Abstract
Although rates of incident dementia have been reported from several populations, the impact of nonparticipation on dementia incidence in studies of cognitive aging is unknown. In 2004, investigators with the Mayo Clinic Study of Aging selected persons aged 70–89 years from an enumeration of all Olmsted County, Minnesota, residents (age- and sex-stratified random sample). Of 4,398 potential participants, 2,050 agreed to undergo an in-person health assessment. Those participants were reevaluated in person using standard diagnostic procedures approximately every 15 months over a median follow-up period of 5.7 years (through September 15, 2013). There were 1,679 persons who refused any participation. A trained nurse abstractor reviewed the medical records of nonparticipants using the Rochester Epidemiology Project's medical record linkage system a median of 3.9 years after refusal. Nonparticipants had a higher prevalence of dementia than participants evaluated in person (6.5% vs. 3.3%; P < 0.0001). The standardized incidence of dementia was not significantly higher among the nonparticipants (23.2 per 1,000 person-years) than in those evaluated in person (19.6 per 1,000 person-years; hazard ratio = 1.17, 95% confidence interval: 0.95, 1.43 (P = 0.13); adjusted for education and sex, with age as the time scale). The small, nonsignificant impact of nonparticipation on rates of incident dementia is reassuring for future studies based on incident dementia cases.
Keywords: aging, cognition, cognitive aging, dementia, epidemiologic methods, incidence, prevalence
Nonparticipation and withdrawal from a study after initial enrollment pose a threat to epidemiologic research on late-life cognitive disorders. Resulting biases may arise in several ways. Persons who agree to participate in a study may do so because of their own perception of risk. For example, persons with a family history of dementia may be more likely to enroll in research studies of cognitive impairment (1, 2). Alternatively, persons who lead a healthy lifestyle may choose to participate in such studies as a reflection of their quest for novelty and stimulation. On the other hand, persons with incipient or overt cognitive impairment may be fearful of participating or may shun involvement in anything unfamiliar. The cognitive status of nonparticipants in recent studies of dementia incidence is unknown (3–7). However, 2 older investigations that were able to determine the cognitive status of initial nonparticipants (8, 9) demonstrated that the nonparticipants had lower cognitive test scores than participants. Little is known about future cognitive outcomes in persons who refuse participation in studies of cognitive aging.
The Mayo Clinic Study of Aging (MCSA) was initiated in 2004 to study the natural history of cognitive impairment in a geographically defined population (10). Over 2,000 nondemented persons were enrolled in the initial cohort. We observed differences between persons who agreed to participate and those who refused (10), but at the time, we had no information about differences in cognitive status among nonparticipants. In the 7 years since the completion of cohort enrollment, we have had the opportunity to use the medical record linkage system of the Rochester Epidemiology Project (11–13) to investigate the prevalence and incidence of dementia among nonparticipants. We report here the incidence rates for dementia from the MCSA, having previously reported on the prevalence (14) and incidence (15) of mild cognitive impairment as well as on the progression from mild cognitive impairment to dementia (16).
METHODS
Study population
The MCSA is a population-based study that was designed to study incident mild cognitive impairment and dementia. The study design has been described in detail elsewhere (10). The sampling frame included all persons aged 70–89 years who were residents of Olmsted County, Minnesota, as of October 1, 2004 (age- and sex-stratified random sample). The medical records of potential participants were formally reviewed prior to contact to exclude those with diagnoses of dementia, those in hospice care, or those considered to have conditions deemed imminently fatal. The rationale for excluding persons with prevalent dementia was that our focus was on the earliest symptomatic manifestations of cognitive impairment. All remaining eligible individuals were contacted. As specified by the Mayo Clinic and Olmsted Medical Center institutional review boards, letters were sent to all eligible persons informing them of the study. The letter allowed the recipient to decline participation by checking a box and returning the letter. A stamped return envelope was included. If we received no written refusal document from a potential participant, study personnel attempted to contact the person by telephone.
Individuals who agreed to participate underwent a 3-hour in-person assessment that included a medical history, an interview with an informant for the participant, cognitive evaluation, and a neurological examination. Participants were assigned diagnoses based on the information collected during the in-person assessments using a consensus conference approach; the consensus conference included the examining physician, the neuropsychologist who reviewed the cognitive testing, and the nurse who completed a Clinical Dementia Rating interview with both the participant and the informant. There were also 669 persons who refused an in-person assessment but agreed to undergo the Telephone Interview of Cognitive Status–Modified (TICS-M) (17). The “telephone-only” participants will not be further considered in this paper, because our focus is on full participants versus complete nonparticipants. The certainty of a diagnosis of cognitive normality among the TICS-M participants was lower than that among those examined in person because of the overlap of TICS-M scores among cognitively normal and cognitively impaired persons in our validation exercise (17).
Active participants were invited to return for reevaluations approximately every 15 months after the initial visit. The same diagnostic procedures were performed during the subsequent visits.
