Abstract
G protein-coupled A2B adenosine receptor (AR) regulates numerous important physiological functions, but its activation by diverse A2BAR agonists is poorly profiled. We probed potential partial and/or biased agonism in cell lines expressing variable levels of endogenous or recombinant A2BAR. In cAMP accumulation assays, both 5′-substituted NECA and C2-substituted MRS3997 are full agonists. However, only 5′-substituted adenosine analogs are full agonists in calcium mobilization, ERK1/2 phosphorylation and β-arrestin translocation. A2BAR overexpression in HEK293 cells markedly increased the agonist potency and maximum effect in cAMP accumulation, but less in calcium and ERK1/2. A2BAR siRNA silencing was more effective in reducing the maximum cAMP effect of non-nucleoside agonist BAY60-6583 than NECA's. A quantitative ‘operational model’ characterized C2-substituted MRS3997 as either balanced (cAMP accumulation, ERK1/2) or strongly biased agonist (against calcium, β-arrestin). N6-Substitution biased against ERK1/2 (weakly) and calcium and β-arrestin (strongly) pathways. BAY60-6583 is ERK1/2-biased, suggesting a mechanism distinct from adenosine derivatives. BAY60-6583, as A2BAR antagonist in MIN-6 mouse pancreatic β cells expressing low A2BAR levels, induced insulin release. This is the first relatively systematic study of structure-efficacy relationships of this emerging drug target.
Keywords: GPCR, adenosine receptor, purines, cyclic AMP, calcium, arrestin
1. Introduction
Structure-efficacy relationships of the A1, A2A and A3 adenosine receptor (AR) agonists have been studied extensively in recent years [1-5]. This knowledge has led to the identification of many partial/biased agonists, including 5′-truncated nucleosides as antagonists and partial agonists for the A3AR [3,6], and as full agonists for A1 [5] and A2A ARs [4], which have potential clinical applications [5,7]. The structure-efficacy relationships of agonists at the A2BAR have not been previously probed.
The A2BAR plays a critical role in many physiological and pathophysiological conditions, such as taste [9,10], inflammation [11-17], cancer [18-22], ischemic conditions [23,24], erectile function [25], diabetes [26-28], stem cell differentiation [29] and oxidative stress [30]. Despite its recently demonstrated importance, the nature of A2BAR activation is still poorly understood. The role of the A2BAR has been reported controversially, e.g. both agonists and antagonists have been demonstrated to have anti-inflammatory effects [31-33]. Those seemingly contradictory results could be, at least in part, because of promiscuous G protein coupling of the A2BAR depending on the tissues and cells investigated or pathways measured; the lack of A2BAR selective agonists until recently; and most importantly, a severe deficiency of understanding of the pharmacological properties of A2BAR agonists used in some early studies. It is known that an agonist in one biological system might be a partial agonist or even an antagonist in other systems. Also, it is possible that different agonists attain variable maximum effects in specific signaling pathways, or a specific agonist has variable maximum effects in different signaling pathways. Biased agonists have been reported for several AR subtypes, but not the A2BAR [34,35].In the present study we investigate various A2BAR agonists of distinct structural classes (Fig. 1) in activating four signaling pathways under the direction of the A2BAR using several cell lines, including T24 cells, a human bladder cancer cell line endogenously expressing the A2BAR, and HEK293 cells expressing both endogenous and recombinant human A2BARs.
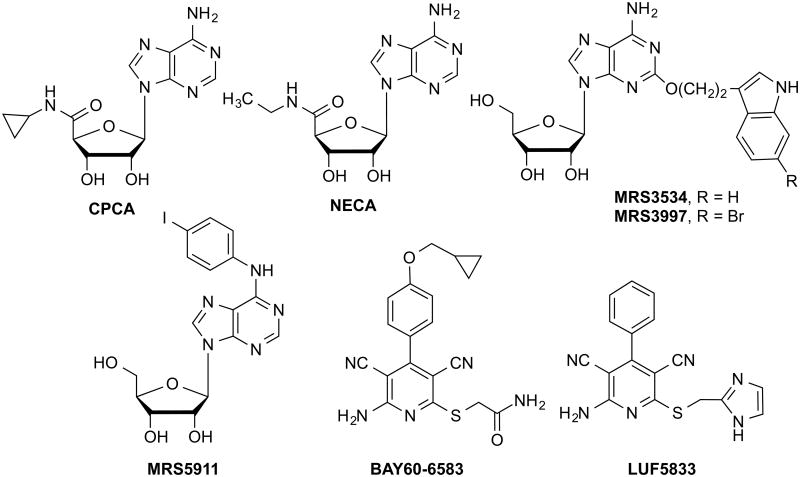
Fig. 1.
Chemical structures of representative agonists used in the present study. Non-nucleoside agonists: BAY and LUF5833; 5′-substituted: NECA, CPCA, 2-substituted: MRS3997 and MRS3534. N6-substituted: MRS5911.
2. Materials and Methods
2.1. Materials
Agonists
We selected four chemical classes of synthetic A2BAR agonists: A. 2-substituted adenosine analogs: MRS3997 [36], MRS3534 [37], CV1808 [38]. B. N6-substituted adenosine derivatives: MRS5911 [39], R-PIA [40]. C. 5′-substituted adenosine analogs, NECA (adenosine-5′-N-ethyluronamide) and CPCA (5-N′-cyclopropyl-carboxamidoadenosine. D. Non-nucleoside 3,5-dicyanopyridine agonists LUF5833 and BAY60-6583 (LUF6210, termed hereafter ‘BAY’) [41,42], which were synthesized at Leiden/Amsterdam Center for Drug Research (Leiden, The Netherlands). MRS3997, MRS3534 and MRS5911 [39] were synthesized at NIDDK, National Institutes of Health (Bethesda, MD, USA). Adenosine, NECA, CPA, CPCA, CV1808, R-PIA and probenecid were from Sigma (St. Louis, MO). Cl-IB-MECA was from Tocris (Minneapolis, MN).
Cell lines
a. Human cell lines (ATCC, Manassas, VA) were selected for endogenous expression of the A2BAR at variable levels: T24 bladder cancer cells; 1321N1 astrocytoma cells; WM266 melanoma cells; PC-3 prostate cancer cells and HEK293 cells expressing both endogenous and recombinant human A2BARs. Other species were represented in: COS-7 monkey kidney cells endogenously expressing the A2BAR (ATCC, Manassas, VA); MIN-6 mouse pancreatic β cells expressing the endogenous mouse A2BAR (obtained from Jürgen Wess, NIDDK, NIH); and PathHunter CHO cells expressing the recombinant mouse A2BAR and an engineered β-arrestin 2 (DiscoverX, Fremont, CA). The reason we used this mouse A2BAR cell line was that a similar version of PathHunter CHO cells from DiscoverX expressing the human A2BAR did not produce a robust response.
Additional materials and kits
Luminescence assay kit for β-arrestin2 translocation was from DiscoveRx (Fremont, CA). Calcium dye kit was from Molecular Devices (Sunnyvale, CA). AlphaScreen cyclic AMP kits and SureFire p-ERK1/2 (Thr202/Tyr204) assay kits were from PerkinElmer (Boston, MA). Superscript III First Strand Synthesis Supermix kit was from Invitrogen (Carlsbad, CA). The gene-specific FAM-labeled MGB Taqman probes were from Applied Biosystems (Life Technologies, Grand Island, NY). All other materials are from commercial sources and are of analytical grade.
2.2. cAMP accumulation assay
Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 Units/ml penicillin, 100 μg/ml streptomycin and 2 μmol/ml glutamine. Cells were plated in 96-well plates in 100 μl medium. After 24 h, the medium was removed and cells were washed three times with 100 μl DMEM, containing 50 mM HEPES, pH 7.4. Cells were then treated for 30 min with agonist and/or test compound in the presence of rolipram (10 μM) and adenosine deaminase (3 units/ml). The reaction was terminated by removal of the supernatant, and cells were lysed upon the addition of 100 μl of lysis buffer (0.3% Tween-20). For determination of cAMP production, an AlphaScreen cAMP kit was used according manufacturer's instructions.
