Abstract
Objective
To analyze the titers of the IgG and IgM antibodies against human herpesvirus 6A/B (HHV-6A/B) in multiple sclerosis (MS) patients treated with different disease modified therapies (DMTs) along two-years of follow-up.
Methods
We collected 2163 serum samples from 596 MS; for 301 MS patients a 2-years follow-up was performed. Serum samples of 337 healthy controls were also analyzed. Anti-HHV-6A/B IgG and IgM were analyzed by ELISA (Panbio).
Results
We found that 129/187 (69.0%) MS patients with a decrease of the anti-HHV-6A/B IgG titers after 2-years with DMTs were free of relapses and progression vs. 46/113 (40.7%) of MS patients with an increase of the anti-HHV-6A/B IgG titers (p = 0.0000015); the higher significance was found for natalizumab. Furthermore, we found that anti-HHV-6A/B IgG titers reached their highest value two weeks before the relapse (p = 0.0142), while the anti-HHV-6A/B IgM titers reached their highest value one month before the relapse (p = 0.0344).
Conclusion
The measurement of the anti-HHV-6A/B IgG titers could be a good biomarker of clinical response to the different DMTs. The increase of the anti-HHV-6A/B IgG and IgM titers predicts the upcoming clinical relapses. However, further longitudinal studies are needed to validate these results.
Introduction
Multiple sclerosis (MS) is an inflammatory and degenerative neurological disease in which damage to the central nervous system causes widespread dysfunction [1]. Early in the course of MS, disease modifying therapies (DMTs), such as interferon-beta (IFN-beta), glatiramer acetate (GA) or natalizumab reduce the relapse rate and the rate of disability progression [2]–[4].
There are increasing evidences that a number of environmental factors are important in the development and course of MS. Although no virus or other environmental agents have been definitively implicated as a causative factor of MS, certain human herpesviruses (HHVs) have been linked with the development of MS [5], especially the Epstein-Barr virus (EBV) [6]–[8], and the formerly known as HHV-6 [9]–[11]; although some authors have described a possible relation between HHV-6B and MS [12], it appears that HHV-6A could be mainly associated with MS [13]–[15]. Different mechanisms have been proposed for these viruses in MS pathogenesis; but, for these viruses or for the other viruses or possible environmental factors that could be involved in MS, a relation with the evolution of the disease and the clinical response to the different DMTs should be demonstrated. Thus, the aim of this study was to analyze the titers of the IgG and IgM antibodies against HHV-6A/B in MS patients treated with different DMTs along two-years of follow-up.
Materials and Methods
Subjects
We collected 2163 serum samples from 596 MS patients in a prospective study (see Table 1). For 301 MS patients a 2-years longitudinal study was performed: a serum sample was collected prior the beginning of a DMT, and each three months (MS patients treated with natalizumab) or six months (MS patients treated with IFN-beta or GA) to complete, at least, two-years of follow up; a serum sample was also collected when the patient suffered a relapse (prior intravenous corticosteroids). Serum samples of 337 healthy controls were also included in the study. For MS patients we collected the following clinical data: the Expanded Disability Status Scale (EDSS) score prior the beginning of the DMT and two years later, and the number of relapses along the two-years of follow-up with the different DMTs.
Table 1. Clinical and demographic characteristics of the samples and subjects included in the study.
| Serum samples of MS patients | 2163 | |
| In relapse (prior intravenous corticosteroids) | 216 | |
| In remission | 1947 | |
| Within the three months before and after a relapse | 278 | |
| % Serum samples collected without treatment | 24.7 | |
| % Serum samples collected during interferon beta treatment | 27.7 | |
| % Serum samples collected during glatiramer acetate treatment | 24.3 | |
| % Serum samples collected during natalizumab treatment | 23.3 | |
| MS patients | 596 | |
| Females | 384 | |
| Males | 212 | |
| MS patients with at least two-years of follow-up* | 301 | |
| Relapsing-remitting MS patients | 279 | |
| Naïve patients | 148 | |
| Secondary progressive MS patients | 22 | |
| Age at the beginning of the study (years) | 36.4 | |
| Duration of the disease (years) | 7.0 | |
| Starting age of the disease (years) | 29.4 | |
| EDSS at the recruitment** | 2.4 | |
| MSSS at the recruitment** | 4.0 | |
| Number of relapses two years before starting the treatment | 2.3 | |
| MS patients treated with interferon beta | 131 | |
| MS patients treated with glatiramer acetate | 89 | |
| MS patients treated with natalizumab | 81 | |
| MS patients with one-year of follow-up | 112 | |
| MS patients with less than one-year of follow-up | 127 | |
| Discontinuations before completing 2-years of follow-up*** | 56 | |
| Healthy controls | 337 | |
| Serum samples of healthy controls | 337 | |
| Sex | Females | 192 |
| Males | 169 | |
| Age at the recruitment | 37.6 | |
*At least two-years of follow-up with the same disease modifying therapy (DMT) from a base line visit (serum sample extracted before starting the DMT) to a 24 months visit.