Passive study of nonparticipants
The combined medical records in the Rochester Epidemiology Project represent virtually all residents of Olmsted County, Minnesota (11). Persons who receive their health care at the Mayo Clinic, at Olmsted Medical Center, or from other local health-care providers are asked to allow their medical records to be used for research purposes; approximately 95% provide permission for the passive use of their medical records in research (12). Beginning in October 2009, nurse abstractors who had extensive experience in medical record review for dementia diagnoses reviewed the medical records of nonparticipants. They also reviewed the medical records of participants who dropped out of the study after baseline participation. The nurse abstractors were aware of the participation status of the persons whose records they were reviewing; the sheer numbers of cases to be reviewed made it impractical to include an equal number of participants.
The medical records were evaluated for all nonparticipants who had provided authorization to use their medical records in research. First, to determine whether the nonparticipant might have dementia, the nurse abstractor reviewed the medical record for any sustained memory or cognitive impairment and for any documented diagnosis of dementia. A screening data sheet was completed for each participant. Any reported memory changes, difficulty with language, executive dysfunction, personality changes, or problems with abstract thinking or construction were recorded. All reports of mental status testing and psychometric testing were reviewed, and scores were recorded. The first onset of cognitive symptoms and the first documented diagnosis of dementia by any physician were recorded. The first documented diagnosis of mild cognitive impairment by a neurologist was also recorded, although there were so few recorded diagnoses of mild cognitive impairment that we were unable to conduct any subgroup analyses. The date of dementia onset was defined as the first date on which symptoms compatible with a diagnosis of dementia were recorded in the medical record.
Diagnostic criteria
A diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria (18). Data for participants who had any cognitive impairment that exceeded simple memory complaints or for whom the information was ambiguous or conflicting were reviewed in consultation with a study neurologist (D.S.K.). We also derived etiologic diagnoses for dementia cases based on standard criteria (for the time period under study) for Alzheimer's disease (19), vascular dementia (20), dementia with Lewy bodies (21), and frontotemporal dementia (22).
We considered dementia as ascertained in the medical record to be prevalent if the diagnosis of dementia onset was made prior to the date of refusal or within 15 months after refusal. There were 2 reasons for imposing a time delay for designating cases ascertained by medical record review as incident. First, because participants evaluated in person could not receive a diagnosis of incident dementia until the first follow-up visit, a similar restriction was needed for dementia cases detected by medical record review. Second, because dementia is often diagnosed at least 1 year after symptom onset (23, 24) and because documentation of dementia in the medical record is unlikely until symptoms have become unmistakably present, it seemed likely that symptoms sufficient to make a dementia diagnosis would have been present at least 15 months earlier in cases detected through medical record review.
After a nonparticipant was confirmed to have dementia through medical record review, the nurse abstractor sought additional information in order to establish an etiological diagnosis for the dementia syndrome. Finally, the medical record was reviewed to ascertain the presence of other medical conditions in order to compute Charlson's comorbidity index (CCI) (25).
Validation study
We previously subjected the medical record review process to a validation based on our in-person assessments. The negative predictive value of a diagnosis of “no dementia” in the medical record was 96.7%. We estimated the sensitivity of diagnoses of dementia based on the medical record review process used here to be 70% (26). Of the persons with dementia based on in-person assessments whose cases were not detected by medical record review, the majority had mild dementia.
Statistical analysis
To facilitate presentation of the data, we defined 3 groups in which we estimated incident dementia. The active surveillance (ACT) group included participants who were evaluated in person initially, were nondemented at the initial evaluation, and returned for at least 1 follow-up visit. The ACT group represents the subgroup of MCSA participants who would be comparable to participants in other studies of incident dementia. The second group included all participants in the ACT group plus those participants who were seen in person initially but then withdrew from subsequent follow-up evaluations, prior to a diagnosis of dementia, and whose dementia status was determined by passive surveillance (PAS). The ACT + PAS group could be conceived of as a “retrieved dropout” sample, to borrow from clinical trial terminology, while the ACT group alone represented “observed cases.” The third group, the refusal (REFUS) group, was a subset of nonparticipants who refused any participation, allowed review of their medical records, and did not have prevalent dementia. The dementia status (prevalent and incident) of the REFUS group was determined entirely by medical record review.
Descriptive statistics for the ACT, ACT + PAS, and REFUS groups were generated. We computed age- and sex-specific incidence rates of dementia to describe the 3 groups. In addition, we used Cox's proportional hazards models to compare the 3 groups. In the Cox models, age was used as the time variable, and all models adjusted for sex and years of education. The Schoenfeld method was used to estimate statistical power to detect differences between groups (27).
