Abstract
Background
Nodal/TGF-Lefty signaling pathway has important effects at early stages of differentiation of human embryonic stem cells in directing them to differentiate into different embryonic lineages. LEFTY, one of transforming growth factors in the Nodal/TGF-Lefty signaling pathway, plays an important role in the development of heart. The aim of this work was to find evidence on whether Lefty variations are associated with congenital heart diseases (CHD).
Methods
We sequenced the Lefty gene for 230 Chinese Han CHD patients and evaluated SNPs rs2295418, rs360057 and g.G169A, which are located within the translated regions of the genes. The statistical analyses were conducted using Chi-Square Tests as implemented in SPSS (version 13.0). The Hardy-Weinberg equilibrium test of the population was carried out using online software OEGE, and multiple-sequence alignments of LEFTY proteins were carried out using the Vector NTI software.
Results
Two heterozygous variants in Lefty1 gene, g.G169A and g.A1035C, and one heterozygous variant in Lefty2 gene, g.C925A, were identified. Statistical analyses showed that the rs2295418 (g.C925A) variant in Lefty2 gene was obviously associated with the risk of CHD (P value = 0.016<0.05). The genotype frequency of rs360057 (g.A1035C) variant in Lefty1 gene was associated with the risk of CHD (P value = 0.007<0.05), but the allele frequency was not (P value = 0.317>0.05).
Conclusions
The SNP rs2295418 in the Lefty2 gene is associated with CHD in Chinese Han populations.
Introduction
Congenital heart diseases (CHD) are a group of common and complex illnesses with high morbidity and mortality. Despite the enormous advances in surgical treatments over the past decades, the genetic etiology is still largely unknown [1]. The incidence of moderate and severe forms of CHD is about 6/1,000 of live births. If tiny muscular ventricular septal defects and other trivial lesions are included, the total incidence is about 75/1,000 of live births [2]. For the CHD patients, about one percent would require intervention [3] and about thirteen percent show recognizable chromosomal variants [4]. Most adult CHD patients are predisposed to cardiac complications, such as coronary heart diseases, arrhythmias or heart failure [5]. Although extensive genetic studies and high-resolution technologies have revealed the genetic defects in many familiar and sporadic CHD cases [6], [7], the genetic abnormalities in the majority of CHD patients remain largely unknown.
In the embryonic development, heart is the first formed organ, strictly controlled by gene regulatory networks, involving transcription factors, signaling pathways, epigenetic factors, and miRNAs [8], [9]. During the last few decades, a variety of CHD-causing gene mutations have been identified, such as those in CITED2 [10], CFC1 [11], GATA4 [12] and TBX1 [13]. These genes play critical roles in cardiac development; mutations in these genes lead to cardiovascular malformations and contribute to CHD [14]. Human embryonic stem (HES) cells may differentiate to various cell types and develop to different embryonic lineages, including those of ectoderm (neurons and epidermal cells), endoderm (hepatocytes and pancreatic cells), and mesoderm (muscle cells and cardiomyocytes cells) under the control of certain factors[15]. LEFTY negatively regulates the Nodal/TGF-Lefty signaling pathway [16] and inhibits cellular proliferation and differentiation [17], [18]. It has been shown that when the Nodal/TGF-Lefty pathway goes wrong, serious malignant transformation may occur. In malignant melanoma cells, for example, LEFTY inhibits the malignant properties of melanoma cells [19] [20], [21]. During the early differentiation of HES cells, LEFTY is expressed in a subset of cells, playing an important role in mesodermal cell differentiation [22]. The Nodal/TGF-Lefty signaling pathway also has an important effect in early stages of HES cell differentiation, directing specific cells into different embryonic lineages. LEFTY, as one of the important transforming growth factors in the Nodal/TGF-Lefty signaling pathway, inhibits the signaling of NODAL, which may play an important role in the development of heart.
To elucidate possible associations of Lefty genes with CHD, we analyzed the transcribed region and splicing sites of the Lefty1 and Lefty2 genes and compared the Lefty gene sequences between 230 Chinese Han CHD patients and 263 controls. We found that the rs2295418 (g.C925A) variant in the Lefty2 gene was closely associated with the risk of CHD.