**EDSS: Expanded Disability Status Scale. MSSS: Multiple Sclerosis Severity Score.
***It was included: adverse events, pregnancy, drug withdrawal for lack of efficacy, and patient decision.
Ethics Statement
This study was approved by our local Ethic Committee (“Comité Ético de Investigación Clínica del Hospital Clínico San Carlos”), and all the participants received and signed the written informed consent before the enrolment.
Samples
One dry tube with blood was collected for each patient in each one of the visits. After collection of the whole blood, we allowed the blood to clot by leaving it undisturbed at room temperature along 30 minutes; then, we removed the clot by centrifuging at 1,500 × g for 10 minutes in a refrigerated centrifuge. Finally, the serum samples were aliquoted in 0.2 ml tubes, and the aliquots were frozen at −80°C during 32.6±14.1 before being analyzed (the mean time needed to complete one plate).
Anti-HHV-6A/B IgG and IgM ELISA
Every serum sample was tested with two tests of Panbio (Inverness, Australia), for the detection of anti-HHV-6A/B IgG and IgM following the manufacturer instructions. In brief, serum antibodies of the IgG or IgM class, when present, combined with an HHV-6 antigen that was attached to the polystyrene surface of the microwells. Residual serum was removed by washing and peroxidase conjugated anti-human IgG or IgM was added. The microwells were washed and a colorless substrate system, tetramethylbenzidine/hydrogen peroxide (TMB/H2O2) was added. The substrate was hydrolyzed by the enzyme and the chromogen changed to a blue color. After stopping the reaction with acid, the TMB became yellow. The color intensity was directly related to the concentration of HHV-6 IgG or IgM antibodies in the test sample. Results were expressed in Panbio units (PU); they were calculated by multiplying the index value by 10 (the index value for each one of the samples = sample absorbance/cut-off value). Samples were analyzed in duplicate for each test, and doubtful samples, those that were between 9 and 11 Panbio units (PU) were tested again: a total of 60 serum samples (49 from MS patients and 11 from healthy controls) had titers between 9–11 PU; 3 of them (that belonged to 3 MS patients) were considered positive after the new analysis: one of them had a titer value of 10.8 PU and other two samples had 10.7 PU; after the new analysis, the samples had titers of 11.1 PU, 11.1 PU and 11.2 PU, respectively (the variation inter-assays was under 5%). The other doubtful serum samples were considered as negative.
Detection of neutralizing antibodies (NAbs) against IFN-beta
NAbs were measured in the serum samples of those MS patients treated with IFN-beta through the cytopathic effect (CPE) assay at 6, 12, 18 and 24 months after starting the treatment, as it has been previously described [16]. The titers were calculated according to Kawade’s formula [17], and expressed in tenfold reduction unit (TRU). Samples were considered positives if titers were >20 TRU/ml.
Statistical analysis
Odds ratios (O.R.) and exact 95 percent confidence intervals (C.I.) were calculated with standard microcomputer software: Epi Info v. 6.02 (CDC, Atlanta, USA) and SPSS Ver. 15.0 (SPSS Inc.). The chi-square or two-tailed Fisher’s exact test was used to test differences in categorical variables. Kruskall-Wallis analysis or the Wilcoxon rank-sum test was used to test differences in continuous variables. We considered statistically significant differences when p<0.05.
Results
Serology of HHV-6A/B in serum samples of MS patients and healthy controls
When we analyzed only those serum samples collected when MS patients were untreated (Table 2), we found that the anti-HHV-6A/B IgG prevalence was 98.0% (295/301) vs. 93.4% (315/337) in healthy controls (p = 0.005), and the mean titer was 26.8 PU vs. 23.1 PU in healthy controls (p = 0.00002). Regarding anti-HHV-6A/B IgM antibodies, the prevalence was 8.3% (25/301) vs. 5.6% (19/337) in healthy controls (p = 0.184) and the mean titer was 4.4 PU vs. 4.0 PU in healthy controls (p = 0.100).