RESULTS
Study sample characteristics and prevalence of dementia
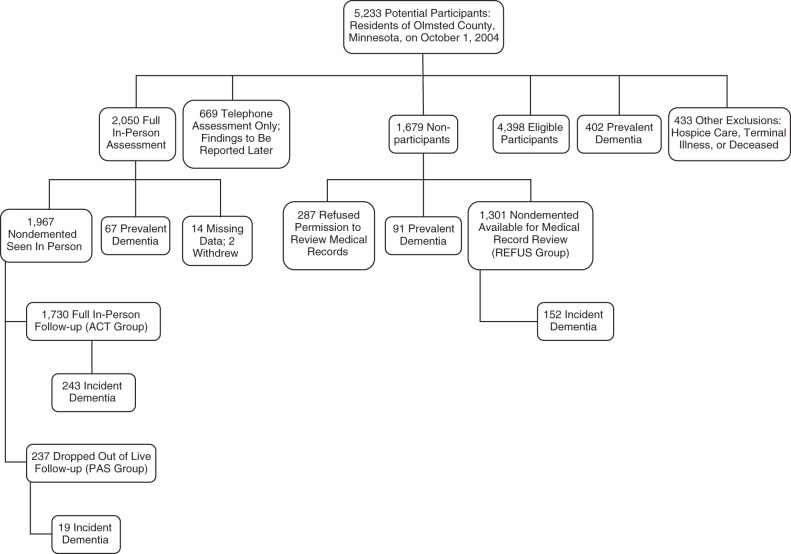
A flow chart delineating participants and nonparticipants in the MCSA is shown in Figure 1. From the initial sampling frame of nearly 10,000 persons who were residents of Olmsted County and were 70–89 years of age on October 1, 2004, we randomly sampled 5,233 persons within age- and sex-specific strata. Through review of medical records, we identified 402 persons (7.7%) with prevalent dementia (26) and 433 persons who died prior to contact, were too ill to participate, or were in hospice care. The remaining 4,398 eligible persons were contacted for participation in the study. As noted above, 669 agreed to complete the TICS-M only.
Figure 1.
Participant flow in the Mayo Clinic Study of Aging, 2004–2013. Two additional participants from the 1,967 nondemented participants who were seen in person subsequently withdrew consent and were excluded. ACT, active surveillance; PAS, passive surveillance; REFUS, refusal (subset of nonparticipants who refused any participation).
We initially evaluated 2,050 participants in person and assigned a diagnosis of “cognitively normal,” “mild cognitive impairment,” or “dementia.” Of these participants, 67 (3.3%) had prevalent dementia, 14 had insufficient data to assign a diagnosis, and 2 initially seen in person later withdrew research authorization. Thus, 1,967 nondemented persons participated. Over the course of follow-up (through September 15, 2013), 237 persons (12%) dropped out (the PAS group), of whom 31 (13.1%) moved out of the area. Of the 1,730 participants who attended in-person follow-up assessments (the ACT group), 86% attended at least 2 follow-up assessments and 58% made at least 4 follow-up visits. The median follow-up time for the ACT + PAS group was 5.7 years (interquartile range, 3.6–6.6). See Table 1 for demographic information on the ACT and PAS groups.
Table 1.
Demographic Characteristics of Participants and Nonparticipants in the Mayo Clinic Study of Aging, 2004–2013
| Characteristic | ACT Group (n = 1,730) |
PAS Group (n = 237) |
P Valuea | ACT + PAS Group (n = 1,967) |
REFUS Groupb (n = 1,301) |
P Valuec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQRd) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | |||
| Age at enrollment visit or refusal/exclusion date, years |
80.1 (75.2–83.8) | 82.2 (76.7–85.3) | <0.0001 | 80.4 (75.4–84.0) | 81.6 (76.3–85.0) | <0.0001 | ||||||||
| Female sex | 847 | 49.0 | 119 | 50.2 | 0.72 | 966 | 49.1 | 683 | 52.5 | 0.06 | ||||
| Education, years | 13 (12.0–16.0) | 12 (12.0–15.0) | 0.015 | 13 (12.0–16.0) | 12 (11.0–14.0) | <0.0001 | ||||||||
| ≤12 years of education | 782 | 45.2 | 126 | 53.2 | 0.021 | 908 | 46.2 | 827 | 63.6 | <0.0001 | ||||
| Charlson comorbidity index score |
3 (2.0–6.0) | 4 (2.0–6.0) | 0.019 | 4 (2.0–6.0) | 3 (1.0–6.0) | <0.0001 | ||||||||
| Years from enrollment or first contact to dementia or censoringe |
6.2 (4.2–6.6) | 2.9 (1.2–3.8) | <0.0001 | 5.2 (3.6–6.6) | 3.9 (2.9–4.5) | <0.0001 | ||||||||
| Incident dementia | 243 | 19 | 262 | 152 | ||||||||||
| Alzheimer's disease | 175 | 77.1 | 12 | 63.2 | 0.02 | 187 | 76.0 | 111 | 74.0 | |||||
| Vascular dementia | 19 | 8.4 | 6 | 31.6 | 25 | 10.2 | 25 | 16.7 | ||||||
| Dementia with Lewy bodies | 15 | 6.6 | 1 | 5.3 | 16 | 6.5 | 14 | 9.3 | ||||||
| Frontotemporal dementia | 3 | 1.3 | 0 | 0.0 | 3 | 1.2 | 0 | 0.0 | ||||||
| Other | 15 | 6.6 | 0 | 0.0 | 15 | 6.1 | 0 | 0.0 | ||||||
Abbreviations: ACT, active surveillance; IQR, interquartile range; PAS, passive surveillance; REFUS, refusal.
