Abstract
During 2012–2013, a total of 4325 host-seeking adult ticks belonging to the genus Ixodes were collected from various localities of Hokkaido, the northernmost island of Japan. Tick lysates were subjected to real-time PCR assay to detect borrelial infection. The assay was designed for specific detection of the Relapsing fever spirochete Borrelia miyamotoi and for unspecific detection of Lyme disease-related spirochetes. Overall prevalence of B. miyamotoi was 2% (71/3532) in Ixodes persulcatus, 4.3% (5/117) in Ixodes pavlovskyi and 0.1% (1/676) in Ixodes ovatus. The prevalence in I. persulcatus and I. pavlovskyi ticks were significantly higher than in I. ovatus. Co-infections with Lyme disease-related spirochetes were found in all of the tick species. During this investigation, we obtained 6 isolates of B. miyamotoi from I. persulcatus and I. pavlovskyi by culture in BSK-M medium. Phylogenetic trees of B. miyamotoi inferred from each of 3 housekeeping genes (glpQ, 16S rDNA, and flaB) demonstrated that the Hokkaido isolates were clustered with Russian B. miyamotoi, but were distinguishable from North American and European B. miyamotoi. A multilocus sequence analysis using 8 genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) suggested that all Japanese B. miyamotoi isolates, including past isolates, were genetically clonal, although these were isolated from different tick and vertebrate sources. From these results, B. miyamotoi-infected ticks are widely distributed throughout Hokkaido. Female I. persulcatus are responsible for most human tick-bites, thereby I. persulcatus is likely the most important vector of indigenous relapsing fever from tick bites in Hokkaido.
Introduction
Borrelia miyamotoi, a member of the relapsing fever group (RF) borreliae, was first discovered from Ixodes persulcatus ticks and the rodent, Apodemus argenteus, in Hokkaido, the northernmost island of Japan [1]. Subsequently, B. miyamotoi has been found in Ixodes scapularis and Ixodes pacificus ticks in North America [2], [3], [4] and Ixodes ricinus in Europe [5], [6]. Cases of human infections with B. miyamotoi were initially reported in Russia [7]. Following this report, several human cases have been confirmed as B. miyamotoi infections in North America [8], [9] and Europe [10]. Recently, tick surveillance for B. miyamotoi was performed in Europe [11] and Russia [7]. The surveys showed that I. ricinus and I. persulcatus in the Eurasian continent consistently harbor B. miyamotoi with low prevalence. However, large-scale tick surveillance has not been conducted in Asian countries where I. persulcatus is distributed.
In Japan, cases of Lyme disease (LD) are clustered in the northernmost island, Hokkaido, because its principal vector, I. persulcatus, is abundant throughout the island [12], [13]. The tick is also a candidate vector for B. miyamotoi [1]. A previous study in Hokkaido detected B. miyamotoi from I. persulcatus ticks and from rodents [14]. However, widespread prevalence data of B. miyamotoi infections are still lacking for populations of ticks, including I. persulcatus and other genus Ixodes species. This basic information on prevalence of B. miyamotoi infections in ticks is urgently required for risk assessment of relapsing fever in Hokkaido.
Ixodes pavlovskyi, a species closely related to I. persulcatus, is also distributed throughout Hokkaido [15], [16], [17]. Compared to the abundant I. persulcatus, I. pavlovskyi is a minor tick species. Nevertheless, the close evolutionary relationship among I. persulcatus and I. pavlovskyi [16], [17] raises concerns about their ability to maintain B. miyamotoi in nature. In this study, large-scale tick surveillance for B. miyamotoi was conducted in Hokkaido to estimate the infection rate of host-seeking adult Ixodes ticks. The tick-derived isolates of B. miyamotoi established in this surveillance were subjected to molecular analyses to characterize their genetic profile. The resultant field and laboratory data will serve as a baseline in understanding the epidemiology of B. miyamotoi in Japan.