2.3. Intracellular calcium mobilization assay
Cells were grown overnight in 100 μl of media in 96-well flat bottom plates at 37°C at 5% CO2 or until approx. 90% confluency. The calcium assay kit was used as directed without washing cells, and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were incubated with 100 μl dye/probenecid for 60 min at room temperature. The compound plate was prepared using dilutions of various compounds in Hank's Buffer (pH 7.4). Samples were run in duplicate using a FLIPR TETRA (Molecular Devices, Sunnyvale, CA) at room temperature. Cell fluorescence (Excitation = 485 and Emission = 525 nm) was monitored following exposure to the compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.4. ERK1/2 activation assay
The method used was essentially as previously described [43]. T24 or HEK293 cells (30,000 cells/100 μl) were seeded in a 96-well plate in complete growth medium. After cell attachment, medium was removed and cells were serum-starved overnight in 90 μl medium without fetal bovine serum. Agonists were stored cold as 5 mM stock solutions in DMSO and diluted in HANK's buffer. DMSO control (final concentration 0.1% corresponding to the highest concentration of agonist that was used) and agonist (10 μl) were added in a 10-fold concentrated solution in Hank's medium, and cells were stimulated for 5 min. Medium was removed and cells were lysed with 1× Lysis buffer (20 μl) (PerkinElmer AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kit). Lysate (4 μl/well) was transferred to a 384 well ProxiPlate Plus (PerkinElmer). Acceptor Beads were diluted 1:50 in a 1:5 mixture of Activation buffer in Reaction Mix and added to the 384-well plate (5 μl/well). The plate was sealed and incubated for 2 h at room temperature. Donor beads (2 μl) diluted 1:20 in Dilution buffer were added, and the plate was incubated for another 2 h at room temperature. The plate was measured using an EnVision multilabel reader using standard AlphaScreen settings.
2.5. β-Arrestin2 translocation assay (mouse A2BAR)
A β-arrestin2 translocation assay was performed using a PathHunter™ β-arrestin assay kit from DiscoveRx (Fremont, CA) as previously described [6]. Briefly, PathHunter CHO cells expressing the mouse A2BAR were grown in 384-well plates for 24 h in Hank's F-12 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 μmol/ml glutamine. Cells were treated with agonists for 90 min before adding detection reagents (mixture of 1 part Galacton Star substrate with 5 parts Emerald II™ Solution, and 19 parts of PathHunter Cell Assay Buffer), and incubated at room temperature for 60 min before luminescence was measured.
2.6. Western blot analysis
Cells used in the assay were lysed at approximately 80% confluency using cell lysis buffer (Cell Signaling Technology, Danvers, MA), and the cell lysates were stored at -80 °C until the assay. Protein concentration was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL). Cell lysates (30 μg protein/well) were analyzed under reducing conditions by SDS-PAGE and proteins were separated on 12 % Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membrane by electroblotting. Membranes were blocked according to the manufacturer's instructions and probed with A2BAR antibody (Alomone Labs, Jerusalem, Israel) overnight at 4 °C. Subsequently, blots were probed with IR dye-conjugated anti-rabbit secondary antibody for 1 h and then analyzed using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). To check the specificity of the antibody, control antigen for the antibody was mixed with A2B antibody and incubated for 1 h with constant agitation. This Ag–Ab mixture was also probed in parallel to antibody only blots as a control.
2.7. Insulin release in MIN6 cells
MIN6 cells were seeded in a 96-well plate at a density of 40,000 cells/well and cultured in DMEM at 37 °C. After 48 h, the medium was replaced with Krebs ringer bicarbonate buffer (KRBB) containing 3.3 mM glucose and 0.3% albumin, for 1 h. Insulin secretion was then induced by supplementing KRBB containing 16.7 mM glucose in the presence or absence of A2BAR ligands in KRBB solution for 1 h at 37 °C. Insulin secretion was determined using an insulin ELISA kit with a mouse insulin standard (Crystal Chem, Inc., Downer's Grove, IL).
2.8. Detection of A2BAR gene expression by quantitative real-time PCR (qRT-PCR)
Total mRNA was isolated following the protocol of the RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription was completed using Superscript III First Strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA). The cDNA was then amplified by PCR with gene-specific FAM-labeled MGB Taqman probes (Applied Biosystems) in 96-well plates using a 7300 Real-Time PCR System (Applied Biosystems) and default thermocycler program. The expression levels of indicated genes were calculated by the 2−ΔΔCT method with GAPDH as the endogenous control.
2.9. Calculation of bias factors
To quantify this bias, a method based on the Black and Leff operational model [44] was used. We first determined τ and KA values based on that model. τ (τ = [Rt]/KE), which is the ligand's efficacy toward a specific pathway, and is defined by the ratio of the total receptor density ([Rt]) over the general equilibrium dissociation constant (KE). KA is defined as the ligand equilibrium dissociation constant of the agonist-receptor complex. Using these parameters, the transduction ratios (τ/KA) and the transduction coefficients [Log (τ/KA)] for agonists were deduced for each signaling pathway. Specifically, bias factors of NECA, MRS5911, MRS3997 and BAY for cAMP accumulation versus ERK1/2 phosphorylation, recruitment of β-arrestin and calcium mobilization were calculated essentially as previously described by Kenakin et al. [45] using the ‘operational model’ of Black and Leff [44]. τ and KA values were calculated from dose-response curves fitted using the operational model-partial agonist function. To obtain the Δlog(τ/KA), which represents the relative ability of agonists to activate a given signaling pathway, the log(τ/KA) values of agonists in each pathway were calculated and subtracted from the log(τ/KA) of NECA. To determine the ΔΔlog(τ/KA), representing the relative preference of a specific agonist for one pathway with respect to another pathway compared with NECA as standard, the ΔLog (τ/KA) values obtained for each signaling pathway (calcium, β-arrestin2) were subtracted from the ΔLog (τ/KA) values calculated for the reference pathway (Gαi-related cAMP pathway). The bias factor is expressed as the anti-Log value of the ΔΔLog (τ/KA). Thus, if the calculated bias is ≫1 for a given compound and associated pathway, it disfavors that particular pathway.
2.10. Statistical and data analyses
Functional parameters were calculated using Prism 6.0 software (GraphPAD, San Diego, CA). Data were expressed as mean ± standard error. Curves were fitted using least squares nonlinear regressions. Statistical significance of the differences was assessed using a Student t test (between two conditions) or a One-Way Analysis of Variance (ANOVA) followed by Bonferroni's multiple comparison tests (between multiple conditions). Differences yielding P values < 0.05 were considered as statistically significant.
3. Results
3.1 A2BAR-mediated cAMP accumulation, intracellular calcium mobilization and ERK1/2 phosphorylation in various types of cell lines
For the assay of cAMP accumulation, all agonists were first tested in T24 cells endogenously expressing the human A2BAR [46]. The 5′-substituted NECA and CPCA and 2-substituted MRS3997 were found to be the most efficacious (Table 1). The maximum effect values, relative to NECA set at 100%, of N6-substituted MRS5911 and two non-adenosine agonists LUF5833 and BAY are 77.7%, 52.8% and 62.9%, respectively. The N6-substituted R-PIA and 2-substituted MRS3534 (Table 1) and CV1808 (EC50 = 9100 ± 5800 nM, maximum effect = 55% of NECA's) are somewhat weak, so it is not clear if they are partial agonists. The four representative agonists from each class (MRS3997, MRS5911, BAY and NECA) were further tested in HEK293 cells endogenously expressing the A2BAR, the maximum effects of which show a pattern that is similar to that in T24 cells. However, all four agonists are full and potent agonists in HEK293 cells overexpressing the A2BAR (Fig. 2A; Table 1), which suggests that some of these and other agonists tested in the A2BAR-overexpressing cells may be partial or even antagonists in some natural systems depending on the expression level of the endogenous A2BAR. The A2AAR selective agonist CGS21680, which is weak at the A2BAR [37], was shown to be nearly a full A2BAR agonist, although still of low potency. The A1AR agonist CPA and A3AR agonist Cl-IB-MECA, although they stimulated cAMP accumulation at high concentrations via the A2B AR, were inactive in inhibition of forskolin-stimulated cAMP accumulation, which excluded the involvement of A1 and A3 ARs.
