Abstract
Cyclin-dependent kinases (CDKs) are key regulators of the cell cycle. In yeasts, only one CDK is sufficient to drive cells through the cell cycle, whereas higher eukaryotes developed a family of related CDKs. Curiously, plants contain a unique class of CDKs (B-type CDKs), whose function is still unclear. We show that the CDKB1;1 gene of Arabidopsis (Arabidopsis thaliana) is highly expressed in guard cells and stomatal precursor cells of cotyledons, suggesting a prominent role for B-type CDKs in stomatal development. In accordance, transgenic Arabidopsis plants with reduced B-type CDK activity had a decreased stomatal index because of an early block of meristemoid division and inhibition of satellite meristemoid formation. Many aberrant stomatal cells were observed, all of them blocked in the G2 phase of the cell cycle. Although division of stomatal precursors was inhibited, cells still acquired stomatal identity, illustrating that stomatal cell differentiation is independent of cellular and nuclear division.
INTRODUCTION
Stomata are essential for plants to survive, and the evolution of genes necessary for the development of stomata has been a key event in the emergence of land plants. The stoma is an epidermal structure that regulates gas and water vapor exchange between the plant and the atmosphere. A pair of stomatal guard cells responds to short-term atmospheric changes by controlling the size of the stomatal pores. Gas exchange also can be influenced by the adjustment of the stomatal density during leaf development depending on the environmental conditions (Brownlee, 2001).
In Arabidopsis (Arabidopsis thaliana), the stoma consists of a pair of guard cells (GCs) and is mostly surrounded by three subsidiary cells, defining an anisocytic stomatal complex (Berger and Altmann, 2000). Stomatal complexes are formed by a series of predictable divisions with a well-defined geometry (reviewed in Serna and Fenoll, 2000a). Three successive types of stomatal precursor cells are involved: meristemoid mother cells (MMCs), meristemoids, and guard mother cells (GMCs), of which the first two divide asymmetrically and the latter symmetrically. Stomatal complex formation begins with an unequal cell division of a protodermal cell (MMC), resulting in the formation of one (first) subsidiary cell and the meristemoid. The meristemoid undergoes up to two further asymmetric divisions, by which a centrally located meristemoid is created that acquires a new shape and assumes GMC identity (Zhao and Sack, 1999). Finally, the GMC divides symmetrically to give rise to two equally sized GCs. The neighboring subsidiary cells, predominantly the most recent, have the potential to divide asymmetrically to form subsidiary or satellite meristemoids (SMs). SMs may undergo up to two further asymmetric divisions, thereby producing secondary anisocytic stomatal complexes (Serna et al., 2002). The newly produced meristemoids are usually positioned away from the preexisting stoma, preventing that stomata are formed in direct contact.
Proper stomatal complex formation implies mechanisms that regulate the correct orientation of the subsidiary cell plane of division to avoid that the new cell plate intersects with the existing stoma (Yang and Sack, 1995; von Groll and Altmann, 2001; Serna et al., 2002). Three Arabidopsis mutants, too many mouths (tmm), stomatal density and distribution1 (sdd1), and four lips (flp), have largely contributed to the understanding of the cellular events associated with the formation of stomatal complexes and the establishment of the stomatal pattern (Yang and Sack, 1995; Berger and Altmann, 2000; Geisler et al., 2000). The gene products of both TMM and SDD1 regulate stomatal patterning and probably act in the same signaling pathway (Nadeau and Sack, 2002; von Groll et al., 2002). By contrast, FLP seems to determine the GMC fate and acts independently of the SDD1 pathway (Yang and Sack, 1995; von Groll et al., 2002).
Stomatal cells have some specific cell cycle characteristics (reviewed in Croxdale, 2000; Geisler and Sack, 2002). In Arabidopsis, trichomes and pavement cells are mostly polyploid, whereas GCs remain diploid (Melaragno et al., 1993). Moreover, although terminally differentiated, mature GCs express genes associated with competence for cell division (Serna and Fenoll, 1997), probably the reason why transgenic Beta vulgaris (sugar beet) can be most easily generated from GC protoplasts (Hall et al., 1996a). In addition, isolated GCs are able to assume different fates and express different gene products when cultured (Taylor et al., 1998).
Our understanding of the cell division cycle is rapidly expanding. Like in other eukaryotic organisms, cell division in plants is regulated by cyclin-dependent kinases (CDKs) (De Veylder et al., 2003; Dewitte and Murray, 2003). In the Arabidopsis genome, 12 CDKs have been found, subdivided into six distinct classes (CDKA to CDKF) (Vandepoele et al., 2002). A-type CDKs comprise functional homologs of the yeast (Schizosaccharomyces pombe) p34cdc2, harboring the PSTAIRE canonical amino acid motif in the cyclin binding domain. Transcripts of A-type CDKs accumulate in a cell cycle phase-independent manner, whereas the associated kinase activity fluctuates during the cell cycle from high in S, G2, and M phases to low in G1 (Mironov et al., 1999).
B-type CDKs are unique to plants and contain a divergent cyclin binding motif. The PSTAIRE motif, characteristic of A-type CDKs, is replaced by either PPTALRE or PPTTLRE, reflecting the existence of the two B1 (CDKB1;1 and CDKB1;2) and B2 (CDKB2;1 and CDKB2;2) subgroups (Boudolf et al., 2001; Vandepoele et al., 2002). In addition, B-type CDKs fail to functionally complement temperature-sensitive cdc2 yeast mutants (Imajuku et al., 1992; Fobert et al., 1996). In contrast with A-type CDKs, B-type CDK transcription is cell cycle regulated. The genes from the two B-type groups differ slightly in their timing of expression: CDKB1 transcripts accumulate during the S, G2, and M phases, and CDKB2 expression is specific to G2 and M phases (Fobert et al., 1996; Segers et al., 1996; Magyar et al., 1997; Umeda et al., 1999; Breyne et al., 2002). The accumulation of CDKB proteins follows their transcription pattern, and the associated kinase activity reaches a maximum in mitosis (Porceddu et al., 2001; Sorrell et al., 2001).
CDKB1;1 has been postulated to play a role in skotomorphogenesis (Yoshizumi et al., 1999). When grown in darkness, transgenic Arabidopsis plants containing an antisense CDKB1;1 construct develop a short hypocotyl and open cotyledons. This short hypocotyl phenotype is related to reduced cell size rather than cell number. In addition, cotyledons of antisense lines fail to green when transferred from darkness to light, which is attributed to the conversion of etioplasts into amyloplasts. These data indicate a function for CDKB1;1 in hypocotyl cell elongation and cotyledon cell development.
Here, we demonstrate that CDKB1;1 plays a key role in plant development. In cotyledons, CDKB1;1 expression was specifically linked to dividing cells associated with the formation of the stomatal complex. To assess its role during stomatal complex formation, transgenic Arabidopsis plants were generated that overexpressed a dominant negative allele of the CDKB1;1 gene. Our results clearly demonstrate that B-type CDK activity is essential for correct stomatal complex development but not for stomatal differentiation.