Table 2. Prevalences and mean titers of the anti-HHV-6A/B IgG and IgM antibodies before and after 2-years of treatment with the different DMTs.
| Anti-HHV-6A/B IgG | ||||||
| Prevalences | Mean Titers (PU) | |||||
| DMTs | Before | 2-years | p | Before | 2-years | p |
| TOTAL | 98.0% (295/301) | 97.0% (292/301) | 0.433 | 26.8 | 25.3 | 0.044 |
| IFN-beta | 96.9% (127/131) | 96.9% (127/131) | 1 | 24.3 | 24.1 | 0.439 |
| GA | 98.9% (88/89) | 96.6% (86/89) | 0.312 | 30.0 | 27.9 | 0.105 |
| NTZ | 97.5% (79/81) | 97.5% (79/81) | 1 | 27.4 | 24.3 | 0.033 |
TOTAL: prevalence and mean titers of all the different disease modifying therapies (DMTs); IFN-beta: interferon beta; GA: glatiramer acetate; NTZ: natalizumab.
Serology of HHV-6A/B and DMTs in MS patients
As can be seen in Table 2, we found a statistical significant reduction of the anti-HHV-6A/B IgG titers after 2-years with the different DMTs (p = 0.044), and above all, with natalizumab (0.033). When we analyzed the anti-HHV-6A/B IgM antibodies (Table 2), we found a trend for the reduction of the prevalence in MS patients treated with natalizumab, and a decrease of the mean titers with the different DMTs (p = 0.003); again, the greatest reduction was for natalizumab (p = 0.024).
Serology of HHV-6A/B and clinical response to the different DMTs
As can be seen in Figures 1A–1E, we found a correlation between the variation of the anti-HHV-6A/B IgG titers and the clinical response (absence of relapses and progression after two-years of follow-up) in MS patients treated with the different DMTs: 129/187 (69.0%) MS patients with a decrease of the anti-HHV-6A/B IgG titers were free of relapses and progression vs. 46/113 (40.7%) of MS patients with an increase of the anti-HHV-6A/B IgG titers (p = 0.0000015); statistical significant differences were found for each one of the DMTs, although the higher significance was found for natalizumab: 45/58 (77.6%) vs. 7/23 (30.4%) (p = 0.00007; O.R. = 7.9), respectively. The percentage of clinical responders was greater among those MS patients with greater reductions of the anti-HHV-6A/B IgG titers, while the percentage of clinical responders in MS patients with an increase in their anti-HHV-6A/B IgG titers was lower when the percentage of increase was greater (Figures 1A–1E).
Figure 1. Percentage of MS patients free of relapses and progression after two years of follow-up from the 301 MS patients included in the longitudinal study.
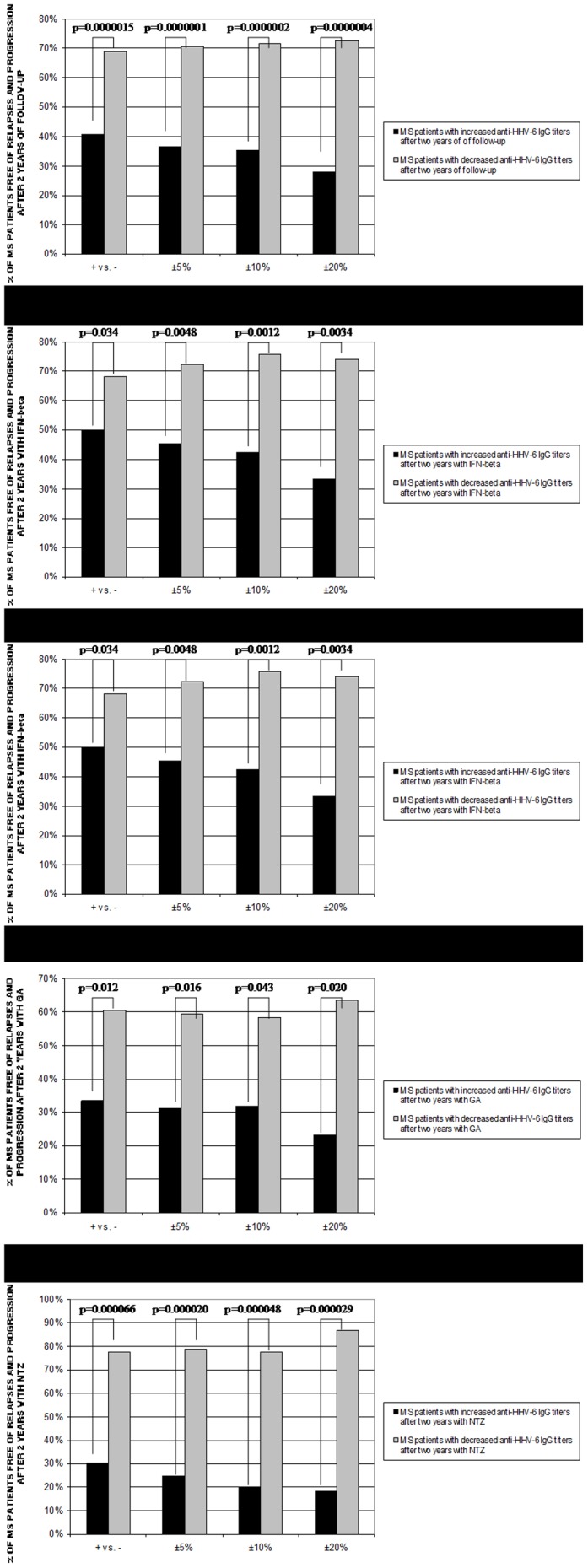
A. DMTs. B. interferon-beta (IFN-beta). C. IFN-beta (only in those MS patients that did not develop NAbs). D. glatiramer acetate (GA). E. natalizumab (NTZ). Four comparisons have been performed for each treatment: MS patients with increased anti-HHV-6A/B IgG titers vs. MS patients with decreased anti-HHV-6A/B IgG titers; MS patients with an increase of the anti-HHV-6A/B IgG titers >5% vs. MS patients with a decrease of the anti-HHV-6A/B IgG titers >5%; MS patients with an increase or decrease of the anti-HHV-6A/B IgG titers >10%; MS patients with an increase or decrease of the anti-HHV-6A/B IgG titers >20%.