Table 1.
Potency (EC50, nM) and maximum effect (% of effect of NECA) of various agonists at four signaling pathways.a
| Ligand | cAMP | Calcium | ERK1/2 | β-arrestin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T24 | HEK | HEK-A2B | T24 | HEK | HEK-A2B | T24 | HEK | HEK-A2b | ||
| MRS3534 | ||||||||||
| EC50 | 2160 ±350 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Max effect | 82.0 ±5.9 | ND | ND | -0.5 ±1.7 | ND | -7.7 ±3.2 | ND | ND | ND | 10.7 ±3.2 |
| MRS3997 | ||||||||||
| EC50 | 745 ±225 | 1020 ±250 | 62.9 ±7.6 | ND | ND | ND | ND | 166 ±32 | 203 ±48 | ND |
| Max effect | 94.9 ±2.2 | 96.2 ±24.3 | 102 ±6.6 | -2.7 ±3.2 | 1.6 ±2.2 | 7.8 ±1.3 | -8.2 ±7.6 | 42.3 ±9.4 | 29.7 ±2.6 | 28.1 ±3.5 |
| MRS5911 | ||||||||||
| EC50 | 899 ±226 | 1130 ±340 | 25.3 ±7.8 | 6170 ±1990 | ND | 7150 ±2360 | ND | 1530 ±340 | 2680 ±460 | 48,100 ±13,900 |
| Max effect | 77.7 ±3.3 | 68.7 ±11.1 | 93.2 ±4.5 | 36.9 ±8.6 | 7.1 ±3.2 | 22.8 ±6.6 | 13.1 ±4.5 | 46.1 ±9.2 | 36.3 ±5.2 | 40.9 ±2.9 |
| R-PIA | ||||||||||
| EC50 | 9750 ±2840 | ND | ND | 13,100 ±4800 | ND | 14,200 ±3600 | ND | ND | ND | ND |
| Max effect | 68.6 ±7.1 | ND | ND | 9.6 ±4.1 | 0.6 ±1.4 | 18.2 ±4.9 | ND | ND | ND | 13.2 ±1.1 |
| LUF5833 | ||||||||||
| EC50 | 9.82 ±2.43 | ND | ND | NA | ND | NA | ND | ND | ND | ND |
| Max effect | 52.8 ±6.6 | ND | ND | -5.6 ±3.8 | ND | 2.8 ±2.1 | ND | ND | ND | 9.7 ±0.6 |
| BAY | ||||||||||
| EC50 | 43.0±17.7 | 296 ±146 | 6.14 ±1.61 | ND | ND | 1100 ±390 | ND | 68.8 ±4.7 | 28.9 ±4.1 | 5620 ±1240 |
| Max effect | 62.9±9.6 | 73.0 ±12.1 | 102± 1 | 2.3± 1.5 | -2.4 ±1.7 | 9.7 ±2.9 | -9.4 ±3.8 | 38.3 ±5.9 | 38.4 ±3.3 | 26.6 ±3.0 |
| CPCA | ||||||||||
| EC50 | 177 ±67 | 267 ±32 | 32.8 ±4.2 | 1930 ±680 | 2620 ±460 | 1490 ±360 | 369 ±32 | 1030 ±230 | 1860 ±240 | 5060 ±1410 |
| Max effect | 98.5 ±1.9 | 102 ±9 | 98.9 ±6.6 | 101 ±7 | 98.7 ±6.6 | 102 ±4 | 101 ±8 | 99.3 ±2.4 | 100 ±6 | 109 ±9 |
| NECA | ||||||||||
| EC50 | 242 ±72 | 331 ±146 | 21.0 ±2.5 | 1480 ±210 | 2460 ±1240 | 1850 ±450 | 478 ±66 | 787 ±168 | 2360 ±370 | 4200 ±730 |
| Max effect | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CGS21680 | ||||||||||
| EC50 | >10,000 | >10,000 | 2920 ±570 | ND | ND | ND | ND | ND | ND | ND |
| Max effect | -7.6 ±6.9 | 2.7 ±2.4 | 107 ±9 | -3.4±2.7 | 0.8 ±1.4 | 4.3 ±2.8 | 2.2 ±0.8 | ND | ND | ND |
| Adenosine | ||||||||||
| EC50 | 4620 ±680 | ND | ND | 11,400 ±5000 | ND | 28,400 ±5700 | ND | ND | ND | ND |
| Max effect | 97.1 ±3.8 | ND | ND | 101 ±9 | ND | 98.8 ±5.6 | ND | ND | ND | ND |
Results were expressed as mean±SEM from at least three independent experiments performed in duplicate or triplicate. Data related to cAMP, calcium and ERK1/2 were from assays in T24 cells and HEK293 cells expressing the human A2BAR. Data related to the β-arrestin2 translocation were from PathHunter CHO cells expressing the mouse A2BAR, as the corresponding human version did not produce a robust response.
ND, not determined or there was not a sufficient effect to determine the parameters.
Fig. 2.
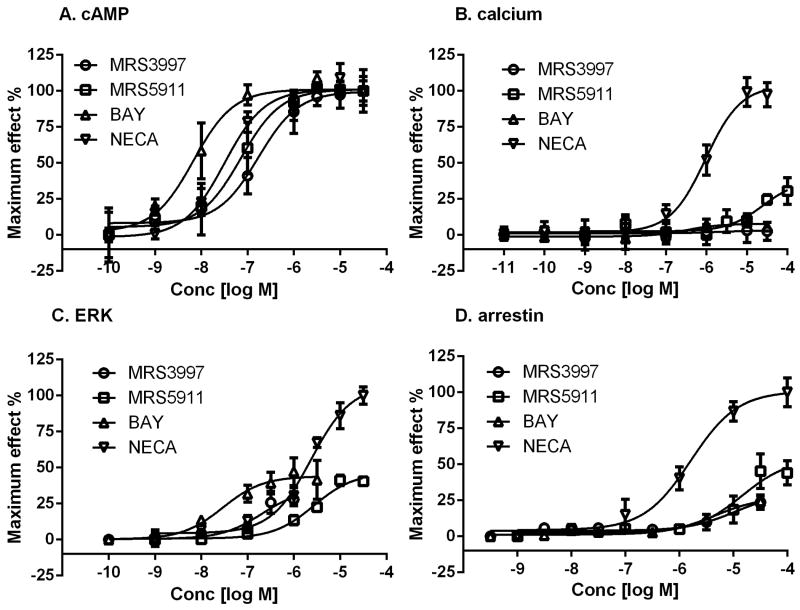
Agonist concentration-response curves in various signaling pathways. A. cAMP accumulation. B. Intracellular calcium mobilization. C. ERK1/2 phosphorylation. D. β-arrestin 2 translocation. Results shown in Figs. 2A, 2B and 2C are from assays in HEK293 cells overexpressing the human A2BAR. Results shown in Fig. 2D are from PathHunter CHO cells expressing the mouse A2BAR. Results are expressed as mean ± SEM and are from at least three independent experiments performed in triplicate. The values of EC50 and maximum effects are listed in Table 1.
In calcium mobilization assays in T24 cells, 5′-substituted NECA and CPCA are the most efficacious, followed by N6-substituted MRS5911 and R-PIA displaying maximum effects of 36.9% and 9.6%, respectively. However, the 2-substituted MRS3997, MRS3534, and CV1808, and the non-adenosine agonists LUF5833 and BAY are very weak and barely induce calcium mobilization. In HEK293 cells, the fluorescence signal induced by NECA and CPCA, although detectable, is weaker than that produced by NECA at T24 cells. In HEK293 cells overexpressing the A2BAR, adenosine, CPCA and NECA are all full agonists in calcium mobilization with MRS5911 being partially efficacious (Table 1). Interestingly, overexpression of the A2BAR did not substantially change the inability of the 2-substituted adenosine analogs and the non-adenosine derivatives to induce calcium mobilization (Fig. 2B), although it significantly enhanced both the potency and efficacy of these agonists in cAMP accumulation. This suggests potential biased agonism of these two classes of A2BAR agonists (Table 1). It is noted that the potent non-adenosine agonist BAY and the C2-substituted adenosine derivative MRS3997 show little if any residual agonist (Ca2+) activity even at high concentrations and in the A2BAR-overexpressing cells.