Materials and Methods
The study population
For this study, a total of 230 CHD patients and 263 control subjects with no reported cardiac phenotypes were recruited from Linyi People's Hospital and the Second Affiliated Hospital of Harbin Medical University, Harbin, China (Table 1). The 263 control subjects were enrolled at the Medical Examination Center of the Second Affiliated Hospital of Harbin Medical University. All these subjects had physical and electrocardiogram examinations and ultrasonic echocardiogram examination, and none of them showed any defects in the heart or other parts of the body. A written informed consent was obtained from each participant, and this work had been approved by the Ethics Committee of Harbin Medical University, consistent with the 1975 Declaration of Helsinki. Detailed records on their medical history, physical examination and chest X-ray examination, electrocardiogram, and ultrasonic echocardiogram were obtained. We deposited our data in the NIH Short Read Archive dataset, with the accession number SRP043439.
Table 1. Clinical characteristics of study population.
| Parameter | CHD | Control |
| Sample (n) | 230 | 263 |
| Male/Female (n) | 142/88 | 171/92 |
| Age (years) | 16.18±10.22 | 8.36±9.98 |
Data are shown as mean±SD.
DNA analysis
Genomic DNA was extracted from peripheral blood leukocytes using standard protocols. The human Lefty1 and Lefty2 genes are located on 1q42.1 and are encoded by four exons. The four exons and the splicing sites of the two genes were amplified by polymerase chain reaction (PCR) with the primers shown in Table 2. PCR products were sequenced using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) and the ABI 3130XL (Applied Biosystems) sequencer for mutational analysis.
Table 2. PCR primers used for Lefty sequence analysis.
| Gene | Exon | Forward primer | Reverse primer | Size | Tm |
| LEFTY1 | 1 | TGCCTGAGACCCTCCTGC | CCCTCACTCAGCCTCCCA | 436 | 59.9 |
| 2 | TTTGCCCCAGAAATAGAACAGG | GACCCAGCGCCGCTTGAG | 499 | 62.1 | |
| 3 | CAACCGCACCTCCCTCAT | CATTCATTCCCACAGCACTC | 513 | 59.2 | |
| 4 | TAAATCTCCATCCCAGACGC | ACCCTCGAACACTTCAGAAACA | 499 | 57.9 | |
| LEFTY2 | 1 | CTCCCTCTTCCCTTCACCC | ACAGCCTCCCACAGAGTCCC | 511 | 60.5 |
| 2 | GCCTGGCTGCCAGCTCAG | GACCCAGCGCCGCTTGAG | 462 | 62.7 | |
| 3 | CAACCGCACCTCCCTCATC | GCAATCGCTGGCATCCTG | 570 | 61.7 | |
| 4 | CCTCCCAGGTGCCCACTA | GGGATGGAGTAACTTGCTAA | 549 | 56.5 |
Rs2295418, Rs360057 and g.G169A Lefty SNP genotyping analysis and Statistical methods
Genotypes of the rs2295418 and rs360057, g.G169A SNPs, within the Lefty2 or Lefty1 genes (Figure 1), were determined using two stage methods. We amplified rs2295418, rs360057 and g.G169A (Table 2, Lefty2exon4; Lefty1exon4 and exon1) and sequenced the PCR products to determine the genotype. The statistical analyses were conducted using Chi-Square Tests to calculate odds ratios and P value as implemented in SPSS (version 13.0). We also used online software OEGE to conduct the Hardy-Weinberg equilibrium test of the CHD and control population.
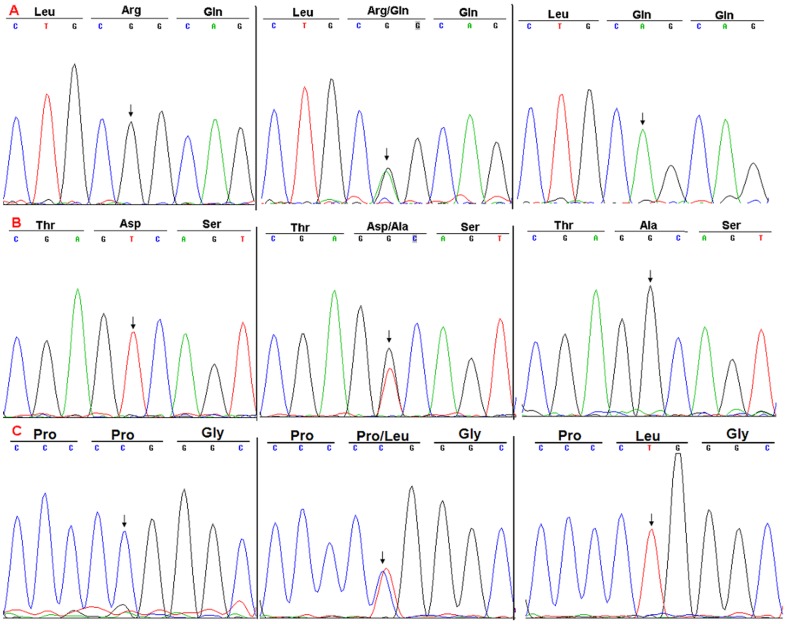
Figure 1. DNA sequence chromatograms of the Lefty-1 and Lefty -2 genes.