Materials and Methods
Tick collection, DNA extraction and borrelial cultivation
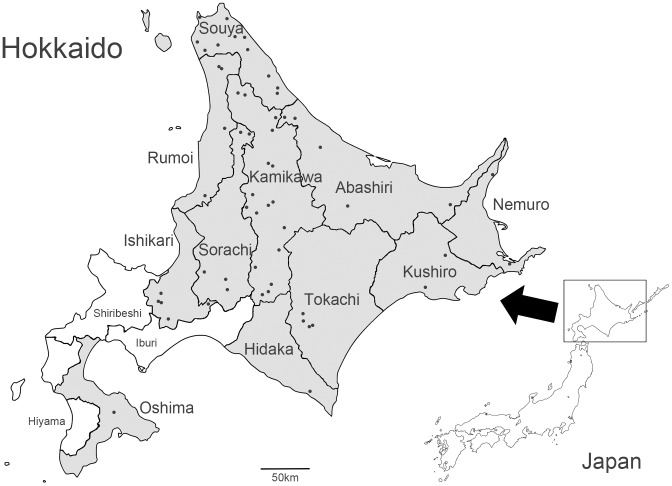
During the spring to summer seasons (April to July) of 2012 and 2013, host-seeking adult ticks of I. persulcatus, I. pavlovskyi and I. ovatus were collected by flagging over vegetation from a total of 57 forested areas in Hokkaido (Figure 1). In these areas, no specific permissions were required for collection of ticks, and this study did not involve endangered or protected species. The collection of I. ovatus was discontinued in 2013 because of its extremely low prevalence of B. miyamotoi infection. The number of ticks examined, and the prevalence of Borrelia spp. among them were calculated for each district.
Figure 1. Tick collection sites in this study.
Ticks were collected in the areas shown by black dots. The gray shading shows district where ticks were collected during this investigation (see Table 1).
All of the ticks collected were subjected to a quantitative real-time PCR (qPCR) assay to detect spirochete DNA. The DNA for PCR templates was prepared from whole or half bodies of each tick by using sodium hydroxide (NaOH), as described previously [18]. Briefly, tick tissues including bacterial cells were lysed in 50 µL of 25 mM NaOH for 10 minutes at 95°C. After adding 4 µL of Tris-HCL (1 M, pH7.5) for neutralization, the lysate was centrifuged at 4°C, and the resulting supernatant was used as template DNA for qPCR. A preliminary study suggested that the sensitivity of qPCR using the lysate was equal to that using DNA extracted by DNeasy Tissue Kit (Qiagen, CA, USA) (Data not shown).
Parts of ticks collected were used to cultivate B. miyamotoi in modified Barbour-Stoenner-Kelly medium (BSK-M: using minimal essential medium alpha [BioWest, Germany] as a substitute for CMRL-1066) under microaerophilic conditions [19], [20] (Table S1). The surface of ticks was sterilized by washing with 0.1% sodium hypochlorite, then with 80% ethyl alcohol. The washed tick was longitudinally bisected using a disposable knife (ELP No. 10, Akiyama Medical MFG. CO., LTD., Tokyo, Japan), and one half was inoculated into BSK-M medium. The remaining half was used to prepare the PCR template, as described above. Tick samples which were shown by qPCR to be positive for B. miyamotoi and negative for LD borreliae, were cultivated at 30°C for 4 weeks, and then the growth of spirochetes was examined by dark-field microscopy every two weeks.
Detection of borrelial DNAs from ticks
As described previously [21], borrelial DNA in the tick lysates was detected by multiplex qPCR targeting the 16 S rRNA gene (16 S rDNA). Based on Barbour et al., a minor groove binder probe with an FAM-label (FAM probe) (Life Technologies Corporation, MD, USA) was designed for LD borreliae, while an minor groove binder probe with a VIC-label (VIC probe) was designed for RF borreliae, including B. miyamotoi. The qPCR was performed using Premix Ex Taq (Probe qPCR), (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. The qPCR was run on an ABI PRISM 7000 system, ABI StepOne system (Life Technologies Corporation), and LightCycler 480 systemII (Roche Diagnostics, Basel, Switzerland). The PCR cycles were set at 40, and the reaction was performed in 12.5 µL (ABI PRISM 7000 system and StepOne system) or 10 µL (LightCycler 480 systemII) in single tubes or plate wells. The sensitivity and specificity of the qPCR was performed as described in the File S1.
PCR, multilocus sequence analysis (MLSA), and phylogeny reconstruction
Tick-derived isolates of B. miyamotoi were characterized by PCR-based DNA sequencing. After DNA extraction, using Wizard genomic DNA purification kit (Promega, WI, USA), Takara Ex Taq (Takara Bio Inc.) and KOD FX (TOYOBO Co., LTD., Osaka, Japan) DNA polymerases were used for all PCR amplifications, as recommended by the manufacturers. Target DNA fragments of the borrelial flagellin gene (flaB), glycerophosphoryl diester phosphodiesterase gene (glpQ), and 16 S rDNA were amplified as described previously [22]. MLSA was performed to further characterize the isolates of B. miyamotoi. For this purpose, the loci from 8 genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) were analyzed [23]. These primers were designed by genome assembling of 4 RF borreliae; Borrelia hermsii (CP000048), Borrelia duttonii (CP000976), Borrelia turicatae (CP000049), and Borrelia recurrentis (CP000993). The PCR thermal conditions to amplify the 8 genes were as follows: 95°C for 30 s, followed by 35 cycles of 95°C for 10 s, 50°C for 30 s, and 72°C for 60 s. All PCR products were purified by High Pure PCR Product Purification Kit (Roche Diagnostics), then directly sequenced using an ABI Prism 3130 or 3130xl Genetic Analyzer (Life Technologies Corporation). All of the PCR primers used in this study are listed in the Table S2.
