Abstract
The vascularly isolated muscles in the hindlimbs of five dogs were perfused with an oxygenated physiological salt solution. The extractions of adenosine and of a nontransported analogue of adenosine, 9-β-d-arabinofuranosyl hypoxanthine (AraH), were determined by the single-pass indicator-dilution technique. A bolus containing [125I]albumin (reference tracer), [14C]adenosine, and [3H]AraH was injected into the artery while samples of venous effluent were collected over the next minute. This injection was repeated with dipyridamole (10–5 M) in the perfusate. Early extractions of AraH (EAra) and adenosine (EAdo) under control conditions were 48 ± 4 and 80 ± 4%, respectively. In the presence of dipyridamole, EAra was unchanged (47 ± 5) while EAdo decreased to 45 ± 7%. Since early extraction reflects primarily the barrier posed by endothelial cells, these results demonstrate significant endothelial uptake of adenosine. Analysis of these data using a mathematical model of blood-tissue exchange indicates that, under the conditions of these experiments, at least 78% of the adenosine taken up by skeletal muscle entered endothelial cells.
Keywords: capillary transport, dipyridamole, multiple indicator-dilution technique
IN SKELETAL MUSCKE, microvascular transport of small water-soluble molecules is usually thought to result from simple diffusion through clefts between adjacent endothelial cells (26). Two lines of evidence suggest that this may be an incomplete view. First, cultured endothelial cells exhibit plasma membrane transport of a number of water-soluble substances including simple sugars (12) and nucleosides (14, 25). Although there are no studies of this type using cultured endothelial cells isolated from capillaries, the evidence available from isolated capillary preparations suggests that capillary endothelium is capable of this type of transport (7). Second, plasma membranes of brain and lung capillary endothelial cells in vivo transport a number of substances including sugars, biogenic amines, prostaglandins, purine nucleosides, amino acids, and free fatty acids (2, 3, 9, 13, 16, 30, 31).
If endothelial cells of skeletal muscle capillaries exhibit similar properties, this could have dramatic effects on transport between plasma and interstitium. Entrance of a substance into endothelial cells in parallel with diffusion through paracellular clefts could facilitate transcapillary movement if the substance is not metabolized or could retard movement if the substance is metabolized in the cytosol of endothelial cells.
We have tested the general hypothesis that cellular uptake of the nucleoside adenosine could be influenced by capillary endothelial cell membrane transport. If this occurs, it would have significance with regard to the regulation of plasma and interstitial adenosine concentration and would critically influence the use of adenosine release into venous effluent as an index of interstitial adenosine concentration. The purpose of this study was to determine the relative importance in skeletal muscle of carrier-mediated adenosine transport into endothelial cells versus permeation of the capillary wall via aqueous channels. Our results indicate that, in this tissue, endothelial membrane transport dominates over interendothelial cleft transport in the removal of adenosine from capillary perfusate.
METHODS
Preparation
Five mongrel dogs were anesthetized with intravenous pentobarbital sodium (30 mg/kg) with supplements given as required. We used a hindlimb skeletal muscle preparation that has been described elsewhere in detail (22). The leg was first skinned from knee to ankle. Circulation to the paw was eliminated by ligating the anterior tibial artery at the ankle and by tightening a hose clamp around the ankle. All tissue was stripped away from the femur at a point just above the knee, except for the femoral artery and vein, which then represent the sole vascular connections. Branches of the femoral vessels not supplying or draining the muscles of interest were ligated. The animal was then heparinized (750 U/kg) prior to cannulation of the femoral artery and vein. The muscles were perfused with a physiological salt solution described below. At this point we completely removed the hindlimb from the animal by sawing through the femur. This eliminated any collateral circulation via the bone marrow.
The muscles were perfused at constant flow (40 ml/min) with a physiological salt solution (PSS) of the following composition (all concentrations are mM): NaCl 117, KCl 4.7, KH2PO4 1.2, NaHCO3 21.0, CaCl2 2.7, MgSO4 · 7H2O 1.2, EDTA (disodium calcium salt) 0.2, dextrose 8.0, and sodium pyruvate 2.0. This solution was passed through a 3-μm filter, placed in a reservoir inside a heated water bath, and bubbled with 95% O2-5% CO2. The perfusate was not recirculated. A 10% solution of bovine serum albumin was infused proximal to the pump at 1% of the PSS flow rate to produce a 0.1% albumin concentration in the perfusate. Albumin at low concentration prevents the development of a high capillary filtration coefficient in crystalloid-perfused tissue (20). Perfusate temperature was monitored near the arterial cannula and maintained at 37–39°C with the aid of a thermostatically controlled heat lamp. Perfusion pressure was monitored at the tip of the arterial cannula using a Statham pressure transducer connected to a Grass polygraph. Arterial and venous PO2, PCO2, and pH were periodically measured on a Corning Instruments model 165/2 blood gas analyzer. Oxygen consumption (ml·min–1 · 100 g–) was calculated as Q·(Pa – Pv)·(0.023/760), where Q is the flow rate per 100 g, Pa and Pv are the arterial and venous PO2S, and 0.023 ml·ml–1 atm–1 is the solubility of O2 in water at 37°C.