RESULTS
CDKB1;1 Promoter Activity in Cotyledons Is Associated with Stomata Formation
Arabidopsis cotyledons are particularly interesting organs for identifying cell cycle genes associated with stomatal cell divisions. Stomata can easily be identified in leaves. However, cells divide at high rates in all tissues of young leaves (De Veylder et al., 2001), whereas in cotyledons, postgerminative cell proliferation in the epidermis is nearly exclusively coupled to the formation of stomatal complexes. The stomatal lineages produce up to 82% of all epidermal (pavement and stomatal) cells in cotyledons (Geisler et al., 2000), whereas the growth of the other cell layers depends almost exclusively on cell expansion (Tsukaya et al., 1994).
Promoter activity in cotyledons of young seedlings of the cell cycle genes CDKA;1, CDKB1;1, CYCB1;1, and CYCA2;1 was characterized by using Arabidopsis lines that expressed the β-glucuronidase (GUS) reporter gene under the control of the corresponding cell cycle gene promoters. After 3 d of imbibition in water, seeds were screened for GUS expression. The promoter activity of CDKA;1 was not specific to any particular cell type in the epidermis of cotyledons (Figure 1C). A faint staining was observed throughout the whole epidermis and was equally visible in stomatal and pavement cells. In the vascular tissues, the staining was more intense. CYCA2;1 promoter activity also was associated with vasculature development, whereas no CYCB1;1 expression could be detected in cotyledons (Figures 1A and 1B). By contrast, CDKB1;1:GUS plants showed a unique expression pattern (Figure 1D) with GUS staining exclusively confined to stomatal precursors and GCs (Figures 1E to 1H). GUS staining could be detected in meristemoids, SMs, GMCs, and GCs but not in cells resembling primary MMCs (small, less sinuous epidermal cells not surrounded by stomatal precursors or GCs). Also, unstained meristemoids and GMCs were observed, which can be explained by the cell cycle phase-dependent expression of CDKB1;1 (Porceddu et al., 2001). Nonexpressing stomatal precursors are probably in G1 phase, where CDKB1;1 is only weakly expressed (Sorrell et al., 2001). Temporal CDKB1;1:GUS expression corresponded entirely with the timing of stomata formation in cotyledons as described by Geisler and Sack (2002). Immediately after root protrusion, strong GUS activity could be detected in the stomatal precursors present in the cotyledons. At this stage, fully developed stomata were nearly absent, but a significant number of GMCs could be observed. The timing of GUS staining differed between the abaxial and adaxial epidermis. In young cotyledons, GUS staining was mainly restricted to the adaxial side, but stomatal precursor cell formation stopped already after 1 week. On the contrary, reporter gene expression could still be detected in the abaxial stomatal precursor cells of cotyledons from 12-d-old seedlings. Additionally, more GUS-stained cells were observed in the abaxial than in the adaxial epidermis, corresponding with the observation that the former has approximately fivefold as many stomata as the latter (Geisler et al., 1998).
Figure 1.
CYCB1;1, CYCA2;1, CDKA;1, and CDKB1;1 Promoter Activity in Arabidopsis Cotyledons.
(A) to (D) GUS expression profile of CYCB1;1, CYCA2;1, CDKA;1, and CDKB1;1 in 3-d-old cotyledons, respectively. Only CDKB1;1 promoter activity was detected in stomatal cells and stomatal precursor cells. Scale bars = 200 μm.
(E) to (H) GUS expression profile of CDKB1;1 in 5-d-old cotyledons. Scale bars = 5 μm. M, meristemoid cell; SC, subsidiary cell.
(E) GUS-expressing meristemoid cell (M). In neighboring subsidiary cell (SC), expression is very faint.
(F) GUS-expressing GMC.
(G) GCs after GMC division.
(H) Stoma and subsidiary meristemoid (SM) cell during stomatal complex formation. SC, subsidiary cell.
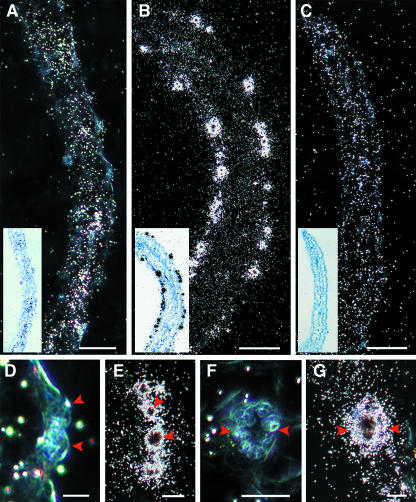
CDKB1;1 expression in stomatal cells of cotyledons was confirmed by mRNA in situ hybridization experiments. In cotyledons harvested 5 d after germination, CDKA;1 hybridization signals were detected in all cell layers but mainly in the vascular bundles (Figure 2A). In stomatal cells, weak or no CDKA;1 expression was detected (Figures 2D and 2F). For CDKB1;1, a strong hybridization signal was observed in several stomata (Figures 2B, 2E, and 2G). The absence of a hybridization signal in stomatal cells of sections hybridized with the control gene coding for neomycin phosphotransferase II confirmed the specificity of CDKB1;1 expression in stomatal cells (Figure 2C).
Figure 2.
In Situ Localization of CDKA;1 and CDKB1;1 mRNA.
(A) to (C) Longitudinal section through cotyledons harvested 5 d after germination. White dots in dark-field images show the hybridization signal. Inset, bright-field image of the same section, with black dots showing the hybridization signal.
(A) CDKA;1 expression.
(B) CDKB1;1 expression.
(C) Expression of neomycin phosphotransferase II gene (control probe).
(D) and (F) CDKA;1 expression in a transverse and a paradermal section through a stoma, respectively.
(E) and (G) CDKB1;1 expression in a transverse and a paradermal section through a stoma, respectively.
Red arrowheads indicate the GCs. Scale bars in (A) to (C) = 50 μm; bars in (D) to (G) = 10 μm.
CDKB1;1.N161 Plants Display an Increased Cell Size and Decreased Stomatal Index
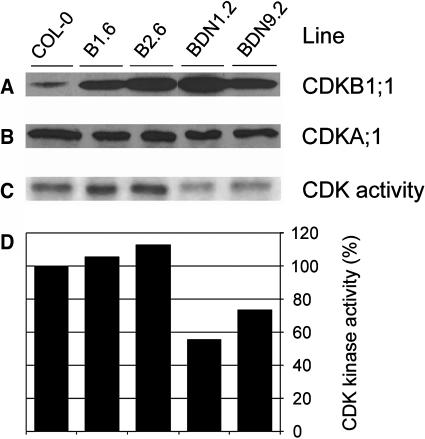
To elucidate the role of CDKB1;1 in stomatal complex formation, transgenic Arabidopsis plants were generated harboring a dominant negative allele of CDKB1;1 (CDKB1;1.N161) under the control of the constitutive 35S promoter of the Cauliflower mosaic virus (CaMV35S). The Asp residue at the amino acid position 161 is required for correct ATP binding by the CDK kinases, and its mutation results in loss of kinase activity. When overexpressed, the mutant CDK has a dominant negative effect, presumably because of competition of the mutant proteins for the association with rate-limiting interacting proteins, such as cyclins (van den Heuvel and Harlow, 1993; Labib et al., 1995). In parallel, Arabidopsis plants were generated overexpressing the wild-type CDKB1;1 gene. Out of multiple transgenic lines, two independent CaMV35S:CDKB1;1 (B1.6 and B2.6) and CaMV35S:CDKB1;1.N161 (BDN1.2 and BDN9.2) lines were selected in which the protein levels exceeded those found in untransformed plants (Figure 3A). Overexpression of neither CDKB1;1 nor CDKB1;1.N161 influenced the level of the CDKA;1 protein (Figure 3B). Because the amount of B-type CDK activity was too low to be detected by immunoprecipitation with a CDKB1;1-specific antibody, total CDK activity was measured by using p10CKS1At affinity purification. CDKB1;1 overproduction led to a slight increase in total CDK activity, whereas that of CDKB1;1.N161 resulted in a decrease of kinase activity to 60% of the level found in control plants (Figures 3C and 3D).