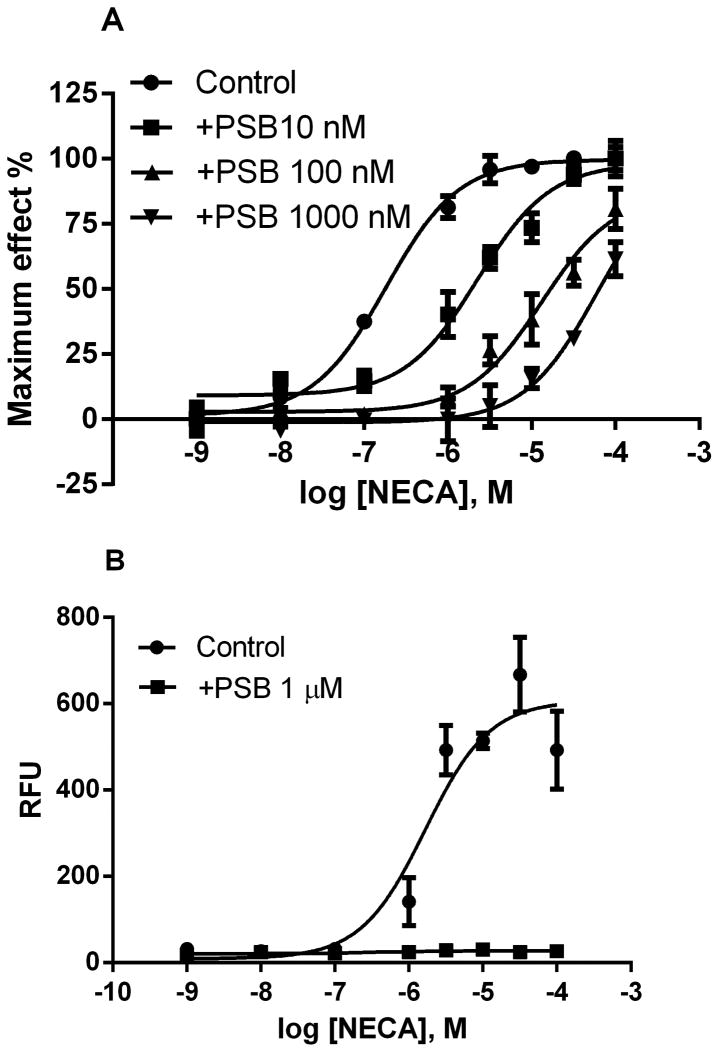
Both cAMP accumulation and calcium mobilization induced by NECA in T24 cells were antagonized by the selective A2BAR antagonist PSB603 (Fig. 3), suggesting that the effects of non-selective A2BAR agonist are solely mediated by the A2BAR. The KB value of PSB603 to antagonize NECA-induced cAMP accumulation was calculated to be 0.31 nM, which is roughly consistent with its affinity for the A2BAR.
Fig. 3.
Effect of the A2BAR antagonist PSB603 on NECA-induced cAMP accumulation (A) and calcium mobilization (B) in T24 bladder cancer cells. Cells were pretreated with PSB603 20 min before the addition of NECA. Results were expressed as mean ± SEM from 3-4 separate experiments performed in duplicate. PSB, PSB603.
The assay of ERK1/2 phosphorylation (Table 1) was initially performed in T24 cell endogenously expressing the human A2BAR. Only NECA and CPCA induced a modest stimulation (within 2-fold of basal value). MRS5911 only produced a ∼13% increase above basal value at 10 μM, while MRS3997 and BAY did not produce any stimulation. In HEK293 cells, however, NECA was able to produce a relatively robust ERK1/2 response (over 3-fold above basal), and all other agonists also produced responses although variable and to a lesser extent. The EC50 values of various agonists are listed in Table 1. In HEK293 cells overexpressing the human A2BAR, NECA was able to produce a much more robust response (10-fold). Interestingly, MRS3997, MRS5911 and BAY behave as partial agonists even in the A2BAR-overexpressing cells (Table 1, Fig. 2C).
Additionally, in all three assays mentioned above, cAMP accumulation, calcium and ERK1/2 activation, the A2A selective agonist CGS21680 was much weaker than NECA (Table 1), further demonstrating that the effect of NECA, CPCA and other agonists is solely via the A2BAR.
3.2. Effect of A2BAR specific siRNA knockdown on the efficacy and potency of agonists
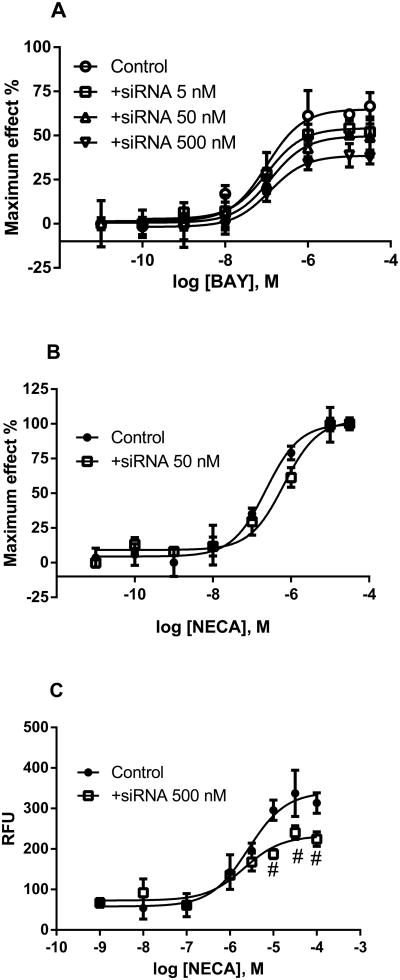
In addition to overexpression of the A2BAR, we also examined the effect of silencing the A2BAR using specific siRNA on the maximum agonist effect in T24 cells. In cAMP accumulation, the knockdown has different effects on NECA and BAY, being more effective in reducing the maximum effect of BAY while only affecting the potency of NECA (Fig. 4), further indicating the partial agonist property of BAY. The maximum agonist effect of BAY in the absence of siRNA was 68 ± 7% (NECA as 100%), which was significantly different from that in the presence of 5, 50 and 500 nM siRNA (54 ± 4%, 48 ± 6% and 36 ± 3%, respectively, P<0.05 compared with the control group). The corresponding EC50 values of BAY in the absence and presence siRNA are 98 ± 22, 102 ± 17, 127 ± 31 and 93 ± 19 nM, respectively. The maximum agonist effect of NECA was not altered by 50 nM A2BAR siRNA (100 ± 5% vs. 99 ± 6%, P>0.05). However, the potency of NECA was decreased ∼3-fold by siRNA silencing (232 ± 37 vs. 724 ± 122 nM). In the calcium mobilization assay, A2BAR siRNA also decreased the maximum effect of NECA (P<0.05 compared with control), although it did not affect the EC50 values (2310 ± 380 vs. 2120 ± 470 nM). This result suggests that even NECA may only produce a partial response in a system with low receptor density, depending on the pathway measured. Indeed, in cAMP accumulation with WM266 cells expressing a low level of the A2BAR, NECA produced a response that was less than 50% of that of forskolin (data not shown). By contrast, in COS-7 cells expressing a relatively high level of the A2BAR, NECA and forskolin are approximately equiefficacious (data not shown).
Fig. 4.
Distinct effects of receptor depletion by siRNA silencing on the A2BAR activation by different agonist in T24 cells. A. Effect on BAY-induced cAMP accumulation in T24 cells. B. Effect on the NECA-induced cAMP accmulation. C. Effect on calcium mobilization. The siRNA was transfected using lipofectamine 2000 as instructed by the manual. Results were expressed as mean ± SEM from three independent experiments performed in duplicate or triplicate. #Significant different from those values in the absence of siRNA (P<0.05).