a P value for comparison of the ACT group with the PAS group.
b The subset of nonparticipants who refused any participation. Incident dementia in the REFUS group was determined entirely by medical record review.
c P value for comparison of the ACT + PAS group with the REFUS group.
d 25th–75th percentiles.
e Censoring events were death, medical record review, or the end of the observation period (September 15, 2013), depending upon the subject group.
As previously described (10), nonparticipants were slightly older, were less educated, and had higher CCI scores than participants at the time of recruitment. Of the 1,679 persons (38.2%) who initially declined participation, 38% declined participation by letter and 57.1% refused participation upon telephone contact. Another 4.9% initially agreed to participate but then cancelled their appointment. Among the 1,679 persons who initially declined to participate, 287 refused to provide authorization for us to review their medical records, leaving 1,392 nonparticipants whose medical records were reviewed. The median lag time between refusal and medical record review or death among nonparticipants was 3.9 years (range, 0.4–8.2 years).
Ninety-one (6.5%) of the 1,392 nonparticipants who allowed review of their medical records had prevalent dementia. The proportion of prevalent dementia in nonparticipants was greater than that in participants (χ2 test; P < 0.0001). Thus, the REFUS group—the subset of nonparticipants who refused any participation, were nondemented based on review of their medical records, and were at risk for incident dementia—numbered 1,301 persons. The nonparticipants with prevalent dementia were older at the time of refusal than the REFUS group (85.5 years vs. 81.6 years; P < 0.0001) and had a higher CCI score (4 vs. 3; P = 0.02) but did not differ by sex or education. With the exception of 1 person who was lost to follow-up at 330 days, all other persons in the REFUS group with less than 1 year of follow-up died.
Table 1 shows the demographic characteristics of the 1,967 initially enrolled nondemented participants (ACT + PAS group), the 1,730 participants who attended all follow-up visits (ACT group), and the 1,301 nondemented nonparticipants in the REFUS group. The REFUS group was slightly older and less educated than the ACT + PAS group.
Incidence of dementia in participation strata
In the ACT group, 243 persons were diagnosed with incident dementia during the course of follow-up. In the ACT + PAS group, there were 262 incident dementia cases (243 plus 19 cases detected by passive surveillance among the dropouts). Incident dementia was diagnosed in 152 persons in the REFUS group.
Rates of incident dementia in the ACT, ACT + PAS, and REFUS groups, by age and sex, are shown in Table 2. A comparison between the ACT + PAS group and the REFUS group is shown in Figure 2. The incidence of dementia in the ACT group, standardized by age and sex to the population of Olmsted County, was 15.9 per 1,000 person-years (95% confidence interval (CI): 12.9, 19.5). The rate rose to 19.6 per 1,000 person-years (95% CI: 16.6, 23.1) in the ACT + PAS group. Incidence rates did not differ between men and women in any group, but they rose sharply with age (Table 2).
Table 2.
