Abstract
Appropriate function of the neocortex depends on timely generation and migration of cells produced in the germinal zones of the neocortex and ganglionic eminence (GE). Failure to accurately complete migration results in cortical dysplasia, a developmental syndrome implicated in many neurologic disorders. We developed a model of cortical dysplasia in ferrets involving administration of methylaxozymethanol acetate (MAM), an antimitotic, to pregnant ferrets on gestational day 33, leading to dramatic reduction of layer 4 in the neocortex. Here, using time-lapse video imaging, we investigate dynamic behavior of migrating cells arising from the GE and cortical ventricular zone (CVZ) in ferrets and the role of GABAA activity. Treatment with MAM significantly reduced migration speed and the relative proportion of cells arising from the GE demonstrating exploratory behavior. To a lesser extent, the behavior of cells leaving the CVZ was affected. Pharmacologic inhibition of GABAA receptors (GABAAR) improved the speed of migration and exploratory ability of migrating MAM-treated cells arising from the GE. Additionally, the expression of α2 and α3 subunits of GABAAR and the potassium chloride co-transporter (KCC2) increased in the neocortex of MAM-treated animals. After MAM treatment, increases in endogenous KCC2 and GABAAR combine to alter the dynamic properties and exploratory behavior of migrating interneurons in ferrets. We show a direct correlation between increased GABAA and KCC2 expression with impaired migration and ability to explore the environment.
Keywords: development, ferret, KCC2, MAM, neuronal migration
Introduction
The laminar organization of the neocortex and its ability to function depends on timely generation and proper migration of cells originating from the ventricular zones of the neocortex (CVZ) and ganglionic eminence (GE). Events that adversely impact the migratory process can lead to cortical dysplasia, a developmental abnormality characterized by aberrant cell clustering, altered gyral patterns, and changes in electrophysiological profile (Taylor et al. 1971; Choi and Mathias 1987; Tassi et al. 2002; Calcagnotto and Baraban 2003; Moroni et al. 2008). The aberrations underlie a vast number of neurological/neuropsychiatric disorders including drug-resistant epilepsy, depression, and schizophrenia (Palmini et al. 1991; Gleeson and Walsh 2000; Zhu and Roper 2000; Ross and Walsh 2001; Calcagnotto et al. 2002). Understanding the process of neuronal movement into the neocortex is critical to comprehending the many disorders that result from disrupted migration.
We developed a model of cortical dysplasia by administering a short acting antimitotic, methylazoxy methanol (MAM), to pregnant ferrets on gestational day 33 (E33). Ferrets are the smallest animal with a convoluted cortex, making them an important model for neocortical development. The tangential expansion of the neocortex, which assists in the formation of the sulci and gyri, relies on proliferation of outer subventricular progenitors, a cell population found in ferrets and humans, but absent in rodents (Fietz et al. 2010; Lui et al. 2011; also see Martinez-Cerdeno et al. 2012 for a different point of view). Our laboratory also found distinctions in the migratory patterns of interneurons in rodents and ferrets, indicating the importance of studying a more developed model of neocortex (Poluch et al. 2008).
Treatment with MAM on E33 coincides with the generation of layer 4 and results in its dramatic reduction and in widespread redistribution of GABAAαR (Noctor et al. 1999; Palmer et al. 2001; Jablonska et al. 2004). Although the morphology and overall number of interneurons in the neocortex is not altered, the laminar position and orientation of interneurons changes in the MAM model, suggesting that these neurons do not migrate into their proper target sites (Poluch et al. 2008). The aberrant redistribution of interneurons and GABAAαR within the neocortex of MAM-treated animals raises important questions. 1) Do changes in the distribution of cells arising from the GE occur as a result of altered dynamic behavior of migrating GE cells, and, if so 2) are these changes influenced by the ambient activity of GABA?
Using real-time video imaging and an in vitro migration assay of organotypic cultures of neonatal ferrets, we observed the dynamic movement patterns of neurons leaving the GE and CVZ. Treatment with MAM impairs the speed and exploratory potential of tangentially migrating interneurons leaving the GE; the impact of MAM treatment on the migration speed of cells arising from the CVZ was minimal and no effect on exploratory behavior was observed. Although cells leaving the GE and CVZ in MAM-treated animals exposed to GABA antagonists showed a significant improvement in the speed of migration, only GE-derived cells displayed improved exploratory activity. This reinforces the idea that E33 MAM treatment alters the migration patterns of GE-derived cells. The changes in the dynamic movement of cells leaving the GE are related to abnormal levels of GABAA-mediated activity, which diminishes the capacity of these cells to explore the environment and effectively migrate to their target within the neocortex.
Materials and Methods
Timed pregnant ferrets were obtained from Marshal Farms (New Rose, NY, USA) and maintained in the animal facilities of the Uniformed Services University of the Health Sciences (USUHS). Pregnant ferrets were injected with 14 mg/kg of MAM (Midwest Research Institute, Kansas City, MO, USA) IP on E33 under isofluorane anesthesia (1–2%). After recovery from anesthesia, ferrets were maintained in the animal facility until their kits were delivered. Handling of animals complied with the USUHS Institutional Animal Care and Use Committee policy on the humane use and treatment of animals.
Preparation of Organotypic Slices
Preparation of organotypic cultures was accomplished as previously described (Palmer et al. 2001). Postnatal day 0 to day 1 (P0–P1) ferret kits were anesthetized with sodium pentobarbital (50 mg/kg), their brains removed and placed in ice-cold artificial cerebrospinal fluid composed of (in mM): 124 NaCl, 3.2 KCl, 2.4 CaCl2, 1.2 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 glucose bubbled with 95% O2 and 5% CO2 under a laminar flow hood. Coronal slices (350–500 µm thick) were prepared from each hemisphere using a tissue chopper (Stoelting, Co., Wood Dale, IL, USA). Brain slices were transferred into 0.4-µm culture plate inserts (Millicell-CM, Bedford, MA, USA) placed in 6-well plates containing Neurobasal media with B27, N2, and G1.2 (containing gentamycin and glutamine) supplements. Slices were incubated at 37 °C under 5% CO2. In some experiments, bicuculline methiodide (BMI; Tocris Bioscience, Park Ellisville, MO, USA) at final concentrations of 1, 10, or 100 µM was added to the media.
Cell Labeling
Two approaches were used to label migrating neurons. In one approach, crystals of 1,l′-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine percholate (DiI, Invitrogen, Carlsbad, CA, USA) were placed in the GE or CVZ using pulled borosilicate glass pipettes. In another approach, we used electroporation to focally transfect cells within the ventricular zone (CVZ) of the GE using a modification of the method described by Flames et al. 2004. Transfection was accomplished using a plasmid that codes for red fluorescent protein (RFP), which was cloned into pCAGGS expression vector (a gift from Dr Tarik Haydar). Between 1 and 3 µL of plasmid DNA (2.7–3.6 µg/µL) was injected into the VZ of the GE of organotypic slices of ferrets. The cathode of a gene paddle electrode (Harvard Apparatus, Inc., Holliston, MA, USA) was placed within the lateral ventricle close to the VZ of the GE while the anode was placed closed to the pial surface in an appropriate position. A pulse of 60 V was applied 4 times, each lasting for 50 ms at intervals of 950 ms using a BTX ECM830 pulse generator (Gal et al. 2006; Harvard Apparatus, Inc., Holliston, MA, USA). Slices were incubated as above for at least 24 h prior to video imaging.