The mean variation of the anti-HHV-6A/B IgG titers (around 20% of increase or decrease) was lower than the mean variation of the anti-HHV-6A/B IgM titers (more than 30% of increase or decrease); no statistical significant correlations were found between the clinical response and the variation of the anti-HHV-6A/B IgM titers after two years with the different DMTs, only a trend for natalizumab when we compared MS patients with increases or decreases >30% (37.5% vs. 69.2%, respectively, p = 0.089). Finally, when we compare those MS patients with an increase of the anti-HHV-6A/B IgG titers>20% and with an increase of the anti-HHV-6A/B IgM titers>30% (both above the mean variation of increase) with those MS patients with a decrease of the anti-HHV-6A/B IgG titers>20% and with a decrease of the anti-HHV-6A/B IgM titers>30% (both above the mean variation of decrease), for all the DMTs, we found a high statistical significance (p = 0.00002; O.R. = 39.9): 1/10 MS patients was free of relapses and progression vs. 31/38 MS patients, respectively.
An association was also found between the increase of the anti-HHV-6A/B IgG titers and the clinical non-response (MS patients with at least two relapses and/or an increase of at least one point in the EDSS score after two years of follow-up with the different DMTs): 35/113 (31.0%) MS patients with an increase of the anti-HHV-6A/B IgG titers were non-responders vs. 33/187 (17.6%) MS patients with a decrease of the anti-HHV-6A/B IgG titers (p = 0.0076; O.R. = 2.1); furthermore, 22/54 (40.7%) MS patients with an increase >20% of the anti-HHV-6A/B IgG titers were non-responders vs. 11/77 (14.3%) MS patients with a decrease >20% (p = 0.0006; O.R. = 4.1). Again, the greater statistical significance was for natalizumab (p = 0.018; O.R. = 3.9).
Serology of HHV-6A/B in MS patients treated with IFN-beta without NAbs
The 32.8% (43/131) of MS patients treated with IFN-beta were positive for NAbs at least once along the two years of follow-up. When we only considered those MS patients treated with IFN-beta that did not develop NAbs in the 2-years of follow-up (Figure 1.C), we found that 16/38 (42.1%) MS patients with an increase of the anti-HHV-6A/B IgG titers were clinical responders vs. 39/50 (78%) MS patients with a decrease of the anti-HHV-6A/B IgG titers (p = 0.0006; O.R. = 4.9); furthermore, 4/21 (19%) MS patients with an increase of the anti-HHV-6A/B IgG titers >20% were clinical responders vs. 13/16 (81.2%) MS patients with a decrease of the anti-HHV-6A/B IgG titers >20% (p = 0.0002; O.R. = 18.4).