3.3. Agonism by various agonists in different cell lines endogenously expressing the A2BAR
In addition to exploring the effect of overexpression and siRNA knockdown, we also tested the potency and maximum effect of representative agonists in various cells (PC-3, T24, COS-7, 1321N1, WM-266 and HEK293) predominantly expressing the A2BAR endogenously, but with variable expression levels. Table 2 shows that the potency and maximum effect of four representative agonists assayed in several cell lines with an endogenous A2BAR at different expression levels (the data from T24 and HEK293 cells are shown in Table 1). It was found that NECA and MRS3997 tend to be full agonists in all cells, while MRS5911 has a somewhat lower maximum effect (Table 2). The maximum effect of BAY is more sensitive to the A2BAR expression level, which is consistent with the siRNA silencing results from T24 cells (Fig. 4).
Table 2.
Potency (EC50, nM) and maximum effect (%) of agonist-induced cAMP accumulation in various cells endogenously expressing the A2BAR.a
| Ligand | 1321N1 | COS-7 | PC-3 | WM266 |
|---|---|---|---|---|
| MRS3997 | ||||
| EC50 | 1260±180 | 226±32 | 480±32 | 1870±320 |
| Max effect | 98.4±3.1 | 100±6 | 98.2±7.7 | 96.2±1.8 |
| MRS5911 | ||||
| EC50 | 1010±180 | 418±22 | 321±66 | 1270±220 |
| Max effect | 89.8±9.2 | 99.3±2.1 | 89.4±2.6 | 48.3±4.9 |
| BAY | ||||
| EC50 | 113±17 | 7.9±1.2 | 45.3±5.9 | ND |
| Max effect | 52.6±3.1 | 98.3±3.7 | 72.6±5.1 | 9.8±1.7 |
| NECA | ||||
| EC50 | 258±25 | 28.5±3.1 | 147±33 | 1280±360 |
| Max effect | 100 | 100 | 100 | 100 |
Data for other 2 cell lines endogenously expressing the human A2BAR, T24 and HEK293, are included in Table 1. Results are expressed as mean±SEM from at least three independent experiments performed in triplicate.
ND, EC50 could not be determined due to insufficient effect.
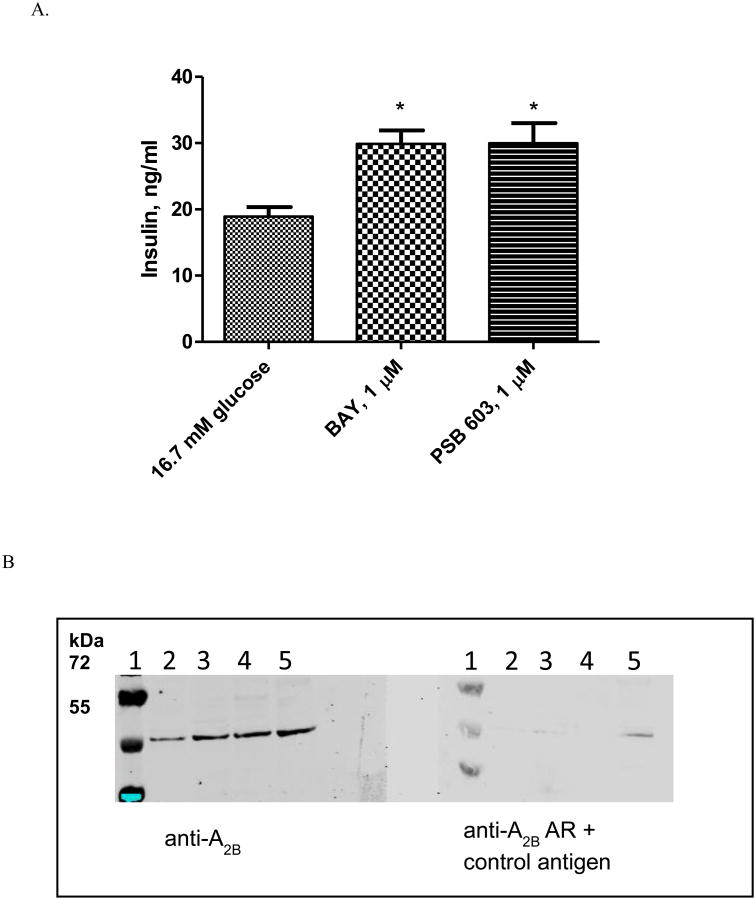
The results above suggest that the maximum effects of some agonists are more sensitive than others to receptor expression levels, suggesting that some agonists such as BAY could be full or partial agonists or even antagonists depending on A2BAR expression levels in cells or tissues. Indeed, in MIN-6 cells that express a low level A2BAR both BAY and the antagonist PSB603 induced insulin secretion (Fig. 5), a demonstrated function of blockade of the A2BAR [47]. Western blot analysis showed that the expression of the A2BAR in MIN-6 cells is much lower than in other cells used, such as HEK293 and COS-7 cells (Fig. 5B).
Fig. 5.
A. Insulin release in MIN-6 cells. Glucose stimulated insulin secretion (GSIS) in MIN6 cells with or without various A2BAR ligands at 16.7 mM glucose concentration. Values are mean ± SEM, for n=3. *P <0.05, when compared to 16.7 mM glucose samples. B. Western blot analyses of the expression of A2BAR in various cell lines. The left side panel was probed only with A2B antibody and right side panel was probed with a mixture of A2B antibody+ its specific antigen and used as a reference. Lane 1, Molecular weight marker; Lane 2, MIN6 cell lysate; Lane 3, 1321N1 astrocytoma cell lysate; Lane 4, COS7 cell lysate; Lane 5, HEK293 cell lysate.
3.4. A2BAR-mediated β-arrestin translocation in PathHunter CHO cells expressing themouse A2BAR
In a β-arrestin2 translocation assay, 5′-substituted NECA and CPCA are full agonists (Table 1; Fig. 2D), and N6-substituted MRS5911 is a partial agonist (40.9% compared with NECA as 100%). The maximum effects of 2-subsitituted MRS3997, and the two non-adenosine agonists BAY and LUF5833 were 28.1%, 26.6% and 9.7%, respectively. The order of maximum effect is similar to that from the calcium assay, but somewhat different from the cAMP assay in T24 cells, further suggesting potentially variable degrees of biased agonism for different agonists. The EC50 and maximum effect values are listed in Table 1.
3.5. A2BAR gene expression levels of various cell lines
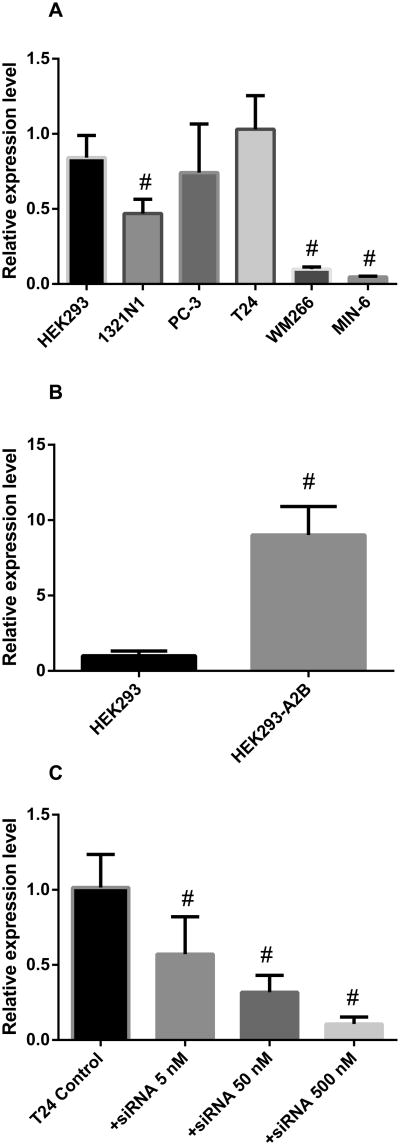
Endogenous A2BAR is highly expressed in T24, HEK293 and PC-3 cells (Fig. 6A). The A2BAR expression level in 1321N1 astrocytoma cells is about 50% of that in T24 cells. In WM266 and MIN-6 cells, the A2BAR expression levels are relatively low (P<0.05, compared with T24 cells). The relative gene expression levels in comparison to T24 (1.00 ± 0.13) are 0.84 ± 0.08, 0.74 ± 0.16, 0.47 ± 0.06, 0.10 ± 0.01 and 0.050 ± 0.003 for HEK293, PC-3, 1321N1, WM266 and MIN-6 cells, respectively. The results from the gene expression assay are roughly consistent with that partially obtained from the Western blot analysis of expressed receptor protein. The expression levels of three other ARs are significantly lower than that of the A2BAR in all cells used (data not shown).