Video Imaging
After incubating organotypic slices for 24 h (CVZ-derived) or 48 h (GE-derived) for analysis, migrating neurons were continuously visualized using an Axiovert 200 inverted microscope fitted with an apotome and Axiovision software (Carl Zeiss AG, Oberkochen, Germany). The microscope was fitted with an incubation chamber and a holder for the slices, which were maintained with humidification at 37 °C and 5% CO2. Serial stacks of images taken through the thickness of the slice were collected using a ×10 objective every 30 min for 24 h. The image stacks were collapsed into a single frame prior to analysis of migration. Migrating cells leaving the GE were analyzed after crossing the corticostriatal boundary into the neocortex.
Analysis of Migration
To measure speed, migrating cells captured in real time were tracked using ImageJ software (http://rsb.info.nih.go/ij) and the tracked distances measured and expressed over time (to obtain the speed in real time) using a program written in R statistical language (R Development Core Team 2009). Also, the orientation of migrating cells leaving the GE cells (either tangential or radial) and exploratory activities were quantified for each migrating cell. Cellular orientation was considered to be either tangential or radial, and determined by the position of a migrating cell in relation to the lateral ventricle or the pia. Migrating cells were considered to be moving tangentially if they were within 20° of a parallel orientation to the lateral ventricle. They were considered to be moving radially if they were within 20° of a vertical orientation to the pia. Cells of other orientations were not considered in the analysis of orientation of movement. The distribution of migrating cells leaving the GE within the neocortex in both normal and MAM-treated animals was determined using Axiovision software. To do this, the neocortex was divided into 3 roughly concentric regions (0–350, 350–700, and >700 μm) from the border of the GE VZ and labeled neurons found within the various bins were counted and expressed as a percentage of the total number of cells.
Western Blot Analysis
The neocortex was dissected from organotypic slices of normal and MAM-treated P0-P1 ferret brains (prepared as described above), frozen on dry ice, and preserved at −82°C prior to use. Tissues were homogenized using RIPA lysis buffer (Santa Cruz Biotech, Santa Cruz, CA, USA) followed by centrifugation at 14 000 g at 4 °C. Protein concentration was estimated using a colorimetric assay. Proteins were separated by SDS-PAGE using 10% Bis-Tris gel and electrophoretically transferred to a PVDF membrane (Invitrogen, Carlsbad, CA, USA). A loading volume of 10 μL containing 1–2 μg of protein was used for each analysis. Membranes were incubated with Casein blocking buffer (PBS (0.5 M NaCl) + 3% Casein + 0.5% Tween-20) overnight, followed by affinity purified rabbit polyclonal antibodies directed against GABAAα2 (1:200; ProSci, Inc., Poway, CA, USA), GABAAα3 (1:2000; Sigma, St. Louis, MO, USA), KCC2 (1:500, Millipore, Billerica, MA), and monoclonal anti-actin (1:3000, NeoMarkers, Fremont, CA) for 24 h. Following several washes with PBS, protein bands were detected using HRP-conjugated anti-rabbit secondary antibodies (1:1000, Jackson Lab., West Grove, PA, USA) and HRP-conjugated anti-mouse (1:3000, Thermo Scientific, Rockford, IL, USA) and visualized using enhanced chemiluminescence detection. Signal intensities were quantified using Image j software (http://rsb.info.nih.go/ij).
Statistics
The χ2 distribution test was used to analyze distribution of GE cells within the neocortex. For all other analysis, Student's t-test or a 1-way analysis of variance followed by a Tukey or least significant difference post hoc test was applied and differences evaluated at P < 0.05.
Results
General Properties of Normal and MAM- Treated Migrating Cells Leaving the GE
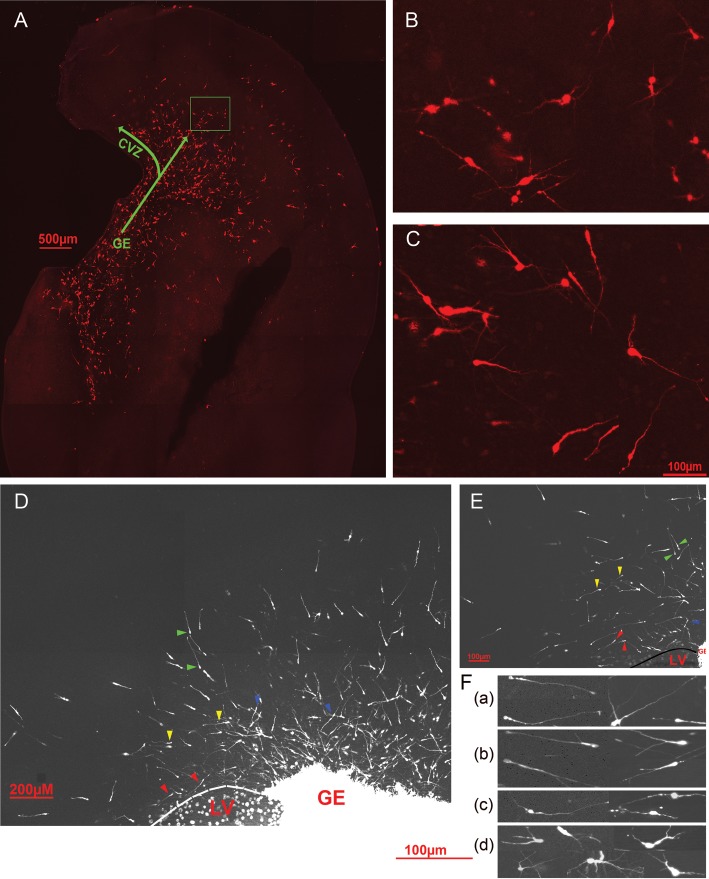
We previously reported that cells leaving the GE in MAM-treated animals exhibit abnormal characteristics, specifically showing orientations that differed from control cells (Poluch et al. 2008). To further characterize the migratory behavior of cells leaving the GE or the CVZ, we applied an in vitro live-video imaging assay to continuously view migrating cells. Our earlier work demonstrates that at P0–P1 in the ferret, the medial and lateral GE are fused, thus we will refer to our label as directed to the fused GE (Poluch et al. 2008). Migrating cells exiting the GE or CVZ of organotypic slices were labeled using electroporation with plasmids that code for RFP or injections of DiI. Both forms of identifying cells were used for analysis. An example of a slice labeled by electroporation can be seen in Figure 1A. Higher power views of cells en route to the cortical plate are shown in Figure 1B,C. These cells have varied orientations as well as morphology; the dynamic features change when observed in real time. After allowing the cells to move away from the GE or CVZ, the dynamic pattern of migration was captured in real time. GE-derived cells moving into the neocortex adopt different orientations and take different routes. A number of migrating neurons course through the VZ or subventricular zone (SVZ), many orient in a tangential direction (Fig. 1D, red arrows). A different group of more superficially labeled GE-derived cells display both tangential (yellow arrows) and radial (green arrows) orientations. Many of the radially oriented cells point in the direction of the pial surface. However, some of the radially oriented cells have their leading edges directed toward the VZ (Fig. 1D,E, blue arrows). These observations support our previous report in the ferret that similar to rodents, tangentially migrating cells leaving the GE follow 3 paths including one close to the VZ, one in the IZ, and one superficially near the marginal zone (not shown in this case) (Poluch et al. 2008). We observe here that migrating cells leaving the GE alter their morphology as they transit from point to point, generating distinct morphological features. In static images, the labeled cells are either unipolar (the cell posses a single leading unbranched process (Fig. 1F-a), display a branched process (Fig. 1F-b), are bipolar (both leading and trailing processes Fig. 1F-c), or multipolar (multiple processes emanate from the soma of the cell (Fig. 1F-d). These features are similar for normal and MAM-treated cells leaving the GE; the images in Figure 1 were obtained from MAM-treated slices, both DiI and electroporated.