Figure 3.
Transgene Expression and CDK Histone H1 Activity in Untransformed and Transgenic Arabidopsis Plants.
Seven-day-old seedlings of transgenic plants overexpressing CDKB1;1 (lines B1.6 and B2.6) or the dominant negative allele CDKB1;1.N161 (lines BDN1.2 and BDN9.2) were compared with untransformed control plants (Col-0) of the same age.
(A) Protein gel blot analysis of total proteins with anti-CDKB1;1 antibodies.
(B) Protein gel blot analysis of total proteins with anti-CDKA;1 antibodies.
(C) Kinase activity in protein complexes purified with p10CKS1At beads.
(D) Relative CDK kinase activity estimated by quantification of the radioactively phosphorylated H1 protein. The control was arbitrarily set at 100%.
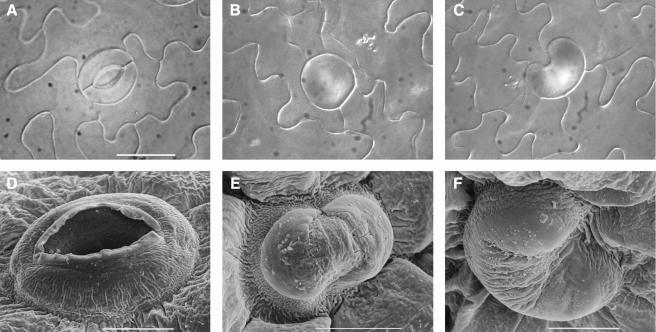
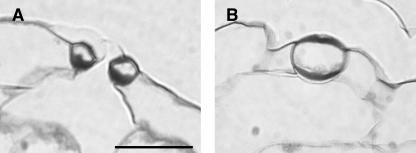
Plants overexpressing the wild-type CDKB1;1 gene were similar in all aspects to untransformed control plants (Tables 1 and 2). The plants overproducing CDKB1;1.N161 were morphologically similar to wild-type control and nontransgenic segregating siblings but with reduced cotyledon and leave sizes (Tables 1 and 2). To address the question of whether the reduction in cotyledon and leaf size was because of inhibition of cell division or cell expansion, the abaxial epidermal cell number and size were determined in cotyledons and first leaves of 3-week-old plants (Tables 1 and 2). The size of cotyledons and first leaves of CDKB1;1.N161 transgenic plants decreased up to 35% and 33%, respectively. In cotyledons, this reduction was accompanied with a 75% lower cell number (43% in leaves), which was partially complemented by a twofold to almost threefold increase in cell size (1.5-fold in leaves). Strikingly, CDKB1;1.N161-overexpressing plants exhibited an up to 66% and 33% decreased stomatal index in cotyledons and leaves, respectively. The important decrease in total cell number could be explained by the fact that stomatal lineages produce ∼67% of all pavement cells in cotyledons (Geisler et al., 2000). In addition to their strongly reduced number, up to 58.5% of the stomata in cotyledons had an aberrant morphology, showing a unicellular round or kidney-shaped appearance and lacking a pore (Figure 4). These aberrant stomatal cells also were frequently found in hypocotyls, but only few were found in leaves (data not shown). The penetrance of the stomatal phenotype is probably correlated with the division rate of the tissue. The dominant negative CDKB1;1 protein has to compete with its endogenous counterpart. CDKB1;1.N161 probably has a higher impact on tissues in which the division rates are low (such as the cotyledons) than on tissues with higher CDKB1;1 levels because of higher division rates (as in leaves). It is expected that plants lacking stomatal pores would not be viable. The transgenic plants obtained probably hold the maximum level of CDKB1;1.N161 tolerated without affecting plant viability.
Table 1.
Abaxial Epidermis Cell Size and Cell Number in Cotyledons of CDKB1;1 and CDKB1;1.N161-Overexpressing Plants
| Abaxial Epidermal Cells
|
Abaxial Stomatal Cells
|
|||||
|---|---|---|---|---|---|---|
| Number of Stomata
|
||||||
| Line | Cotyledon Size (mm2) | Size (μm2) | Estimated Number | Stomatal Index | Normal | Aberrant |
| Col-0 | 6.4 ± 0.3 | 1867 ± 113 | 4622 ± 292 | 32.7 ± 0.7 | 1137 ± 67 | None |
| CDKB1;1 B1.6 | 6.1 ± 0.3 | 1847 ± 164 | 4765 ± 365 | 31.8 ± 2.5 | 1132 ± 67 | None |
| CDKB1;1 B2.6 | 5.9 ± 0.3 | 2024 ± 202 | 4197 ± 542 | 30.8 ± 1.6 | 994 ± 144 | None |
| CDKB1;1.N161 BDN1.2 | 4.2 ± 0.2 | 3797 ± 287 | 1122 ± 137 | 16.9 ± 1.7 | 176 ± 28 | 75 ± 26 |
| CDKB1;1.N161 BDN9.2 | 4.8 ± 0.2 | 5335 ± 420 | 1176 ± 103 | 11.1 ± 1.8 | 134 ± 27 | 189 ± 37 |
All measurements were performed on 3-week-old cotyledons. The indicated values are means ± se (n = 6 to 10).
Table 2.
Abaxial Epidermis Cell Size and Cell Number in First Leaves of CDKB1;1 and CDKB1;1.N161-Overexpressing Plants
| Abaxial Epidermal Cells
|
Abaxial Stomatal Cells
|
|||||
|---|---|---|---|---|---|---|
| Number of Stomata
|
||||||
| Line | Leaf Size (mm2) | Size (μm2) | Estimated Number | Stomatal Index | Normal | Aberrant |
| Col-0 | 14.2 ± 0.5 | 1460 ± 90 | 17628 ± 874 | 27.6 ± 1.4 | 3816 ± 292 | None |
| CDKB1;1 B1.6 | 13.3 ± 0.5 | 1416 ± 132 | 18829 ± 1228 | 25.7 ± 0.9 | 3842 ± 284 | None |
| CDKB1;1 B2.6 | 15.2 ± 0.3 | 1542 ± 110 | 17805 ± 788 | 26.5 ± 1.0 | 3735 ± 236 | None |
| CDKB1;1.N161 BDN1.2 | 9.5 ± 0.5 | 2017 ± 110 | 10060 ± 653 | 21.3 ± 1.7 | 1759 ± 159 | ND |
| CDKB1;1.N161 BDN9.2 | 10.1 ± 0.5 | 2189 ± 137 | 10089 ± 738 | 19.1 ± 0.9 | 1628 ± 154 | ND |
All measurements were performed on 3-week-old first leaves. The indicated values are means ± se (n = 6 to 10). ND, not determined.