A: g.G169A (p.Arg33Gln); B: g.A1035C-rs360057 (p.Asp322Ala); C: g.C925A-rs2295418 (p.Pro286Leu).
Multiple sequence alignments
From the NCBI website (http://www.ncbi.nlm.nih.gov/), the LEFTY protein sequences of various species were obtained, and using the Vector NTI software, multiple-sequence alignments of LEFTY proteins were carried out.
Results
Patients
Clinical diagnosis of the recruited patients was confirmed in Linyi People's Hospital and The Second Affiliated Hospital of Harbin Medical University. There was no history of other systemic abnormalities in these CHD patients, and their mothers did not have a history of taking medicines or attracting infections during pregnancy. The 230 CHD patients contained 12 pulmonary stenosis, 14 tetralogy of Fallot, 14 patent ductus arteriosus, 22 mitral valve insufficiency, 41 atrial septal defect, 95 ventricular septal defect and 32 other complex congenital heart diseases.
Lefty gene analysis
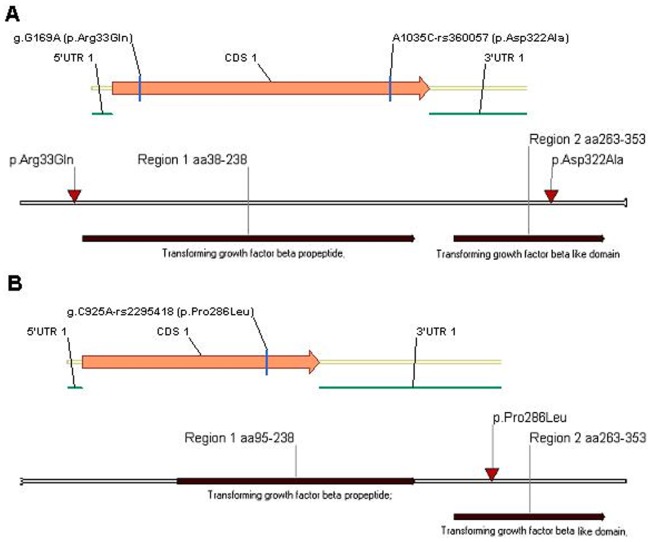
We sequenced Lefty to test the hypothesis that germline common genetic variants in Lefty may confer susceptibility to CHD. We first compared the transcribed region and splicing sites of Lefty and found two variations in the Lefty1 gene [g.G169A (p.Arg33Gln) and g.A1035C-rs360057 (p.Asp322Ala)] and one variation in the Lefty2 gene [g.C925A-rs2295418 (p.Pro286Leu)] in the CHD cases (Figure 1). These variations were located within the translated region of the genes, and the g.A1035C-rs360057 and g.C925A-rs2295418 variations were located within the transforming growth factor-β-like domain of LEFTY protein (Figure 2).
Figure 2. Schematic diagrams of rs2295418 and rs360057 locations within the translated region of Lefty-2 and Lefty -1 genes and transforming growth factor-β-like domain of the proteins.
A: Lefty-1; B: Lefty-2.
Rs2295418, Rs360057 and g.G169A Lefty SNP genotyping and Statistical analysis
To further test any possible associations between Lefty and CHD, we conducted SNP analyses and found that the rs2295418 (g.C925A) variant in Lefty2 gene was obviously associated with the risk of CHD; the genotype frequency of the rs360057 (g.A1035C) variant in Lefty1 gene was associated with the risk of CHD, but there was no statistical significance in the allele frequency. The g.G169A variant in Lefty1 gene was not associated with the risk of CHD in the Chinese Han population (Tables 3, 4). We also conducted the Hardy-Weinberg equilibrium test for the CHD patients and controls and our results were in line with the Hardy-Weinberg equilibrium.