Figure 1.
Images of migrating neurons leaving the ganglionic eminence (GE) of a normal ferret. (A) Example of an organotypic culture obtained from a P0 ferret labeled by electroporation using a plasmid that codes for red fluorescent protein (RFP). (B,C) Higher power views of cells en route to the cortex revealing varied orientations and morphologies of migrating GE cells. B is taken from the boxed in region of A and C is from a different normal slice culture. The arrows indicate the path of the migrating cells. GE: ganglionic eminence; CVZ: cortical ventricular zone. (D) Migrating GE-derived neurons labeled with DiI in an organotypic culture of a MAM-treated animal display different orientations and positions within the neocortex: tangentially migrating GE cells within the VZ/SVZ region (red arrows), tangentially migrating GE cells within the intermediate zone (yellow arrows), radially oriented GE cells in the intermediate zone (green arrows) and radially oriented GE cell directed toward the ventricle (blue arrows). (E) A different organotypic culture of a MAM-treated animal labeled with DiI, showing similar orientations of migrating cells. Arrows mark the different orientations and positions of cells within the neocortex as described above. (F) Higher power images of cells obtained from several labeled organotypic cultures from both normal and MAM-treated animals; various morphologies can be seen including those with a single leading unbranched process (a), those with single leading but branched processes (b), bipolar cells (c), and cells with multiple leading processes (d). The images shown in (d) are from an electroporated slice, while those from (a) to (c) are from DiI-injected slices. GE, ganglionic eminence; LV, lateral ventricle. Scale is for F.
Although the general trend of movement for cells leaving the GE is towards the neocortex, individual cells move in all directions and often alter their course of direction (Supplementary material Movie 1). Cells may emit one or more processes and move in the direction of the new branch (e.g. Supplementary material Movie 2), or emit branches and continue in the same direction or not move at all, but only explore the environment for a time (Supplementary material Movie 3). We also frequently observed nucleokinesis in which the nucleus moves within the cytoplasm of a cell in the direction of the leading process (as described by others such as Nadarajah et al. 2001, Martini et al. 2009, Supplementary material Movies 2 and 3). This process usually precedes the point-to-point translocation of the cell during migration. These general patterns of movement are also similar for normal and MAM-treated GE-derived cells.
Dynamic Behavior of Cells Exiting the GE in Normal and MAM-Ttreated Slices
Our earlier work indicates that after treatment with MAM, in addition to changes in orientation while migrating into the cortical plate, several subtypes of interneurons are redistributed in the neocortex of juvenile ferrets (Poluch et al. 2008). To assess the factors contributing to the altered distribution, we evaluated specific dynamic parameters of cells leaving the GE in normal and MAM-treated animals, including the speed, orientation of movement, and exploratory behavior (defined as extension of new processes and changes in the direction of movement).
Speed and Orientation of Movement
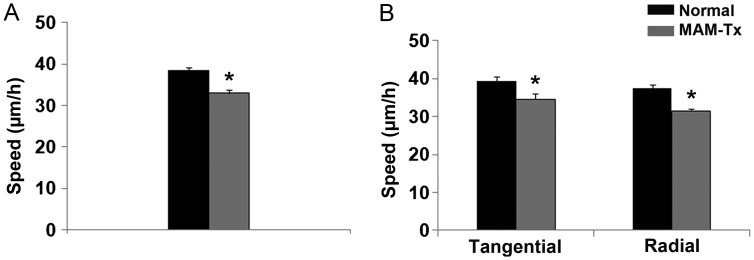
The migratory speed of GE-derived cells was measured as described in the Materials and Methods section. Cells leaving the GE in organotypic cultures of normal ferrets migrated significantly faster (37.435 μm ± 0.87/h) compared with similar cells in animals treated with MAM (32.985 μm ± 0.73/h) (Fig. 2A). Because migrating interneurons from the GE move in both tangential and radial orientations (e.g., Fig. 1D and also Ang et al. 2003; Tanaka et al. 2003, 2006), we examined the relationship of speed to these 2 orientations during movement and assessed distinctions between normal and MAM treatment for radial versus tangential travel. Cells were considered to be traveling tangentially when they oriented within 20° parallel to the lateral ventricle; examples can be seen in cells identified by red and yellow arrows in Figure 1D. We determined cells to be moving radially when their leading edge oriented toward and perpendicular to the pial surface, also within 20° of this measurement. After this analysis, we observed that normal cells traveling in both radial and tangential directions migrated significantly faster than MAM-treated cells (Fig. 2B).
Figure 2.
Effect of MAM treatment on the speed and orientation of migrating interneurons leaving the ganglionic eminence and traveling to the neocortex. (A) Neurons leaving the GE travel faster in normal slices compared with those in MAM-treated slices, n = 435 cells (normal) and 458 cells (MAM-treated). (B) Cells traveling in either the radial or tangential direction travel slower in MAM-treated slices. Tangentially migrating cells: n = 129 (normal), 105 (MAM-treated); radially migrating cells: n = 223 (normal), 348 (MAM-treated). Significance determined using Student's t-test; *P < 0.05. Error bars = standard error of mean.
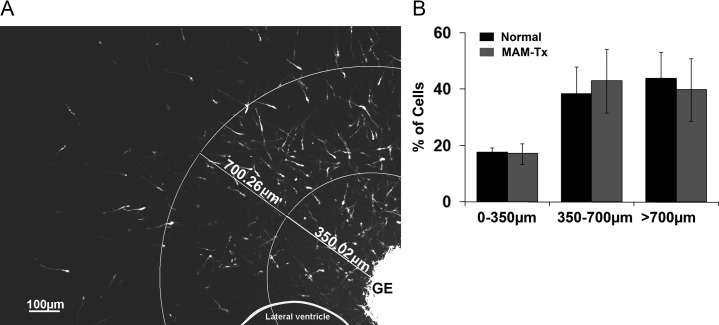
To investigate if changes in the speed of migration affect the final distribution of migrating neurons, we assessed their positions in organotypic slices of normal and MAM-treated animals after 48 h of incubation. We divided the neocortex into 3 roughly concentric regions (0–350, 350–700 and >700 µm) from the point of DiI injection in the GE using Axiovision software (Fig. 3A). The total number of migrating GE cells in each sector was computed; the amount of cells in each sector of the normal and MAM-treated organotypic slices were compared and presented as percentages of the total. Although slightly more cells in normal organotypic slices migrated further away from the GE as revealed by the higher percent in the region 700 µm from the GE, the overall distribution across the 3 sectors was not significantly different (Fig. 3B). In other words, the cells leaving the MAM-treated GE moved slower, but eventually situate at similar distances away from the GE.
Figure 3.
Distribution of cells leaving the GE and traveling to the neocortex. (A) To determine if cells migrated different distances after treatment with MAM, roughly concentric regions were created to count the neurons that traveled up to 350 μm from the border of the GE VZ, from 350 to 700 μm from that border, or greater than 700 μm from the center of DiI injection. (B) After 48 h in culture, there were no significant differences in the overall distribution of cells leaving the GE in normal slices versus those in MAM-treated slices, A χ2 distribution showed no significant differences. n = 12 slices (MAM-treated), 10 slices (normal). Error bars = standard error of mean. GE, ganglionic eminence.
