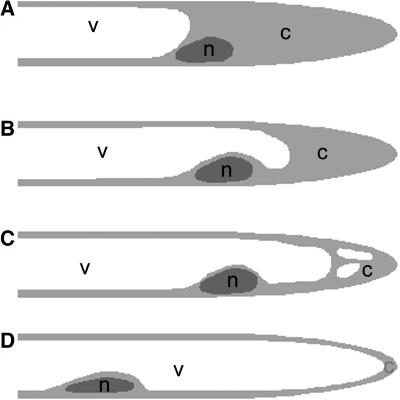
Figure 1.
Cartoon of Root Hair Cytoarchitecture.
Simple representations of the cytoarchitecture of developmental stages of root hairs.
(A) Growing root hair. The subapex of the root hair is filled with cytoplasm (c), and the nucleus (n) is at the base of this area. The shank of the root hair is filled with the central vacuole (v) and cortical cytoplasm.
(B) Early growth-terminating root hair. The first sign of growth termination is that the central vacuole overtakes the nucleus and, therefore, that the subapical region with dense cytoplasm is getting shorter.
(C) Late growth-terminating root hair. The central vacuole expands more and more into the subapex and smaller vacuoles, or extensions of the central vacuole appear into the remaining cytoplasm.
(D) Full-grown root hair. The nucleus has lost its fixed position in the root hair. The vacuole completely fills the root hair and is surrounded by a thin layer of cytoplasm.
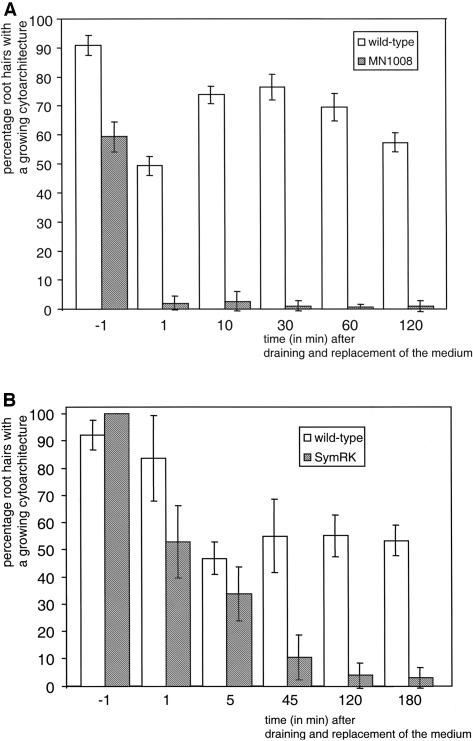
We scored cytoarchitecture of root hairs of wild-type and dmi2-1 seedlings growing in the same slide after using this draining and replacement method (Figure 2). Before medium change, 98% of the wild-type and dmi2-1 root hairs in the root cone with growing hairs had the appropriate cytoarchitecture of a growing root hair (Figure 1A). Immediately after medium change, 25% of the wild-type root hairs had kept this cytoarchitecture. In dmi2-1, the effect of medium change was more drastic: Only 2.7% of the root hairs had kept the original cytoarchitecture of a growing root hair. The remaining 75% (wild type) and 97.3% (dmi2-1) had obtained a (late) growth-termination type of cytoarchitecture (Figure 1C). Approximately 50 to 60% of the wild-type root hairs recovered the growing root hair cytoarchitecture, whereas in dmi2-1 the percentage of root hairs with the proper cytoarchitecture remained at a level of 2 to 5%. This shows that the mutant does not show any recovery of the cytoarchitecture (Figure 2).
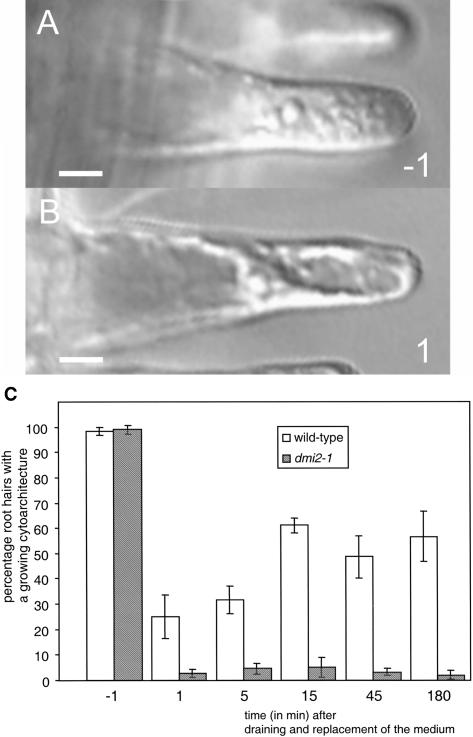
Figure 2.
Medium Change–Induced Touch Response in M. truncatula.
(A) Young growing M. truncatula dmi2-1 root hair just before medium change.
(B) M. truncatula dmi2-1 root hair in the same region on the root as the one in (A), just after medium change. In just 1 min, the cytoplasm has reorganized such that it evenly surrounds the central vacuole, creating a cytoarchitecture of a full-grown root hair.
(C) Percentage of M. truncatula root hairs with a cytoarchitecture of a growing root hair before and after medium change. Before medium change, this percentage in the wild type and dmi2-1 is ∼100%. After medium change, this percentage decreases in both the wild type and dmi2-1. In time, the wild type shows recovery, whereas in dmi2-1, the typical cytoarchitecture does not recover. Monitored were six roots per wild type and dmi2-1, respectively, >50 root hairs per root. Bars in (A) and (B) = 10 μm.
To prevent the disruption of the cytoarchitecture by changing the medium, we developed a much gentler way of refreshing the medium. Before medium refreshment, the Fåhraeus slides with seedlings were carefully placed on the microscope stage and were left to recover for at least half an hour from their transfer from the culture room to the microscope stage. For medium replacement, the slide was left on the microscope stage, and the medium in the slides was replaced by adding fresh medium on one side of the horizontally placed slide and slowly pipetting away on the other side. This was done in such a way that the ∼800 μL of medium in the slide was completely refreshed in ∼1 min. After this gentle medium perfusion, root hair growth continued in the wild type and dmi2-1, no disruption of root hair cytoarchitecture occurred, and no subsequent morphological response was observed (Figure 3).
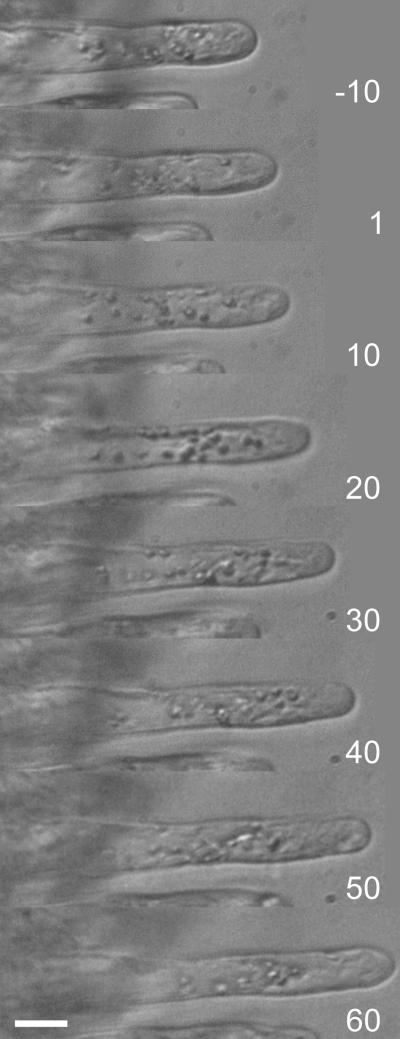
Figure 3.