Table 3. The genotype and allele frequency of SNP rs2295418, rs360057 and g.G169A in 230 Chinese Han CHD patients and 263 non-CHD controls.
| SNP | Group | Genotype frequency (%) | Allele frequency (%) | ||||
| rs2295418 | Genotype | G/G | G/A | A/A | G | A | |
| CHD | 230 | 173(75.2) | 45(19.6) | 12(5.2) | 391(85.0) | 69(15.0) | |
| Controls | 263 | 223(84.8) | 35(13.3) | 5(1.9) | 481(91.4) | 45(8.6) | |
| rs360057 | Genotype | T/T | T/G | G/G | T | G | |
| CHD | 230 | 148(64.3) | 62(27.0) | 20(8.7) | 358(77.8) | 102(22.2) | |
| Controls | 263 | 167(63.5) | 89(33.8) | 7(2.7) | 423(80.4) | 103(19.6) | |
| g.G169A | Genotype | G/G | G/A | A/A | G | A | |
| CHD | 230 | 179(77.8) | 47(20.4) | 4(1.7) | 405(88.0) | 55(12.0) | |
| Controls | 263 | 203(77.2) | 56(21.3) | 4(1.5) | 462(87.8) | 64(12.2) | |
Table 4. SNP rs2295418, rs360057 within Lefty-2 and Lefty-1 associated with the risk of congenital heart diseases in Chinese populations.
| Genotyped SNP | Associated gene | Pearson Chi-square | Pearson's R | |||||||
| Value | Min counta | df | Asymp. Sig. (2-sided) | Value | Asymp. Std. errorb | Approx. Tc | Approx. Sig | |||
| rs2295418 | LEFTY2 | Genotype | 8.274 | 7.93 | 2 | 0.016 | −0.129 | 0.044 | −2.893 | 0.004d |
| Allele | 9.968 | 53.18 | 1 | 0.002 | −0.101 | 0.032 | −3.170 | 0.002d | ||
| rs360057 | LEFTY1 | Genotype | 10.069 | 12.60 | 2 | 0.007 | −0.044 | 0.045 | −0.966 | 0.334d |
| Allele | 1.001 | 95.64 | 1 | 0.317 | −0.032 | 0.032 | −1.000 | 0.318d | ||
| g.G169A | LEFTY1 | Genotype | 0.086 | 3.73 | 2 | 0.958 | 0.005 | 0.045 | 0.100 | 0.920d |
| Allele | 0.010 | 55.52 | 1 | 0.919 | 0.003 | 0.032 | 0.101 | 0.919d | ||
a: The minimum expected count;
b: Not assuming the null hypothesis;
c: Using the asymptotic standard error assuming the null hypothesis;
d: Based on normal approximation.
Conservation of the protein in evolution
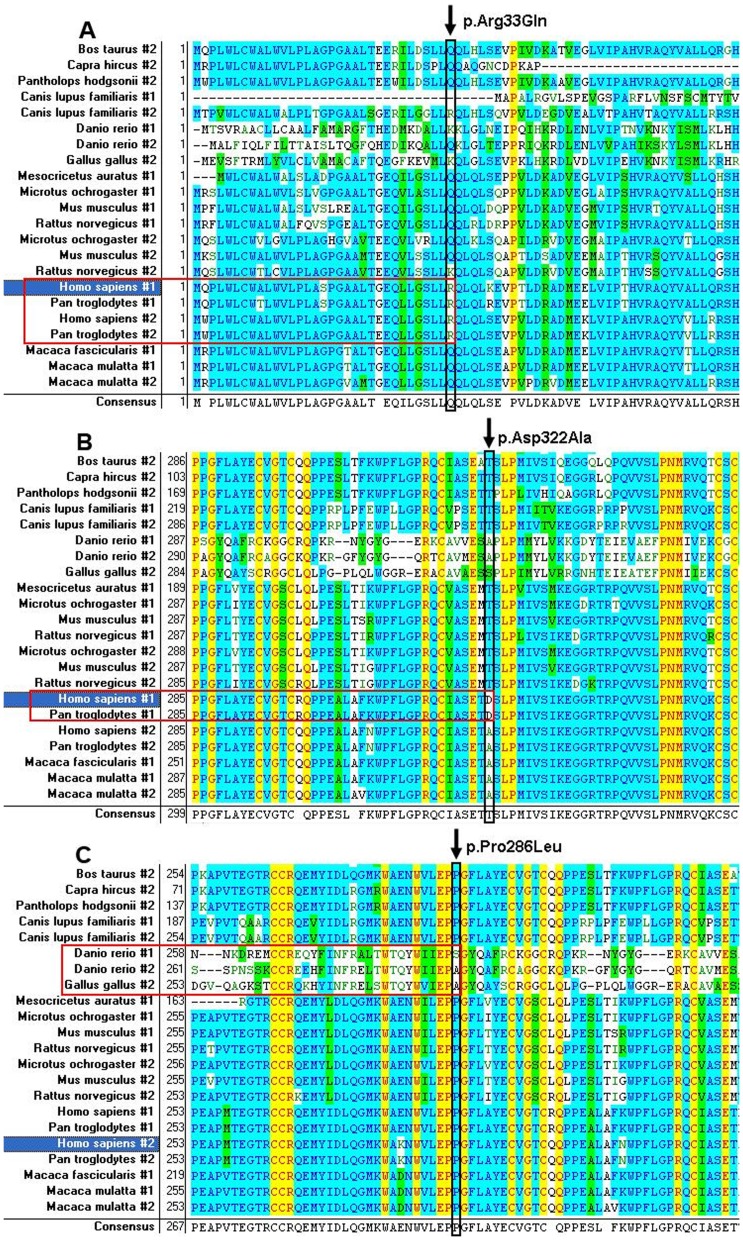
Comparison of the LEFTY1 and LEFTY2 protein sequences from species including birds, fishes and mammals by multiple-sequence alignment analysis showed that the 286Pro residue in LEFTY2 was highly conserved among the mammals but the 33Arg and 322Asp residues in LEFTY1 were just conserved in Chimpanzee and Humans (Figure 3).
Figure 3. Multiple-sequence alignment of Lefty-1(#1) and -2(#2) from birds, fishes and mammals (including Homo sapiens, Pan troglodytes, Macaca mulatta etc.).
A: p.Arg33Gln; B: p.Asp322Ala; C: p.Pro286Leu.
Discussion