Exploratory Behavior of GE-Derived Neurons in Normal and MAM-Treated Slices
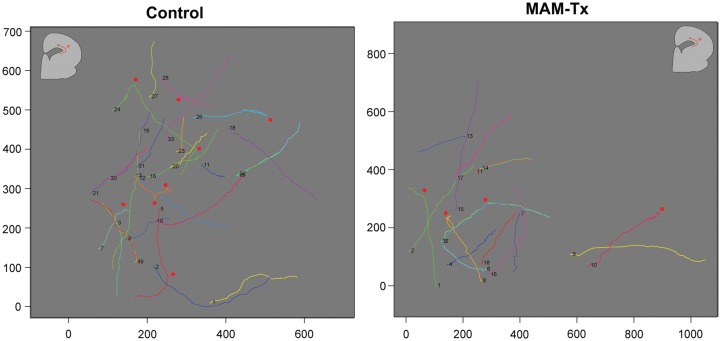
Migrating GE-derived cells are influenced by a variety of signaling molecules that induce stereotypic migratory activity, which we refer to as exploratory behavior. Two parameters defined exploratory behavior in our study: 1) process extension or the ability of migrating cells to extend more than one leading process and 2) turns or changes in direction of movement, in which a deviation >45° from the initial course of movement occurred. In Figure 4A, the green arrow indicates a cell-changing direction, while the red arrow shows a cell branching and also changing its direction of movement. To determine if treatment with MAM alters the ability of migrating GE-derived cells to explore the neocortical environment, migrating cells were observed during live-video imaging for 24 h. For several examples, the path of multiple migrating cells was traced using ImageJ. The movement was tracked for a period of at least 8 h and the position of each cell recorded. Figure 5A,B illustrate that the general pattern of movement in the MAM-treated tracks of cells (Fig. 5B) show fewer changes in their direction when compared with the normal patterns of movement (Fig. 5A). Changes in the trajectory of movement are indicated in the images with red dots. The quantification of movement patterns is seen in Figure 4B, which demonstrates that the tracks of normal cells completed more changes of direction in their movement patterns (i.e., turns) compared with the MAM-treated cells. The percent of cells displaying process extension or turning in each slice was calculated for normal and MAM-treated organotypic cultures. Cells were scored negative if they did not display any of these behaviors during the observed period of time. In MAM-treated slices, fewer cells leaving the GE extended more than one leading process in comparison with cells in normal animals. In addition, the percent of cells that performed turns was fewer after MAM treatment (Fig. 4B).
Figure 4.
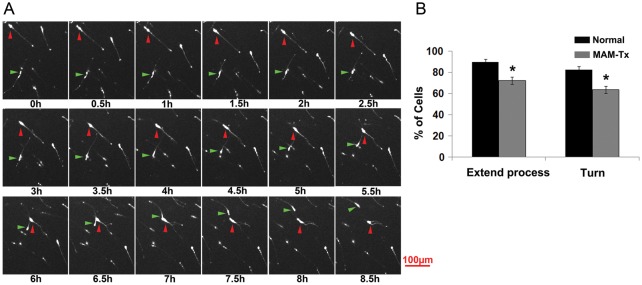
Effect of MAM treatment on the ability of migrating GE cells to extend multiple leading processes and initiate a change in direction of movement. (A) Sequence of images of DiI-labeled GE cells during time-lapse imaging for 8.5 h. Two cells are indicated with either a red or green arrow and followed over time. The cell labeled with the red arrow moves diagonally from the upper left to the approximate center of the image. At 6.5 h it branches and moves toward the right. The cell labeled with a green arrow remains relatively still from 0 to0.4 h. At this point, it begins to move upward and curves to the left, moving out of view at 8.5 h. (B) The percent of migrating cells extending new processes was evaluated in normal and MAM-treated slices. After MAM treatment, migrating cells extended fewer new processes and displayed fewer turns/changes in the direction of movement. n = 23 slices/366 cells (MAM-treated), 21 slices/239 cells (normal); *P ≤ 0.05, Student's t-test. Error bars = standard error of mean.
Figure 5.
Tracks of cells in normal and MAM-treated slices. (A) The positions of migrating cells were tracked every 30 min over a period of 24 h. Each cell is indicated with a different color and a number. This is an example taken from a normal cortex. (B) The tracks of cells leaving the ganglionic eminence (GE) of MAM-treated cortex are indicated here. The position of each cell is illustrated with a different color and number. The cells leaving the normal GE show more variability in their route, compared with those leaving the GE of MAM-treated brains. The numbers on the x- and y-axes indicate the size of the area of the neocortex analyzed while the length of each track indicates a rough estimate of the distance traveled in micrometers. The outline of ferret cortex in A and B indicates the approximate path of cell movement (red arrow). Each red dot indicates where a traveling cell made a change in the direction of movement that was at least 45° from the original path.
Interference with GABAA-Mediated Activity Affects Migratory Behavior of GE-Derived Cells in MAM-Treated Animals
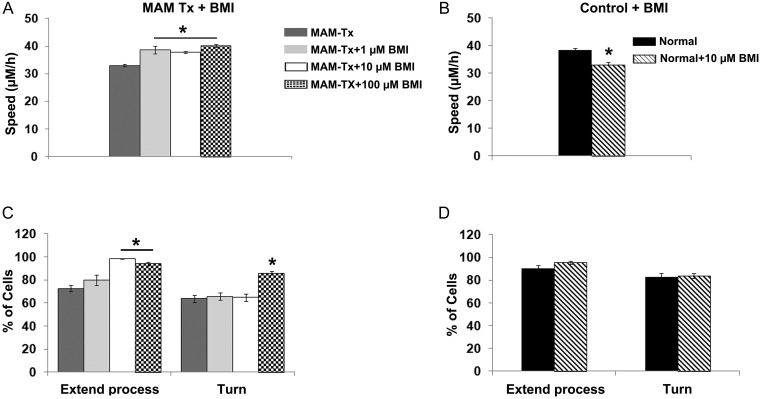
The migratory behavior of interneurons entering the neocortex is regulated by a variety of signaling molecules including GABA (Lopez-Bendito et al. 2003, 2008; Cuzon et al. 2006; Heck et al. 2007; Bortone and Polleux 2009, Inada et al. 2011; Lysko et al. 2011). We previously demonstrated that treatment with MAM leads to a widespread redistribution of GABAAα receptors; increasing GABAAR-mediated activity in normal organotypic cultures also interferes with the orientation of interneurons as they migrate into the cortical plate, similar to the effect of MAM treatment (Jablonska et al. 2004; Poluch et al. 2008). These findings suggest that GABA activity is unusually high after MAM treatment and manipulating the level of GABA activity may alter the positioning of neocortical interneurons. We predicted that treatment with GABAA antagonists would shift the dynamic behavior of neurons originating from MAM-treated slices toward normal. The speed and exploratory behavior of migrating cells in normal and MAM-treated slices was evaluated as described previously after adding the GABAA antagonist BMI to the media. To test the relationship between dose and response to BMI, we used concentrations of 1, 10, and 100 µM added to the media. All the concentrations of BMI significantly increased the speed of migration in the MAM-treated group to be closer to normal (Fig. 6A). To evaluate exploratory behavior, we observed that exposing organotypic slices of E33 MAM-treated brains to varying concentrations of BMI resulted in the 2 higher concentrations of BMI causing rescue of process extension. The percent of migrating cells extending more than one leading process increased at these concentrations and was nearly identical to those of normal cells migrating away from the GE (Fig. 6C). The number of turns, or alterations in the direction of movement, however, only changed after adding the highest concentration of BMI to the media. The increase in turning behavior was only significant at this highest level (Fig. 6C). This matches our prediction that decreasing GABAA activity in MAM-treated brains results in more normal behavior (Fig. 6A,B). When blockade of GABAAR was tested in normal organotypic cultures, we found no changes in exploratory behavior, however, BMI decreased the migration speed of cells generated in the GE (Fig. 6B,D).