Serology of HHV-6A/B and EDSS
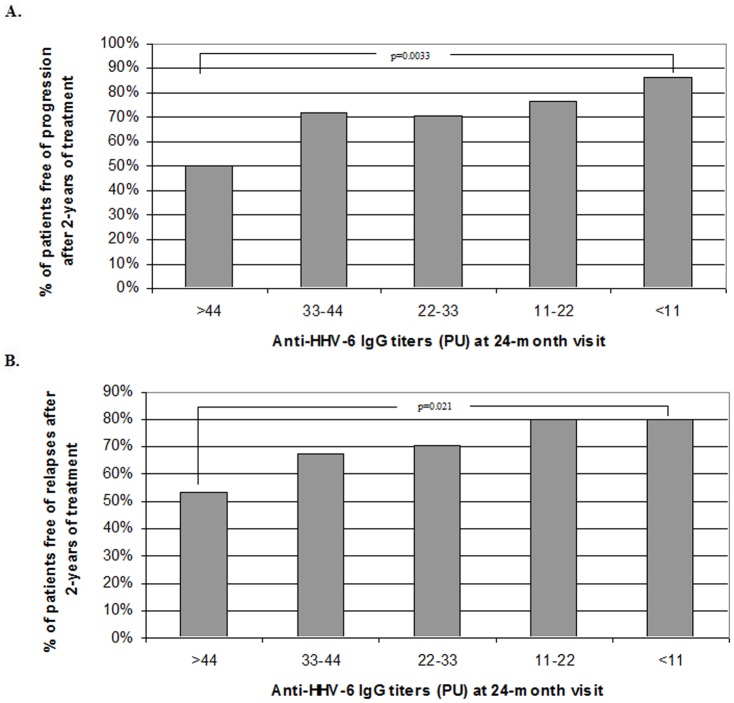
Only the 28.2% (53/188) of MS patients that did not experienced an increase in the EDSS score (the EDSS was equal or lower after 2-years of treatment) had an increase of the anti-HHV-6A/B IgG titers; however, among those MS patients that experienced an increase in the EDSS after two years of treatment, the 62.9% (44/70) had an increase of the anti-HHV-6A/B IgG titers (p = 0.0000003; O.R. = 4.3). Furthermore, as we can see in Figure 2.A, the lower anti-HHV-6A/B IgG titers at 24-months visit, the greater the percentage of patients free of progression after two years of treatment.
Figure 2. Association between the anti-HHV-6A/B IgG titers at the 2-years visit with the progression of the disease (A) and the activity of the disease (B) after two years of treatment with the different DMTs.
A. 9/18 MS patients with titers >44 PU after 2-years of treatment were free of progression vs. 39/54 with titers between 33–44 PU vs. 74/104 with titers between 22–33 PU vs. 82/108 with titers between 11–22 PU vs. 15/17 with titers <11 PU. B. 10/18 MS patients with titers >44 PU after 2-years of treatment were free of relapses vs. 37/54 with titers between 33–44 PU vs. 74/104 with titers between 22–33 PU vs. 80/108 with titers between 11–22 PU vs. 14/17 with titers <11 PU.