Time Series of a Growing M. truncatula dmi2-1 Root Hair before and after Medium Refreshment via Gentle Perfusion.
The gentle way of refreshing the growth medium does not disturb root hair cytoarchitecture and growth and, therefore, root hair morphology. Images were taken every 10 min, except of the one 1 min after medium refreshment. Bar = 15 μm.
From the above-described experiment, it seems as if dmi2-1 root hairs have a nonsymbiotic phenotype, which is caused by an increased susceptibility to mechanical manipulation. Both wild-type and dmi2-1 root hairs reacted identically to application of an osmotic shock or cold treatment (data not shown). However, in an experiment in which we applied touch to single growing root hairs, they reacted differently. A microinjection needle was positioned below a root hair growing in agarose, and the needle was moved up and down again, such that the root hair was bent and returned to its original position. After this touch, the root hair cytoarchitecture was followed in time. In the wild type (Figure 4A), this touch did not result in any change in cytoarchitecture (n = 10 out of 11 root hairs from three plants), whereas in dmi2-1, the cytoarchitecture changed from a growing into that of a late growth-terminating root hair (n = 15 out of 16 root hairs from four plants; Figure 4B). We conclude that dmi2-1 root hairs are more sensitive to touch than root hairs of wild-type plants.
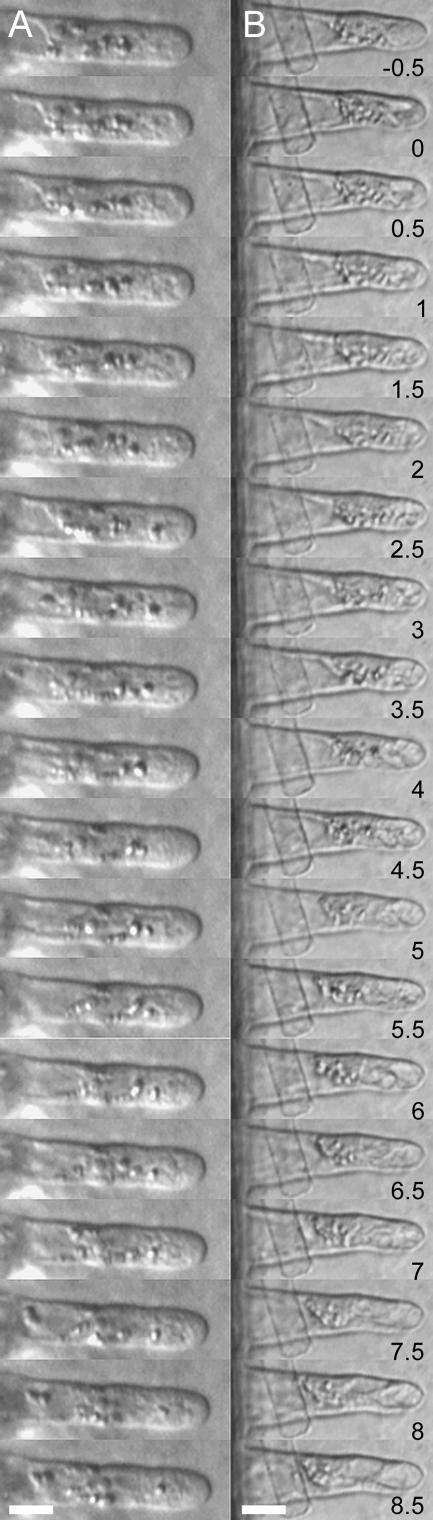
Figure 4.
Time Series of a M. truncatula Wild-Type and dmi2-1 Root Hair before and after Touch with a Needle.
(A) Wild-type root hair. Time 0 is the root hair just after needle touch. Needle touch does not affect the cytoarchitecture and/or root hair growth.
(B) dmi2-1 root hair. Immediately after touch with the needle, vacuoles appear, which increase in time, and the root hair stops growing. Images were taken every 30 s. Bars = 15 μm.
Root Hair Cytoarchitecture as Determinant for RHD by NF
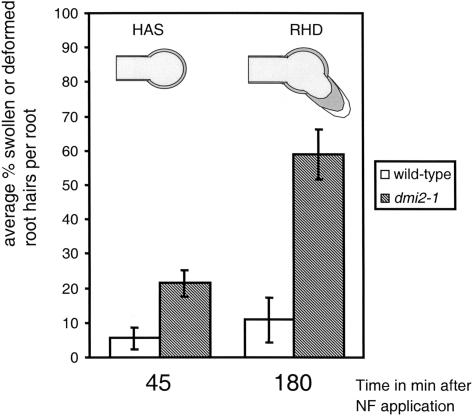
We replaced the culture medium with medium containing 10−9 M NF using the method that caused maximal disturbance. The immediate response to this draining and replacement (i.e., disruption of the growing root hair cytoarchitecture, followed by a recovery in the wild type and not in dmi2-1) was similar to that described above. Forty-five minutes after NF addition, 27% of the dmi2-1 root hairs, which had a growing root hair cytoarchitecture just before draining and replacement, showed root hair swelling (Has), whereas in the wild type this was only 6% (Figure 5). Three hours after NF treatment, 59% of dmi2-1 root hairs, which had a growing root hair cytoarchitecture at the time of application, showed complete RHD with swelling and outgrowth, whereas in the wild type, only 12% showed root hair deformations in this area of the root (Figure 5).
Figure 5.
Percentage of Wild-Type and dmi2-1 Root Hairs Showing Has and RHD in Hairs That Were Growing at the Time of Rough NF Application.
Forty-five minutes after application, there is already a clear difference between the wild type and dmi2-1. A significant higher percentage dmi2-1 root hairs shows swelling of the root hair tip. At 180 min after application, 59% of the dmi2-1 root hairs, which were growing at the time of NF application (but became growth terminating because of the medium change), show NF-induced RHD. In the wild type, only 12% of the root hairs, which were growing at the time of NF application, show NF-induced RHD. Monitored were six roots for the wild type and dmi2-1, respectively, >50 root hairs per root.
From these experiments, we conclude that mechanical manipulation via draining and replacement of the medium induces growth termination in the root hairs that were short and growing at the time of NF application. This made them respond to the NF with complete RHD (i.e., swelling and outgrowth), like root hairs of that cytoarchitecture normally do.