We quantified the capillary extraction of both adenosine and a structural analogue of adenosine, 9-β-d-arabinofuranosyl hypoxanthine (AraH), using the single-pass multiple indicator-dilution technique (5, 10). [125I]-albumin (New England Nuclear) was used as the reference tracer. Aliquots of [U-14C]adenosine (Amersham, 528 μCi/pmol) in 2% ethanol were evaporated to dryness and redissolved in PSS on the day of the experiment. [3H]AraH was prepared by deamination of [3H]arabinofuranosyl adenine ([3H]AraA, 38 Ci/mmol, ICN). One millicurie (25 μmol) of [3H]AraA in 20% ethanol was evaporated to dryness and redissolved in 1 ml of phosphate buffer. Deamination was achieved by adding 120 units of adenosine deaminase (Sigma type III) and incubating at room temperature for 0.5 h. The deaminase was inactivated by the addition of perchloric acid. After neutralization, the AraH fraction of this solution was isolated using high-pressure liquid chromatography (HPLC). This fraction was dried, resuspended in PM, and frozen for later use. This AraH solution did not exhibit any residual adenosine deaminase activity.
AraH uptake experiments
The purpose of these experiments was to verify that AraH is not transported by the membrane nucleoside carrier. This was predicted from the observation that arabinofuranosyladenine does not inhibit cardiac adenosine uptake (24). [3H]AraH (0.5 μCi, 0.01 nmol) was added to 4 ml fresh canine blood samples, both with and without dipyridamole (3 μM). Blood samples were incubated for 20 min at 37°C then centrifuged. Plasma aliquots were deproteinized and neutralized as described previously (19), and neutralized extracts (100 μl) were analyzed for [3H]AraH as described below.
Experimental protocol
After perfusion with PSS was begun, we allowed a 0.5-h equilibration period. During this time the muscles were gently massaged periodically to remove trapped red blood cells. Indicator-dilution curves were obtained by injecting a bolus (0.25 ml) of PSS containing [125I]albumin (~0.5 μCi, 0.2 μg), [14C]-adenosine (~2 μCi, 5 nmol), and [3H]AraH (~5 μCi, 0.15 nmol) through the tubing near the arterial cannula. Immediately following this injection, the venous effluent was collected into 30 tared test tubes during the next 2 min. The sampling interval was approximately 0.5 s for the first 15 tubes, 5 s for tubes 15–25, and 10 s for tubes 25–30. All tubes contained either 250 μl (tubes 1–15) or 500 μl (tubes 16–30) of “collecting solution,” which consisted of 32 μM dipyridamole and 3 μM erythro-9-(2-hydroxy-3-nonyl)adenine HCl (EHNA). The purpose of the collecting solution was to prevent deamination or cellular uptake of adenosine by the few red blood cells appearing in the tubes. Two indicator-dilution curves were obtained in every animal. The first curve was a control curve, and a second curve was obtained during dipyridamole infusion. Dipyridamole (Persantin, Boeh-ringer Ingelheim) was infused into the arterial line proximal to the pump to achieve a perfusate concentration of 10–5 M.
Injectate solution containing [125I]albumin, [14C]adenosine, and [3H]AraH was prepared on the day of the experiment. Each of the two arterial injections consisted of 0.25 ml of this solution. To determine the total injected dose of [14C]adenosine and [3H]AraH, at least three aliquots of this solution were diluted 1:100 and separated by HPLC. Total injected [125I]albumin counts were determined by distributing 0.25 ml of injectate solution into approximately 40 tubes, which were then counted on a Packard Instruments autogamma scintillation spectrometer, and the counts were added together. Two 0.25-ml aliquots were counted in this fashion in each experiment.
At the conclusion of the experiment the muscles were excised and weighed. These weights were compared with the weights of the contralateral muscles to estimate edema formation.
Distribution of flow
The regional distribution of perfusate flow was measured in two preparations to account for heterogeneity of flow in the analysis of the data (5). At the conclusion of these experiments a 0.2- to 0.5-ml bolus of 95Nb-labeled microspheres (New England Nuclear) was injected into the arterial cannula. The muscles were sectioned into 1- to 3-g segments that were counted for 95Nb, The distribution of local flows was taken to be the same as the deposition densities of the microspheres and was analyzed as described in detail by King et al. (17)
Sample analysis
Immediately after collection, the sample tubes were shaken (to mix the collecting solution and the venous effluent), centrifuged in the cold to eliminate the few remaining red blood cells, and weighed for the calculation of sample volume. A 50- or 100-μl aliquot of supernatant was removed from each tube for 125I counting. In two experiments, duplicate 125I samples were taken from each tube and the counts were averaged. The remainder of the supernatant was transferred to a separate tube and frozen for later analysis by HPLC.