Fig. 6.
Quantification of A2BAR gene expression using qRT-PCR. A. A2BAR mRNA expression level in various cells. B. Comparison of the A2BAR gene expression levels of control HEK293 and A2B overexpressing HEK293 cells. C. A2BAR gene expression level in T24 cells in the absence and presence of various concentrations of A2BAR specific siRNA. All data were normalized based on endogenous GAPDH expression level and were expressed as relative expression level. Data were from 3-4 separate experiments providing similar results performed in triplicate. #Significantly different from T24 or HEK293 control groups (P<0.05 compared with control).
In the A2BAR-overexpressing HEK293 cells, the mRNA expression is about 8-fold higher than the endogenous A2BAR mRNA expression in HEK293 cells (9.02 ± 1.09 vs. 1.09 ± 0.19) (Fig. 6B). A2B siRNA significantly and progressively diminished the A2BAR expression in T24 cells (Fig. 6C). The relative gene expression levels in the absence and presence of siRNA (5, 50, 500 nM) were 1.0 ± 0.13, 0.57 ± 0.14, 0.32± 0.07 and 0.11 ± 0.03, respectively.
3.6. Analysis of the partial agonism and/or degree of bias at different signaling pathways based on the operational model
As described above, we applied two methods, siRNA silencing and receptor overexpression, to accommodate the operational model of receptor activation and analyze the potential partial agonism. The analysis from both methods in two types of cells, T24 and HEK293, gave similar τ values for BAY (1.19 and 1.54), indicating the partial agonist activity in comparison to NECA (τ > 20).
It is interesting to note that the 2-substituted adenosine derivative MRS3997 has a high maximum effect compared with the N6-substituted MRS5911 and the non-adenosine BAY in cAMP accumulation (in both T24 and HEK293 cells), but MRS5911 has a higher maximum effect than MRS3997 and BAY in the calcium mobilization assay. These three agonists are almost equally efficacious in ERK1/2 phosphorylation. Clearly, these results cannot be readily explained by partial agonism. Hence, we quantified the potential biased agonism at the A2BAR-overexpressing HEK293 cells to distinguish it from the partial agonism in the low receptor-expression systems. The calculated bias factors of agonists in various signaling pathways, cAMP accumulation, calcium mobilization, ERK1/2 phosphorylation and β-arrestin translocation, are listed in Table 3. Results suggest that MRS3997 is a relatively balanced agonist for cAMP accumulation and ERK1/2 phosphorylation, but strongly biased against calcium mobilization and β-arrestin translocation. MRS5911 is weakly biased against ERK1/2 (with a bias factor of 1.86), but strongly biased against calcium mobilization and β-arrestin translocation. Interestingly, analysis suggests that BAY is an ERK1/2-biased agonist, weakly against cAMP accumulation but strongly against calcium mobilization and β-arrestin translocation (Table 3).
Table 3.
Calculation of bias factors of four representative agonists (5′, C2, and N6 modified and nonnucleoside) at different signaling pathways.a
| Pathway/agonist | τ | KA (M) | log (τ/KA) | Δlog (τ/Ka) | ΔΔlog (τ/KA) | Bias |
|---|---|---|---|---|---|---|
| cAMP | ||||||
| NECA | 238 | 9.07E-6 | 7.42 ± 0.24 | 0.00 ± 0.21 | ||
| MRS3997 | 45.1 | 6.70E-6 | 6.83 ± 0.45 | 0.59 ± 0.08 | ||
| MRS5911 | 116 | 7.76E-6 | 7.17 ± 0.68 | -0.25 ± 0.02 | ||
| BAY60-6583 | 100 | 7.05E-7 | 8.15 ± 0.38 | 0.73 ± 0.04 | ||
| ERK1/2 | ||||||
| NECA | 25.0 | 5.87E-5 | 5.63 ± 0.31 | 0.00 ± 0.02 | 0.00 | 1.00 |
| MRS3997 | 0.44 | 3.62E-7 | 6.08 ± 0.89 | 0.45 ± 0.03 | 0.14 | 1.38 |
| MRS5911 | 0.68 | 5.26E-6 | 5.11 ± 0.52 | -0.52 ± 0.04* | 0.27 | 1.86 |
| BAY60-6583 | 0.62 | 5.95E-8 | 7.02 ± 0.44 | 1.39 ± 0.17* | -0.66 | 0.22 |
| Calcium | ||||||
| NECA | 39.0 | 3.56E-5 | 5.04 ± 0.21 | 0.00 ± 0.09 | 0.00 | 1.0 |
| MRS3997b | 0.02 | 7.10E-6 | 3.35 ± 0.44 | -3.47 ± 0.05 | 4.06 | 11500 |
| MRS5911 | 0.59 | 8.47E-5 | 3.84 ± 0.19 | -1.20 ± 0.13* | 1.93 | 85 |
| BAY60-6583b | 0.08 | 1.67 E-6 | 4.66 ± 0.35 | -3.49 ± 0.38 | 4.22 | 16600 |
| β-Arrestin | ||||||
| NECA | 38.9 | 7.32E-5 | 5.73 ± 0.51 | 0.00 ± 0.11 | 0.00 | 1.00 |
| MRS3997 | 0.27 | 1.81E-5 | 4.17 ± 0.23 | -1.56 ± 0.18* | 2.15 | 141 |
| MRS5911 | 1.04 | 8.34E-5 | 4.10 ± 0.22 | -1.63 ± 0.20* | 1.38 | 24 |
| BAY60-6583 | 0.25 | 1.23E-5 | 4.30 ± 0.17 | -1.43 ± 0.09* | 2.16 | 145 |
Results are expressed as mean ± SEM from at least three independent experiments.
The respective maximum agonist efficacies of MRS3997 and BAY are only about 8% and 10% in comparison to NECA (100%).
Significantly different from control (P <0.05). The calculation was as described in references 43 and 44.
4. Discussion
In the present study, we demonstrated both partial agonism and biased agonism at the A2BAR by various agonists of distinct chemical classes, which should be useful for understanding the important function of the A2BAR and for explaining some of the contradictory and controversial results previously reported.
The A2BAR has long been classified as a Gs-coupled receptor due to its ability to stimulate cAMP accumulation. The potential coupling to many other effectors, such as MAP kinases and calcium via other G proteins, has also been reported [48-50]. The A2BAR has also been previously reported to mediate arrestin signaling [51]. However, agonists from different chemical classes have not been applied in those previous studies and thus, some A2BAR functions reported previously may be due to the effect of a specific agonist in a specific type of cell or tissue and may be applicable only for a specific signaling pathway studied. Indeed, both agonists and antagonists have been demonstrated to have anti-inflammatory effects [31-33].
It is noted that the A2BAR expression level clearly had an impact on maximum agonist effect or on the potency (EC50 values). Considering this, we compared the agonist effects and analyzed the potential biased agonism using HEK293 cells overexpressing the A2BAR. One of the important findings from the present study is that BAY, unlike NECA, MRS3997 and MRS5911, is an ERK1/2-biased agonist. This is in line with the earlier results from Yang et al [52] who showed that in HEK293 cells expressing the A2BAR, the selective A2BAR agonist BAY increased ERK phosphorylation and cAMP levels but did so via a distinct mechanism. Furthermore, it was also shown that in cardiomyocytes, BAY did not affect cAMP levels but blocked the increased superoxide generation mediated via ERK1/2 [52]. On the other hand, the lack of stimulation of ERK1/2 phosphorylation in T24 cells (in contrast to HEK 293 cells) by BAY and MRS3997 may also indicate a different mechanism to induce ERK1/2 activation in this cell line than previously suggested [49,50], which should be a topic of interest to explore in the future. Of course, it should also be noted that all the assays were performed under somewhat different experimental conditions, which may have an impact on the results.