Figure 6.
Administration of bicuculline (BMI) at different doses (1, 10, and 100 μM) to MAM-treated slices results in an increase in the speed and exploratory behavior of migrating cells for all doses tested. MAM-treated slices were placed in culture with BMI added to the medium. (A) The addition of BMI significantly increased the speed of migration in the treated slices at all doses tested. Speeds were significantly different from the MAM-treated condition. One-way ANOVA followed by the Tukey post hoc test, *P ≤ 0.05. (B) Adding 10-μM BMI to the media of normal organotypic cultures decreases the speed of migration. Student's t-test, *P ≤ 0.05. (C) The addition of BMI also improved the ability of MAM-treated cells to extend processes. One-way ANOVA followed by the Tukey post hoc test, *P ≤ 0.05. The number of turns in the direction of movement after MAM treatment increased only with the highest dose of BMI. ANOVA followed by the least significant difference post hoc test, *P < 0.05. (D) Adding 10-μM BMI to the media of normal organotypic cultures does not affect exploratory behavior: extending a process or turning. Significant differences are shown for comparison with the MAM-treated condition (A,C) or with the normal condition (C,D). Speed: n = 467 cells (MAM-treated),179 cells (MAM-treated + 1 μM BMI), 350 cells (MAM-treated + 10 μM BMI), 204 cells (MAM-treated + 100 μM BMI), 411 (normal + 10 μM BMI); exploratory behavior: n = 23 slices/366 cells (MAM-treated), 8 slices/103 cells (MAM-treated + 1 μM BMI), 22 slices/161 cells (MAM-treated + 10 μM BMI), 12 slices/116 cells (MAM-treated + 100 μM BMI), and 23 slices (normal + 10 μM BMI). Error bars = standard error of mean.
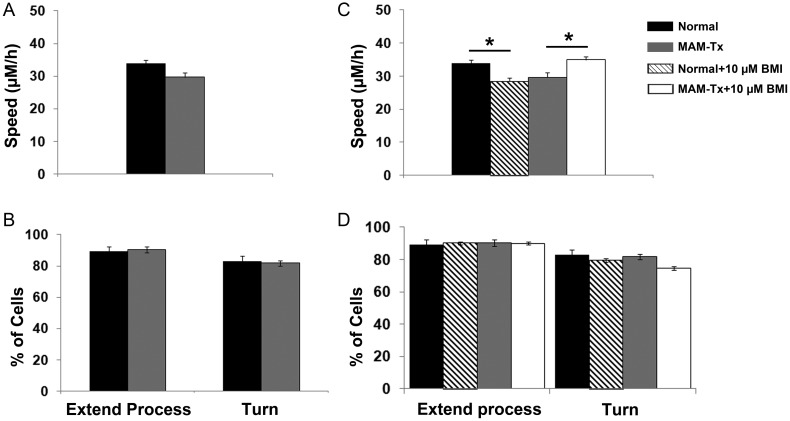
Treatment with MAM has Minimal Impact on Migratory Behavior of Neurons Originating from the Cortical Ventricular Zone
Our previous observations indicated that tangentially migrating GABAergic neurons were specifically disoriented in MAM-treated ferrets (Poluch et al. 2008). To test if the changes in the dynamic behavior of migrating interneurons were limited to the neurons originating from the GE, we examined the migration of neurons from the CVZ to the neocortex using live-video imaging. Organotypic slices were injected with DiI in the neocortical VZ and only slices in which the DiI label was confined to the VZ were used for analysis. In this regard we limited the labeling of cells arising from the GE in the vicinity of the CVZ. We hoped to insure that the labeled cells migrating away originated entirely from the CVZ. An example of an injection site and cells migrating away from the CVZ can be seen in Supplementary Figure S1. We measured the speed and exploratory behavior of cells leaving the CVZ in normal and MAM-treated slices. We found no significant differences in the parameters analyzed for exploratory behavior between the 2 groups; therefore, no distinctions in turning or branching occurred between normal or MAM-treated cells leaving the CVZ (Fig. 7B). This is also true when we added BMI to the organotypic cultures of CVZ-derived cells and assessed exploratory behavior; there were no differences between normal and MAM-treated cells leaving the CVZ (Fig. 7D). Analysis of migration speed, however, elicited distinctions between the 2 groups (Fig. 7C). Comparison between normal and MAM-treated cells leaving the CVZ showed that treatment with MAM results in slower moving cells, but the difference in speed did not quite reach significance (P < 0.06). Adding BMI to the media resulted in an increase of speed for the MAM-treated CVZ-derived cells, similar to the result seen for GE-derived cells. For the cells leaving the normal CVZ, however, BMI treatment caused a decrease in the speed of migration of normal cells leaving the CVZ (Fig. 7C). This result fits our overall hypothesis that altered GABAAR activity interferes with normal migration as was seen for the control cells leaving the GE (Fig. 6C).
Figure 7.
Effect of MAM treatment on the speed of migration and exploratory behavior of cells leaving the cortical ventricular zone (CVZ) to the neocortex. (A) There were no significant differences in the speed of migration of cells leaving the CVZ from either normal or MAM-treated slices, although the cells leaving the CVZ in MAM-treated slices tended to move more slowly (P < 0.06, Student's t-test). n = 191 cells (normal) and 185 cells (E33 MAM). (B) No differences were observed in the ability of cells leaving the CVZ to extend processes or change the direction of movement or turn. Exploratory behavior: n = 13 slices/85 cells (normal) and n = 12 slices/75 cells (MAM-treated). (C) Treating normal cells leaving the CVZ with BMI showed that 10-μM BMI caused a significant reduction of speed. Adding 10 μM of BMI to the MAM-treated organotypic cultures, however, resulted in an increase in migration speed of the cells leaving the CVZ. n = 273 cells (normal + 10 μM BMI), n = 226 cells (MAM-treated + 10 μM BMI). One-way ANOVA followed by the Tukey post hoc test, *P ≤ 0.05. (D) The cells leaving the CVZ showed no differences between normal and MAM treated in their ability of extend a process or turn. Adding BMI did not result in any significant differences between groups. n = 25 slices/192 cells (normal + 10 μM BMI) and n = 18 slices/142 cells (MAM-treated + 10 μM BMI).
Treatment with MAM Increases the Expression of GABAAα2, GABAAα3 Receptor Subunits, and KCC2
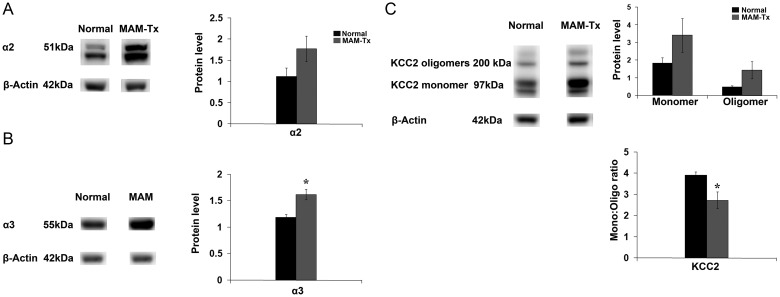
Our previous studies strongly suggest that GABAA receptors and GABA activity are increased in the neocortex of MAM-treated animals (Jablonska et al. 2004; Poluch et al. 2008). Here, we also show that blockade of GABAA-mediated activity ameliorates the deficits in dynamic activity after MAM treatment. To further assess the role of GABA in mediating the movements of migrating cells leaving the GE following treatment with MAM, we determined the level of expression of GABAAα2 and GABAAα3 receptor subunits using western blots. GABAAα2 and GABAAα3 show high expression in both the neocortex and GE early in development, and may play important roles in migration and other developmental events in GE cells (Laurie et al. 1992; Poulter et al. 1992; Fristchy et al. 1994; Wang and Kriegstein 2009; Carlson and Yeh 2010). In our study, both α2 and α3 subunits are increased in MAM-treated cortex compared with normal, furthering support for increased GABAA-mediated activity in our model animals (Fig. 8A,B).