M. sativa (Alfalfa) Wild-Type and MNNN-1008 and L. japonicus Wild-Type and SYMRK Root Hairs Respond to Medium Change in a Fashion Similar to That of M. truncatula Wild-Type and dmi2-1 Root Hairs
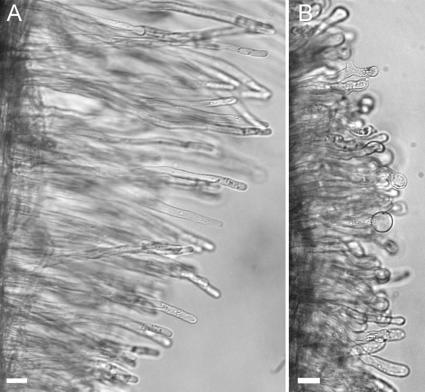
Because we observed the increased sensitivity to medium change in M. truncatula dmi2-1 root hairs, we performed similar medium draining and replacement experiments on the M. sativa MNNN-1008 (Dudley and Long, 1989) and the L. japonicus cac41.5 SYMRK (Stracke et al., 2002) mutants, orthologs of M. truncatula DMI2 (Endre et al., 2002; Stracke et al., 2002). For each species, two wild-type and two mutant seedlings were grown together in the same Fåhraeus slide, and growing root hair cytoarchitecture (Figure 1A) was scored before and after medium change (monitored were six roots for M. sativa wild type and MNNN1008 and four roots for L. japonicus wild type and cac41.5 SYMRK, respectively, >50 root hairs per root). As shown in Figure 6A, the effect of draining and replacement of the medium in the M. sativa wild type and MNNN-1008 mutant was comparable to the M. truncatula wild type and dmi2-1. Immediately after medium change, the percentage of root hairs with the proper cytoarchitecture dropped from 59% to 2% (Figure 6A) and did not recover. In the wild type, a decline in the percentage of growing root hairs can be seen at the moment of medium change, followed by an immediate recovery. For L. japonicus roots, a difference between the wild-type and the mutant root hairs was already visible before medium refreshment. In the mutant, the root hairs were swollen and branched, whereas wild-type root hairs looked normal (Figure 7). To score the effect of medium change, only those root hairs with a growing cytoarchitecture were followed in time. The effect of medium change for these L. japonicus root hairs was similar to the comparable root hairs in M. truncatula and M. sativa; wild-type root hairs showed recovery, whereas in the mutant the percentage of root hairs with the growing cytoarchitecture decreased in time (Figure 6B).
Figure 6.
Medium Change–Induced Touch Response in M. sativa and L. japonicus NORK mutants.
Medium change experiment as described in Figure 2, but with other species and their respective NORK mutant. Both for M. sativa (A) and L. japonicus (B), the same trend is visible. Medium change induces a decrease in the percentage of root hairs with a cytoarchitecture of a growing root hair, from which the wild types are able to recover and the NORK mutants not. Monitored were six roots for M. sativa wild type and MNNN1008 and four roots for L. japonicus wild type and Cac41.5, respectively, >50 root hairs per root.
Figure 7.
Difference of L. japonicus Wild Type and Cac41.5 When Grown in Liquid Medium between Glass Slides.
(A) Wild-type root. The root hairs have a normal diameter and show a normal elongation pattern.
(B) L. japonicus Cac41.5 root before medium change. Most of the root hairs are short, swollen, and/or branched. Only root hairs with a proper shape and cytoarchitecture similar to the M. truncatula root hair in Figure 2A were followed during the medium change experiments. Bars = 30 μm.
We conclude that the NORK mutation in the described receptor kinase (Endre et al., 2002) produces a consistent nonsymbiotic phenotype of enhanced susceptibility to medium change in the three legumes mutated in this protein.
Growing M. truncatula dmi2-1 Root Hairs Show Wild-Type Responses to NF Spot Application
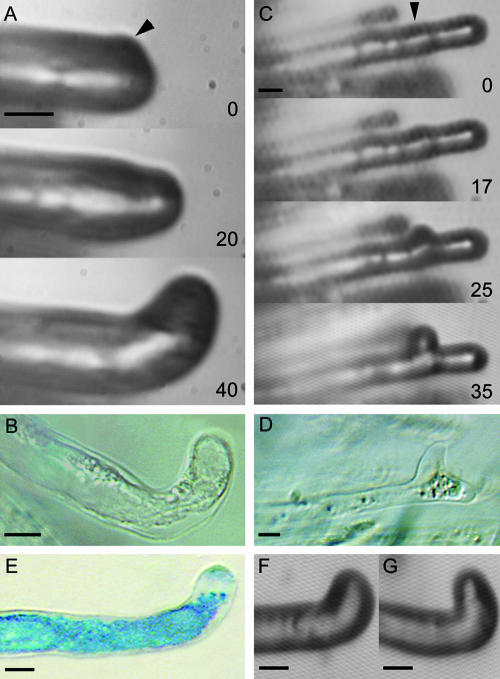
Because the responses of wild-type and M. truncatula dmi2-1 root hairs to gentle global application of NF are identical, we hypothesized that growing dmi2-1 root hairs should respond to spot application of purified NF (Esseling et al., 2003) similar to the wild type, with reorientation of the root hair growth axis toward the site of application and root hair branching when applied 30 μm below the root hair tip. In addition, to better understand the position of DMI2 in relation to DMI1 and DMI3, we also used the C71 (dmi1-1) and TRV25 (dmi3-1) mutants in our spot application experiments. Spot application of NF at the side of the tip of growing root hairs resulted for all mutant root hairs (dmi2-1, n = 13 out of 13, Figure 8A; dmi1-1, n = 16 out of 16, Figure 8F; and dmi3-1, n = 12 out of 12, Figure 8G) in reorientation of the growth axis toward the site of application in a time course similar to that for the wild type (Esseling et al., 2003). Spot application 30 μm below the tip of the growing dmi2-1 root hair (n = 5 out of 5) resulted in the formation of a root hair branch at the site of application (Figure 8C). In accordance with what has been reported for the dmi2-1 mutant (Vernoud et al., 1999; Catoira et al., 2000), the promoter of MtENOD11 coupled to β-glucuronidase (ProMtENOD11:GUS) expression could not be detected in reoriented (Figure 8B) and branched dmi2-1 root hairs (Figure 8D), whereas the wild-type root hairs showed ProMtENOD11:GUS expression (Figure 8E), as has been reported before (Esseling et al., 2003). The endogenous ProMtENOD11:GUS expression in the root cap of wild-type and dmi2-1 roots was sustained (data not shown).
Figure 8.
Responses of M. truncatula dmi2-1 ProMtENOD11:GUS Root Hairs to Spot Application of 10−9 M NF.
(A) Time series of reorientation of root hair growth after spot application of 10−9 M NF on the side of the tip of a growing M. truncatula dmi2-1 root hair. At 20 min, the reorientation is visible, and at 40 min, it is pronounced.
(B) Reoriented M. truncatula dmi2-1 root hair carrying the ProMtENOD11:GUS reporter gene, 60 min after spot application and 24 h after GUS reaction. In all of 13 root hairs, no GUS was detected, showing that the root hair does not express MtENOD11 after NF spot application.
(C) Time series of the formation of a root hair branch after spot application of 10−9 M NF 30 μm below the tip of a growing M. truncatula dmi2-1 root hair. At 17 min after application, the first sign of the formation of a branch is visible, which becomes more pronounced later in time.
(D) The same root hair as in (C), 60 min after spot application and 24 h after GUS reaction. In all five root hairs, no GUS was detected, showing that the root hairs do not express ProMtENOD11:GUS after NF spot application.
(E) ProMtENOD11:GUS expression in a wild-type root hair, 60 min after NF spot application and 24 h after GUS reaction. Clearly, the reorientation toward the site of application and the GUS expression are visible.
(F) Growing C71 (dmi1-1) root hair, 45 min after NF spot application.