Raw sequence data were assembled by Sequencher 5.1 (Gene Codes Corporation, MI, USA). Sequences of each gene were aligned by ClustalW (v.1.6), and Neighbor-joining trees were generated by 1000 bootstrap repetitions under the Kimura 2-parameter by MEGA 5.2 [22], [24]. All positions containing alignment gaps and missing nucleotides were eliminated only in pairwise sequence comparisons (the pairwise deletion option was used).
Statistical tests for prevalence data
Prevalence data of borrelial infections in tick groups from different categories (e.g. species, sex and locale) were analyzed by the Fisher's exact tests. A p-value less than 0.01 was considered to be statistically significant.
Results
Sensitivity and specificity of the qPCR
The sensitivity and specificity of the qPCR were examined, as shown in the supporting information. The limit of the DNA detection consistently observed was a minimum of 10 plasmid copies (data not shown). Regarding the specificity of qPCR, in the case of the FAM probe, 85% (11/13) of LD borreliae were detected, whereas Borrelia valaisiana Am501T was barely detectable, Borrelia lusitaniae PotiB2T was not detected, and none of the RF borreliae were detected. On the other hand, the VIC probe detected B. miyamotoi, B. hermsii, and Borrelia coriaceae Co53T. However, for species with a 1 bp mismatch with the probe, such as B. duttonii Ly and Borrelia sp. AGRF [25], amplification was delayed, resulting in a reduced final concentration that just barely reached our threshold. None of the reptile associated borreliae were detected by either probe. Therefore, the FAM probe widely detects B. burgdorferi sensu lato, which has been isolated from Ixodes ticks in Japan, including pathogenic borreliae of humans, such as B. garinii, Borrelia bavariensis and Borrelia afzelii, and non-pathogenic borreliae, Borrelia japonica, Borrelia tanuki and Borrelia turdi, whereas the VIC probe detects a narrow range of relapsing fever borreliae, of which the only one reported in Japan is B. miyamotoi.
Prevalence of borreliae in Ixodes ticks
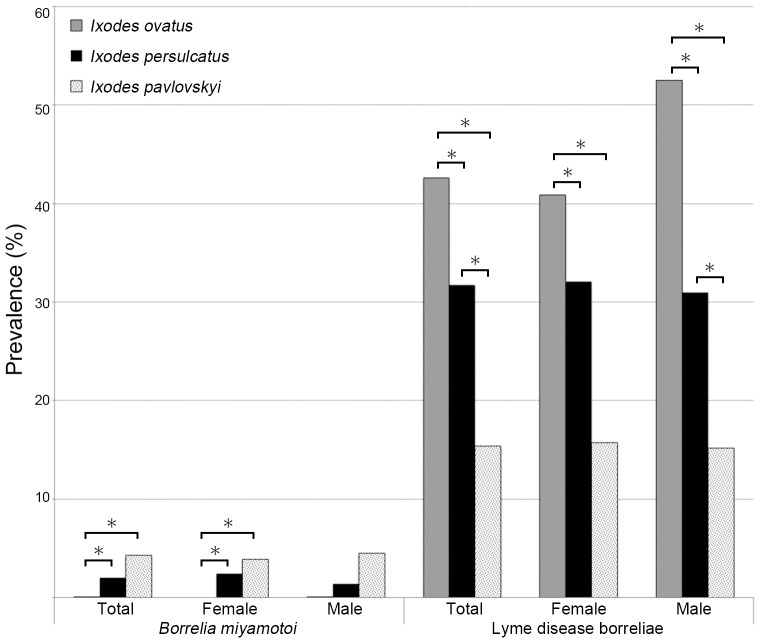
A total of 4325 adult ticks were collected from various localities covering 11 out of the total 14 districts of Hokkaido (Figure 1). As shown in Table 1, ticks harboring B. miyamotoi were found from 8 districts in northern, eastern and central Hokkaido. There was no significant statistical difference in the prevalence among the 8 localities. Overall prevalence of B. miyamotoi in ticks of Hokkaido was 2% in I. persulcatus, 4.3% in I. pavlovskyi, and 0.1% in I. ovatus, respectively (Table 1). There was no statistical difference between I. persulcatus and I. pavlovskyi. The prevalences among both of these tick species were statistically higher than that of I. ovatus (Figure 2). The overall prevalence of LD borreliae was 31.7% in I. persulcatus, 15.4% in I. pavlovskyi, and 42.6% in I. ovatus, respectively (Table 1). Co-infections of B. miyamotoi and LD borreliae in adult ticks were observed in 25 of 3532 I. persulcatus (0.7%), 1 of 117 I. pavlovskyi (0.9%), and 1 of 676 I. ovatus (0.1%).