Age- and Sex-Specific Incidence Rates of Dementia (Per 1,000 Person-Years) in the Mayo Clinic Study of Aging, 2004–2013a
| Subgroup and Sex |
Age Range, years |
Total |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70–74 |
75–79 |
80–84 |
85–89 |
||||||||||||
| No. | IR, % | 95% CI | No. | IR, % | 95% CI | No. | IR, % | 95% CI | No. | IR, % | 95% CI | No. | IR, % | 95% CI | |
| ACT group | |||||||||||||||
| Menb | 11 | 9.5 | 4.7, 17.0 | 18 | 15.0 | 8.9, 23.8 | 43 | 34.1 | 24.7, 45.9 | 26 | 53.0 | 34.6, 77.7 | 98 | 17.8 | 13.7, 23.1 |
| Womenc | 4 | 3.9 | 1.1, 10.1 | 13 | 12.4 | 6.6, 21.2 | 37 | 28.2 | 19.9, 38.9 | 32 | 46.3 | 31.7, 65.4 | 86 | 14.6 | 10.8, 19.7 |
| Both sexes | 15 | 5.9 | 3.2, 10.7 | 31 | 13.5 | 9.4, 19.4 | 80 | 30.2 | 24.0, 38.1 | 58 | 48.7 | 37.5, 63.2 | 184 | 15.9 | 12.9, 19.5 |
| ACT + PAS group | |||||||||||||||
| Mend | 21 | 15.6 | 9.6, 23.8 | 22 | 15.4 | 9.6, 23.3 | 57 | 36.8 | 27.9, 47.7 | 41 | 62.2 | 44.6, 84.4 | 141 | 21.6 | 17.5, 26.8 |
| Womene | 5 | 4.2 | 1.4, 9.9 | 22 | 18.0 | 11.3, 27.3 | 49 | 30.7 | 22.7, 40.6 | 45 | 49.8 | 36.4, 66.7 | 121 | 18.3 | 14.4, 23.2 |
| Both sexes | 26 | 7.6 | 4.5, 12.8 | 44 | 16.8 | 12.5, 22.6 | 106 | 32.8 | 26.8, 40.1 | 86 | 54.0 | 43.5, 67.1 | 262 | 19.6 | 16.6, 23.1 |
| REFUS groupf | |||||||||||||||
| Meng | 3 | 5.6 | 1.1, 16.2 | 12 | 26.1 | 13.9, 44.7 | 32 | 39.4 | 26.9, 55.6 | 25 | 62.6 | 40.5, 92.4 | 72 | 21.2 | 14.8, 30.2 |
| Womenh | 6 | 12.1 | 4.5, 26.4 | 11 | 23.7 | 11.9, 42.5 | 28 | 33.3 | 22.3, 47.8 | 33 | 51.4 | 35.4, 72.2 | 78 | 24.8 | 18.3, 33.6 |
| Both sexes | 9 | 8.6 | 4.4, 16.9 | 23 | 24.0 | 15.9, 36.3 | 60 | 34.9 | 26.7, 45.6 | 58 | 55.2 | 42.6, 71.6 | 150 | 23.2 | 18.4, 29.2 |
Abbreviations: ACT, active surveillance; CI, confidence interval; IR, incidence rate; PAS, passive surveillance; PY, person-years; REFUS, refusal.
a Incidence rates were directly standardized by age and sex to the Olmsted County, Minnesota, population on October 1, 2004, whenever it was applicable.
b Denominators were as follows (by age group): 70–74 years, 1,161 PY; 75–79 years, 1,198 PY; 80–84 years, 1,262 PY; 85–89 years, 491 PY.
c Denominators were as follows (by age group): 70–74 years, 1,013 PY; 75–79 years, 1,052 PY; 80–84 years, 1,312 PY; 85–89 years, 691 PY.
d Denominators were as follows (by age group): 70–74 years, 1,349 PY; 75–79 years, 1,430 PY; 80–84 years, 1,549 PY; 85–89 years, 659 PY.
e Denominators were as follows (by age group): 70–74 years, 1,179 PY; 75–79 years, 1,220 PY; 80–84 years, 1,594 PY; 85–89 years, 903 PY.
f The subset of nonparticipants who refused any participation.
g Denominators were as follows (by age group): 70–74 years, 540 PY; 75–79 years, 498 PY; 80–84 years, 813 PY; 85–89 years, 400 PY.
h Denominators were as follows (by age group): 70–74 years, 494 PY; 75–79 years, 464 PY; 80–84 years, 871 PY; 85–89 years, 642 PY.
Figure 2.
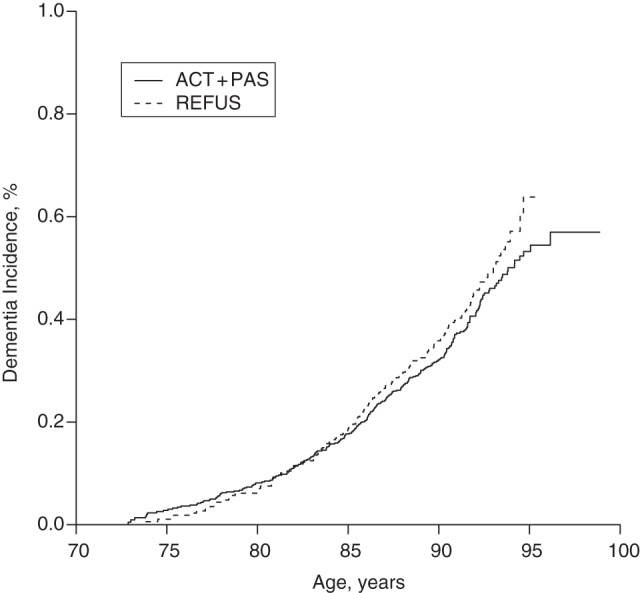
Kaplan-Meier plot of dementia incidence in the Mayo Clinic Study of Aging, 2004–2013. The active surveillance (ACT) plus passive surveillance (PAS) group (ACT + PAS) included all participants who were seen in person initially and at all follow-up visits until censoring by the end of the observation period (September 15, 2013) or dementia onset (ACT group), or who were seen in person initially but then withdrew from subsequent follow-up evaluations, prior to an in-person diagnosis of dementia, and whose dementia status was determined by passive surveillance (PAS group). The refusal (REFUS) group was a subset of nonparticipants who refused any participation, allowed review of their medical records, and did not have prevalent dementia. Incident dementia in the REFUS group was determined entirely by medical record review.