In the calcium mobilization assay, NECA and CPCA produced a robust response in T24 cells, but not in HEK293 cells, via an endogenous A2BAR, which is consistent with an early report by Phelps et al. [44], although both express similar levels of the A2BAR. This suggests that potentially different mechanisms are involved in A2BAR-induced calcium mobilization in different cells. It seems that the calcium response is not only dependent on A2BAR expression level, but also on the effector coupling. Even in the presence of siRNA, NECA can still induce a substantial, albeit lower, calcium response in T24 cells (Fig. 4C). Also, the overexpression of the A2BAR in HEK293 cells enhanced the robustness of the calcium response induced by NECA and MRS5911, but failed to increase the agonist potency of NECA or the ability of MRS3997 and BAY to produce a robust response. This can be explained with the biased agonism but not the partial agonist activity of these compounds. In contrast to T24 cells, only a minimal response of calcium mobilization is observed in COS-7 and 1321N1 cells endogenously expressing the A2BAR, or in CHO cells expressing the recombinant human A2BAR (data not shown). This is possibly due to a different effector coupling and should be a topic of future investigation. Nevertheless, we demonstrated that both in T24 cells and in HEK293 cells overexpressing the A2BAR, 5′-substituted NECA and CPCA are full agonists, while N6-substituted MRS5911 and R-PIA are partial agonists. The 2-substituted MRS3997 and MRS3534 and the non-adenosine agonists have little if any residual efficacy in calcium mobilization.
The class of non-adenosine agonists has been demonstrated to be partial agonists with a variable maximum agonist effect at the A2BAR [41]. We noted in an earlier study that BAY may possess partial agonist activity in PC-3 cells expressing the A2BAR [53]. Consistent with these early findings, a recent paper by Hinz et al. [42] confirmed the partial agonist activity of this agonist. Hinz et al. [42] further demonstrated that BAY could antagonize NECA-induced cAMP accumulation in Jurkat-T cells. However, the potential biased agonism of BAY and other agonists has not been previously explored.
Regarding N6-substituted adenosine derivatives, R-PIA has long been known as a low potency A2BAR agonist. The present study demonstrated that the relatively more potent agonist MRS5911 is a full agonist in cAMP accumulation in HEK293 cells overexpressing the A2BAR but is a partial agonist in calcium mobilization and β-arrestin translocation. The results from the present study are consistent with an earlier finding that the N6- and 5′-disubstituted N6-benzyl NECA showed partial agonist activity in calcium mobilization in HEK293 cells expressing the A2BAR [54]. In addition, our preliminary data from label-free measurements in HEK293 cells, which is a kind of end point of detection of receptor activation, also suggest that NECA is more efficacious than BAY, MRS3997 and MRS5911 (unpublished results).
Although the choice of an appropriate method for quantification of biased agonism is still controversial [55,56], it seems that the results from analysis of biased agonism and partial agonism using the operational model and the method described by Kenakin et al. [44,45] are quite reasonable. Concerning biased agonism, in some reported cases the clear dissection of partial and biased agonism is still quite ambiguous; the results from the present study evidently cannot be explained solely by either partial agonism or biased agonism. Additionally, our results suggest that each class of agonists may have a different activation profile in different cells or tissues, which may be related to the receptor expression level, down-stream signaling protein expression level, or different downstream signaling molecules.
In conclusion, both biased agonism and partial agonism at the A2BAR are shown in the present study of four signaling pathways, which should be useful in the further demonstration and explanation of the important function of the A2BAR. Effects on both potency and maximal efficacy were studied, and characteristic patterns were associated with each structural class of agonist. N6-Substituted agonists were biased in favor of the cAMP pathway and biased against calcium, β-arrestin and to a lesser extent ERK1/2. Non-nucleoside agonists appear to be ERK1/2-biased, and surprisingly, one such 3,5-dicyanopyridine displayed antagonism in pancreatic β-islet cells that express a A2BAR associated with inhibiting insulin release. C2 substitution tends to preserve cAMP and ERK1/2 effects and to disfavor Ca2+ and arrestin. Only 5′-substituted adenosine analogs are full agonists in all pathways examined.
This signaling divergence has implications for various pathophysiological conditions (including cancer, diabetes, inflammation and hypoxia), for which treatment by A2BAR agonists is being considered. The choice of agonist vs. antagonist of the A2BAR for treatment of the associated disease states is still controversial, and we have added another important variable for agonists, i.e. signaling bias. These characteristics of standard compounds can now be used to probe the involvement of specific A2BAR-related pathways in pathological states.
Acknowledgments
Supported the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA. The authors thank Ad IJzerman for providing BAY and LUF5833, Ray Olsson for providing MRS3534, Hayamitsu Adachi for MRS3997 and Dilip K. Tosh for CPCA.
Abbreviations
- BAY60-6583 (BAYLUF6210)
2-[[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]-2-pyridinyl]thio]acetamide
- cyclic AMP (cAMP)
3′,5′-cyclic adenosine monophosphate
- CGS21680
2-[p-(2-carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine
- CHO
Chinese hamster ovary
- CPA
N6-cyclopentyladenosine
- CPCA
adenosine-5′-N-cyclopropyluronamide
- CV1808
2-phenylaminoadenosine
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular-signal-regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G protein-coupled receptor
- HEK
human embryonic kidney
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- IB-MECA
N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- KRBB
Krebs ringer bicarbonate buffer
- LUF5833
2-aminophenyl-6-(1H-imidazol-2-ylmethylsulfanyl)pyridine-3,5-dicarbonitrile
- MRS3534
2-(2-(indol-3-yl)ethyloxy)adenosine
- MRS3997
2-(2-(6-bromo-indol-3-yl)ethyloxy)adenosine
- MRS5911
N6-(4-iodophenyl)adenosine
- NECA
adenosine-5′-N-ethyluronamide
- R-PIA
R-N6-(phenylisopropyl)adenosine
- PSB603
8-[4-[4-(4-chlorophenyl)piperazide-1-sulfonyl)phenyl]]-1-propylxanthine
Footnotes
These results have been presented in part in an abstract form in ASPET 4th GPCR Symposium of 2013 (Boston, MA) and in the 2014 Experimental Biology Meeting (San Diego, CA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhan-Guo Gao, Email: zg21o@nih.gov.
Kenneth A. Jacobson, Email: kajacobs@helix.nih.gov.