Reverse-phase HPLC was used to separate [125I]albumin, [3H]AraH, [14C]adenosine, and [14C]labeled adenosine metabolites (hypoxanthine and inosine) in the venous effluent samples. We used a Waters Associates C18 μ Bondapak radial compression column, automatic injector, absorbance detector, system controller, and data module. Separation was accomplished using 4 mM KH2PO4 (solution A, pH 4.5) and 70% methanol (solution B) in a 15min gradient that started with 100% solution A and finished with 100% solution B. Flow rate was 3.9 ml/min. Under these conditions the labeled compounds eluted in the following order: albumin, hypoxanthine, inosine plus AraH (co-eluted), adenosine. The albumin fraction was discarded. The remaining fractions were evaporated to dryness, redissolved in 500 μl water, added to scintillation cocktail, and counted on a Packard Instruments liquid scintillation counter. Counting rates were corrected for efficiency and, in the inosine plus AraH fraction, for 3H and 14C overlap. When known amounts of tracer were added to venous effluent, recovery was 101 ± 2% for adenosine and 100 ± 2% for AraH.
Data analysis
[125I]albumin, [3H]AraH, [14C] adenosine, [14C]inosine, and [14C] hypoxanthine activities in each venous effluent sample were converted to relative concentrations by expressing them as percent of injected dose per milliliter of effluent. Relative concentrations were then plotted versus the time after tracer injection. Extractions of adenosine and AraH were calculated using the formula E(t) = 1 - Cd(t)/Cr(t), where E(t) is the extraction at time t, Cr is the concentration of the intravascular reference tracer albumin, and Cd is the concentration of the diffusible indicator (either adenosine or AraH). An average early extraction of adenosine or AraH was calculated from the samples taken up to the peak of the [125I]albumin concentration-time curve. The average number of such samples per curve was 7 ± 1.
The paired t statistic was used to determine the effect of dipyridamole on the extractions and permeability-surface area (PS) products of adenosine and AraH. P values less than 0.05 were considered statistically significant. For the parenchymal cell PS product of adenosine, the sign test was used because the very large and variable changes due to dipyridamole made the t test inappropriate. Values stated in the text are means ± SE.
Muthematical model
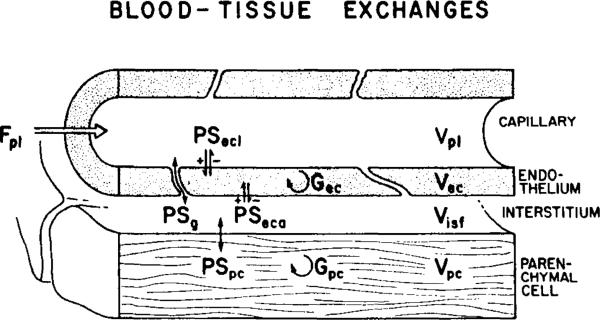
The data were analyzed further via a model for blood-tissue exchange that accounts for transport across the capillary wall and uptake and retention by cells. The model, described by Bassingthwaighte et al. (6), is composed of a set of blood-tissue exchange units in parallel to account for the heterogeneity of flow as defined by the microsphere deposition densities. Each unit was in turn composed of flowing capillary perfusate, the capillary wall composed of endothelial cells with aqueous gaps separating them, interstitial space, and a muscle cell (Fig. 1). The intravascular transport was defined by the measured flows, the microsphere deposition density giving the probability density function of flows and the outflow dilution curve for the reference tracer albumin. On the basis of the definition of intravascular transport so defined, the AraH curves were used to provide estimates of the permeability surface area product, PSg, for the gaps between the endothelial cells of the capillary for this nonmetabolized tracer. Because AraH and adenosine have the same molecular weight, the value of PSk for adenosine must be identical to that for AraH and its volume of distribution in the interstitium likewise the same. The adenosine curves were then analyzed on the basis of the parameter values already fixed from the albumin and AraH and microsphere data; the remaining three parameters were the cellular permeability-surface area products, the intracellular volumes of distribution, and the intracellular rate of transformation. The PS products are better defined than the volumes of distribution or the consumption rates since these last two tend to influence the early parts of the curves in the same general fashion. The value for the PS product of the luminal surface of the endothelial cell, PSecl, should be quite well defined by these experimental data, since PSecl along with PSg governs the initial rate of escape of the tracer from the capillary plasma.
Fig. 1.
Representation of model used for analysis of indicator-dilution curves. Fpl, plasma (perfusate) flow; PS, permeability-surface areas for adenosine passage through endothelial cell luminal membrane (PSecl); water-filled channels between endothelial cells (PSg); endothelial cell abluminal membrane (PSeca); and parenchymal cell membrane (PSpc). G, intracellular consumption (metabolism) of adenosine by endothelial cells (Gec) or by parenchymal cells (Gpc). V, volume of plasma (VPl), endothelial cell (VISF), interstitial (VISF), and parenchymal cell (Vpc) spaces.
Data were fitted using a previously described parameter-optimizing program (18), employing the following constraints.
Adenosine but not AraH can enter endothelial cells and parenchymal cells (PSecl and PSpc for AraH = 0).
The PS product for paracellular diffusion (PSg) is equivalent for AraH and adenosine, because their molecular weights are the same.
The microsphere data, reflecting heterogeneity of flow, were fit by a truncated Gaussian function (17) with a relative dispersion of 0.55 spread from 0.15 to 1.8 times the mean flow.
The apparent volumes of distribution (V′) were constrained in accordance with anatomic values. Capillary volume was fixed at 0.01 ml · g–1 (8), and total volume of distribution was fixed from 0.80–0.82 ml · g–l with , and (ISF, interstital fluid) free to adjust within this constraint.