Table 1. Prevalence of borreliae in ticks.
| Location in Hokkaido | Tick species | Tick Number | B. miyamotoi positive No. (%)* | LD borreliae positive No. (%)* | |||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |||
| North | Souya | I. persulcatus | 223 | 266 | 489 | 6 (2.7) | 5 (1.9) | 11 (2.2) | 42 (18.8) | 61 (22.9) | 103 (21.1) |
| I. pavlovskyi | 1 | 0 | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Rumoi | I. persulcatus | 13 | 14 | 27 | 0 (0) | 0 (0) | 0 (0) | 4 (30.8) | 5 (35.7) | 9 (33.3) | |
| I. ovatus | 3 | 21 | 24 | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 11 (52.4) | 12 (50) | ||
| Kamikawa | I. persulcatus | 499 | 986 | 1485 | 5 (1) | 20 (2) | 25 (1.7) | 165 (33.1) | 308 (31.2) | 473 (31.9) | |
| I. pavlovskyi | 25 | 24 | 49 | 1 (4) | 2 (8.3) | 3 (6.1) | 7 (28) | 4 (16.7) | 11 (22.4) | ||
| I. ovatus | 40 | 401 | 441 | 1 (2.5) | 0 (0) | 1 (0.2) | 18 (45) | 144 (35.9) | 162 (36.7) | ||
| East | Abashiri | I. persulcatus | 250 | 280 | 530 | 7 (2.8) | 9 (3.2) | 16 (3) | 107 (42.8) | 123 (43.9) | 230 (43.4) |
| I. pavlovskyi | 0 | 3 | 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | ||
| I. ovatus | 12 | 54 | 66 | 0 (0) | 0 (0) | 0 (0) | 7 (58.3) | 32 (59.3) | 39 (59.1) | ||
| Nemuro | I. persulcatus | 91 | 95 | 186 | 1 (1.1) | 2 (2.1) | 3 (1.6) | 24 (26.4) | 23 (24.2) | 47 (25.3) | |
| Kushiro | I. persulcatus | 143 | 186 | 329 | 0 (0) | 6 (3.2) | 6 (1.8) | 60 (42) | 79 (42.5) | 139 (42.2) | |
| Tokachi | I. persulcatus | 76 | 72 | 148 | 1 (1.3) | 3 (4.2) | 4 (2.7) | 17 (22.4) | 21 (29.2) | 38 (25.7) | |
| I. pavlovskyi | 0 | 3 | 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| I. ovatus | 44 | 101 | 145 | 0 (0) | 0 (0) | 0 (0) | 26 (59.1) | 49 (48.5) | 75 (51.7) | ||
| Central | Sorachi | I. persulcatus | 67 | 73 | 140 | 0 (0) | 2 (2.7) | 2 (1.4) | 6 (9) | 13 (17.8) | 19 (13.6) |
| Hidaka | I. persulcatus | 7 | 9 | 16 | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 1 (11.1) | 2 (12.5) | |
| Ishikari | I. persulcatus | 63 | 116 | 179 | 0 (0) | 4 (3.4) | 4 (2.2) | 14 (22.2) | 35 (30.2) | 49 (27.4) | |
| I. pavlovskyi | 40 | 21 | 61 | 2 (5) | 0 (0) | 2 (3.3) | 3 (7.5) | 3 (14.3) | 6 (9.8) | ||
| South | Oshima | I. persulcatus | 2 | 1 | 3 | 0 (0) | 0 (0) | 0 (0) | 0(0) | 0(0) | 0(0) |
| Total | I. persulcatus | 1434 | 2098 | 3532 | 20 (1.4) | 51 (2.4) | 71 (2) | 444 (31) | 674 (32.1) | 1118 (31.7) | |
| I. pavlovskyi | 66 | 51 | 117 | 3 (4.5) | 2 (3.9) | 5 (4.3) | 10 (15.2) | 8 (15.7) | 18 (15.4) | ||
| I. ovatus | 99 | 577 | 676 | 1 (1) | 0 (0) | 1 (0.1) | 52 (52.5) | 236 (40.9) | 288 (42.6) | ||
*The numbers include mixed infections.
Figure 2. The prevalence of B. miyamotoi and LD borreliae in Ixodes ticks in Hokkaido.
The asterisk shows significant difference: * P<0.01, by Fisher's exact test.