(G) Growing TRV25 (dmi3-1) root hair, 55 min after NF spot application. Both the C71 and TRV25 root hairs show root hair reorientation toward the site of application in a timeframe comparable to wild-type root hairs.
Arrowheads point to the site of application, and bars = 10 μm.
We conclude that dmi2-1 root hairs still have the property to reorient their growth axis when challenged with NF, which we have shown to be a prerequisite for root hair curling (Esseling et al., 2003), and ask the question whether the NORK-mutated root hairs indeed can curl around bacteria.
M. truncatula dmi2-1 Root Hairs Curl but Are Unable to Entrap Sinorhizobium meliloti
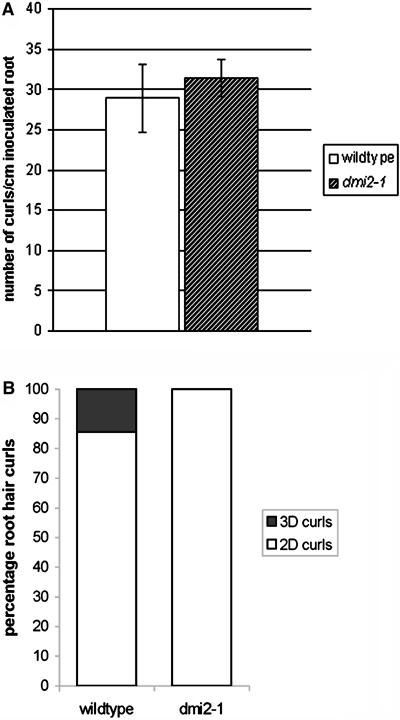
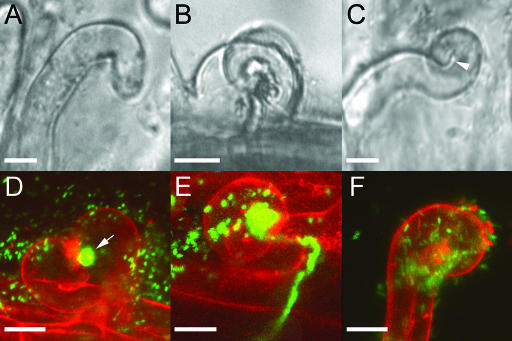
To test whether dmi2-1 root hairs have the ability to curl around and entrap rhizobia, we inoculated 8-d-old wild-type and dmi2-1 plants with their symbiotic partner S. meliloti. Five days after inoculation, the inoculated parts of the root were cut off. The parts that were not in contact with the agar were microscopically examined for curled root hairs. Interestingly, for wild-type and dmi2-1 roots, a comparable amount of curled root hairs per centimeter of inoculated root was counted (28.9 ± 4.2 sd for the wild type and 31.4 ± 2.3 sd for dmi2-1; Figure 9). For curl evaluation, we excluded reoriented (deformed) root hairs, such as that in Figure 10A, and defined two categories of curls: single-faceted two-dimensional 180° curls and multifaceted three-dimensional >180° curls. The latter arise because the tip of the hair continues growing after it touches upon its own shank at 180° curling (Figure 10C, arrowhead). Upon examination with Hoffman modulation contrast microscopy and confocal laser scanning microscopy, it appeared that 14.5% of the curled wild-type root hairs were of the multifaceted three-dimensional type and that only in these curls were bacteria entrapped (Figure 10D) and infection threads had grown (Figures 10B and 10E). From these data, we conclude that a multifaceted root hair pocket is a requirement for infection thread formation in the wild type. Multifaceted three-dimensional curls in which bacteria were entrapped, as well as infection threads, were absent in dmi2-1 root hairs (Figures 10C and 10F). The root hairs stopped curling (growing) when the tip of the root hair touched its own shank.
Figure 9.
Number of Root Hair Curls per Centimeter Inoculated Root.
(A) For the wild type and dmi2-1, a similar number of curled root hairs per centimeter of inoculated root were counted. Error bars are standard deviations.
(B) Of the wild-type curled root hairs, 14.5% had a complex three-dimensional bacteria-entrapping structure. The other 85.5% were single-faceted two-dimensional 180° curls without entrapped bacteria. On dmi2-1, all root hair curls were single-faceted two-dimensional 180° curls without entrapped bacteria.
Figure 10.
M. truncatula Wild-Type and dmi2-1 Root Hairs Curl around S. meliloti 2011-GFP.
(A) Reoriented wild-type root hair in the presence of S. meliloti.
(B) Bright-field image of a wild-type root hair curl with an infection thread. Note the complex multifaceted three-dimensional structure of the curl.
(C) Bright-field image of a curled dmi2-1 root hair. Arrowhead points to the furrow at 180° curling.
(D) Projection of 30 images from a confocal laser scanning microscope Z-stack of a wild-type curled root hair, entrapping a GFP-expressing bacterial colony (arrow). The cell wall (red) was counterstained with 0.1% propidium iodine. Note the multifaceted three-dimensional structure of the root hair curl.
(E) Projection of 35 images of a Z-stack from a wild-type curled root hair with a bacterial colony in a closed pocket.
(F) Projection of 20 images of a Z-stack from a dmi2-1 root hair, curling in the presence of bacteria but unable to entrap them. Note the bacteria on the outside of the root hair. Monitored were four roots per wild type and dmi2-1. Bars = 15 μm.
Because dmi2-1 root hairs lack NF-induced ProMtENOD11:GUS expression (Vernoud et al., 1999; Catoira et al., 2000; this study) but have the ability to curl in the presence of rhizobia, we conclude that there is an early branch in the signaling cascade upstream of DMI2/NORK. One branch leads to root hair curling, the other toward ProMtENOD11:GUS expression.
DISCUSSION
Cytoarchitecture as Determinant of the Morphological Response of Legume Root Hairs to NF
Typical for a root hair–bearing root is the upside-down Christmas tree, the cone that the root hairs form along the root. Along this cone, one can find bulges, growing root hairs, growth terminating root hairs, and full-grown root hairs (M. truncatula; Sieberer and Emons, 2000). To better describe the effects of NF on V. sativa root hairs, Heidstra et al. (1994) suggested a zonation of root hairs in this cone. According to this zonation, zone I corresponds to young growing hairs that do not deform upon NF application, zone II root hairs are those responding to NF with RHD, and zone III is the full-grown root hairs. From this zonation, one would easily expect a correlation between position of the root hair on the root and the ability to deform upon NF application. However, pretreatment of V. sativa root hairs with the ethylene inhibitors aminoethoxyvinyl glycine (AVG) or Ag+ changed the susceptibility of the zone I hairs to NF, in that they were able to deform upon NF application (Heidstra et al., 1997). Pretreatment with AVG or Ag+ induced growth termination; the cytoarchitecture of zone I hairs changed into that of zone II hairs (see Figure 1 in Heidstra et al., 1997), indicating that the ability to deform is correlated with the cytoarchitecture and not with the position of the root hair on the root. Further analysis has shown that for V. sativa (de Ruijter et al., 1998) and for M. truncatula (Sieberer and Emons, 2000; this study), the cytoarchitecture (i.e., its developmental stage) is the only microscopic characteristic determining whether a root hair will respond to NF with RHD or not. Therefore, position of the root hair along the root should not be used as an absolute determinant to predict NF-induced root hair behavior.