Serology of HHV-6A/B and relapses
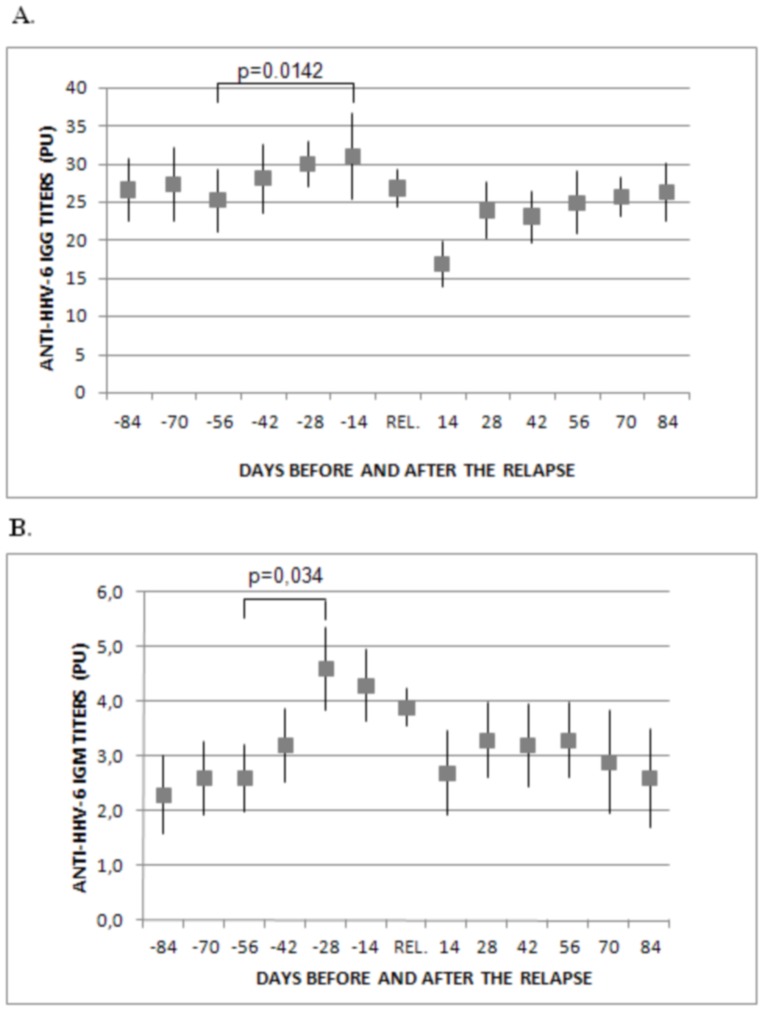
For the study of the anti-HHV-6A/B IgG and IgM response in relapses we included 216 serum samples collected in relapse and 278 serum samples collected within the three months before and after a relapse, from the total of 2163 serum samples collected from the 596 MS patients included in the study (Table 1). As can be seen in Figure 3.A, when we analyzed the anti-HHV-6A/B IgG response in relapses, we found that two months before the relapse the IgG titers began to increase and they reached their highest value two weeks before the relapse (p = 0.0142); then, they began to decrease and reached the lowest value two weeks after the relapse. Similarly, the anti-HHV-6A/B IgM titers increased around two months before the relapse, they reached their highest value one month before the relapse (p = 0.0344), two weeks before the IgG response (Figure 3.B). Furthermore, similarly to what happened to the progression, we found that higher titers of anti-HHV-6A/B IgG at 24-month visit was associated with a higher likelihood of relapses during the two years of treatment (Figure 2.B).
Figure 3. Anti-HHV-6A/B IgG and IgM titers in 216 serum samples collected in relapse (REL.) and in 278 serum samples collected three months before and after the relapse (n = 21 at day–84, n = 20 at day –70, n = 23 at day –56, n = 23 at day –42, n = 25 at day –28, n = 31 at day –14, n = 26 at day 14, n = 21 at day 28, n = 22 at day 42, n = 24 at day 56, n = 23 at day 70, n = 19 at day 84).
A. Anti-HHV-6A/B IgG response. B. Anti-HHV-6A/B IgM response.
Discussion
The results of this study show a good correlation between the variation of the anti-HHV-6A/B IgG titers and the clinical response to the different DMTs in MS patients after two years of follow up (Figures 1A–1E). We found that MS patients with higher titers after two-years of treatment had higher likelihood of progression and relapses (Figure 2); and furthermore, we have found an association between the increase of the anti-HHV-6A/B IgG and IgM titers (Figure 3) with the upcoming relapses (however, as IgM antibodies to HHV-6 from Panbio can cross react with a number of other viral antigens such as CMV and EBV, the serology of these viruses should be also analyzed).
In previous studies of our group, we have reported that the presence of HHV-6A/B DNA in blood and serum during IFN-beta treatment could be a good marker of poor response [18]–[20]. Similar results were found by other authors in MS patients treated with IFN-beta when serum cell-free DNA of HHV-6A/B was analyzed [21]. IFN-beta is a cytokine naturally expressed in response to viral infections [22], however, its mechanism of action in MS is not completely understood, but it has been speculated that, besides its immunomodulatory properties, the efficacy of IFN-beta may be related to its antiviral properties [23]. However, it has been observed consistently that a proportion of patients (2% to 47%) develop NAbs directed against IFN beta as a consequence of the treatment [24], [25]. In our study, the 32.8% of MS patients was positive for NAbs; as NAbs are associated with a loss of efficacy of IFN-beta treatment and a reduced bioavailability, they appeared at any time of the four scheduled visits and while some NAbs positive patients remained positive along the IFN-beta treatment, others patients became negative, we decided to evaluate the variation of the anti-HHV-6A/B IgG titers only in an homogenous cohort of MS patients without NAbs. As we have previously mentioned, the correlation between the anti-HHV-6A/B IgG variation and the clinical response was greatly enhanced when we only considered those MS patients treated with IFN-beta that did not develop NAbs (p = 0.0006) instead of the whole population of IFN-beta treated MS patients (p = 0.034). Therefore, it would be important to distinguish between MS patients with and without NAbs in future studies of other viruses and possible biomarkers in MS patients treated with IFN-beta, to obtain more accurate and reliable results.