RESULTS
The average perfusate flow rate in these muscles was 31.4 ± 2 ml · min–1 · 100 g–1, and the perfusion pressure was 22 ± 5 mmHg. Arterial PO2, PCO2, and pH were 569 ± 11 mmHg, 34 ± 2 mmHg, and 7.38 ± 0.01, respectively. Venous PO2, PCO2, and pH averaged 234 ± 15 mmHg, 42 ± 2 mmHg, and 7.30 ± 0.03. Oxygen consumption was 0.31 ± 0.01 ml O2 · min–1 100 g–1. This compares with an O2 consumption of 0.26 ± 0.09 ml O2 · min–1 · 100 g–1 in this same preparation when perfused with blood (27). At the conclusion of the experiment, the ratio of experimental muscle weight to contralateral muscle weight was 1.20 ± 0.07, indicating edema formation.
AraH uptake experiments
After a 20-min incubation in blood from three different dogs, we recovered 97.8 ± 0.3% of the original [3H]AraH in the plasma. This result was unaffected by the presence of dipyridamole. Under identical conditions in one experiment, we recovered only 3% of added adenosine, which agrees closely with our previous observation that adenosine disappears from dog plasma at a rate of approximately 20%/min (19). We conclude from these results that AraH is not transported by the membrane nucleoside carrier in the formed elements of dog blood.
Single-pass indicator-dilution experiments
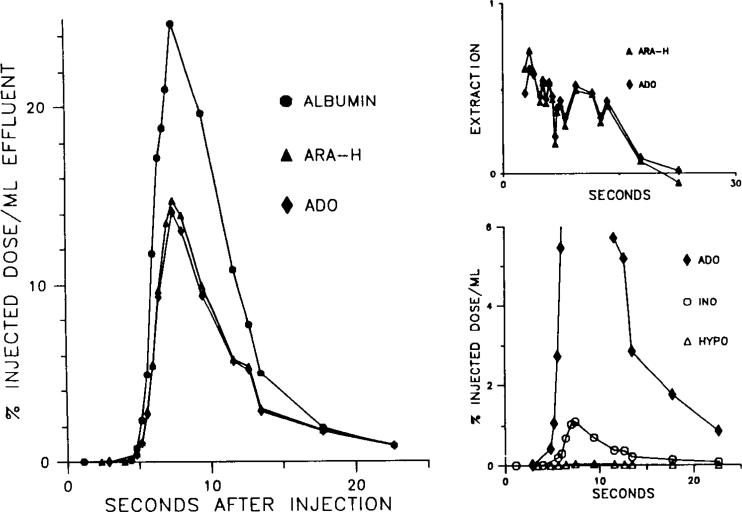
The multiple indicator-dilution curves from a representative experiment are shown in Figs. 2 and 3. As shown in Fig. 2, under control conditions the relative venous concentration of adenosine at any given time is considerably lower than that of both AraH and albumin. This translates into a higher capillary extraction of adenosine (top right panel). Early extraction of AraH in five experiments averaged 0.48 ± 0.04, whereas adenosine extraction was 0.80 ± 0.04 (P < 0.05). The higher extraction of adenosine compared with AraH is presumably due to carrier-mediated uptake of adenosine. The results after dipyridamole, shown in Fig. 3, support this interpretation. During dipyridamole infusion, the concentrations and extractions of adenosine and AraH and virtually indistinguishable. Early AraH extraction was 0.47 ± 0.05, and adenosine extraction was 0.45 ± 0.07.
Fig. 2.
Appearance of adenosine (Ado), arabinofuranosyl hypoxanthine (AraH), and albumin in venous effluent following multiple-tracer injection. Note relatively low concentration of Ado in venous effluent when compared with AraH. This leads to high extraction for Ado relative to AraH shown in upper right graph. Also note that extraction of Ado is steady, whereas extraction of AraH falls off due to back diffusion of this extracellular tracer from interstitial space. Lower right panel shows expanded view of Ado, inosine (ino), and hypoxanthine (hype) curves.
Fig. 3.
Appearance of injected tracers in venous effluent in the presence of dipyridamole (10–5 M). Data are from same preparation shown in Fig. 2. Note that adenosine (Ado) and arabinofuranosyl hypoxanthine (AraH) curves overlap and that both tracers now exhibit a falloff in extraction due to back diffusion. Tracer inosine appearance persists in presence of dipyridamole, but hypoxanthine appearance is virtually eliminated.
Under control conditions, the total number of counts appearing in the venous effluent over the entire collection period (as a percentage of the injected adenosine counts) was 10.6 ± 2.2% for adenosine, 1.8 ± 0.7% for inosine, and 4.8 ± 2.8% for hypoxanthine. During dipyridamole infusion these values were 71.8 ± 8.2% for adenosine, 2.3 ± 0.7% for inosine, and 0.47 ± 0.2% for hypoxanthine. Representative outflow curves for inosine and hypoxanthine before and after dipyridamole are shown in the lower right hand panels of Figs. 2 and 3. Hypoxanthine was measured in only three of the five experiments.