Figure 4.
Microscopic Analysis of CDKB1;1.N161-Overexpressing Arabidopsis Plants.
(A) Bright-field micrograph of normal stoma.
(B) and (C) Bright-field micrographs of different aberrant stomatal cells.
(D) Scanning micrograph of normal stoma.
(E) and (F) Scanning micrographs of different aberrant stomatal cells.
Scale bar in (A) = 20 μm ([A] to [C], same magnification); bars in (D) to (F) = 10 μm.
CDKB1;1.N161-overexpressing plants were crossed with either wild-type Columbia (Col-0) plants (as a control), a CDKB1;1-overexpressing line, or a line overexpressing CDKA;1 (Hemerly et al., 1995) to find out whether the observed stomatal phenotype was attributable to a specific inhibition of B-type CDK activity or to a partial inhibition of the A-type CDKA;1. The fidelity of the crosses was confirmed by PCR analysis (data not shown). As can be seen in Table 3, overexpression of the wild-type CDKB1;1 gene totally suppressed the aberrant phenotype triggered by CDKB1;1.N161. These data confirm that CDKB1;1.N161 operates as a dominant negative kinase by sequestering factors needed for the activation of the endogenous CDKB1;1 protein. Increasing the pool of CDKB1;1 by crossing in the CDKB1;1-overexpressing construct counteracted the dominant effect of CDKB1;1.N161, and the normal stomatal phenotype was restored. By contrast, CDKA;1 overexpression was not able to rescue the mutant stomatal phenotype (Table 3), demonstrating that this phenotype is specifically attributable to an inhibition of the B-type CDK activity.
Table 3.
Stomatal Index and Number in the Abaxial Epidermis of CDKB1;1.N161 Double Transgenic Plants
| Number of Stomata
|
|||
|---|---|---|---|
| Line | Stomatal Index | Normal | Aberrant |
| Col-0 | 26.2 ± 0.8 | 523.0 ± 31 | None |
| CDKB1;1.N161 x Col-0 | 16.2 ± 2.4 | 123.0 ± 22 | 84 ± 14 |
| CDKB1;1.N161 x CDKB1;1 | 28.8 ± 1.0 | 547.5 ± 56 | None |
| CDKB1;1.N161 x CDKA;1 | 15.0 ± 1.1 | 163.1 ± 15 | 109 ± 13 |
All measurements were performed on 11-d-old cotyledons. The indicated values are means ± se (n = 5).
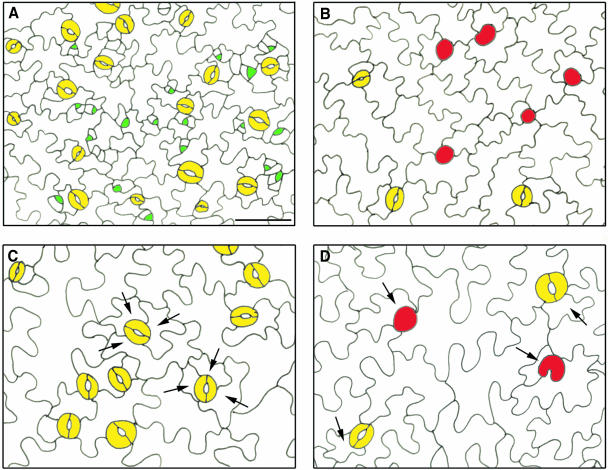
In nontransgenic Arabidopsis plants, 40 to 66% of the stomatal complexes are anisocytic, resulting from three consecutive asymmetric divisions (Geisler et al., 2000; Serna and Fenoll, 2000b) (Figure 5C). By contrast, both normal and aberrant stomata in CDKB1;1.N161-overproducing plants were mostly accompanied by only one small subsidiary cell (Figure 5D), suggesting that only the first asymmetric division of the MMC was accomplished. Moreover, the activation of SMs, originating from MMCs located next to a preexisting stoma or precursor, was strongly inhibited. In young seedlings of control plants, the stomata were surrounded by dividing meristemoids (Figure 5A), whereas in transgenic plants, small dividing SMs could hardly be observed (Figure 5B). As a result, in wild-type plants, most stomata belong to higher order stomatal complexes, meaning that they derive from the same protodermal cell through SM formation (Figures 5A and 5C) (Serna et al., 2002), whereas in CDKB1;1.N161 transgenic plants, mostly isolated stomata were formed (Figures 5B and 5D).
Figure 5.
Stomatal Complexes in Wild-Type and CDKB1;1.N161-Overexpressing Plants.
(A) Cotyledon epidermis of a control plant showing meristemoid and SM formation 4 d after germination.
(B) Cotyledon epidermis of a CDKB1;1.N161-overexpressing transgenic plant 4 d after germination.
(C) Mature cotyledon epidermis of a control plant showing anisocytic higher order stomatal complexes.
(D) Mature cotyledon epidermis of CDKB1;1.N161-overexpressing transgenic plant showing normal and aberrant stomata neighbored with one small subsidiary cell.
Arrows indicate subsidiary cells. Yellow, GCs; green, meristemoids; red, aberrant stomata. Scale bar = 40 μm ([A] to [D], same magnification).
CDKB1;1 Activity Is Not Required for Stomatal Differentiation
Acquisition of the typical spherical shape of stomata starts at the GMC stage with the occurrence of cell wall thickening that probably restricts local elongation of the cell wall (Zhao and Sack, 1999). In addition, stomata maturation is characterized by starch accumulation in plastids and deposition of cellulose microfibril arrays that radiate out of the pore. When normal and abnormal stomata were compared by electron scanning microscopy, anomalous cuticle features were seen resembling those found in differentiating stomata (Figures 4D to 4F). The aberrant stomatal cells had central thickenings similar to those that give rise to the stomatal pore (Figure 4E) and, as seen on transverse sections, had cell wall thickenings at the upper and lower parts of the cell (Figure 6). Moreover, as for wild-type stomata, air space in the underlying mesophyll could be observed (Figure 6). Also, starch accumulation, a typical marker for stomatal differentiation, was found in these aberrant stomatal cells (data not shown). These observations suggest that GC identity does not rely on CDKB1;1 activity and, thus, that final stomatal differentiation is seemingly independent from cell cycle activity.
Figure 6.
Transverse Sections through Cotyledons of Untransformed and Transgenic Arabidopsis Plants.
(A) Stoma sectioned from the cotyledon epidermis of a wild-type plant.
(B) Stoma sectioned from the cotyledon epidermis of a CDKB1;1.N161-overexpressing transgenic plant.
Scale bar = 20 μm ([A] and [B], same magnification).