In this study, we analyzed the transcribed regions and splicing sites of the Lefty genes in large cohorts of CHD patients and controls and found that two variants, rs2295418 (g.C925A) and rs360057 (g.A1035C), were associated with the risk of CHD in the Chinese Han population, demonstrating the involvement of the Lefty genes in the CHD etiology.
The formation of the human heart starts on day 18 or 19 in the mesoderm after fertilization and involves strict temporal, spatial, and sequential gene expressions. Nodal/TGF-Lefty signaling pathway acts upon gastrulation, which develops to progenitor cells of the mesoderm and endoderm [22]. In mice, the formation of mesendoderm was affected by the expression level of Nodal/TGF-Lefty signaling pathway [23], and mutations in the Nodal gene can affect the formation of primitive streak, which is formed by mesendoderm progenitor cells. The vascular systems of the mouse arise from extraembryonic mesoderm that migrate through the primitive streak to the presumptive yolk sac [24]. At later stages of embryonic development, Nodal expression initiates a series of signal transduction and induces its own and Lefty gene expression, and the LEFTY negatively regulates the Nodal/TGF-Lefty signaling pathway [16], [22].
We analyzed genes of the Nodal/TGF-Lefty signaling pathway, which has been demonstrated to play vital roles in mouse mesoderm differentiation and heart formation; such genes are also temporally expressed in the differentiation of the HES cells [22]. We here demonstrated that the rs2295418 (g.C925A) and the rs360057 (g.A1035C) variants in Lefty2 and Lefty1 genes were associated with the risk of CHD in the Chinese Han population. These nucleotides were conserved only between Chimpanzee and man among the species compared. SNP-rs1904589 within the Nodal gene, which we also analyzed in the study, was not found to be significantly associated with the risk of CHD in the population (data not show).
Of great interest, although the translated regions of the two genes are 97.18% similar in nucleotide sequence, Lefty2 plays a more central role in the mesoderm differentiation [25], which may at least partly explain why the rs2295418 variants in Lefty2 gene were so closely associated with the risk of CHD. In contrast to Lefty2, Lefty1 seems to be less involved: although the genotype frequency of the rs360057 variant in Lefty1 gene was apparently associated with the risk of CHD, its allele frequency was not. Further work will be needed on the Nodal/TGF-Lefty signaling pathway for their involvement in the pathogenesis of CHD at the molecular level.