Mathematical model
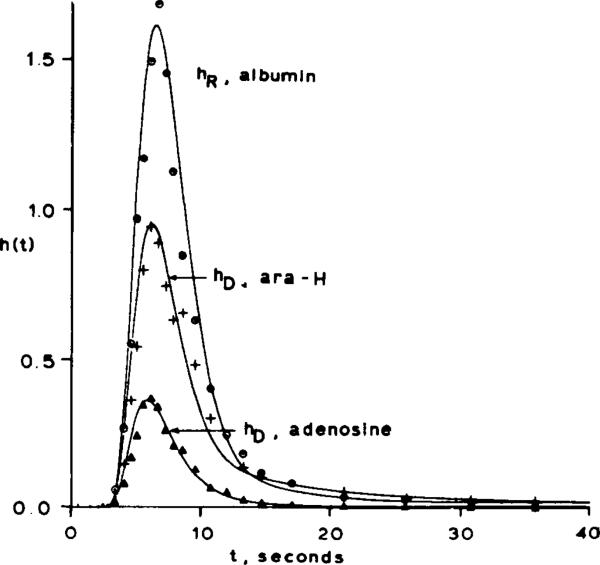
The results of the computer fits of the indicator-dilution curve data are shown in Fig. 4 and Table 1. Under control conditions was estimated from the albumin and AraH curves to be 0.19 ml·g–1, which is in agreement with the anatomic value (1). The constraint that PSg must be the same for both AraH and adenosine forced the optimizer to fit the rising and peak portions of the curves (which are very sensitive to endothelial cell uptake) by adjusting PSecl for adenosine. Since the volume of endothelial cells is so small (0.004 ± 0.001 ml·g–l), increasing PSecl alone does not produce a fit of the curves, and therefore endothelial cell metabolism of adenosine (Gec) has to be invoked. Increasing the values of the parameters describing parenchymal cell transport (PSpc) and metabolism (Gpc) does not affect the early portions of the model curves because these parameters exert most of their influence on later portions of the curves. The PSecl for adenosine at the luminal membrane averaged 0.55 ± 0.07, whereas the PS for diffusion between cells (PSg) was 0.15 ± 0.04 ml·min–1g–1. Thus roughly 78% of the adenosine leaving the plasma entered endothelial cells. Some of the injected adenosine reaching the interstitium can be expected to enter the endothelial cells via the abluminal membrane. Seventy-eight percent therefore represents the minimum fraction of adenosine entering endothelial cells. During dipyridamole infusion, PSecl for adenosine was adjusted to zero by the optimizer in order to fit the data.
Fig. 4.
Representative fit of model simulation (indicated by solid lines) to experimental data points under control conditions. The h(t) values on ordinate are relative concentrations of tracer expressed as percent of injected dose appearing per second.
TABLE 1.
Blood-tissue exchange parameters for adenosine
| PS g | PS ecl | PS pc | Gec | Gpc | ||
|---|---|---|---|---|---|---|
| Control | 0.15±0.04 | 0.55±0.07 | 19.3±10.7 | 37.0±16.7 | 40.4±19 | 0.19±0.004 |
| Dipyridamole, 10–5 M | 0.24±0.03 | 0.0* | 0.68±0.5† | 0.19±0.05 |
Summary of parameter values (±SE) providing best model fit of experimental data (n = 5). Units of all permeability-surface area products (PS) and intracellular consumption terms (G) are ml·min–1·g–1. Units of are ml·g–1. Subscript meanings are as follows: g, gaps between endothelial cells; ecl, endothelial cell luminal membrane; pc, parenchymal cell; ec, endothelial cell; ISF, interstitial fluid. PSg was determined from the albumin and arabinofuranosyl hypoxanthine (AraH) curves and was assumed to be the same for AraH and adenosine. Other PS and G values refer to adenosine. PSecl, PSpc, Gec, and Gpc were assumed to be zero for AraH. Zero value for adenosine PSecl during dipyridamole infusion provides the best fit of the data and was not imposed as a constraint. During dipyridamole infusion, Gec and Gpc for adenosine are indeterminate because of the very low PS values for entry into cells.
P < 0.05 compared with control value, paired t test.
P = 0.06 compared with control, sign test.
The coefficient of variation for the curve fitting averaged 0.25 ± 0.05 for AraH curves and for adenosine curves in the presence of dipyridamole. This appears to be due to the noisiness of the data rather than to a systematic deviation between the model and the data. The coefficient of variation for control adenosine curves was 0.35 ± 0.05, which reflects a consistent deviation between the model and data points in the tail of the curve.
DISCUSSION
These experiments provide evidence that a large portion of the adenosine extracted during a single transit through canine skeletal muscle enters endothelial cells. This conclusion is based on the observations that 1) the early extraction of adenosine is considerably higher than that of a structural analogue that is not transported by the membrane nucleoside carrier, and 2) the early extractions of adenosine and its analogue become indistinguishable after inhibition of the adenosine carrier by dipyridamole. Analysis of these data using a mathematical model that describes both paracellular diffusion and cellular uptake of adenosine (6) indicates that, under control conditions in these experiments, at least 78% of the extracted adenosine entered endothelial cells.