Phylogenetic analysis of isolated strains
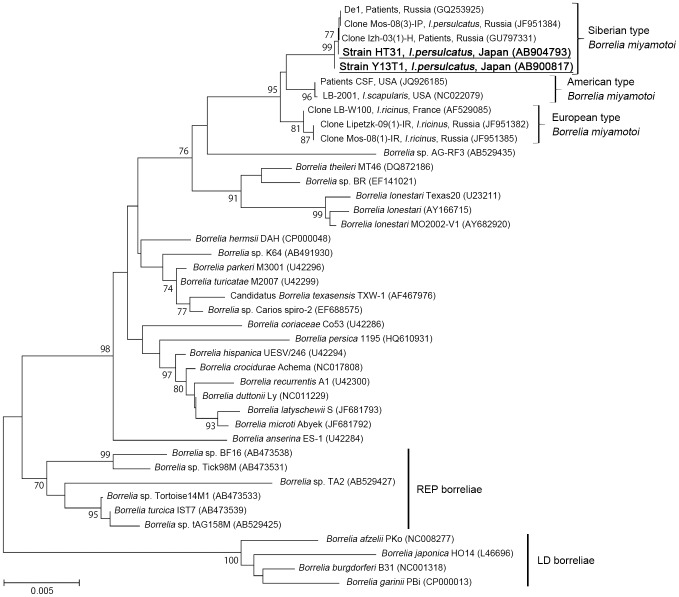
In this study, 6 isolates of B. miyamotoi derived from I. persulcatus and I. pavlovskyi were established in culture using BSK-M medium (Table 2). These isolates were utilized for molecular characterization, together with 5 isolates previously established in Hokkaido [1]. In the initial step of molecular analyses, DNA sequences of housekeeping genes (glpQ, 16 S rDNA, and flaB) were determined for all of the Hokkaido isolates, and there were no nucleotide substitutions in all 11 isolates. Therefore, the isolate HT31 (the type strain of B. miyamotoi) was selected as a representative for phylogenetic analysis. Neighbor-joining phylogenetic trees inferred from each of the housekeeping genes clearly showed that HT31 was clustered with B. miyamotoi from Russia, but was distinguishable from B. miyamotoi found in North American and B. miyamotoi detected from most I. ricinus ticks in Europe (Figures 3, S1, and S2). These three clusters of B. miyamotoi were temporally designated as “Siberian,” “American” and “European” types, in this study (Figure 3).
Table 2. Borrelia miyamotoi strains used in this study.
| Strain name | Isolation source | Location | Isolated year | Reference |
| HT31T | I. persulcatus, female | Shiretoko, Hokkaido, Japan | 1990 | 1 |
| HT24 | I. persulcatus, female | Shiretoko, Hokkaido, Japan | 1990 | 1 |
| FR64b | Apodemus argenteus, whole blood | Furano, Hokkaido, Japan | 1991 | 1 |
| Hk004 | I. persulcatus, nymph | Sibecha, Hokkaido, Japan | 1993 | 1 |
| NB103-1 | I. persulcatus, nymph on Emberiza spodocephala | Nemuro, Hokkaido, Japan | 1991 | 1 |
| MYK1 | I. pavlovskyi, female | Shibetsu, Hokkaido, Japan | 2012 | in this study |
| MYK2 | I. persulcatus, female | Nayoro, Hokkaido, Japan | 2012 | in this study |
| MYK3 | I. persulcatus, female | Obihiro, Hokkaido, Japan | 2012 | in this study |
| MYK4 | I. persulcatus, female | Simukapp, Hokkaido, Japan | 2012 | in this study |
| MYK5 | I. persulcatus, female | Takinoue, Hokkaido, Japan | 2012 | in this study |
| Y13T1 | I. persulcatus, male | Oumu, Hokkaido, Japan | 2013 | in this study |
Figure 3. Phylogenetic analysis of RF borreliae based on 16 S rDNA of Borrelia spp.
The phylogenetic tree of 16 S rDNA was constructed. The phylogenetic branches were supported in >70% by the bootstrap analysis. The bar indicates the percentage of sequence divergence. Sequences in this study were shown in bold type. If possible, clone or strain name, isolation source, and country were described in the case of B. miyamotoi. Numbers in parentheses indicate Accession Numbers in GenBank.
The molecular typing by MLSA using 8 genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) revealed that all of the Hokkaido isolates were identical to each other. Sequence similarity between the Hokkaido isolates (Siberian type) and American type of B. miyamotoi (LB-2001 [26], Acc. No. NC_022079) were 98% in concatenated sequences of the 8 genes (4776 bp), but the values ranged from 97.5 to 99.1% in individual genes. A phylogenetic tree constructed using concatenated sequences is shown in the supporting information (Figure S3). Nucleotide sequences of B. miyamotoi isolates from Hokkaido were deposited in DDBJ/EMBL/GenBank databases as the following accession numbers: AB900797–807 and AB904793 for HT31T, AB900808–16 for MYK1, and AB900817 for Y13T1.