The incidence of dementia in the REFUS group, standardized by age and sex to the Olmsted County population, was 23.2 per 1,000 person-years (95% CI: 18.4, 29.2). The difference between the REFUS group and the ACT group was not significant (hazard ratio = 1.16, 95% CI: 0.95, 1.43; P = 0.15), nor was the difference between the REFUS group and the combined ACT + PAS group (hazard ratio = 1.17, 95% CI: 0.95, 1.43; P = 0.13). With the sample size and event rates described above, we had 80% power to detect hazard ratios of 1.33 or higher, and we had at least 98% power to detect moderate and larger effect sizes (hazard ratios greater than 1.5).
We tabulated specific dementia etiologies for participants and nonparticipants (Table 2). We found only minimal differences in proportions of persons with diagnoses of Alzheimer's disease between groups. There was a slight excess of vascular dementia in the PAS and REFUS groups, but the biggest difference between groups was “other” diagnoses in the ACT group.
Within the REFUS group, younger age (P-trend < 0.01 for risk of incident dementia) and higher (≥6) CCI score (hazard ratio = 1.50, 95% CI: 1.06, 2.11) were associated with higher hazard ratios for incident dementia. There was no difference in dementia incidence between men and women in the REFUS group.
DISCUSSION
We used the unique resources of the Rochester Epidemiology Project's medical record linkage system to address the impact of nonparticipation and dropout on epidemiologic research in cognitive aging. There was a slightly higher frequency of prevalent dementia in the REFUS group, which was not surprising given the older age and greater disability of nonparticipants (10). After we accounted for prevalent dementia, the REFUS group (nondemented nonparticipants who allowed their medical records to be reviewed) had a 17% higher incidence rate of dementia than the ACT group, but the difference was not statistically significant. We can be virtually certain that the rate of incident dementia in the REFUS group was no more than 50% greater than that in the ACT + PAS group and that it was unlikely to be more than 33% higher than that in the ACT + PAS group. The fact that nonparticipation had no impact on dementia incidence rates or, at worst, a small impact on dementia incidence rates was reassuring, particularly because of the greater restrictions on access to potential participants in epidemiologic research imposed by regulatory bodies in the past few decades.
The strength of this study resided in the opportunity to ascertain dementia diagnoses among individuals who were not seen in person. The medical record linkage system of the Rochester Epidemiology Project, plus the relative geographic isolation of Olmsted County's health-care system from other health-care systems, made it possible for us to detect incident dementia through passive surveillance without substantial loss to follow-up. The quality of medical care in Olmsted County and our experience with medical record diagnoses of dementia also facilitated our effort. The strengths and limitations of the Rochester Epidemiology Project's medical record linkage system are reasonably well understood (28). While the population of Olmsted County is largely European in origin and their access to health care may be greater than that in other populations, the availability of medical records on virtually the entire population has major advantages for studying a condition like dementia.
There are also limitations to detecting dementia by passive surveillance. Because of the uncertainty of assigning a date of dementia onset by medical record review and the necessity of defining a time postrefusal for distinguishing between prevalent and incident dementia, we may have either over- or underestimated the number of prevalent cases. In order to match the experience of the active participants, we labeled all dementia cases detected by medical record review that occurred within 15 months of refusal as “prevalent.” The delay for declaring cases as “incident” dementia was consistent with the earliest time point at which a diagnosis of dementia could be made for participants evaluated in person. In addition, the lag between (symptomatic) dementia onset and dementia diagnosis has typically been greater than 1 year (23, 24). Indeed, in our own analyses of progression to dementia among MCSA participants, the rate of progression from cognitive normality to dementia was very low compared with the progression from mild cognitive impairment to dementia (1.9 per 1,000 person-years vs. 71.3 per 1,000 person-years) (15). Third, the majority of the cases of prevalent dementia detected among participants evaluated in person were mild cases of dementia, consistent with the contention that passive surveillance for dementia underestimates mild dementia (26). Therefore, dementia patients whose cases were detected by passive surveillance were likely to have been sufficiently impaired to qualify for a dementia diagnosis at least 15 months earlier. Fourth, differences in dementia etiology between the ACT group and the PAS or REFUS group should be viewed with caution because of the differences in diagnostic information available for active surveillance versus passive surveillance.
One other limitation should be noted: There were 287 persons (5.6% of the entire study group) who refused both participation in the active study and access to their medical records. Their cognitive status was unknown.