There are not previous studies on GA and viruses in MS. GA is an acetate salt of a random polymer of four amino acids that shares some cross-reactivity with myelin basic protein (MBP) [26]. The mechanisms by which GA exerts its effects in MS patients are not fully elucidated: generating suppressor cells, inducing tolerance, expanding regulatory T-cell populations, and altering antigen-presenting cells have all gained favor for a time as the mechanism for the immunomodulatory effects of GA [27]. In our study, we found a significant correlation between the reduction of the anti-HHV-6A/B IgG titers after 2-years of treatment and the clinical response.
Natalizumab is a humanized monoclonal antibody that selectively binds to the α4-integrin component of adhesion molecules found on lymphocytes, monocytes, and eosinophils; thus, natalizumab inhibits the interaction of α4β1 with VCAM-1, and because VCAM-1 is expressed on inflamed cerebrovascular endothelial cells, α4β1 is believed to be the critical target of natalizumab in preventing leukocyte migration into the central nervous system in MS [28]. Furthermore, it has been published that the treatment leads to a significant decrease in serum IgM and IgG levels in patients with MS; these findings might support the hypothesis that natalizumab interferes with homing of B cells, possibly leading to impaired differentiation into plasma cells and subsequently disturbed immunoglobulin synthesis [29]. In our study, we have also found a significant decrease of the anti-HHV-6A/B IgG and IgM titers after two-years of treatment; but, what it is most interesting, we have found a high correlation between the variation of the anti-HHV-6A/B IgG titers and the clinical response to this treatment (Figure 1.E).
The relationship with the clinical response for all the DMTs was found both for the progression and for the relapses. Furthermore, the increase of the anti-HHV-6A/B IgG and IgM titers predicts the upcoming clinical relapses. Recently, Simpson et al. [30] published that HHV-6A/B infection or the immune response to HHV-6A/B antigens may have an effect on the risk of MS relapses, since the observed effect was directly related to anti-HHV-6A/B IgG titers and may indicate that either HHV-6A/B infection or factors associated with an altered humoral immune response to HHV-6A/B may have an effect on MS clinical course.
Then, we can conclude that the measurement of the anti-HHV-6A/B IgG titers could be a good biomarker of clinical response to the different DMTs. However, without magnetic resonance imaging (MRI) data, we really don’t have a full picture about disease activity and therapeutic responses of the MS patients included in this study; further longitudinal studies that include MRI data are needed to validate these results.
Acknowledgments
We are indebted to María Concepción Ramírez and María Jesús Díez who collected the specimens.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data set is not suitable for public deposition as it contains clinical patient information. Requests for the data may be sent to the corresponding author.