Acknowledgments
The authors thank the patients and family members for their cooperation and participation in this study.
Patient consent. Obtained.
Ethics approval. Ethics Committee of Harbin Medical University.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper. We deposited our data in the NIH Short Read Archive dataset, with the accession number SRP043439.
Funding Statement
This work was supported by a grant from Heilongjiang Innovation Research Foundation for Graduate Studies (YJSCX2012-199HLJ) to XD; a grant from Heilongjiang Province (ZD200917) to KJY; a grant from Pharmacy College of Harbin Medical University to FFL for training undergraduate students; and grants of National Natural Science Foundation of China (NSFC81271786, 81110378, 30970119, 81030029) to SLL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, et al. (2010) Mortality in adult congenital heart disease. Eur Heart J 31: 1220–1229. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39: 1890–1900. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman JI, Kaplan S, Liberthson RR (2004) Prevalence of congenital heart disease. Am Heart J 147: 425–439. [DOI] [PubMed] [Google Scholar]
- 4. Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, et al. (2007) Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115: 3015–3038. [DOI] [PubMed] [Google Scholar]
- 5. van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, et al. (2011) The changing epidemiology of congenital heart disease. Nat Rev Cardiol 8: 50–60. [DOI] [PubMed] [Google Scholar]
- 6. Bruneau BG (2008) The developmental genetics of congenital heart disease. Nature 451: 943–948. [DOI] [PubMed] [Google Scholar]
- 7. Richards AA, Garg V (2010) Genetics of congenital heart disease. Curr Cardiol Rev 6: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buckingham M, Meilhac S, Zaffran S (2005) Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 6: 826–835. [DOI] [PubMed] [Google Scholar]
- 9. van Weerd JH, Koshiba-Takeuchi K, Kwon C, Takeuchi JK (2011) Epigenetic factors and cardiac development. Cardiovasc Res 91: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sperling S, Grimm CH, Dunkel I, Mebus S, Sperling HP, et al. (2005) Identification and functional analysis of CITED2 mutations in patients with congenital heart defects. Hum Mutat 26: 575–582. [DOI] [PubMed] [Google Scholar]
- 11. Wang B, Wang J, Liu S, Han X, Xie X, et al. (2009) CFC1 mutations in Chinese children with congenital heart disease. Int J Cardiol. [DOI] [PubMed] [Google Scholar]
- 12. Butler TL, Esposito G, Blue GM, Cole AD, Costa MW, et al. (2010) GATA4 Mutations in 357 Unrelated Patients with Congenital Heart Malformation. Genet Test Mol Biomarkers. [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Chen D, Ma L, Meng H, Liu Y, et al. (2012) Genetic analysis of the TBX1 gene promoter in ventricular septal defects. Mol Cell Biochem 370: 53–58. [DOI] [PubMed] [Google Scholar]
- 14. Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, et al. (2001) Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet 38: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuldiner M, Benvenisty N (2003) Factors controlling human embryonic stem cell differentiation. Methods Enzymol 365: 446–461. [DOI] [PubMed] [Google Scholar]
- 16. Tabibzadeh S, Hemmati-Brivanlou A (2006) Lefty at the crossroads of "stemness" and differentiative events. Stem Cells 24: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 17. Ikushima H, Miyazono K (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 18. Heldin CH, Landstrom M, Moustakas A (2009) Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol 21: 166–176. [DOI] [PubMed] [Google Scholar]
- 19. Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, et al. (2008) Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A 105: 4329–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, et al. (2009) Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics 1: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malchenko S, Galat V, Seftor EA, Vanin EF, Costa FF, et al. (2010) Cancer hallmarks in induced pluripotent cells: new insights. J Cell Physiol 225: 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dvash T, Sharon N, Yanuka O, Benvenisty N (2007) Molecular analysis of LEFTY-expressing cells in early human embryoid bodies. Stem Cells 25: 465–472. [DOI] [PubMed] [Google Scholar]
- 23. Schier AF (2003) Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19: 589–621. [DOI] [PubMed] [Google Scholar]
- 24. Barroso-delJesus A, Lucena-Aguilar G, Sanchez L, Ligero G, Gutierrez-Aranda I, et al. (2011) The Nodal inhibitor Lefty is negatively modulated by the microRNA miR-302 in human embryonic stem cells. FASEB J 25: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 25. Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, et al. (1999) Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell 4: 287–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper. We deposited our data in the NIH Short Read Archive dataset, with the accession number SRP043439.