Aberrant Stomatal Cells of the CDKB1;1.N161 Mutant Are Blocked in G2 Phase
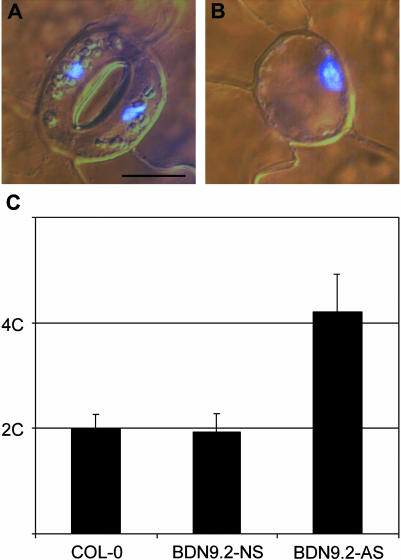
The cell cycle stage in which the aberrant stomatal cells were arrested was determined by fluorescence densitometry performed on leaves stained with 4′,6-diamidino-2-phenylindole. All phenotypically normal stomata, either in transgenic or in wild-type plants, consisted of one nucleus per GC (Figure 7A). Also, in all aberrant stomatal cells, only one, but larger, nucleus was observed (Figure 7B). Quantification of the fluorescence level showed that the nucleus of aberrant GCs was exactly twice as large as that of normal cells (Figure 7C). Previously, it has been demonstrated that nuclei of GCs of Arabidopsis have a 2C DNA content (Melaragno et al., 1993). Therefore, we can conclude that the aberrant stomatal cells hold a 4C DNA level, illustrating that the cell cycle is arrested in the G2 phase. In none of the aberrant stomatal cells was any morphological indication ever observed for the initiation of cytokinesis, such as cell plate formation. In addition, the nucleus of the aberrant stomatal cell was mostly positioned asymmetrically in the cell (Figure 7B).
Figure 7.
Microscopic Analysis of Cotyledons of Plants Overexpressing CDKB1;1.N161 Stained with 4′,6-Diamidino-2-Phenylindole.
(A) Wild-type stoma.
(B) Aberrant stomatal cell of a CDKB1;1.N161 transgenic plant.
(C) Quantification of 4′,6-diamidino-2-phenylindole intensity of nuclei in normal GCs of control plants (COL-0, n = 46), CDKB1;1.N161 transgenic plants (BDN9.2-NS, n = 82), and in aberrant stomatal cells of CDKB1;1.N161 transgenic plants (BDN9.2-AS, n = 60).
Scale bar = 10 μm ([A] and [B], same magnification).
DISCUSSION
Based on its disability to complement yeast CDK mutants and the presence of a unique cyclin binding motif not found in CDKs from other eukaryotes, the B-type class of CDKs is assumed to regulate plant-specific aspects of the cell cycle. Although the exact role of CDKB1;1 in plant development is still enigmatic, we propose that it has a function in stomatal development because its expression in cotyledons coincides with the formation of stomatal precursors and GCs. Moreover, overexpression of CDKB1;1.N161 in Arabidopsis has a dramatic impact on the formation of stomata, probably by inhibiting division of stomatal precursors, resulting in an important decrease in stomatal number and formation of stomata with aberrant morphology. Furthermore, we have illustrated that the aberrant stomatal cells are blocked in G2, but could still acquire stomatal identity, independently from nuclear division and cellular division.
Cell Cycle Gene Expression in GCs and Stomatal Precursors
When focusing on cotyledons, CDKB1;1 promoter activity correlates almost exclusively with the formation of meristemoids, GMCs, and GCs, whereas the expression of CDKA;1 is not specifically confined to a particular cell type. We believe that the kinase activity of CDKB1;1 is crucial to complete the correct stomatal complex formation pathway because CDKB1;1.N161 overexpression results in a severe decrease in the stomatal index and many aberrant stomatal cells. By contrast, no aberrant stomatal cells or reduced stomatal index were found in plants overexpressing the CDKA;1-specific kinase inhibitor KRP2, although cell division is much more inhibited in these plants than in CDKB1;1.N161 transgenic plants (De Veylder et al., 2001). CDKB1;1 might be part of a CDK/cyclin complex with a specific role in cell divisions associated with the formation of the stomatal complex. Because no promoter activity of CYCB1;1 and CYCA2;1 could be detected in stomatal precursors, other cyclin partners than the two tested are probably involved in the stomata-specific cell cycle events.
In leaves, the expression pattern of CDKA;1, CYCB1;1, CYCA2;1, and CDKB1;1 was comparable in both pavement cells and stomatal cells (Hemerly et al., 1993; Ferreira et al., 1994; Serna and Fenoll, 1997; Burssens et al., 2000; data not shown). Nevertheless, also in leaves of the CDKB1;1.N161 transgenic plants, a clear reduction in stomatal index was observed, illustrating that CDKB1;1 regulates cell divisions associated with the formation of stomatal complexes even in rapidly dividing tissues where the expression levels of different cell cycle genes are high.
Curiously, CDKB1;1 is not only expressed in the stomatal precursor cells, but also can be detected in differentiated GCs. Previously, Serna and Fenoll (1997) have found that CDKA;1 and CYCB1;1 are transcribed in mature GCs as well and have suggested that the expression of cell cycle genes in mature GCs might reflect the competence of these cells to divide. This hypothesis is illustrated by the ease by which calli can regenerate from GC protoplasts of B. vulgaris and Nicotiana tabacum (tobacco) but also from intact GCs from B. vulgaris and not from other leaf epidermal cells (Sahgal et al., 1994; Hall et al., 1996a, 1996b).
CDKB1;1.N161 Overexpression Blocks Meristemoid Division and Secondary Meristemoid Formation
Stomata formation is blocked at a very early stage during plant development in CDKB1;1.N161 transgenic plants. In 4-d-old CDKB1;1.N161 transgenic plants, 70% of all (normal and abnormal) stomata eventually formed were already present, whereas this was <40% in control plants (data not shown). Because it is difficult to make dental resin impressions on very young cotyledons without damaging the tissues, we could not use this technique to trace the divisions preceding the stomatal development in the transgenic plants. Although the observed aberrant stomatal cells display GC and GMC morphology, they may correspond to blocked meristemoids that have converted into GMCs, which differentiated partly into GCs. This assumption is based on the observation that meristemoids are the first stomatal precursor cells in which high CDKB1:1-GUS promoter expression could be detected, which means that CDKB1;1 activity is probably required for meristemoid mitosis. In addition, both normal and aberrant stomata are surrounded by fewer subsidiary cells in CDKB1;1.N161-overexpressing plants than in wild-type plants (Figures 5C and 5D). Mostly, only one small subsidiary cell is observed, indicating that only the first MMC division has been fulfilled. As such, most of the stomata in the transgenic lines derive from meristemoids formed by the first division of primary MMCs. However, because aberrant stomatal cells are sometimes surrounded by more than one subsidiary cell, stomatal precursor cell division could occasionally be blocked at a later stage during stomatal development. In most aberrant stomatal cells, the nucleus resides at one side of the cell. MMCs and meristemoids display an asymmetrically located nucleus before division, whereas GMCs have a more centrally located nucleus (Zhao and Sack, 1999). Therefore, the asymmetrical localization of the nucleus also suggests that most of the asymmetric meristemoid divisions are blocked. However, it cannot be excluded that the occurrence of a noncentrally localized nucleus is a consequence of the presence of aberrant cytoskeleton arrays in the pore-lacking GCs.