Enhanced Touch Response in DMI2/NORK-Mutated Legumes
To cope with the environment in which they are growing, plants need to be able to respond to a myriad of external stimuli, such as temperature, drought, touch, and gravity. The sensitivity to external stimuli is extremely high, which is nicely shown by Fasano et al. (2002). A transient touch stimulation by a 2-s-short pulse of growth medium, expelled from a micropipette positioned adjacent to an Arabidopsis root, is already enough to induce a rapid signaling, leading to a cytosolic Ca2+ wave in the root (see Figure 3D in Fasano et al., 2002). Related to this, we now show that direct touch with a needle has a dramatic effect on the cytoarchitecture of growing M. truncatula dmi2-1 root hairs, and that draining and replacement of the growth medium induces a quick change of cytoarchitecture in legume root hairs, a change that could be prevented with a very careful medium replacement. The difference between wild-type and NORK-mutated legumes is the ability to recover from these touch responses. The sensitivity of the polarly organized cytoplasm to mechanical stress may be causal to the tendency to show spikes and exaggerated oscillations in the level of cytoplasmic Ca2+ in untreated dmi2 root hairs (Shaw and Long, 2003a) and might indicate where to search for the mechanism of NORK activity in the root hair. From our results, we conclude that NORK is a component that is not essential for tip growth itself but protects the tip growth process against environmental disturbance. Whether or not related to this, at the same time NORK is essential for NF signal transduction toward ProMtENOD11:GUS expression. The differences in the responses between the three NORK mutants could depend on the nature of the mutation (Endre et al., 2002; Stracke et al., 2002).
The Absence of Multifaceted Curls in M. truncatula dmi2-1 and Their Requirement for Infection in the Wild Type
NF spot application onto the hemisphere of dmi2-1 root hairs induces reorientation of the root hair growth axis toward the site of application, or when applied 30 μm below the tip, it induces the formation of a root hair branch. For wild-type root hairs, these responses have been shown to mimic root hair curling (Esseling et al., 2003).
Inoculation of M. truncatula wild-type and dmi2-1 roots with S. meliloti 2011 resulted in a similar amount of root hairs, which curled at least 180°, per centimeter of root. A noticeable difference was that in dmi2-1 root hairs, the hairs did not curl further and, therefore, did not form multifaceted three-dimensional structures. Apparently, as soon as the tip of the root hair touched its own shank, the root hair stopped growing and, therefore, curling. Because the dmi2 root hairs have been shown to be very responsive to touch (this study), we think that the touch of the root hair tip to its own shank is enough to induce growth arrest and, therefore, also arrest curling before a tight pocket has been formed around the bacteria.
In addition, this experiment shows that in wild type the formation of a three-dimensional multifaceted curl is necessary for infection thread formation. A closed root hair pocket is formed, in which the growing colony of bacteria is entrapped, and because it grows it most probably exerts force. This has been suggested a long time ago (Dart, 1974). Inhibition of the production of this multifaceted three-dimensional curl in the mutant does not seem to be a sufficient cause for inhibition of infection thread formation because the mutant also lacks NF-induced ENOD-11 expression.
A Split in NF-Induced Signal Transduction
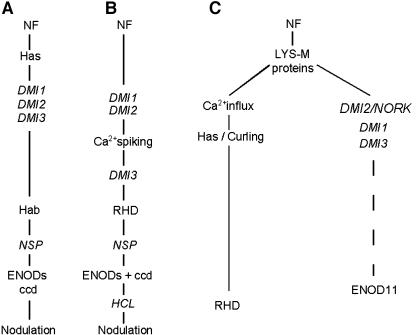
Analysis of M. truncatula nodulation-defective mutants resulted in genetic dissections of NF-induced signaling (Catoira et al., 2000; Wais et al., 2000; reviewed in Geurts and Bisseling, 2002). Based on the observed morphological responses of the root hairs to global NF application, a linear pathway was suggested (Figure 11A), in which DMI1, DMI2/NORK, and DMI3 were placed between Has and root hair branching, followed by NSP, subsequent ENOD expression, cortical cell division (ccd), and nodulation (Catoira et al., 2000). Studies on the behavior of NF-induced Ca2+ spiking in these and other nodulation defective mutants, led to a refinement of this linear NF pathway (Wais et al., 2000). In this scheme (Figure 11B), Has was left out, and DMI1/DMI2 preceded Ca2+ spiking, followed by DMI3, RHD (i.e., swelling and outgrowth), NSP, expression of ENODs and cortical cell division, HCL, and nodulation.
Figure 11.
Putative NF-Induced Signal Transduction Pathways.
(A) Pathway proposed by Catoira et al. (2000).
(B) Pathway proposed by Wais et al. (2000). The difference between these two pathways is the positioning of Ca2+ spiking between DMI1-DMI2 and DMI3, and the combination of Has and root hair branching into RHD.
(C) New pathway, in which our results are integrated into the already published results. From our results, we can conclude that DMI2/NORK, DMI1, DMI3, and its downstream ProMtENOD11:GUS expression are not required for the early NF-induced morphological responses. Therefore, we propose that very soon after NF perception via LysM proteins and before DMI2/NORK, DMI1, and DMI3, the pathway forms a split. One part, including the rapid NF-induced Ca2+ influx, branches off to root hair reorientation/Has and RHD, and the other part via DMI2/NORK to ProMtENOD11:GUS expression. Because we only used ProMtENOD11:GUS as a NF reporter gene, we can only make a statement about this gene, and we do not want to exclude the possibility that in DMI2/NORK mutants other NF-induced genes are expressed.
We now show that NORK/DMI2 and its downstream products are not required in the root hair for the early NF-induced morphological responses. Gentle global NF application results in complete RHD of growth-terminating dmi2-1 root hairs, and NF spot application results in root hair reorientation or root hair branching, depending on the position of application. Moreover, dmi2-1 root hairs can curl in the presence of rhizobia. Therefore, we propose an early split in NF-induced signal transduction in the root hair (Figure 11C). One branch of the pathway leads to the morphological responses: RHD, root hair branching, or root hair reorientation and curling, depending on the assay. The other branch includes DMI2/NORK and ProMtENOD11:GUS expression. Basically, it follows the pathways that have been proposed by Catoira et al. (2000) and Wais et al. (2000), with the important difference that the morphological responses branch off soon after NF perception. Because growing dmi1-1 and dmi3-1 root hairs also show root hair reorientation upon NF spot application, we would like to conclude that DMI1 and DMI3 are also not required for NF induced morphological responses, and we include them in the branch that leads to ProMtENOD11:GUS expression.
Interestingly, work has been published recently that also points in the direction of a dual signaling pathway (Shaw and Long, 2003a, 2000b). By measuring Ca2+ levels in growing M. truncatula root hairs before and after NF application, these authors found an uncoupling between the rapid NF-induced Ca2+ influx (Felle et al., 1998) at the root hair tip (Cárdenas et al., 1999; reviewed in Cárdenas et al., 2000) and NF-induced Ca2+ spiking (Ehrhardt et al., 1996; Wais et al., 2000; Walker et al., 2000). They show that in ∼50% of the tested DMI2 root hairs, the rapid NF-induced Ca2+ influx at the tip is sustained. In addition, the basal H2O2 production, which is thought to activate Ca2+ channels in the root hair tip during normal root hair tip growth (Foreman et al., 2003), goes down after NF application in M. truncatula wild-type and DMI2 root hairs (Shaw and Long, 2003b). As we discussed before (Esseling et al., 2003), this NF-induced Ca2+ influx at the tip is expected to be required for sustained root hair elongation needed for root hair curling, a process that is not impaired in DMI2 root hairs (this study). NF-induced lowering of the endogenous H2O2-induced Ca2+ influx at the existing root hair tip, combined with the NF-induced local Ca2+ influx, could give the directional information for reorientation of root hair tip growth (Figure 11C).