The greater extraction of adenosine than AraH can only be explained by permeation of the endothelial luminal plasma membrane. Uptake of adenosine by parenchymal muscle cells can only occur after adenosine reaches the interstitial fluid (ISF). The difference between the adenosine and AraH curves cannot be explained by less return flux of adenosine than AraH from ISF to capillary, since the ISF concentration is still essentially zero for both in the first few seconds. Although parenchymal cell uptake cannot contribute to the extraction of tracers in these early samples, it does influence the concentration in samples taken after the peak of the reference tracer curve when back diffusion of the extracellular tracer becomes apparent (4, 15, 30). The large extraction of adenosine relative to AraH occurring in the early samples demonstrates a high rate of permeation into endothelial cells, with PSecl/PSg ≅ 4.
This qualitative analysis is supported by the more quantitative analysis provided by fitting the curves using a parameter-optimizing routine in which the parameters describe a model that allows endothelial cell uptake and metabolism of adenosine (6). This model makes use of the whole curves (not just early extraction) to estimate the contributions of paracellular diffusion and cellular uptake. This model also incorporates heterogeneity of flow, which can have a large influence on the calculation of PS values (5). Significant endothelial cell adenosine uptake (high PSecl) is required to fit the experimental data. The parameters for paracellular diffusion (PSg) and endothelial cell luminal uptake (PSecl) are estimated with a high degree of confidence because these parameters dominate the early portion of the indicator-dilution curves where the signal-to-noise ratio is highest. Parameters that primarily influence the tails of the curves [e.g., transport by the abluminal endothelial cell membrane (PSeca), PSpc, , Gec] are estimated with less confidence, due to the effects of noise in the data, and partially overlapping influences of these parameters on the tails of the curves. For example, although our data suggest that PSeca could be even larger than PSecl, the data are not conclusive because the model cannot distinguish from outflow curves alone between uptake of interstitial adenosine by parenchymal cells (PSpc) versus endothelial cells (PSeca). Quantitative autoradiography in these studies would be necessary to distinguish accurately between PSeca and PSpc.
Although this model of capillary transport predicts the rising and peak portions of the adenosine curves, it does not accurately predict the tails of the adenosine curves in the absence of dipyridamole. The model consistently predicts that the tail of the adenosine curve is above the actual data points. This may be because the model as presently constructed dues not adequately describe the heterogeneities present in skeletal muscle. For example, we assume that although flow is heterogeneous, all capillaries have the same PSg and PSecl. These poor fits of the adenosine curve tails, however, do not influence our conclusions about endothelial cell uptake based on earlier portions of the curve where the fit was quite good.
We chose AraH as an extracellular marker because of its structural similarity to adenosine. The lack of AraH uptake by dog blood cells supports our assumption that AraH does not enter cells. Dog red blood cells, however, do not possess the nucleoside carrier (11). Presumably, then, the dipyridamole-sensitive adenosine uptake by dog blood cells must take place in leukocytes or platelets. It could be argued that we would have observed AraH transport by the nucleoside carrier if we had studied a preparation where more carriers are present. Our indicator-dilution curves do not support this contention. If AraH is transported by the membrane nucleoside carrier of endothelial cells, dipyridamole should have greatly decreased the extraction of AraH (and PSg). Because dipyridamole had no effect on these parameters, we conclude that AraH is not transported by the adenosine carrier in dog skeletal muscle endothelial cells. Our data do not rule out the possibility that some uptake of both AraH and adenosine takes place via a mechanism not sensitive to dipyridamole and not present in dog blood cells. If this is the case, the difference between AraH and adenosine extractions represents not the total endothelial cell uptake, but only uptake by the dipyridamole-sensitive carrier. This would cause us to underestimate the degree of adenosine uptake by endothelium.
We used 15-μm microspheres to estimate the distribution of flow at the level of the exchange vessels. To the extent that microspheres do not accurately measure this distribution, this will result in inaccuracies. For example, our poor fit of the tails of the adenosine curves might result from neglecting some pathways with very low flow and high adenosine extractions. Better estimates of flow heterogeneity at the microcirculatory level might improve our ability to fit the experimental data.
We chose a crystalloid-perfused muscle preparation for this study because of the ease of sample analysis as compared with blood and because it eliminated the problems uf adenosine uptake and degradation by blood during transit through the muscle. Although the O2 content of the perfusate is far lower than that of blood, at these flow rates it is adequate to meet the resting O2 demand of the muscles. O2 consumption in these experiments was equivalent to that in previous experiments in which this preparation was perfused with blood. In two preliminary experiments using blood-perfused preparations, at least 56% of the extracted adenosine entered endothelial cells, which is similar to the results presented here.
The results of this study confirm and extend the observations of carrier-mediated nucleoside transport in cultured endothelial cells (14, 25) and in lung (2, 9, 16). Adenosine uptake was inhibited by dipyridamole in all of these preparations. In addition, autoradiograms from isolated guinea pig hearts perfused with low concentrations of labeled adenosine reveal that virtually all of the grains are within endothelial cells (23).
Although dipyridamole greatly increased the amount of tracer adenosine exiting the muscle and decreased the amount of hypoxanthine, it had no significant effect on tracer inosine release. This result indicates the presence of extracellular adenosine deaminase, possibly as an ectoenzyme of endothelial cells (16). Since nucleoside phosphorylase is found primarily within endothelial cells (21), the effect of dipyridamole on tracer hypoxanthine release can be explained by the inhibition of adenosine uptake.