SMs arise from the asymmetric division of subsidiary cells formed by the asymmetric meristemoid division. In wild-type plants, 75% of all stomata originate from SMs (Geisler et al., 2000), by which the stomatal number gradually increases over time. As illustrated in Figure 5, hardly any SM is present in CDKB1;1.N161 transgenic plants 4 d after germination, which may explain why only a small number of stomata have been formed after this time point. The lack of SM formation can be attributed to the reduction in subsidiary cells, from which SMs originate, but also to the presumable role played by CDKB1;1 in subsidiary MMC division. An early block in stomata formation probably also accounts for the strongly reduced number of pavement cells because stomatal lineages produce ∼67% and 48% of all pavement cells in cotyledons and leaves, respectively (Geisler et al., 2000).
GC Identity Is Acquired Independently from Nuclear and Cellular Division
Blocked meristemoids are arrested in the G2 phase of the cell cycle, corresponding with the need of CDKB1;1 kinase activity to progress through the G2-to-M transition (Porceddu et al., 2001). Surprisingly, aberrant cells still acquire stomatal identity, as observed by the GC shape formation, cell wall thickening, and starch accumulation, which are all features typical for developing GMCs and mature stomata (Zhao and Sack, 1999). These data illustrate that CDKB1;1.N161 overexpression blocks cell division, but not cell differentiation of the stomata, and suggests that the decision to differentiate into GMCs has already been taken before or during the time point at which cells are arrested by CDKB1;1.N161. Previously, in cytokinesis-defective mutants, GMCs, which are unable to divide, have been shown to acquire cell wall thickenings on the outer cell wall as well (Yang et al., 1999; Söllner et al., 2002). Likewise, treatment of maize (Zea mays) seedlings with caffeine interferes with cell plate formation but does not prevent deposition of a wall thickening in the middle of the periclinal walls (Galatis and Apostolakos, 1991). Taken together, these results indicate that GC identity is acquired independently from nuclear and cellular divisions.
Antisense versus Dominant Negative
The stomata phenotype described here has not been observed in plants expressing a CDKB1;1 antisense gene (Yoshizumi et al., 1999). Surprisingly, for these antisense transgenic plants no effect on cell division had been seen. The differences must relate to the dissimilar experimental setup. Because no CDK activity measurements have been reported for the CDKB1;1 antisense lines, it is difficult to determine which strategy is most efficient in inhibiting B-type CDK activity. Two classes of B-type CDKs exist in plants (B1 and B2). The available experimental data suggest different roles for the two classes. First, both types of CDKs are differentially expressed during the cell cycle (Magyar et al., 1997; Breyne et al., 2002). Second, a large-screen two-hybrid interactome experiment illustrated that B1- and B2-type CDKs associate with distinct partners (L. De Veylder, V. Boudolf, D. Inzé, and P. Hilson, unpublished data). Therefore, overexpression of the dominant negative CDKB1;1.N161 allele most probably affects the class of B1-type CDKs only. By contrast, an antisense strategy might have an effect on both classes simultaneously.
Plants overexpressing CDKB1;1.N161 also differ from the CDKB1;1 antisense lines in their lack of a cell elongation defect in dark-grown seedlings and the conversion of amyloplasts into etioplasts (data not shown). Previously, a large fraction of CDKB1;1 proteins could be traced back in high molecular mass complexes displaying negligible kinase activity (Porceddu et al., 2001). Because almost no cell division occurs in hypocotyls grown in the dark, it is temping to postulate that inactive CDKB1;1 might be part of a protein complex that regulates cell elongation. An antisense strategy is supposed to pull CDKB1;1 from these complexes. By contrast, overexpression of the dominant negative variant CDKB1;1.N161 is not expected to interfere with the function of such a complex and, thus, not to affect cell elongation of dark-grown seedlings. Analysis of knockout plants could provide important data that might reconcile the apparent discrepancies in phenotype between antisense and dominant negative genotypes.
Future Perspectives
In the literature, a possible relationship between cell cycle and stomatal patterning in dicotyledonous plants has been discussed but not proven yet (Croxdale, 2000; Geisler and Sack, 2002). The division of an MMC located adjacent to a stomatal cell or precursor cell is regulated in a position-dependent manner (Geisler et al., 2000). The new SM is invariably formed away from the preexisting stomatal cell or precursor cell. Because the formation of SMs is strongly inhibited in the CDKB1;1.N161 transgenic plants, it is difficult to say whether CDKB1;1 is directly involved in stomatal patterning. The relation between the known patterning-related genes (TMM and SDD1) and CDKB1;1 still needs to be investigated. Based on the specific expression of CDKB1;1 in stomatal precursor cells, it is tempting to propose that CDKB1;1 acts downstream of genes involved in stomatal development. It will be of great interest to further unravel the interplay of the cell cycle activity and cell differentiation processes unique to plants.
METHODS
Expression Analysis of pCDKB1;1:GUS Transgenic Plants
Cotyledons were collected daily from seedlings that were sown simultaneously from transgenic plants harboring CDKA;1 (Hemerly et al., 1993), CYCB1;1 (Ferreira et al., 1994), CYCA2;1 (Burssens et al., 2000), and CDKB1;1 (de Almeida Engler et al., 1999) promoter-GUS fusions. Seeds were placed on wet germination paper or agar, transferred to darkness at 4°C overnight, and then moved to photoperiodic light, the moment at which the seedling age was scored.
The histochemical GUS assays were performed according to standard protocols (Jefferson et al., 1987) with minor modifications. The young seedlings were incubated in 90% acetone for 2 h at 4°C. After the material had been washed in phosphate buffer, it was immersed in the enzymatic reaction mixture (1 mg/mL of 5-bromo-4-chloro-3-indolyl β-d-glucuronide, 2 mM ferricyanide, and 0.5 mM of ferrocyanide in 100 mM phosphate buffer, pH 7.4). The reaction was performed at 37°C in the dark for 4 h to overnight, and the material was cleared with chlorolactophenol (chloral hydrate:phenol:lactic acid, 2:1:1) and observed under a light microscope or stereoscope.
mRNA in Situ Hybridization
Almost all steps of the in situ hybridization method were performed as described by de Almeida Engler et al. (2001). Seedlings of Arabidopsis (ecotype Columbia) were germinated on K1 medium (Valvekens et al., 1988). Samples of plant material were fixed in 2.5% glutaraldehyde, dehydrated, and embedded in paraffin. Sections (10 μm thick) were fixed to 3-aminopropyltriethoxy-silane–coated slides. Gene-specific antisense probes of CDKA;1 and CDKB1;1 were synthesized from PCR products flanked by T7 and Sp6 promoters, and 15 × 106 cpm/slide was applied (75 ng/mL). Exposure times varied, and images were created with a digital Axiocam (Zeiss, Jena, Germany) under standard dark- and bright-field optics.