We now show that physical manipulation/touch of growing M. truncatula root hairs induces the nonsymbiotic phenotype in dmi2-1 root hairs. Therefore, we would like to leave the absence of NF-induced Ca2+ spiking in dmi2-1 root hairs (Wais et al., 2000; Shaw and Long, 2003a) as unresolved. The initial Ca2+-spiking experiments on dmi2 mutants showed that dmi2-3 was leaky for Ca2+ spiking, and the experiments were performed on excised root segments (Wais et al., 2000). Excision of the root could be enough to induce the nonsymbiotic phenotype in dmi2-1 root hairs. Although the later Ca2+-spiking experiments were performed on intact seedlings and the cytoarchitecture of the injected wild-type root hairs seems normal (Shaw and Long, 2003a), it might be that the iontophoresis is enough to reveal the (mild form of the) nonsymbiotic phenotype in the dmi2-1 root hairs, which could result in the absence of NF-induced Ca2+ spiking.
The described combination of cell biology and mutant analysis has contributed to a better understanding of the signaling processes required for the symbiosis between rhizobia and legumes. The challenge is now to find out if, and how, the enhanced sensitivity to touch is related to infection thread formation.
METHODS
Seed Preparation
M. truncatula wild-type and TR25 with ProMtENOD11:GUS seeds were removed from the pods and scarified in concentrated sulfuric acid for 10 min. M. sativa wild-type and MNNN-1008 seeds were scarified for 2 min, and L. japonicus wild-type Gifu and Cac 41.5 (SYMRK) seeds for 10 min in concentrated sulfuric acid. After scarification, all seeds were extensively washed with running demineralized water and sterilized in a mixture of 30% (w/v) H2O2 and 96% (w/v) ethanol (1:1 [w/v]) for 2 min. Subsequently, seeds were washed with sterile demineralized water (three times for 2 min and three times for 30 min) and transferred to a small glass jar containing fresh sterile demineralized water. The capped jar was stored at 4°C overnight. Imbibed seeds were then transferred onto 0.8% (w/v) agar plates prepared in sterile demineralized water. The plates were sealed with Parafilm, wrapped in aluminum foil, and stored at 4°C until root protrusion.
Spot Application Assay
Spot applications were performed as described before (Esseling et al., 2003). In short, a water pressure microinjection device (water pressure device, Gilmont, Barrington, IL; needle holder, Eppendorf, Merck Eurolab BV, Amsterdam, The Netherlands) was used to apply microdroplets of purified NF [NodRm-IV(C16:2, Ac, S)] diluted with Millipore water (Bedford, MA) to a final NF concentration of 10−9 M to one side of growing root hair tips. Subsequent growth axis reorientation was recorded with a video camera (Hitachi, Tokyo, Japan) linked to an inverted Nikon Diaphot TMD microscope (Nikon, Tokyo, Japan).
Global NF Application Procedure
One- to 1.5-cm-long seedlings were transferred to sterile Fåhraeus slides containing sterile plant growth medium (PGM) and grown for 36 h in a climate chamber (25°C, 16 h light/8 h dark). Before observation, the Fåhraeus slides were gently placed on the microscope stage and were left to recover for at least half an hour. For this purpose, the microscope was placed into the culture room. For gentle NF application, the PGM in the Fåhraeus slides was replaced by PGM containing 10−9 M NF by adding PGM with NF on one side of the slide and slowly pipetting away on the other side. For NF application via draining and replacement, the PGM in the Fåhraeus slides was drained away by placing the bottom of the slide on a piece of tissue filter paper, and PGM with NF was pipetted in the slide using a blue-tip 1000-μL pipette (Socorex, Lausanne, Switzerland). Images were taken from the area on the root hair cone, which had root hairs with the appropriate cytoarchitecture before medium change, and were recorded with a Sony CCD camera (Sony, Tokyo, Japan) linked to an upright Nikon Optiphot DIC microscope.
Needle-Touch Experiment
After germination, M. truncatula wild-type and TR25 seedlings were placed on 0.3% (w/v) agarose in PGM. The square Petri dishes were closed with Parafilm and left horizontal in a climate chamber (25°C, 16 h light/8 h dark). One day later, the roots had grown into the agarose, and root hairs were formed. To prepare for the touch experiment, the Parafilm was removed from the plates, and with the lid still on, the plates with the seedlings were gently placed on a Nikon Diaphot 200 inverted microscope, with Hoffman modulation optics. After half an hour of acclimatization, the lid of the plate was removed, and the plate was again left for half an hour. Thereafter, a microinjection needle usually used for spot application or microinjection was gently positioned under the root hair of interest. For another 10 min, the root hair of interest was followed to make sure that this needle positioning did not disturb root hair cytoarchitecture and/or growth. Touch was applied by moving the needle up and down, in such a way that the root hair was bent in both directions. Time-lapse recording of the root hair was performed with a Hamamatsu CCD camera, coupled to an Argus-20 low-light enhancement image processor (Hamamatsu Photonics, Hamamatsu City, Japan). Images were recorded every 30 s. To prevent an ambiguous approach, the experiment was performed blind without knowing which plate contained the wild-type or dmi2-1 seedlings, before and at the moment of the experiment.
S. meliloti Inoculation Procedure
After germination, seedlings of ∼1- to 1.5-cm long were transferred to agar plates in buffered Nod medium (BNM; Ehrhardt et al., 1992) with 1 μM AVG. The plates were sealed with Parafilm, leaving the top of the plates opened for ventilation, and stored vertically in a climate chamber (20°C, 50% relative humidity, 16 h light/8 h dark). After 8 d, the root of each seedling was inoculated at the bulge area with a green fluorescent protein (GFP)–expressing S. meliloti 2011 (pHC60; Limpens et al., 2003) culture, diluted to an OD600 of 0.01 with sterile Millipore water. After sealing with Parafilm, the plates were placed back into the climate chamber until observation. Before observation, the roots were cut off just above the inoculation spot and placed into 0.1% (w/v) propidium iodide for 15 min to stain the cell wall. Fluorescence images were acquired with 488 nm and 568 nm laser light on a Zeiss LSM510 coupled to a Zeiss axiovert microscope (Carl Zeiss, Jena, Germany). Bright-field images were obtained on a Nikon Diaphot 200, equipped with Hoffman modulation contrast, and acquired with a Hamamatsu CCD camera coupled to an Argus-20 low-light enhancement image processor.
ProMtENOD11:GUS Expression
ProMtENOD11:GUS expression was assessed by incubating the seedlings for 24 h in the β-glucuronidase (GUS) substrate X-Gluc (2 mM 5-bromo-4-chloro-3-indolylglucuronide, 1% [w/v] dimethylformamide, 0.1 mM K3[Fe(CN)6], 0.1 mM, K4[Fe(CN6)]·3H2O, 1 mM EDTA, and 50 mM KH2PO4, pH 7.0) at 37°C (Journet et al., 1994). Images were recorded with a CCD camera (Sony) linked to an upright Nikon Optiphot DIC microscope.