References
- 1.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45:4471–84. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao ZG, Blaustein JB, Gross AS, Melman N, Jacobson KA. N6-Substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. J Med Chem. 2003;46:3775–7. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong LS, Pal S, Choe SA, Choi WJ, Jacobson KA, Gao ZG, Klutz AM, Hou X, Kim HO, Lee HW, Lee SK, Tosh DK, Moon HR. Structure-activity relationships of truncated D- and l-4′-thioadenosine derivatives as species-independent A3 adenosine receptor antagonists. J Med Chem. 2008;51:6609–13. doi: 10.1021/jm8008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou X, Kim HO, Alexander V, Kim K, Choi S, Park SG, Lee JH, Yoo LS, Gao ZG, Jacobson KA, Jeong LS. Discovery of new human A2A adenosine receptor agonists: design, synthesis, and binding mode of truncated 2-hexynyl-4′-thioadenosine. ACS Med Chem Lett. 2010;1:516–520. doi: 10.1021/ml1001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosh DK, Paoletta S, Deflorian F, Phan K, Moss SM, Gao ZG, Jiang X, Jacobson KA. Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity. J Med Chem. 2012;55:8075–90. doi: 10.1021/jm300965a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res. 2008;57:303–11. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paoletta S, Tosh DK, Finley A, Gizewski ET, Moss SM, Gao ZG, Auchampach JA, Salvemini D, Jacobson KA. Rational design of sulfonated A3 adenosine receptor-selective nucleosides as pharmacological tools to study chronic neuropathic pain. J Med Chem. 2013;56:5949–63. doi: 10.1021/jm4007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbah HN, Gupta RC, Kohli S, Wang M, Rastogi S, Zhang K, Zimmermann K, Diedrichs N, Albrecht-Küpper BE. Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure. Circ Heart Fail. 2013;6:563–71. doi: 10.1161/CIRCHEARTFAILURE.112.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 2012;32:322–30. doi: 10.1523/JNEUROSCI.4070-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kataoka S, Baquero A, Yang D, Shultz N, Vandenbeuch A, Ravid K, Kinnamon SC, Finger TE. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS One. 2012;7(1):e30032. doi: 10.1371/journal.pone.0030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladwin MT. Sickle cell disease: is kind of inflammatory disease. Adenosine receptor crossroads in sickle cell disease. Nat Med. 2011;17:38–40. doi: 10.1038/nm0111-38. [DOI] [PubMed] [Google Scholar]
- 12.Belikoff BG, Vaickus LJ, Sitkovsky M, Remick DG. A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. J Immunol. 2012;189:3707–13. doi: 10.4049/jimmunol.1201207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalla RV, Zablocki J, Tabrizi MA, Baraldi PG. Recent developments in A2Badenosine receptor ligands. Handb Exp Pharmacol. 2009;(193):99–122. doi: 10.1007/978-3-540-89615-9_4. [DOI] [PubMed] [Google Scholar]
- 14.Haskó G, Csóka B, Németh ZH, Vizi ES, Pacher P. A2B adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–70. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ham J, Rees DA. The adenosine A2B receptor: its role in inflammation. Endocr Metab Immune Disord Drug Targets. 2008;8:244–54. doi: 10.2174/187153008786848303. [DOI] [PubMed] [Google Scholar]
- 16.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei W, Du C, Lv J, Zhao G, Li Z, Wu Z, Haskó G, Xie X. Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J Immunol. 2013;190:138–46. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, Smit MA, Geiger TR, Laoukili J, Iskit S, Rodenko B, Zwart W, Evers B, Horlings H, Ajouaou A, Zevenhoven J, van Vliet M, Ramaswamy S, Wessels LF, Peeper DS. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110:5139–44. doi: 10.1073/pnas.1222085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2B adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–9. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–52. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–95. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Methner C, Krieg T. A2B adenosine receptors in cardioprotection: timing is everything. J Mol Cell Cardiol. 2011;50:582–3. doi: 10.1016/j.yjmcc.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nürnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J. 2011;25:2823–30. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csóka B, Koscsó B, Töro G, Kókai E, Virág L, Németh ZH, Pacher P, Bai P, Haskó G. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2013;63:850–66. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG, LeBrasseur N, Ravid K. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012;7(7):e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, Kim JK, LaNoue KF, Linden J. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–79. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih YR, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC, Vecchio KS, Chien S, Lee OK, Varghese S. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci U S A. 2014;111:990–5. doi: 10.1073/pnas.1321717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Hoeven D, Wan TC, Gizewski ET, Kreckler LM, Maas JE, Van Orman J, Ravid K, Auchampach JA. A role for the low affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J Pharmacol Exp Ther. 2011;338:1004–12. doi: 10.1124/jpet.111.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–70. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–15. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem. 2008;51:2267–78. doi: 10.1021/jm7014815. [DOI] [PubMed] [Google Scholar]
- 34.Verzijl D, IJzerman AP. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011;7:171–92. doi: 10.1007/s11302-011-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao ZG, Jacobson KA. Allosteric modulation and functional selectivity of G protein-coupled receptors. Drug Discov Today Technol. 2013;10:e237–e243. doi: 10.1016/j.ddtec.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi H, Palaniappan KK, Ivanov AA, Bergman N, Gao ZG, Jacobson KA. Structure-activity relationships of 2,N6,5′-substituted adenosine derivatives with potent activity at the A2B adenosine receptor. J Med Chem. 2007;50:1810–27. doi: 10.1021/jm061278q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao ZG, Mamedova LK, Chen P, Jacobson KA. 2-Substituted adenosine derivatives: affinity and efficacy at four subtypes of human adenosine receptors. Biochem Pharmacol. 2004;(68):1985–93. doi: 10.1016/j.bcp.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brackett LE, Daly JW. Functional characterization of the A2b adenosine receptor in NIH 3T3 fibroblasts. Biochem Pharmacol. 1994;47:801–14. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 39.de Zwart M, de Groote M, van der Klein PAM, van Dun S, von Frijtag Drabbe Künzel JK, IJzerman AP. Phenyl-substituted N6-phenyladenosines and N6-phenyl-5′-N-ethylcarboxamidoadenosines with high activity at human adenosine A2B receptors. Drug Devel Res. 2000;49:85–93. [Google Scholar]
- 40.Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem Pharmacol. 2001;61:657–63. doi: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beukers MW, Chang LCW, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, IJzerman AP. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J Med Chem. 2004;47:3707–9. doi: 10.1021/jm049947s. [DOI] [PubMed] [Google Scholar]
- 42.Hinz S, Lacher SK, Seibt B, Müller CE. BAY60-6583 acts as a partial agonist at adenosine A2B receptors. J Pharmacol Exp Ther. 2014;349:427–36. doi: 10.1124/jpet.113.210849. [DOI] [PubMed] [Google Scholar]
- 43.Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, IJzerman AP, Jacobson KA. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol. 2011;82:658–68. doi: 10.1016/j.bcp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 45.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci. 2012;3:193–203. doi: 10.1021/cn200111m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelps PT, Anthes JC, Correll CC. Characterization of adenosine receptors in the human bladder carcinoma T24 cell line. Eur J Pharmacol. 2006;536:28–37. doi: 10.1016/j.ejphar.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 47.Rüsing D, Müller CE, Verspohl EJ. The impact of adenosine and A2B receptors on glucose homoeostasis. J Pharm Pharmacol. 2006;58:1639–45. doi: 10.1211/jpp.58.12.0011. [DOI] [PubMed] [Google Scholar]
- 48.Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A2B adenosine receptors: radioligand binding, western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–13. [PubMed] [Google Scholar]
- 49.Cohen MV, Yang XM, Liu Y, Solenkova NV, Downey JM. Cardioprotective PKG-independent NO signaling at reperfusion. Am J Physiol Heart Circ Physiol. 2010;299:H2028–36. doi: 10.1152/ajpheart.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulte G, Fredholm BB. The G(s)-coupled adenosine A2B receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp Cell Res. 2003;290:168–76. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 51.Mundell SJ, Matharu AL, Kelly E, Benovic JL. Arrestin isoforms dictate differential kinetics of A2B adenosine receptor trafficking. Biochemistry. 2000;39:12828–36. doi: 10.1021/bi0010928. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Xin W, Yang XM, Kuno A, Rich TC, Cohen MV, Downey JM. A2B adenosine receptors inhibit superoxide production from mitochondrial complex I in rabbit cardiomyocytes via a mechanism sensitive to Pertussis toxin. Br J Pharmacol. 2011;163:995–1006. doi: 10.1111/j.1476-5381.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei Q, Costanzi S, Balasubramanian R, Gao ZG, Jacobson KA. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013;9:271–80. doi: 10.1007/s11302-012-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel H, Porter RH, Palmer AM, Croucher MJ. Comparison of human recombinant adenosine A2B receptor function assessed by Fluo-3-AM fluorometry and microphysiometry. Br J Pharmacol. 2003;138:671–7. doi: 10.1038/sj.bjp.0705091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajagopal S. Quantifying biased agonism: understanding the links between affinity and efficacy. Nat Rev Drug Discov. 2013;12(6):483. doi: 10.1038/nrd3954-c1. [DOI] [PubMed] [Google Scholar]
- 56.Kenakin T, Christopoulos A. Measurements of ligand bias and functional affinity. Nat Rev Drug Discov. 2013;12:205–16. doi: 10.1038/nrd3954-c2. [DOI] [PubMed] [Google Scholar]