Our estimates of incident dementia in the elderly reflect the general pattern of a doubling of the incidence rate for every 5 years of age. The incidence of dementia in the MCSA participants ranged from less than 1 per 100 person-years in participants aged 70–74 years to nearly 5 per 100 person-years in participants aged 85–89 years, similar to other recent studies (3–6, 29–32). Although the rates of incident dementia were comparable in the younger groups to our prior estimates from Olmsted County based on passive surveillance (33, 34), the current analysis found rates that were 20%–40% higher in the older 2 age quinquennia.
The MCSA participation rate was comparable to that in investigations on the prevalence of Alzheimer's disease conducted after 2000 (35, 36). Our participation rate was lower than the 79% participation obtained in a survey completed in the 1990s (7), possibly reflecting the greater restrictions on contacting potential participants imposed by local and federal regulations more recently. It is unclear whether participation rates in the 70%–90% range would minimize the impact of prevalent dementia among nonparticipants. However, as we have shown, nonparticipation has much less of an impact on incident dementia.
It is likely that almost all population-based studies of cognitive function would find that nonparticipants were older, frailer, and less educated than participants, but few studies document the differences (5, 10, 37). In 2 analyses in which nonparticipants were reapproached and then evaluated, the nonparticipants were more impaired than their peers who had initially agreed to participate (8, 9).
Nonparticipation and premature withdrawal are problems that will continue to challenge studies of cognitive impairment in the elderly. There was an excess of prevalent dementia among the nonparticipants in our population-based study. Improved means of passive surveillance for dementia in elderly persons would be very helpful in order to account for all persons with prevalent dementia. These individuals are the very ones who could benefit from treatments and services. One solution would be to require cognitive screening in primary-care settings, something that is now required, in principle, as part of the Medicare Annual Wellness Visit. Unfortunately, active surveillance for dementia in primary-care settings is evolving, with no standard methods being recommended at present. It is to be hoped that this situation will improve and that more accurate assessments of cognitive impairment will ensue.
ACKNOWLEDGMENTS
Author affiliations: Department of Neurology, College of Medicine, Mayo Clinic, Rochester, Minnesota (David S. Knopman, Rosebud O. Roberts, Walter A. Rocca, Ronald C. Petersen); Mayo Alzheimer's Disease Research Center, Mayo Clinic, Rochester, Minnesota (David S. Knopman, Bradley F. Boeve, Ronald C. Petersen); Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota (Rosebud O. Roberts, Walter A. Rocca, Michelle M. Mielke, Ronald C. Petersen); Division of Biostatistics and Bioinformatics, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota (V. Shane Pankratz, Ruth H. Cha); Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota (Eric G. Tangalos); Division of Psychology, Department of Psychiatry, College of Medicine, Mayo Clinic, Rochester, Minnesota (Robert J. Ivnik); Department of Psychiatry, College of Medicine, Mayo Clinic, Scottsdale, Arizona (Yonas E. Geda); and Department of Neurology, College of Medicine, Mayo Clinic, Scottsdale, Arizona (Yonas E. Geda).
This work was supported by the National Institutes of Health (NIH) (grants U01 AG006786, P50 AG016574, K01 MH068351, RR024150, and K01 AG028573), the Robert Wood Johnson Foundation, and the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program and was made possible by the Rochester Epidemiology Project (NIH grant R01 AG034676).
We thank the Mayo Clinic Study of Aging nurse abstractors: Mary Dugdale, Connie Fortner, Julie Gingras, and Joan LaPlante.
Dr. David S. Knopman serves as Deputy Editor for Neurology and serves on a Data and Safety Monitoring Board for Lundbeck Pharmaceuticals (Copenhagen, Denmark) and for the Dominantly Inherited Alzheimer Network at Washington University in St. Louis (St. Louis, Missouri). He has served on a Data and Safety Monitoring Board for Lilly Pharmaceuticals (Indianapolis, Indiana), served as a consultant to TauRx Pharmaceuticals Ltd. (Singapore, Singapore), was an investigator in clinical trials sponsored by Baxter International, Inc. (Deerfield, Illinois) and Elan Pharmaceuticals, Inc. (Dublin, Ireland), and receives research support from the NIH. Dr. Rosebud O. Roberts receives research support from the NIH, Abbvie Health Economics and Outcomes Research (North Chicago, Illinois), and the Walter S. and Lucienne Driskill Foundation (Chicago, Illinois). Dr. V. Shane Pankratz receives research support from the National Institute on Aging (NIA), NIH. Dr. Walter A. Rocca receives research support from the NIH. Dr. Michelle M. Mielke receives research support from the NIA and the Driskill Foundation. Dr. Bradley F. Boeve receives publishing royalties for The Behavioral Neurology of Dementia (Cambridge University Press, New York, New York, 2009) and research support from Cephalon, Inc. (Frazer, Pennsylvania), Allon Therapeutics, Inc. (Vancouver, Canada), GE Healthcare (Milwaukee, Wisconsin), the NIA, and the Harry