Acknowledgments
We thank David Barker and Etienne Journet (Laboratory of Plant Microbe Interactions, Institut National de la Recherche Agronomique (INRA)-Centre National de la Recherche Scientifique, Castanet-Tolosan, France) for providing M. truncatula C71 and TR25 seeds carrying the ProMtENOD11:GUS construct and the TRV25 mutant, and Gérard Duc (INRA, Dijon, France) for permission to use the mutant lines. We also thank Sharon Long (Stanford University, Stanford, CA) for providing us with the M. sativa MNNN1008, Martin Parniske (John Innes Centre, Norwich, UK) for the L. japonicus Cac 41.5 SYMRK mutant and wild-type Gifu. Furthermore, we thank René Geurts (Wageningen University and Research Center, Wageningen, The Netherlands) for providing us with the S. meliloti 2011 GFP bacteria and for stimulating comments on the manuscript. J.J.E. was supported by the Dutch Organization for Scientific Research, Division of Earth and Life Sciences (NWO-ALW; ALW 805-33-342). F.G.P.L. was supported by a grant from the European Commission Training and Mobility of Researchers program (Grant FMRX-CT98-0239) and a postdoctoral fellowship from the Region Haute-Normandie.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Anne Mie C. Emons (annemie.emons@wur.nl).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.019653.
References
- Ardourel, M., Demont, N., Debellé, F., Maillet, F., De Billy, F., Promé, J.-C., Dénarié, J., and Truchet, G. (1994). Rhizobium meliloti lipooligosaccharide nodulation factors: Different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6, 1357–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. III Vie 316, 1194–1199. [Google Scholar]
- Ben Amor, B., Shaw, S.L., Oldroyd, G.E.D., Maillet, F., Penmetsa, R.V., Cook, D., Long, S.R., Dénarié, J., and Gough, C. (2003). The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34, 495–506. [DOI] [PubMed] [Google Scholar]
- Cárdenas, L., Feijó, J.A., Kunkel, J.G., Sánchez, F., Holdaway-Clarke, T., Hepler, P.K., and Quinto, C. (1999). Rhizobium Nod factors induce increases in intracellular free calcium influxes in bean root hairs. Plant J. 19, 347–352. [DOI] [PubMed] [Google Scholar]
- Cárdenas, L., Holdaway-Clarke, T.L., Sánchez, F., Quinto, C., Feijó, J.A., Kunkel, J.G., and Hepler, P.K. (2000). Ion changes in legume root hairs responding to Nod Factors. Plant Physiol. 123, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas, L., Vidali, L., Dominguez, J., Pérez, H., Sánchez, F., Hepler, P.K., and Quinto, C. (1998). Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 116, 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira, R., Galera, C., de Billy, F., Penmetsa, R.V., Journet, E.P., Maillet, F., Rosenberg, C., Cook, D., Gough, C., and Denarié, J. (2000). Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell 12, 1647–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compaan, B., Yang, W.-C., Bisseling, T., and Franssen, H. (2001). ENOD40 expression in the pericycle precedes cortical cell division in Rhizobium-legume interaction and the highly conserved internal region of the gene does not encode a peptide. Plant Soil 230, 1–8. [Google Scholar]
- Dart, P.J. (1974). The infection process. In The Biology of Nitrogen Fixation, A. Quispel, ed (Amsterdam, The Netherlands: North Holland), pp. 382–429.
- de Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). Rhizobium Nod factors induce an increase in subapical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant Microbe Inter. 12, 829–832. [Google Scholar]
- de Ruijter, N.C.A., Rook, M.B., Bisseling, T., and Emons, A.M.C. (1998). Lipochito-oligosaccharides reinitiate root hair tip growth in Vicia sativa with high [Ca2+]c and spectrin-like antigen at the tip. Plant J. 13, 341–350. [Google Scholar]
- D'Haeze, W., and Holsters, M. (2002). Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 12, 79–105. [DOI] [PubMed] [Google Scholar]
- Driever, W., Solnica-Krezel, L., Schier, A.F., Neuhauss, S.C., Malicki, J., Stemple, D.L., Stainier, D.Y., Zwartkruis, F., Abdelilah, S., Rangini, Z., Belak, J., and Boggs, C. (1996). A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46. [DOI] [PubMed] [Google Scholar]
- Dudley, M.E., and Long, S.R. (1989). A non-nodulating Alfalfa mutant displays neither root hair curling nor early cell division in response to Rhizobium meliloti. Plant Cell 1, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Atkinson, E.M., and Long, S.R. (1992). Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256, 998–1000. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Wais, R., and Long, S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85, 673–681. [DOI] [PubMed] [Google Scholar]
- Endre, G., Kereszt, A., Kevei, Z., Mihacea, S., Kaló, P., and Kiss, G.B. (2002). A receptor kinase gene regulating symbiotic nodule development. Nature 417, 962–966. [DOI] [PubMed] [Google Scholar]
- Esseling, J., de Ruijter, N.C.A., and Emons, A.M.C. (2000). The root hair actin cytoskeleton as backbone, highway, morphogenetic instrument and target for signalling. In Root Hairs: Cell and Molecular Biology, R.W. Ridge and A.M.C. Emons, eds (Tokyo, Japan: Springer), pp. 29–52.
- Esseling, J.J., Lhuissier, F.G.P., and Emons, A.M.C. (2003). Nod Factor induced root hair curling: Continuous polar growth towards the point of Nod Factor application. Plant Physiol. 132, 1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus, G. (1957). The infection of white clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 16, 374–381. [DOI] [PubMed] [Google Scholar]
- Fasano, J.M., Massa, G.D., and Gilroy, S. (2002). Ionic signaling in plant responses to gravity and touch. J. Plant Growth Regul. 21, 71–88. [DOI] [PubMed] [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1995). Nod signal-induced plasma membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipochitooligosaccharide. Plant J. 7, 939–947. [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1996). Rapid alkalinization in alfalfa root hairs in response to rhizobia lipochitooligosaccharide signals. Plant J. 10, 295–301. [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1998). The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J. 13, 455–463. [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1999. a). Elevation of the cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal in alfalfa. Plant Physiol. 121, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1999. b). Nod factors modulate the concentration of cytosolic free calcium differentially in growing and non-growing hairs of Medicago sativa L. Planta 209, 207–212. [DOI] [PubMed] [Google Scholar]
- Fisher, R.F., and Long, S.R. (1992). Rhizobium-plant signal exchange. Nature 357, 655–660. [DOI] [PubMed] [Google Scholar]
- Foreman, J., Demidchik, V., Bothwell, J.H.F., Mylona, P., Miedema, H., Angel Torres, M., Linstead, P., Costa, S., Brownlee, C., Jones, J.D.G., Davies, J.M., and Dolan, L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Geurts, R., and Bisseling, T. (2002). Rhizobium Nod factor perception and signalling. Plant Cell 14 (suppl.), S239–S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter, P., et al. (1996). The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36. [DOI] [PubMed] [Google Scholar]
- Heidstra, R., Geurts, R., Franssen, H., Spaink, H.P., van Kammen, A., and Bisseling, T. (1994). Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 105, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra, R., Yang, W.C., Yalcin, Y., Peck, S., Emons, A.M., van Kammen, A., and Bisseling, T. (1997). Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 124, 1781–1787. [DOI] [PubMed] [Google Scholar]
- Journet, E.P., El-Gachtouli, N., Vernoud, V., De Billy, F., Pichon, M., Dedieu, A., Arnould, C., Morandi, D., Barker, D.G., and Gianinazzi-Pearson, V. (2001). Medicago truncatula ENOD11: A novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol. Plant Microbe Inter. 14, 737–748. [DOI] [PubMed] [Google Scholar]
- Journet, E.P., Pichon, M., Dedieu, A., de Billy, F., Truchet, G., and Barker, D.G. (1994). Rhizobium meliloti Nod factors elicit cell-specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J. 6, 241–249. [DOI] [PubMed] [Google Scholar]
- Kijne, J.W. (1992). The Rhizobium infection process. In Biological Nitrogen Fixation, G. Stacey, R. Burris, and H. Evans, eds (New York and London: Chapman and Hall), pp. 349–398.