The implication of these findings is that uptake and metabolism by endothelial cells is critically important in determining the magnitude of adenosine movement between plasma and interstitium. The extent to which endothelial cells serve as a barrier to adenosine depends on the intracellular fate of the adenosine taken up. If all of this adenosine enters the intracellular adenine nucleotide pool or is converted to inosine or hypoxanthine and subsequently released, then endothelial cells are an adenosine (“sink” and represent a formidable barrier. If, on the other hand, the intracellular metabolism of adenosine proceeds slowly and a nucleoside carrier exists on the abluminal membrane, the net effect might be to facilitate adenosine movement across the capillary. Several lines of evidence suggest that endothelial cells behave as an adenosine sink. First, a large component for endothelial cell sequestration (metabolism) of adenosine (Gec) must be included in the capillary transport model to fit our data. Autoradiography studies have also demonstrated selective entrapment of adenosine (or its metabolites) within endothelium (23, 29). Finally, adenosine taken up by cultured endothelial cells and by lung is rapidly incorporated into the adenine nucleotide pool (2, 16, 25). On the basis of these considerations, it seems likely that, at physiological concentrations, very little of the adenosine entering an endothelial cell is able to leave in the form of adenosine.
Studies measuring adenosine release from an organ as an index of interstitial adenosine concentration must be reevaluated in light of the present findings. For release rate to serve as a valid index of interstitial concentration, the amount of adenosine reaching the venous effluent must vary in a predictable way with interstitial concentration. If the released adenosine comes from the inter-stitium, uptake by endothelial cells means that estimates based on venous effluent concentration or release rate may greatly underestimate the interstitial concentration unless such uptake is taken into account (27, 28). Until more is known about the source of the released adenosine and the capillary transport of adenosine, adenosine release rates should therefore be interpreted with caution. Similarly, the response to infused adenosine gives an unreliable estimate of the vasodilator potency of interstitial adenosine unless endothelial cell uptake is taken into account.
Acknowledgments
We deeply appreciate the contributions to this study by Greg Romig, Joel Silver, Dr. Carl Thompson, and I. S. Chan.
REFERENCES
- 1.Aukland K, Nicolaysen G. Interstitial fluid volume: local regulatory mechanisms. Physiol. Rev. 1981;61:556–643. doi: 10.1152/physrev.1981.61.3.556. [DOI] [PubMed] [Google Scholar]
- 2.Bakhle YS, Chelliah R. Metabolism and uptake of adenosine in rat isolated lung and its inhibition. Br. J. Pharmacol. 1983;79:509–515. doi: 10.1111/j.1476-5381.1983.tb11025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhle YS, Vane JR. Pharmacokinetic function of the pulmonary circulation. Physiol. Rev. 1974;54:1007–1045. doi: 10.1152/physrev.1974.54.4.1007. [DOI] [PubMed] [Google Scholar]
- 4.Bassingthwaighte JB, Chaloupka M. Sensitivity functions in the estimation of parameters of cellular exchange. Federation Proc. 1984;43:180–184. [PMC free article] [PubMed] [Google Scholar]
- 5.Bassingthwaighte JB, Goresky CA. Handbook of Physiology. The Cardiovascular System. The Microcirculation. IV. American Physiol. Soc.; Bethesda, MD: 1984. Modeling in the analysis of solute and water exchange in the microvasculature. pp. 549–626. sect. 2. chapt. 13. [Google Scholar]
- 6.Bassingthwaighte JB, Sparks HV, Chan IS, DeWitt DF, Gorman MW. Modeling of transendothelial transport. Federation Proc. 1985;44:2623–2626. [PMC free article] [PubMed] [Google Scholar]
- 7.Betz AL, Goldstein GW. Developmental changes in metabolism and transport properties of capillaries isolated from rat brain. J. Physiol. Lond. 1981;312:365–376. doi: 10.1113/jphysiol.1981.sp013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casley-Smith JR, Green HS, Harris JL, Wadey JP. The quantitative morphology of skeletal muscle capillaries in relation to permeability. Microvasc. Res. 1975;10:43–64. doi: 10.1016/0026-2862(75)90019-9. [DOI] [PubMed] [Google Scholar]
- 9.Catravas JD. Removal of adenosine from the rabbit pulmonary circulation, in vivo and in vitro. Circ. Res. 1984;54:603–611. doi: 10.1161/01.res.54.5.603. [DOI] [PubMed] [Google Scholar]
- 10.Chinard FP, Vosburgh GJ, Enns T. Transcapillary exchange of water and other substances in certain organs of the dog. Am. J. Physiol. 1955;183:221–234. doi: 10.1152/ajplegacy.1955.183.2.221. [DOI] [PubMed] [Google Scholar]