Regeneration and Molecular Analysis of CDKB1;1- and CDKB1;1.N161-Overexpressing Plants
The cDNA for CDKB1;1.N161 was obtained by mutating the codon GAT (Asp-161) for AAT (Asn-161) by in vitro mutagenesis (Porceddu et al., 1999). The coding regions of CDKB1;1 and CDKB1;1.N161 were amplified by PCR with the primers 5′-GGCCATGGAGAAGTACGAGAAGC-3′ and 5′-GGGGATCCTCAGAACTGAGACTTGTCAAGG-3′. The obtained PCR fragments were digested with NcoI and BamHI. Subsequently, the restriction fragments were cloned between the CaMV35S promoter and the nopaline synthase (NOS) 3′ untranslated region in the NcoI and BamHI sites of the pH35S vector (Hemerly et al., 1995), resulting in the pH35SCDKB1;1 and pH35SCDKB1;1.N161 vectors. The CaMV35S/CDKB1;1/NOS and CaMV35S/CDKB1;1.N161/NOS cassettes were released by EcoRI and SalI and cloned into the EcoRI and SalI sites of pBinPLUS (van Engelen et al., 1995) to obtain the pBINCDKB1;1 and pBINCDKB1;1.N161 vectors. Both pBINCDKB1;1 and pBINCDKB1;1.N161 were mobilized by the helper plasmid pRK2013 into the Agrobacterium tumefaciens C58C1RifR strain, harboring the plasmid pMP90 (Koncz and Schell, 1986). Arabidopsis was transformed by the floral dip method (Clough and Bent, 1998). Transgenic CaMV35S/CDKB1;1 and CaMV35S/CDKB1;1.N161 plants were obtained on kanamycin-containing medium. For all analyses, plants were grown under a 16-h-light/8-h-dark photoperiod at 22°C on germination medium (Valvekens et al., 1988). The obtained transformants were analyzed by protein gel blotting and CDK activity measurements as described before (De Veylder et al., 1997).
Histological Analysis of Abaxial Epidermis of Leaf and Cotyledon
Untransformed and transgenic plants were grown on the same Petri dish to exclude different growth conditions. To avoid errors resulting from discrepancies in germination timing, seedlings that germinated at the same time were marked and harvested 3 weeks after sowing. Leaves and cotyledons were placed overnight in ethanol to remove chlorophyll and were subsequently cleared and stored in lactic acid for microscopy. Cell density and stomatal index were determined as described by De Veylder et al. (2001). For scanning electron microscopy, cotyledons from 3-week-old seedlings were processed and analyzed as described (De Veylder et al., 2002).
Nuclei were stained fluorescently by fixing 3-week-old cotyledons in a mixture of 9:1 ethanol:acetic acid (v/v). After the samples had been rinsed, they were stained for 24 h with 0.1 μg/mL of 4′,6-diamidino-2-phenylindole, mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and observed under a 63× oil immersion objective on a Zeiss Axioskop equipped with an Axiocam CCD camera (Zeiss). Images were obtained using the Axiovision software and were analyzed in gray-scale with the public domain image analysis program ImageJ (version 1.28; http://rsb.info.nih.gov/ij/). Relative fluorescence units were reported as integrated density, which is the product of the area and the average fluorescence of the selected nucleus.
Acknowledgments
The authors thank the members of the cell cycle group for fruitful discussions and useful suggestions, Martine De Cock for help in preparing the manuscript, and Peter Chaerle, Ive De Smet, and Ruth De Groodt for help with microscopy and scanning micrographs. This work was supported by grants from the Interuniversity Poles of Attraction Programme-Belgian Science Policy (P5/13), the European Union (ECCO QLG2-CT1999-00454), and Crop Design (0235). V.B. is indebted to the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen for a predoctoral fellowship. L.D.V. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek—Vlaanderen.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Lieven De Veylder (lieven.deveylder@psb.ugent.be).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.021774.
References
- Berger, D., and Altmann, T. (2000). A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14, 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Boudolf, V., Rombauts, S., Naudts, M., Inzé, D., and De Veylder, L. (2001). Identification of novel cyclin-dependent kinases interacting with the CKS1 protein of Arabidopsis. J. Exp. Bot. 52, 1381–1382. [PubMed] [Google Scholar]
- Breyne, P., Dreesen, R., Vandepoele, K., De Veylder, L., Van Breusegem, F., Callewaert, L., Rombauts, S., Raes, J., Cannoot, B., Engler, G., Inzé, D., and Zabeau, M. (2002). Transcriptome analysis during cell division in plants. Proc. Natl. Acad. Sci. USA 99, 14825–14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee, C. (2001). The long and the short of stomatal density signals. Trends Plant Sci. 6, 441–442. [DOI] [PubMed] [Google Scholar]
- Burssens, S., de Almeida Engler, J., Beeckman, T., Richard, C., Shaul, O., Ferreira, P., Van Montagu, M., and Inzé, D. (2000). Developmental expression of the Arabidopsis thaliana CycA2;1 gene. Planta 211, 623–631. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Croxdale, J.L. (2000). Stomatal patterning in angiosperms. Am. J. Bot. 87, 1069–1080. [PubMed] [Google Scholar]
- de Almeida Engler, J., De Groodt, R., Van Montagu, M., and Engler, G. (2001). In situ hybridization to mRNA of Arabidopsis tissue sections. Methods 23, 325–334. [DOI] [PubMed] [Google Scholar]
- de Almeida Engler, J., De Vleesschauwer, V., Burssens, S., Celenza, J.L., Jr., Inzé, D., Van Montagu, M., Engler, G., and Gheysen, G. (1999). Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11, 793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder, L., Beeckman, T., Beemster, G.T.S., de Almeida Engler, J., Ormenese, S., Maes, S., Naudts, M., Van Der Schueren, E., Jacqmard, A., Engler, G., and Inzé, D. (2002). Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa/DPa transcription factor. EMBO J. 21, 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder, L., Beeckman, T., Beemster, G.T.S., Krols, L., Terras, F., Landrieu, I., Van Der Schueren, E., Maes, S., Naudts, M., and Inzé, D. (2001). Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13, 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder, L., Joubès, J., and Inzé, D. (2003). Plant cell cycle transitions. Curr. Opin. Plant Biol. 6, 536–543. [DOI] [PubMed] [Google Scholar]
- De Veylder, L., Segers, G., Glab, N., Casteels, P., Van Montagu, M., and Inzé, D. (1997). The Arabidopsis Cks1At protein binds to the cyclin-dependent kinases Cdc2aAt and Cdc2bAt. FEBS Lett. 412, 446–452. [DOI] [PubMed] [Google Scholar]
- Dewitte, W., and Murray, J.A.H. (2003). The plant cell cycle. Annu. Rev. Plant Biol. 54, 235–264. [DOI] [PubMed] [Google Scholar]
- Ferreira, P.C.G., Hemerly, A.S., de Almeida Engler, J., Van Montagu, M., Engler, G., and Inzé, D. (1994). Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobert, P.R., Gaudin, V., Lunness, P., Coen, E.S., and Doonan, J.H. (1996). Distinct classes of cdc2-related genes are differentially expressed during the cell division cycle in plants. Plant Cell 8, 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatis, B., and Apostolakos, P. (1991). Microtubule organization and morphogenesis of stomata in caffeine-affected seedlings of Zea mays. Protoplasma 165, 11–26. [Google Scholar]