T. Mangurian, Jr. Foundation (Fort Lauderdale, Florida). Dr. Eric G. Tangalos serves on a Data and Safety Monitoring Board for Eli Lilly and Company (Indianapolis, Indiana); serves as a consultant for Purdue Pharma L.P. (Stamford, Connecticut) and Amgen, Inc. (Thousand Oaks, California); serves on the editorial boards of MD Net Guide, Journal of the American Medical Directors Association, and IM News; has received honoraria for slide development from Takeda Pharmaceutical Company Ltd. (Tokyo, Japan), Novartis International AG (Basel, Switzerland), and Ortho Biotech Products, L.P. (Bridgewater, New Jersey) (now Janssen Biotech, Inc., Horsham, Pennsylvania); receives research support from Baxter International and Elan Pharmaceuticals; and serves as a consultant to Novartis International. Dr. Robert J. Ivnik serves on the editorial boards of The Clinical Neuropsychologist and Aging, Neuropsychology, and Cognition; receives publishing royalties for Clinical Interpretation of the WAIS-III and WMS-III (Academic Press, Inc., New York, New York, 2003); and receives research support from the NIA. Dr. Yonas E. Geda receives research support from the NIH, the Mayo Center for Translational Science Activities (Rochester, Minnesota), and the Robert Wood Johnson Foundation (Princeton, New Jersey) (Harold Amos Scholar). Dr. Ronald C. Petersen serves on scientific advisory boards for the National Advisory Council on Aging (NIA), Pfizer, Inc. (New York, New York) data monitoring committees, and Janssen Alzheimer Immunotherapy (New Brunswick, New Jersey); is a consultant for Elan Pharmaceuticals, GE Healthcare, Roche, Inc. (Basel, Switzerland), and Merck, Inc. (Darmstadt, Germany); receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, New York, New York, 2003); and receives research support from the NIA. Ruth H. Cha reports no potential conflicts of interest.
REFERENCES
- 1.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuang D, Kukull W, Sheppard L, et al. Impact of sample selection on APOE epsilon 4 allele frequency: a comparison of two Alzheimer's disease samples. J Am Geriatr Soc. 1996;44(6):704–707. doi: 10.1111/j.1532-5415.1996.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 3.Matthews F, Brayne C Medical Research Council Cognitive Function and Ageing Study Investigators. The incidence of dementia in England and Wales: findings from the five identical sites of the MRC CFA Study. PLoS Med. 2005;2(8):e193. doi: 10.1371/journal.pmed.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 8.Norton MC, Breitner JC, Welsh KA, et al. Characteristics of nonresponders in a community survey of the elderly. J Am Geriatr Soc. 1994;42(12):1252–1256. doi: 10.1111/j.1532-5415.1994.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 9.Launer LJ, Wind AW, Deeg DJ. Nonresponse pattern and bias in a community-based cross-sectional study of cognitive functioning among the elderly. Am J Epidemiol. 1994;139(8):803–812. doi: 10.1093/oxfordjournals.aje.a117077. [DOI] [PubMed] [Google Scholar]
- 10.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–325. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knopman DS, Roberts RO, Geda YE, et al. Validation of the Telephone Interview for Cognitive Status–Modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Task Force on DSM-IV, American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 22.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 23.Knopman D, Donohue JA, Gutterman EM. Patterns of care in the early stages of Alzheimer's disease: impediments to timely diagnosis. J Am Geriatr Soc. 2000;48(3):300–304. doi: 10.1111/j.1532-5415.2000.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones RW, Mackell J, Berthet K, et al. Assessing attitudes and behaviours surrounding Alzheimer's disease in Europe: key findings of the Important Perspectives on Alzheimer's Care and Treatment (IMPACT) survey. J Nutr Health Aging. 2010;14(7):525–530. doi: 10.1007/s12603-010-0263-y. [DOI] [PubMed] [Google Scholar]
- 25.Buntinx F, Niclaes L, Suetens C, et al. Evaluation of Charlson's comorbidity index in elderly living in nursing homes. J Clin Epidemiol. 2002;55(11):1144–1147. doi: 10.1016/s0895-4356(02)00485-7. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Petersen RC, Rocca WA, et al. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement. 2011;7(1):53–60. doi: 10.1016/j.jalz.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. [PubMed] [Google Scholar]
- 28.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fratiglioni L, Launer LJ, Andersen K, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 suppl 5):S10–S15. [PubMed] [Google Scholar]
- 30.Gao S, Hendrie HC, Hall KS, et al. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55(9):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51(3):728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 32.The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology. 2000;55(1):66–73. [PubMed] [Google Scholar]
- 33.Knopman DS, Petersen RC, Cha RH, et al. Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol. 2006;63(2):218–221. doi: 10.1001/archneur.63.2.218. [DOI] [PubMed] [Google Scholar]
- 34.Edland SD, Rocca WA, Petersen RC, et al. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59(10):1589–1593. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- 35.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5(3):207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]