- 11.Clanachan AS, Paterson ARP, Hammond JR, Jarvis SM. Species differences in nucleoside transport by mammalian erythrocytes (Abstract). In: Berne RM, Rall TW, Rubio R, editors. Regulatory Function of Adenosine. Nijhoff; Boston: 1983. p. 505. [Google Scholar]
- 12.Corkey RF, Corkey BE, Gimbrone MA., Jr Hexose transport in normal and SV40-transformed human endothelial cells in culture. J. Cell Physiol. 1981;106:425–434. doi: 10.1002/jcp.1041060312. [DOI] [PubMed] [Google Scholar]
- 13.Crone C. Facilitated transfer of glucose from blood into brain tissue. J. Physiol. Lond. 1965;181:103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieterle Y, Ody C, Ehrensburger A, Stalder H, Junod AF. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ. Res. 1978;42:869–876. doi: 10.1161/01.res.42.6.869. [DOI] [PubMed] [Google Scholar]
- 15.Duran WN, Yudilevich DL. Capillary and cellular barriers to ouabain transport in the heart. Microvasc. Res. 1974;7:84–88. doi: 10.1016/0026-2862(74)90039-9. [DOI] [PubMed] [Google Scholar]
- 16.Hellewell PG, Pearson JD. Metabolism of circulating adenosine by the porcine isolated perfused lung. Circ. Res. 1983;53:1–7. doi: 10.1161/01.res.53.1.1. [DOI] [PubMed] [Google Scholar]
- 17.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ. Res. 1985;57:285–295. doi: 10.1161/01.res.57.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin M, Kuikka J, Bassingthwaighte JB. Sensitivity analysis in optimization of time-distributed parameters for a coronary circulation model. Med. Prog. Technol. 1980;7:119–124. [PMC free article] [PubMed] [Google Scholar]
- 19.Manfredi JP, Sparks HV. Adenosine's role in coronary vasodilation induced by atria1 pacing and norepinephrine. Am. J. Physiol. 1982;243:H536–H545. doi: 10.1152/ajpheart.1982.243.4.H536. Heart Circ. Physiol. 12. [DOI] [PubMed] [Google Scholar]
- 20.Mason JC, Curry FE, Michel CC. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc. Res. 1977;13:185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- 21.Mentzer RM, Rubio R, Berne RM. Release of adenosine by hypoxic canine lung tissue and its possible role in pulmonary circulation. Am. J. Physiol. 1975;229:1625–1631. doi: 10.1152/ajplegacy.1975.229.6.1625. [DOI] [PubMed] [Google Scholar]
- 22.Mohrman DE, Sparks HV. Resistance and venous oxygen dynamics during sinusoidal exercise of dog skeletal muscle. Circ. Res. 1973;33:337–345. doi: 10.1161/01.res.33.3.337. [DOI] [PubMed] [Google Scholar]
- 23.Nees S, Herzog V, Bock M, Gerlach E. Vasoactive adenosine perfused through isolated hearts is selectively trapped within endothelial cells (Abstract). Federation Proc. 1984;43:900. [Google Scholar]
- 24.Olsson RA, Khouri EM, Bedynek JL, Jr., McLean J. Coronary vasoactivity of adenosine in the conscious dog. Circ. Res. 1979;45:468–478. doi: 10.1161/01.res.45.4.468. [DOI] [PubMed] [Google Scholar]
- 25.Pearson JD, Carleton JS, Hutchings A, Gordon JL. Uptake and metabolism of adenosine by pig aortic endothelial and smooth muscle cells in culture. Biochem. J. 1978;170:265–271. doi: 10.1042/bj1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renkin EM. Handbook of Physiology. The Microcirculation. IV. Am. Physiol. Soc.; Bethesda, MD: 1984. Control of microcirculation and blood-tissue exchange. pp. 627–688. sect. 2. chapt. 14. [Google Scholar]
- 27.Sparks HV, Gorman MW, Belloni FL, Fuchs BD. Endothelial uptake of adenosine: implications for vascular control. In: Hunyor S, Ludbrook J, Shaw J, McGrath M, editors. The Peripheral Circulation. Elsevier; Baltimore, MD: 1984. pp. 23–32. [Google Scholar]
- 28.Sparks HV, Manfredi JP, Phair RD. A compartmental model of changes in interstitial adenosine associated with increased myocardial adenosine. In: Merrill GF, Weiss HR, editors. Calcium Ion Antagonists. Urban & Schwarzenberg; Baltimore, MD: 1983. pp. 189–205. [Google Scholar]
- 29.Stirling CE. Autoradiographic localization of 3H-adenosine (Abstract). In: Berne RM, Rall TW, Rubio R, editors. Regulatory Function of Adenosine. Nijhoff; Boston: 1983. p. 542. [Google Scholar]
- 30.Syrota A, Girault M, Pocidalo J, Yudilevich DL. Endothelial uptake of amino acids, sugars, lipids, and prostaglandin in rat lung. Am. J. Physiol. 1982;243:C20–C26. doi: 10.1152/ajpcell.1982.243.1.C20. Cell Physiol. 12. [DOI] [PubMed] [Google Scholar]
- 31.Yudilevich DL, DeRose N, Sepulveda FV. Facilitated transport of amino acids through the blood-brain barrier of the dog studied in a single capillary circulation. Brain Res. 1972;44:569–578. doi: 10.1016/0006-8993(72)90319-8. [DOI] [PubMed] [Google Scholar]