Figure 8.
Expression of GABAA receptor subunits and KCC2 following treatment with MAM. (A) Treatment with MAM results in an increase in the expression of GABAAα2, and (B) GABAAα3 receptor subunits in the neocortex as demonstrated by western blots. (C) Both monomeric and oligomeric forms of KCC2 are increased (top bar graph) with significant elevation in the ratio of KCC2 oligomer:monomer (bottom graph). Protein levels were determined by densitometric analysis using ImageJ software. For both normal and MAM-Tx animals, n = 4. Significance determined using the Student's t-test, P ≤ 0.05 Error bars = standard error of mean.
To further evaluate the potential of altered environmental GABA to influence migration, we established the amount of KCC2 in normal and MAM-treated cortex. During development, KCC2 maintains a high extracellular Cl− gradient and induces hyperpolarization of cells in response to GABA, playing a role in the developmental switch from the GABA-induced depolarization to hyperpolarization, which may also terminate cell migration (Rivera et al. 1999; Bortone and Polleux 2009). After MAM treatment, both monomeric and oligomeric forms of KCC2 were increased in the neocortex as demonstrated using western blots (Fig. 8C, right upper panel, also see Fig. 9). Furthermore, ratiometric analysis reveals a decrease in KCC2 monomer:oligomer ratio (Fig. 8C, right lower panel).
Figure 9.
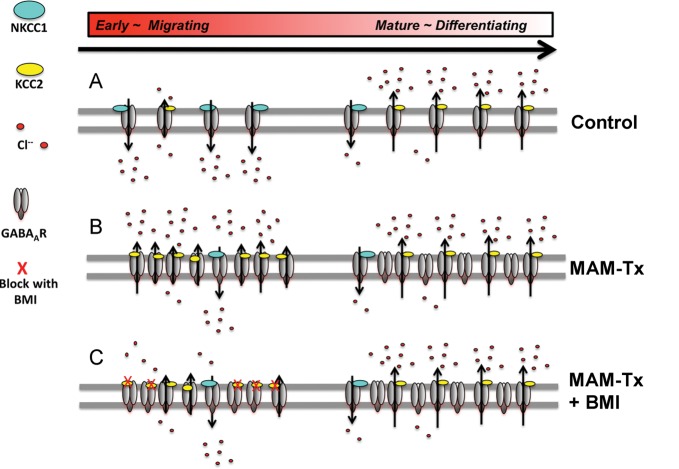
Examples of GABAAR function in the development of normal and MAM-treated cortex. (A) depicts normal receptors that show higher expression of NKCC1 during early development and low expression of KCC2 (on the left). As they mature, the receptors express higher levels of KCC2 and lower levels of NKCC1 (on the right). The high NKCC1 levels allow easier migration of cells and the excitatory action of GABAAR. As the cells mature, the shift to KCC2 slows migration and results in the inhibitory action of GABAARs. (B) shows a hypothetical cell that expresses GABAAR after MAM-treatment. This cell shows high levels of GABAAR and KCC2, which slows migration and encourages the inhibitory action of GABAAR. The expression levels of KCC2 during development (left) are similar to those as the cells mature (right). (C) Blockade of GABAAR with BMI lowers the action of KCC2 and results in more normal actions during development in MAM-treated cells (on the left). As the cells mature, the GABAAR activity remains high (on the right). Levels of ambient chloride levels as a result of the expression of 2 cation-chloride transporters, KCC2 or NKCC1 are indicated.
Discussion
The dynamic behavior of migrating interneurons, influenced by both intrinsic and environmental factors, plays an important role in the proper positioning of these cells in the neocortex. We developed a model of cortical dysplasia in ferrets by interrupting the development of layer 4 cells, resulting in abnormal placement of specific populations of interneurons (Noctor et al. 1999; Poluch et al. 2008). In this study, we show that transient and layer-specific interference with corticogenesis leads to: 1) alterations in the basal activity of GABAA and in KCC2 expression and that 2) this change directly influences the migratory behavior of cells leaving the GE and CVZ-derived cells to a lesser extent. These findings directly and uniquely implicate increased GABAAR and KCC2 activity in impairing dynamic aspects of migration and in the ability of GE-derived cells to actively explore the environment during migration to the cerebral cortex. We demonstrate that details of migration not shown before involving active exploration are strongly influenced by ambient GABAAR and KCC2 activity. This may underlie the modified migratory behavior of later born interneurons.
Ferrets signify a valuable developmental model. As the smallest mammal with a convoluted cortex, they represent an important link between studying lissencephalic rodents and nonhuman primates. Several groups identified important developmental features in ferrets, including our previous findings that many neurons populating the neocortex are generated in lateral portions of the GE (Poluch et al. 2008). Several recent articles describe a distinct outer sub-VZ (oSVZ) in ferrets, a region found in primates, and suggest that this region may be important for generating cells that populate and expand the gyrencephalic neocortex (Fietz et al. 2010; Lui et al. 2011; Reillo and Borrell 2011; Reillo et al. 2011). The oSVZ contains a population of cells called outer radial glia (oRG; also called tRG), which appear to be the cell type responsible for generating additional cortical cells leading to the expansion of the developing cortex. This idea is contested recently by a group suggesting that the outer SVZ in ferrets does not differ substantially from that region in the rat (Martinez-Cerdeno et al. 2012). Nevertheless, these examples demonstrate the importance of studying developmental processes using several animal models.
MAM Treatment Alters the Dynamic Behavior of Cells Migrating from the GE
The ability of migrating GE-derived cells to reach appropriate positions within the neocortex depends on the concerted action of extrinsic factors within the neocortex and intrinsic signaling mechanisms that inhibit, facilitate, and guide migrating neurons (e.g., Anderson et al. 1997; Powell et al. 2001; Lopez-Bendito et al. 2003, 2008; Polleux et al. 2003; Stumm et al. 2003; Alifragis et al. 2004; Flames et al. 2004; Cuzon et al. 2006; Cobos et al. 2007; Liodis et al. 2007; Friocourt et al. 2008). Events leading to changes in neocortical architecture may cause variability in the environment that alters the pattern of migration of neuronal cells and results in abnormal cell positioning and cortical dysplasia. In our model of cortical dysplasia, layer 4 of ferret neocortex is diminished resulting in widespread termination of thalamocortical afferents (Noctor et al. 2001; Palmer et al. 2001), redistribution of GABAA receptors and parvalbumin and calbindin positive interneurons (Jablonska et al. 2004; Poluch et al. 2008), and changes in the sequence of information transfer in the somatosensory cortex (McLaughlin and Juliano 2005). To better understand the mechanisms contributing to the redistribution of interneurons, we evaluated several parameters of migratory behavior in GE-derived cells. Cells leaving the GE in normal animals migrated significantly faster compared to similar cells in MAM-treated animals. In addition, GE-generated neurons in MAM-treated animals exhibited less exploration of the neocortical environment. Exploratory behavior, including extension of multiple leading processes and changes in direction of movement, results from coordinated signaling from various sites, which communicate cues that determine the final location of these cells. The change in the speed of migration and exploratory activity of interneurons following treatment with MAM therefore has important implications on the overall positioning of cells within the neocortex.