Discussion
It is known that I. persulcatus principally serves as a tick vector for LD borreliae in Hokkaido. Hokkaido has an area of approximately 83,000 km2, and the total population of Hokkaido, according to the National Census 2010, is 5.5 million people (http://www.stat.go.jp/english/index.htm). However, 42% of the population inhabits the plains of Ishikari district (Figure 1). Similar to the taiga zone in Russia, I. persulcatus ticks are abundant mainly in forested areas of northern and eastern Hokkaido, which are thinly populated. Human tick bites and tick-borne LD, therefore, occur sporadically in Hokkaido [27]. In this study, we conducted large-scale tick surveillance for B. miyamotoi in Hokkaido, because human cases of B. miyamotoi infections have been confirmed in Russia [7]. Moreover, human B. miyamotoi infection, determined through retrospective analysis, has been reported in Hokkaido (http://www.nih.go.jp/niid/ja/relapsing-fever-m/relapsing-fever-iasrs/3877-pr4046.html). Our study demonstrated that B. miyamotoi is widely distributed throughout Hokkaido with low prevalence (0–3%) in I. persulcatus (Table 1, Figure 2). The prevalence is similar to those reported in Russian and European I. persulcatus [7], [11]. Besides I. persulcatus, B. miyamotoi was found from 4.3% (within 0–6.1%) of I. pavlovskyi, and this is the first report of detection and isolation of B. miyamotoi from I. pavlovskyi. Moreover, we detected B. miyamotoi from an I. ovatus male. However, given that the prevalence in I. ovatus was significantly lower than that in I. persulcatus and I. pavlovskyi, the potential vectors of B. miyamotoi in Hokkaido are thought to be I. persulcatus and I. pavlovskyi. Female ticks of I. persulcatus are responsible for most human tick-bites in Hokkaido [27]. The abundance of B. miyamotoi-infected female ticks is, therefore, directly linked to human risk. Although the prevalence of B. miyamotoi is relatively low in rural communities of Hokkaido, B. miyamotoi is important for public health as an agent of fever and variable symptoms. Moreover, I. pavlovskyi can serve as an additional vector for B. miyamotoi. I. persulcatus and I. pavlovskyi are closely related morphologically, ecologically and phylogenetically [17], [28]. The larvae and nymphs of both tick species have a wide range of feeding hosts, such as small mammals and birds. On the other hand, unlike I. persulcatus, adult I. pavlovskyi mainly infests birds. The potential for I. pavlovskyi to bite humans is still unclear, however, its public health significance is negligible given the far lower abundance.
MLSA is an efficient tool to resolve phylogenetic relationships of LD borreliae on an intraspecies level [23]. In this study, we obtained for MLSA, 11 isolates, including 5 strains isolated in the early 1990's. These isolates were 100% identical in 8 loci, even from different tick and vertebrate sources. This result suggests that Japanese B. miyamotoi are clonal. The reason, however, remains unclear. Further analysis, such as the examination of MLSA using European B. miyamotoi or B. miyamotoi from the main island of Japan may contribute to the elucidation of its evolutionary history, e.g. clonal expansion.
In previous studies, suspected human cases of co-infection with B. miyamotoi and LD borreliae have been reported from North America and Russia [6], [7]. In this study, we also found co-infection of B. miyamotoi and LD borreliae in 3 tick species. Of 1118 I. persulcatus ticks which were infected with LD borreliae, 25 (2.2%) were co-infected with B. miyamotoi. It, therefore, seems likely that approximately 2% of LD patients might be co-infected with B. miyamotoi in Hokkaido. On the other hand, it is unclear when, during feeding, B. miyamotoi is transmitted to vertebrates through saliva. Whereas LD borreliae are known to require several days (∼48 hours) for transmission from ticks to vertebrates [29], soft tick-borne RF borreliae were efficiently transmitted through saliva within minutes [30]. In a previous study, we demonstrated that Borrelia sp. AGRF, which is phylogenetically related to B. miyamotoi, is maintained in the salivary gland of unfed hard-ticks, suggesting that the borrelial transmission to vertebrate hosts occurs rapidly when the ticks start to feed blood [25]. Thus, we speculate that B. miyamotoi may be transmitted to humans more rapidly than LD borreliae, thereby increasing the risk of infection. Further studies will be required to confirm this.
In conclusion, the present surveillance showed that B. miyamotoi-infected ticks are widely distributed throughout Hokkaido, and that I. persulcatus is likely the most important vector of indigenous relapsing fever-like spirochetes transmitted by ticks in Hokkaido. Our subsequent genetic analyses also demonstrated that the Hokkaido isolates of B. miyamotoi are clonal.