- Lerouge, P., Roche, P., Faucher, C., Maillet, F., Truchet, G., Promé, J.C., and Dénarié, J. (1990). Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acetylated glucosamine oligosaccharide signal. Nature 344, 781–784. [DOI] [PubMed] [Google Scholar]
- Lhuissier, F.G.P., de Ruijter, N.C.A., Sieberer, B.J., Esseling, J.J., and Emons, A.M.C. (2001). Time course of cell biological events evoked in legume root hairs by Rhizobium Nod Factors: State of the art. Ann. Bot. 87, 289–302. [Google Scholar]
- Limpens, E., Franken, C., Smit, P., Willemse, J., Bisseling, T. and Geurts, R. (2003). LysM domain receptor kinases regulating rhizobial nod factor-induced infection. Science 302, 630–633. First published on August 28, 2003; 10.1126/science.1090074 (Science Express Reports) [DOI] [PubMed] [Google Scholar]
- Madsen, E.B., Madsen, L.H., Radutoiu, S., Olbryt, M., Rakwalska, M., Szczygłowski, K., Sato, S., Kaneko, T., Tabata, S., Sandal, N., and Stougaard, J. (2003). A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425, 637–640. [DOI] [PubMed] [Google Scholar]
- Meinke, D.W., Cherry, J.M., Dean, C., Rounsley, S.D., and Koornneef, M. (1998). Arabidopsis thaliana: A model plant for genome analysis. Science 282, 662–682. [DOI] [PubMed] [Google Scholar]
- Miller, D.D., de Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). The role of actin in root hair morphogenesis: Studies with lipochito-oligosaccharides as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17, 141–154. [Google Scholar]
- Miller, D.D., de Ruijter, N.C.A., and Emons, A.M.C. (1997). From signal to form: Aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J. Exp. Bot. 48, 1881–1896. [Google Scholar]
- Nusslein-Volhard, C., and Wieschaus, E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Penmetsa, R.V., and Cook, D.R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial host. Science 275, 527–530. [DOI] [PubMed] [Google Scholar]
- Penmetsa, R.V., and Cook, D.R. (2000). Production and characterization of diverse developmental mutants in Medicago truncatula. Plant Physiol. 123, 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon, M., Journet, E.P., Dedieu, A., De Billy, F., Truchet, G., and Barker, D.G. (1992). Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. Plant Cell 4, 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingret, J.L., Journet, E.P., and Barker, D.G. (1998). Rhizobium Nod factor signaling: Evidence for a G protein-mediated transduction mechanism. Plant Cell 10, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radutoiu, S., Madsen, L.H., Madsen, E.B., Felle, H.H., Umehara, Y., Grønlund, M., Sato, S., Nakamura, Y., Tabata, S., Sandal, N., and Stougaard, J. (2003). Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425, 585–592. [DOI] [PubMed] [Google Scholar]
- Roche, P., Debelle, F., Maillet, F., Lerouge, P., Faucher, C., Truchet, G., Dénarié, J., and Promé, J.-C. (1991). Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell 67, 1131–1143. [DOI] [PubMed] [Google Scholar]
- Sagan, M., Morandi, M., Tarenghi, E., and Duc, G. (1995). Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis. Plant Sci. 111, 63–71. [Google Scholar]
- Scheres, B., van de Wiel, C., Zalensky, A., Horvath, B., Spaink, H., van Eck, H., Zwartkruis, F., Wolters, A.M., Gloudemans, T., van Kammen, A., and Bisseling, T. (1990). The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell 60, 281–294. [DOI] [PubMed] [Google Scholar]
- Shaw, S.L., and Long, S.R. (2003. a). Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 131, 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, S.L., and Long, S.R. (2003. b). Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132, 2196–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer, B., and Emons, A.M.C. (2000). Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma 214, 118–127. [Google Scholar]
- Somerville, C., and Somerville, S. (1999). Plant functional genomics. Science 285, 380–383. [DOI] [PubMed] [Google Scholar]
- Spaink, H.P., Sheeley, D.M., van Brussel, A.A.N., Glushka, J., York, W.S., Tak, T., Geiger, O., Kennedy, E.P., Reinhold, V.N., and Lugtenberg, B.J.J. (1991). A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354, 125–130. [DOI] [PubMed] [Google Scholar]
- Stracke, S., Kistner, C., Yoshida, S., Mulder, L., Sato, S., Kaneko, T., Tabata, S., Sandal, N., Stougaard, J., Szczygłowski, K., and Parniske, M. (2002). A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417, 959–962. [DOI] [PubMed] [Google Scholar]
- Szczygłowski, K., Shaw, R.S., Wopereis, J., Copeland, S., Hamburger, D., Kasiborski, B., Dazzo, F.B., and de Bruijn, F.J. (1998). Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol. Plant Microbe Interact. 11, 684–697. [Google Scholar]
- Timmers, A.C.J., Auriac, M.-C., and Truchet, G. (1999). Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126, 3617–3628. [DOI] [PubMed] [Google Scholar]
- Truchet, G., Roche, P., Lerouge, P., Vasse, J., Camut, S., de Billy, F., Promé, J.-C., and Dénarié, J. (1991). Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351, 670–673. [Google Scholar]
- Vernoud, V., Pingret, J.-L., Chabaud, M., Dedieu, A., de Carvalho Niebel, F., Journet, E.P., and Barker, D.G. (1999). Nod factor signal transduction in the Medicago truncatula Nod−/Myc− mutants TR25/26. In Biology of Plant-Microbe Interactions, Vol. 2, P.J.G.M. de Wit, T. Bisseling, and W.J. Stiekema, eds (St. Paul, MN: International Society for Molecular Plant-Microbe Interactions), pp. 114–119.
- Wais, R.J., Galera, C., Oldroyd, G., Catoira, R., Varma Penmetsa, R., Cook, D., Gough, C., Dénarié, J., and Long, S.R. (2000). Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc. Natl. Acad. Sci. USA 97, 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S.A., Viprey, V., and Downie, J.A. (2000). Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97, 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W.-C., Katinakis, P., Hendriks, P., Smolders, A., De Vries, F., Spee, J., Van Kammen, A., Bisseling, T., and Franssen, H. (1993). Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 3, 573–585. [DOI] [PubMed] [Google Scholar]