Inhibiting GABAAR-Mediated Activity Improves Migration of Interneurons in MAM-Treated Animals
GABA is an important influence on migrating cells, although its precise role in the migration of telencephalic cells is complex. GABA receptors are important during different phases of migration and mediate aspects of the initiation, continuation, and termination of cellular movement (Behar et al. 1996, 2001; Bolteus and Bordey 2004; Heck et al. 2007; Bortone and Polleux 2009). Cuzon et al. (2006) report that GABAA activity is present throughout the path of migration of mouse cells moving from the GE into the neocortex, but increases in activity as neurons approach and enter their cortical target. Our studies suggest that MAM treatment results in increased ambient GABAA-mediated activity, which may interfere with migration of cells exiting the GE as they move en route to the neocortex before the final phase identified by Cuzon et al. 2006. This is supported by our original observation of expanded GABAAα immunoreactivity in MAM-treated cortex (Jablonska et al. 2004) and the current finding of increased GABAAα2 and GABAAα3 receptor subunits as demonstrated by western blot. Both α2 and α3 subunits of the GABAA receptor are expressed early during development and may mediate the initial roles of GABA (Laurie et al. 1992; Poulter et al. 1992; Fristchy et al. 1994; Carlson and Yeh 2010). This assists in explaining our finding that elevated levels of GABA interfere with migration of cells leaving the GE to slow the pace but not prevent their movement.
To investigate the involvement of GABAA-mediated transmission in the migration of interneurons, we manipulated the level of GABAA activity in MAM-treated organotypic cultures using BMI to reduce the ambient GABAA activity. We predicted that limiting the increased GABAA activity would change the cellular actions in MAM-treated cortex to be more like normal. In fact, blocking GABAA activity in MAM-treated animals resulted in significant enhancement of the migration speed of cells leaving the GE as well as increasing the percentage of MAM-treated GE-derived cells extending more than one leading process changing their direction of movement. The property of migration speed was more susceptible to manipulation than exploratory behavior. All concentrations of BMI increased the speed of MAM-treated migrating GE-derived cells, while only the highest BMI concentration resulted in the improvement of turning behavior. These distinctions reflect differences in the sensitivity of migratory behavior to variations in GABAAR activity. Overall, however, these findings suggest that reducing the effectiveness of abnormally high ambient GABAAR in the MAM-treated ferret encourages migratory activity to become more normal.
In control animals, blockade of GABAAR activity impairs the speed of migration without significant impact on the exploratory behavior. This suggests that an optimum level of GABAAR activity is important for proper migration of cells originating from the GE, since both abnormally high levels of GABAAR (as in MAM-treated animals) and pharmacological inhibition of GABAAR in normal animals (which contain an optimal level of GABAAR activity) reduced the migratory speed. Inada et al. (2011) also report that activation of GABAAR causes increased motility of interneurons, while blockade of GABAAR results in longer periods of inactivity, a finding that correlates with our results in normal cells.
Increased GABA Signaling Interferes with Migration
Both GABAAα2 and GABAAα3 expression are increased in MAM-treated animals compared with controls. Does increased GABAAα expression in MAM-treated animals alter the dynamic behavior of GE-derived cell migration? It has been known for several years that GABA acts as an excitatory neurotransmitter early in development, which helps facilitate cell migration, but switches to mediate inhibition as cells mature (Plotkin et al. 1997; Clayton et al. 1998; Rivera et al. 1999). The dual role of GABA is attributed to the temporal expression of 2 cation-chloride transporters, sodium-potassium-chloride (NKCC1), and KCC2. Migrating neurons initially express NKCC1, which imports chloride ions (Cl−) into the cell to maintain a higher intracellular Cl− concentration (Clayton et al. 1998). In response to GABAA activation, the outward movement of Cl− results in depolarization of the cells and triggers Ca2+-mediated events that influence the cytoskeleton and stimulate migration (Behar et al. 2001; Soria and Valdeolmillos 2002). As cells mature, NKCC1 downregulates and coincides with increased expression of KCC2 (Plotkin et al. 1997). KCC2 is a Cl− exporter that maintains a higher extracellular gradient of Cl−. Consequently, GABAA activation when KCC2 is increased results in hyperpolarization and cell inhibition (Payne 1997; Rivera et al. 1999). Interestingly, in dissociated hippocampal cell cultures, activation of GABAAR induces the expression of NKCC1 and KCC2 (Ganguly et al. 2001). In addition, pharmacologic blockade of GABAA delayed the expression of KCC2, while increased activity of GABAAR induces precocious expression of the transporter. Premature expression of KCC2 as a result of a heightened state of GABAA-mediated activity, as we see here, may be responsible for the migration defects observed in MAM-treated ferrets. KCC2 is implicated in several important processes, including decreased motility as a neuron migrates, which leads to cell slowing, stopping, and differentiating. As a consequence, a premature switch of the GABAA response may occur and produce early arrest of migrating interneurons (Fig. 9). Bortone and Polleux 2009 demonstrated that depolarization through GABAA receptors stimulates motility of GABAergic migrating neurons while hyperpolarization of GABAA receptors, induced by KCC2 expression, provides a stop signal for migrating cells. We observed increased expression of both KCC2 monomer and oligomers in the neocortex of young MAM-treated animals. KCC2 is initially expressed as monomeric protein; monomeric KCC2 gradually forms oligomers, which represent the functional unit of the protein and allow the characteristic inhibition to occur (Blaesse et al. 2006). The ratio of KCC2 monomer to oligomer correlates with the functional state of the protein; more mature cells have higher levels of oligomers. In our study, the ratio of KCC2 monomer to oligomer was “reduced” in MAM-treated animals (i.e., indicating a higher level of oligomers), which suggests greater potential for activity of this transporter in our model animals. Oligomerization of KCC2 is an important step in the activation and function of KCC2 as a Cl− exporter (Blaesse et al. 2006). Although others have demonstrated that GABAAR and KCC2 activity are involved in neuronal migration, we demonstrate here that high endogenous levels of these substances directly interfere with migration. In addition, they appear specifically important in dynamic and exploratory behavior in cells leaving the GE.
MAM Treatment has Minimal Effect on the Profile of Migrating Cortical VZ Derived Cells
To determine whether the effect of MAM treatment on the dynamics of migration is restricted to interneurons alone, we also investigated the migratory behavior of neurons arising from the cortical VZ. Our earlier study suggested that the primary migration defect in MAM-treated animals was in interneurons (Poluch et al. 2008). Our findings here suggest that a high level of GABAARs interferes with migration, whether the cells originate from the neocortex or the GE. The cells leaving the GE are the most likely to be affected and show the most obvious defect. When we assessed the migration speed of CVZ cells in MAM-treated animals, it was reduced but the deficit was not significant. Adding BMI to the media of control or MAM-treated organotypic cultures caused the speed of the control cells to decrease, while that of MAM-treated CVZ-derived cells increased. This result is similar to our observation of changes in the speed of normal and MAM-treated cells leaving the GE. We interpret this to mean that since the level of GABAAR in MAM-treated animals is high in the neocortex, interference with GABAAR activity causes the MAM-treated cells leaving the CVZ to move at a normal speed, while the normal cells are slowed down by the blockade of GABAAR, similar to the findings of Inada et al. (2011). In regard to exploratory behavior of cells leaving the CVZ, we did not observe any significant differences between cells in normal animals and those that received MAM treatment, indicating the impact might be restricted to tangentially migrating interneurons. Although it is not entirely clear why MAM-treatment differentially affects the migration of GE- versus CVZ-derived cells, a number of reports emphasize the more extensive and tortuous course of interneurons, which may influence their migration patterns. The differential impact of MAM-treatment on the dynamic behavior of GE- and CVZ-derived cells relative to normal cells may also reflect the different neurogenic origin of the 2 cell populations that migrate under the influence of similar, but not identical, signaling mechanisms (Marin and Rubenstein 2003).