Funding Statement
Roberto Alvarez-Lafuente is recipient of a research contract of the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (Feder) (CP07/00273). Arias-Leal and Garcia-Martinez are recipients of a technician contract from “REEM: Red Española de Esclerosis Múltiple” (RETICS-REEM RD12/0032/0001; www.reem.es). This work was financially supported by grants from the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (Feder) (FIS PI12/02349), “Fundación Mutua Madrileña”, and “Fundación LAIR”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple Sclerosis. N Engl J Med 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 2. Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, et al. (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 3. Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, et al. (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8 987–997. [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 5. Tselis A (2011) Evidence for viral etiology of multiple sclerosis. Semin Neurol 31: 307–316. [DOI] [PubMed] [Google Scholar]
- 6. Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, et al. (2001) Epstein-Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 286: 3083–3088. [DOI] [PubMed] [Google Scholar]
- 7. Levin LI, Munger KL, Rubertone MV, Peck CA, Lennette ET, et al. (2005) Temporal relationship between elevation of epstein-barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 293: 2496–2500. [DOI] [PubMed] [Google Scholar]
- 8. Salvetti M, Giovannoni G, Aloisi F (2009) Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol 22: 201–206. [DOI] [PubMed] [Google Scholar]
- 9. Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, et al. (1995) Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A 92: 7440–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodman AD, Mock DJ, Powers JM, Baker JV, Blumberg BM (2003) Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis 187: 1365–1376. [DOI] [PubMed] [Google Scholar]
- 11. Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, et al. (1997) Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med 3: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 12. Höllsberg P, Kusk M, Bech E, Hansen HJ, Jakobsen J, et al. (2005) Presence of Epstein-Barr virus and human herpesvirus 6B DNA in multiple sclerosis patients: associations with disease activity. Acta Neurol Scand 112: 395–402. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez-Lafuente R, Martínez A, Garcia-Montojo M, Mas A, De Las Heras V, et al. (2010) MHC2TA rs4774C and HHV-6A active replication in multiple sclerosis patients. Eur J Neurol 17: 129–135. [DOI] [PubMed] [Google Scholar]
- 14. Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, et al. (2000) Tissue distribution and variant characterization of human herpesvirus (HHV)-6: Increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis 182: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 15. Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S (2000) Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol 47: 306–313. [PubMed] [Google Scholar]
- 16. Garcia-Montojo M, Dominguez-Mozo MI, De las Heras V, Bartolome M, Garcia-Martinez A, et al. (2010) Neutralizing antibodies, MxA expression and MMP-9/TIMP-1 ratio as markers of bioavailability of interferon beta treatment in multiple sclerosis patients. A two years follow-up study. Eur J Neurol 17: 470–478. [DOI] [PubMed] [Google Scholar]
- 17. Kawade Y (1986) Quantitation of neutralization of interferon by antibody. Methods Enzymol 119: 558–573. [DOI] [PubMed] [Google Scholar]
- 18. Álvarez-Lafuente R, De las Heras V, Bartolomé M, Montojo-García M, Arroyo R (2006) Human Herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathology 16: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Álvarez-Lafuente R, De las Heras V, Bartolomé M, Picazo JJ, Arroyo R (2004) Beta interferon treatment reduce HHV-6 viral load in multiple sclerosis relapses but not in remission. Eur Neurol 52: 87–91. [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Montojo M, De Las Heras V, Dominguez-Mozo M, Bartolome M, Garcia-Martinez MA, et al. (2011) Human herpesvirus 6 and effectiveness of interferon beta 1b in multiple sclerosis patients. Eur J Neurol 18: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 21. Hong J, Tejada-Simon MV, Rivera VM, Zang YC, Zhang JZ (2002) Anti-viral properties of interferon beta treatment in patients with multiple sclerosis. Mult Scler 8: 237–242. [DOI] [PubMed] [Google Scholar]
- 22. Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, et al. (2007) Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6: 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson KP (1997) The historical development of interferons as multiple sclerosis therapies. J Mol Med 75: 89–94. [DOI] [PubMed] [Google Scholar]
- 24. Bertolotto A, Deisenhammer F, Gallo P, Sölberg Sørensen P (2004) Immunogenicity of interferon beta: differences among products. J Neurol 251: II15–II24. [DOI] [PubMed] [Google Scholar]
- 25. Hurwitz BJ (2008) Important sources of variability in clinical studies of neutralizing antibodies against interferon beta. J Neurol Sci 272: 8–19. [DOI] [PubMed] [Google Scholar]
- 26. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, et al. (1995) Copolymer-1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double blind, placebo-controlled trial. The Copolymer-1 Multiple Sclerosis Study Group. Neurology 45: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 27. Racke MK, Lovett-Racke AE, Karandikar NJ (2010) The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology 74: S25–S30. [DOI] [PubMed] [Google Scholar]
- 28. Rice GP, Hartung HP, Calabresi PA (2005) Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64: 1336–1342. [DOI] [PubMed] [Google Scholar]
- 29. Selter RC, Biberacher V, Grummel V, Buck D, Eienbröker C, et al. (2013) Natalizumab treatment decreases serum IgM and IgG levels in multiple sclerosis patients. Mult Scler 19: 1454–1461. [DOI] [PubMed] [Google Scholar]
- 30. Simpson S Jr, Taylor B, Dwyer DE, Taylor J, Blizzard L, et al. (2012) Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult Scler 18: 799–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data set is not suitable for public deposition as it contains clinical patient information. Requests for the data may be sent to the corresponding author.