Supporting Information
Phylogenetic analysis of RF borreliae based on flaB of Borrelia spp. The phylogenetic branches were supported in >70% by the bootstrap analysis. The bar indicates the percentage of sequence divergence. Sequences in this study are shown in bold type. The number in parentheses indicates Accession Number in GenBank.
(TIF)
Phylogenetic analysis of RF borreliae based on glpQ of Borrelia spp. The phylogenetic branches were supported in >70% by the bootstrap analysis. The bar indicates the percentage of sequence divergence. Sequences in this study are shown in bold type. The number in parentheses indicates Accession Number in GenBank.
(TIF)
Bayesian phylogenetic inference of concatenated housekeeping gene sequences of RF borreliae. The phylogenetic tree was constructed based on Bayesian phlylogenetic inference as previously described by Margos et al [23]. The posterior probability values of the clades are provided. Bars labeled 0.02 depict 2% divergence. The LD borreliae (ST1 [B. burgdorferi B31], ST84 [B. garinii PBi], ST70 [B. afzelii VS461] were downloaded from the MLST website; www.mlst.net) were used as outgroups (data not indicated). The number in parentheses indicates Accession Number in GenBank.
(TIF)
The composition of BSK-M medium.
(DOC)
The Primer list used in this study.
(DOC)
The sensitivity and specificity of the qPCR.
(DOC)
Acknowledgments
We would like to thank M. Fukunaga (Fukuyama University) and T. Masuzawa (Chiba Institute of Science) for providing Borrelia strains, C. Miyoshi, R. Sakai, A. Naniwa and A. Okuda (Field Science Center for Northern Biosphere, Hokkaido University) for providing information about collecting ticks, Y. Ito (Yamaguchi University) for her excellent technical assistance, and S. Yamamoto (Miyazaki prefectural Institute for Public Health and Environment) for technical advice on DNA extraction from ticks. We appreciate K. R. Taylor (University of Florida, Department of Infectious Diseases and Pathology) for his critical comments on this manuscript. The authors would like to acknowledge the technical expertise of The DNA Core facility of the Center for Gene Research, Yamaguchi University, supported by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All accession number of sequences are deposited in DDBJ/EMBL/GenBank databases as the following accession numbers: AB900797-817 and AB904793.
Funding Statement
This work was supported in part by a grant for research on emerging and reemerging infectious diseases from the Japanese Ministry of Health, Labor, and Welfare (H24-Shinko-Ippan-008) (http://www.mhlw.go.jp/seisakunitsuite/bunya/hokabunya/kenkyujigyou/hojokin-koubo-h25/gaiyo/19.html), and Program to Disseminate Tenure Tracking System, MEXT, Japan (http://www.jst.go.jp/tenure/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, et al. (1995) Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45: 804–810. [DOI] [PubMed] [Google Scholar]
- 2. Scoles GA, Papero M, Beati L, Fish D (2001) A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis 1: 21–34. [DOI] [PubMed] [Google Scholar]
- 3. Mun J, Eisen RJ, Eisen L, Lane RS (2006) Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol 43: 120–123. [DOI] [PubMed] [Google Scholar]
- 4. Salkeld DJ, Cinkovich S, Nieto NC (2014) Tick-borne Pathogens in Northwestern California, USA. Emerg Infect Dis 20: 493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fraenkel CJ, Garpmo U, Berglund J (2002) Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol 40: 3308–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richter D, Schlee DB, Matuschka FR (2003) Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis 9: 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, et al. (2011) Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17: 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, et al. (2013) Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med 159: 21–27. [DOI] [PubMed] [Google Scholar]
- 9. Gugliotta JL, Goethert HK, Berardi VP, Telford SR 3rd (2013) Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, et al. (2013) A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382: 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geller J, Nazarova L, Katargina O, Järvekülg L, Fomenko N, et al. (2012) Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS One 7: e51914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyamoto K, Nakao M, Uchikawa K, Fujita H (1992) Prevalence of Lyme borreliosis spirochetes in ixodid ticks of Japan, with special reference to a new potential vector, Ixodes ovatus . J. Med. Entomol 29: 216–220. [DOI] [PubMed] [Google Scholar]
- 13. Masuzawa T (2004) Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn J Infect Dis 57: 229–235. [PubMed] [Google Scholar]
- 14. Taylor KR, Takano A, Konnai S, Shimozuru M, Kawabata H, et al. (2013) Borrelia miyamotoi infections among wild rodents show age and month independence and correlation with Ixodes persulcatus larval attachment in Hokkaido, Japan. Vector Borne Zoonotic Dis 13: 92–97. [DOI] [PubMed] [Google Scholar]