- Geisler, M., Nadeau, J., and Sack, F.D. (2000). Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell 12, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, M., Yang, M., and Sack, F.D. (1998). Divergent regulation of stomatal initiation and patterning in organ and suborgan regions of the Arabidopsis mutants too many mouths and four lips. Planta 205, 522–530. [DOI] [PubMed] [Google Scholar]
- Geisler, M.J., and Sack, F.D. (2002). Variable timing of developmental progression in the stomatal pathway in Arabidopsis cotyledons. New Phytol. 153, 469–476. [DOI] [PubMed] [Google Scholar]
- Hall, R.D., Riksen-Bruinsma, T., Weyens, G., Lefèbvre, M., Dunwell, J.M., and Krens, F.A. (1996. b). Stomatal guard cells are totipotent. Plant Physiol. 112, 889–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R.D., Riksen-Bruinsma, T., Weyens, G.J., Rosquin, I.J., Denys, P.N., Evans, I.J., Lathouwers, J.E., Lefèbvre, M.P., Dunwell, J.M., van Tunen, A., and Krens, F.A. (1996. a). A high efficiency technique for the generation of transgenic sugar beets from stomatal guard cells. Nat. Biotechnol. 14, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Hemerly, A., de Almeida Engler, J., Bergounioux, C., Van Montagu, M., Engler, G., Inzé, D., and Ferreira, P. (1995). Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 14, 3925–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly, A.S., Ferreira, P., de Almeida Engler, J., Van Montagu, M., Engler, G., and Inzé, D. (1993). cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajuku, Y., Hirayama, T., Endoh, H., and Oka, A. (1992). Exon-intron organization of the Arabidopsis thaliana protein kinase genes CDC2a and CDC2b. FEBS Lett. 304, 73–77. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz, C., and Schell, J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204, 383–396. [Google Scholar]
- Labib, K., Craven, R.A., Crawford, K., and Nurse, P. (1995). Dominant mutants identify new roles for p34cdc2 in mitosis. EMBO J. 14, 2155–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar, Z., et al. (1997). Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell 9, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno, J.E., Mehrotra, B., and Coleman, A.W. (1993). Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov, V., De Veylder, L., Van Montagu, M., and Inzé, D. (1999). Cyclin-dependent kinases and cell division in higher plants—The nexus. Plant Cell 11, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, J.A., and Sack, F.D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296, 1697–1700. [DOI] [PubMed] [Google Scholar]
- Porceddu, A., De Veylder, L., Hayles, J., Van Montagu, M., Inzé, D., and Mironov, V. (1999). Mutational analysis of two Arabidopsis thaliana cyclin-dependent kinases in fission yeast. FEBS Lett. 446, 182–188. [DOI] [PubMed] [Google Scholar]
- Porceddu, A., Stals, H., Reichheld, J.-P., Segers, G., De Veylder, L., De Pinho Barrôco, R., Casteels, P., Van Montagu, M., Inzé, D., and Mironov, V. (2001). A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. J. Biol. Chem. 276, 36354–36360. [DOI] [PubMed] [Google Scholar]
- Sahgal, P., Martinez, G.V., Roberts, C., and Tallman, G. (1994). Regeneration of plants from cultured guard cell protoplasts of Nicotiana glauca (Graham). Plant Sci. 97, 199–208. [Google Scholar]
- Segers, G., Gadisseur, I., Bergounioux, C., de Almeida Engler, J., Jacqmard, A., Van Montagu, M., and Inzé, D. (1996). The Arabidopsis cyclin-dependent kinase gene cdc2bAt is preferentially expressed during S and G2 phases of the cell cycle. Plant J. 10, 601–612. [DOI] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (1997). Tracing the ontogeny of stomatal clusters in Arabidopsis with molecular markers. Plant J. 12, 747–755. [DOI] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (2000. a). Stomatal development in Arabidopsis: How to make a functional pattern. Trends Plant Sci. 5, 458–460. [DOI] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (2000. b). Stomatal development and patterning in Arabidopsis leaves. Physiol. Plant 109, 351–358. [Google Scholar]
- Serna, L., Torres-Contreras, J., and Fenoll, C. (2002). Clonal analysis of stomatal development and patterning of Arabidopsis leaves. Dev. Biol. 241, 24–33. [DOI] [PubMed] [Google Scholar]
- Söllner, R., Glässer, G., Wanner, G., Somerville, C.R., Jürgens, G., and Assaad, F.F. (2002). Cytokinesis-defective mutants of Arabidopsis. Plant Physiol. 129, 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell, D.A., Menges, M., Healy, J.M.S., Deveaux, Y., Amano, X., Su, Y., Nakagami, H., Shinmyo, A., Doonan, J.H., Sekine, M., and Murray, J.A.H. (2001). Cell cycle regulation of cyclin-dependent kinases in tobacco cultivar Bright Yellow-2 cells. Plant Physiol. 126, 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J.E., Abram, B., Boorse, G., and Tallman, G. (1998). Approaches to evaluating the extent to which guard cell protoplasts of Nicotiana glauca (tree tobacco) retain their characteristics when cultured under conditions that affect their survival, growth, and differentiation. J. Exp. Bot. 49, 377–386. [Google Scholar]
- Tsukaya, H., Tsuge, T., and Uchimiya, H. (1994). The cotyledon: A superior system for studies of leaf development. Planta 195, 309–312. [Google Scholar]
- Umeda, M., Umeda-Hara, C., Yamaguchi, M., Hashimoto, J., and Uchimiya, H. (1999). Differential expression of genes for cyclin-dependent protein kinases in rice plants. Plant Physiol. 119, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens, D., Van Montagu, M., and Van Lijsebettens, M. (1988). Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 85, 5536–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, S., and Harlow, E. (1993). Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054. [DOI] [PubMed] [Google Scholar]
- Vandepoele, K., Raes, J., De Veylder, L., Rouzé, P., Rombauts, S., and Inzé, D. (2002). Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14, 903–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen, F.A., Molthoff, J.W., Conner, A.J., Nap, J.-P., Pereira, A., and Stiekema, W.J. (1995). pBINPLUS: An improved plant transformation vector based on pBIN19. Transgenic Res. 4, 288–290. [DOI] [PubMed] [Google Scholar]
- von Groll, U., and Altmann, T. (2001). Stomatal cell biology. Curr. Opin. Plant Biol. 4, 555–560. [DOI] [PubMed] [Google Scholar]
- von Groll, U., Berger, D., and Altmann, T. (2002). The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell 14, 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Nadeau, J.A., Zhao, L., and Sack, F.D. (1999). Characterization of a cytokinesis defective (cyd1) mutant of Arabidopsis. J. Exp. Bot. 50, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Yang, M., and Sack, F.D. (1995). The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7, 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi, T., Nagata, N., Shimada, H., and Matsui, M. (1999). An Arabidopsis cell cycle-dependent kinase-related gene, CDC2b, plays a role in regulating seedling growth in darkness. Plant Cell 11, 1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., and Sack, F.D. (1999). Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Am. J. Bot. 86, 929–939. [PubMed] [Google Scholar]