Summary
A plethora of signaling molecules influence migrating interneurons. GABA initially appears to act as a stimulating signal to facilitate migration and then as a stop signal to arrest the migratory process, an effect that correlates with the sequential expression of NKCC1 and KCC2. An appropriate level of GABA is necessary for the proper migration and positioning of interneurons. Our data suggest that following treatment with MAM, the basal level of GABAA activity increases, and the elevated level of GABAA activity impairs the migration of interneurons, and to a lesser extent cells arising from the CVZ, through premature expression of KCC2. Thus, treatments that decrease the level of GABAA activity enhance the migration of MAM-treated interneurons in part by interfering with the action of KCC2.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by funding from the National Institute of Neurological Disorders and Stroke, grant number RO1 24014.
Supplementary Material
Notes
We thank Sylive Poluch, Tarik Haydar, and Marcin Gierdalski for their helpful discussions and scientific assistance. We also thank Latoya Hyson for excellent animal care and technical assistance. Conflict of Interest: None declared.
References
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–5648. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AA, Eisenstat SL, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Ang ESBC, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23(13):5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL. GABAB receptors mediate motility signals for migrating embryonic cortical cells. Cereb Cortex. 2001;11(8):744–753. doi: 10.1093/cercor/11.8.744. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, Nothwang HG. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26(41):10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Baraban SC. An examination of calcium current function on heterotopic neurons in hippocampal slices from rats exposed to methylazoxymethanol. Epilepsia. 2003;44:315–321. doi: 10.1046/j.1528-1157.2003.41102.x. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Paredes MF, Scott C, Baraban SC. Heterotopic neurons with altered inhibitory synaptic function in an animal model of malformation associated epilepsy. J Neurosci. 2002;22(17):7596–7605. doi: 10.1523/JNEUROSCI.22-17-07596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson VCC, Yeh HH. GABAA receptor subunit profiles of tangentially migrating neurons derived from the medial ganglionic eminence. Cereb Cortex. 2010;21:1792–1802. doi: 10.1093/cercor/bhq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Mathias SC. Cortical dysplasia associated with massive ectopia of neurons and glial cells within the subarachnoid space. Acta Neuropathol (Berl) 1987;73:105–109. doi: 10.1007/BF00693774. [DOI] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl− cotransporter expression in rat neocortex. Brain Res Dev Brain Res. 1998;109:281–292. doi: 10.1016/S0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PWL, Cheng Q, Yeh HH. Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex. 2006;16(10):1577–1588. doi: 10.1093/cercor/bhj084. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JLR, Maŕın O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, Raguenes O, Chelly J, Ferec C, Nakajima K, Parnavelas JG. Cell autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–5805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristchy J-M, Paysan J, Enna A, Mohlerl H. Switch in the expression of rat GABA,-receptor subtypes during postnatal development: an immunohistochemical study. J Nerosci. 1994;14(9):5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26(3):1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the Developmental-switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/S0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gleeson GJ, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 2000;23:352–359. doi: 10.1016/S0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, Luhmann HJ. GABAA receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb Cortex. 2007;17:138–148. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- Inada H, Watanabe M, Uchida T, Ishibashi H, Wake H, Nemoto T, Yanagawa Y, Fukuda A, Nabekura J. GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS one. 2011;6(12):1–11. doi: 10.1371/journal.pone.0027048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B, Smith AL, Palmer SL, Noctor SC, Juliano SL. GABAA receptors reorganize when layer 4 in ferret somatosensory cortex is disrupted by methylazoxymethanol (MAM) Cereb Cortex. 2004;14(4):432–440. doi: 10.1093/cercor/bhh005. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Nerosci. 1992;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Luján R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABAB receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sanchez-Alcaniz JA, Pla R, Borrell V, Pico E, Valdeolmillos M, Marin O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko DE, Putt M, Golden JA. SDF1 regulates leading process branching and speed of migrating interneurons. J Neurosci. 2011;5:1739–1745. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marın O, Rubenstein JLR. Cell migration in the forebrain. Ann Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor C. Comparative analysis of the subventricular zone in rat, ferret and macaque:evidence for an outer subventricular zone in rodents. Plos one. 2012;7(1):1–37. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini FJ, Valiente M, Lopez-Bendito G, Szabo G, Moya F, Valdeolmillos M, Marin O. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- McLaughlin DF, Juliano S. Disruption of layer 4 development alters laminar processing in ferret somatosensory cortex. Cereb Cortex. 2005;15:1791–1803. doi: 10.1093/cercor/bhi056. [DOI] [PubMed] [Google Scholar]
- Moroni RF, Inverardi F, Regondi MC, Panzica F, Spreafico R, Frassoni C. Altered spatial distribution of PV-cortical cells and dysmorphic neurons in the somatosensory cortex of BCNU-treated rat model of cortical dysplasia. Eplepsia. 2008;49(5):872–887. doi: 10.1111/j.1528-1167.2007.01440.x. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Palmer SL, Juliano SL. Interference with the development of early generated neocortex results in the disruption of radial glia and abnormal formation of cortical layers. Cerebral Cortex. 1999;9:121–136. doi: 10.1093/cercor/9.2.121. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Palmer SL, McLaughlin DF, Juliano SL. Disruption of layers 3 and 4 during development results in altered thalamocortical projections in ferret somatosensory cortex. J Neurosci. 2001;21:3184–3195. doi: 10.1523/JNEUROSCI.21-09-03184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SL, Noctor SC, Jablonska B, Juliano SL. Laminar specific alterations of thalamocortical projections in organotypic cultures following layer 4 disruption in ferret somatosensory cortex. Eur J Neurosci. 2001;13:1559–1571. doi: 10.1046/j.0953-816x.2001.01519.x. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, Wright G. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol. 1991;30:741–749. doi: 10.1002/ana.410300602. [DOI] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(SICI)1097-4695(19971120)33:6<781::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2003;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Poluch S, Jablonska B, Juliano SL. Alteration of interneuron migration in a ferret model of cortical dysplasia. Cereb Cortex. 2008;18(1):78–92. doi: 10.1093/cercor/bhm032. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Barker JL, O'Carroll AM, Lolait SJ, Mahan LC. Differential and transient expression of GABAA receptor α-subunit mRNAs in the developing rat CNS. J Neurosci. 1992;12(8):2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30:79–89. doi: 10.1016/S0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2009. ISBN 3-900051-07-0, Available from: URL http://www.R-project.org . [Google Scholar]
- Reillo I, Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex. 2011;9:2039–2054. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2011;21:1674–1694. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtenin H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Ross EM, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu Rev Neurosci. 2001;24:1041–70. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- Soria JM, Valdeolmillos M. Receptor-activated calcium signals in tangentially migrating cortical cells. Cereb Cortex. 2002;12(8):831–839. doi: 10.1093/cercor/12.8.831. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cossu M, Ferrario A, Galli C, Bramerio M, Citterio A, Spreafico R. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, Corsellis JAN. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. J Neurophys. 2009;587(9):1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Roper SN. Reduced inhibition in an animal model of cortical dysplasia. J Neurosci. 2000;20(23):8925–8931. doi: 10.1523/JNEUROSCI.20-23-08925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.