- 15. Nakao M, Miyamoto K, Kitaoka S (1992) A new record of Ixodes pavlovskyi Pomerantzev from Hokkaido, Japan (Acari: Ixodidae). Japanese Journal of Sanitary Zoology 43: 229–234. [Google Scholar]
- 16. Fukunaga M, Yabuki M, Hamase A, Oliver JH Jr, Nakao M (2000) Molecular phylogenetic analysis of ixodid ticks based on the ribosomal DNA spacer, internal transcribed spacer 2, sequences. J Parasitol 86: 38–43. [DOI] [PubMed] [Google Scholar]
- 17. Takano A, Fujita H, Kadosaka T, Takahashi M, Yamauchi T, et al. (2014) Construction of a DNA database for ticks collected in Japan: application of molecular identification based on the mitochondrial 16S rDNA gene. Med Entomol Zool 65: 13–21. [Google Scholar]
- 18. Yamazaki-Matsune W, Taguchi M, Seto K, Kawahara R, Kawatsu K, et al. (2007) Development of a multiplex PCR assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis . J Med Microbiol 56: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 19. Barbour AG (1984) Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57: 521–525. [PMC free article] [PubMed] [Google Scholar]
- 20. Takano A, Nakao M, Masuzawa T, Takada N, Yano Y, et al. (2011) Multilocus sequence typing implicates rodents as the main reservoir host of human-pathogenic Borrelia garinii in Japan. J Clin Microbiol 49: 2035–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, et al. (2009) Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg 81: 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takano A, Fujita H, Kadosaka T, Konnai S, Tajima T, et al. (2011) Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and Relapsing fever Borrelia spp. Environ Microbiol Rep 3: 632–637. [DOI] [PubMed] [Google Scholar]
- 23. Margos G, Gatewood AG, Aanensen DM, Hanincová K, Terekhova D, et al. (2008) MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi . Proc Natl Acad Sci U S A 105: 8730–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takano A, Sugimori C, Fujita H, Kadosaka T, Taylor KR, et al. (2012) A novel relapsing fever Borrelia sp. infects the salivary glands of the molted hard tick, Amblyomma geoemydae . Ticks Tick Borne Dis 3: 259–261. [DOI] [PubMed] [Google Scholar]
- 26. Hue F, Langeroudi AG, Barbour AG (2013) Chromosome Sequence of Borrelia miyamotoi, an Uncultivable Tick-Borne Agent of Human Infection. Genome Announc 1: e00713–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashimoto Y, Kinouchi M, Takahashi H, Kawagishi N, Kishiyama K, et al. (2002) An epidemic study of 700 cases with tick bites in Hokkaido prefecture during the past 6 years: Relationship to Lyme Disease. Jpn J Dermatol 112: 1467–1473 (In Japanese). [Google Scholar]
- 28. Korenberg EI, Nefedova VV, Romanenko VN, Gorelova NB (2010) The tick Ixodes pavlovskyi as a host of spirochetes pathogenic for humans and its possible role in the epizootiology and epidemiology of borrelioses. Vector Borne Zoonotic Dis 10: 453–458. [DOI] [PubMed] [Google Scholar]
- 29. Schwan TG, Piesman J (2002) Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis 8: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbour AG (2005) Relapsing fever. In: Goodman JL, Dennis DT, Sonenshine DE, editors.Tick-borne diseases of humans: ASM Press. pp. 268–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis of RF borreliae based on flaB of Borrelia spp. The phylogenetic branches were supported in >70% by the bootstrap analysis. The bar indicates the percentage of sequence divergence. Sequences in this study are shown in bold type. The number in parentheses indicates Accession Number in GenBank.
(TIF)
Phylogenetic analysis of RF borreliae based on glpQ of Borrelia spp. The phylogenetic branches were supported in >70% by the bootstrap analysis. The bar indicates the percentage of sequence divergence. Sequences in this study are shown in bold type. The number in parentheses indicates Accession Number in GenBank.
(TIF)
Bayesian phylogenetic inference of concatenated housekeeping gene sequences of RF borreliae. The phylogenetic tree was constructed based on Bayesian phlylogenetic inference as previously described by Margos et al [23]. The posterior probability values of the clades are provided. Bars labeled 0.02 depict 2% divergence. The LD borreliae (ST1 [B. burgdorferi B31], ST84 [B. garinii PBi], ST70 [B. afzelii VS461] were downloaded from the MLST website; www.mlst.net) were used as outgroups (data not indicated). The number in parentheses indicates Accession Number in GenBank.
(TIF)
The composition of BSK-M medium.
(DOC)
The Primer list used in this study.
(DOC)
The sensitivity and specificity of the qPCR.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All accession number of sequences are deposited in DDBJ/EMBL/GenBank databases as the following accession numbers: AB900797-817 and AB904793.